Abstract
An intravenous (i.v.) formulation of itraconazole was evaluated in disseminated fungal infection models in guinea pigs. In acute disseminated Candida albicans and Aspergillus fumigatus infections, treatment at 5 mg/kg of body weight twice a day (b.i.d.) significantly prolonged survival. In these models and in animals with chronic disseminated cryptococcosis, itraconazole given i.v. at 2.5 and 5 mg/kg b.i.d. greatly reduced the proportions of organs with culture-detectable fungal burdens. The efficacy of i.v. itraconazole in these animal models justifies its further investigation for the treatment of life-threatening mycoses in humans.
Itraconazole is a broad-spectrum antifungal agent used clinically for a variety of serious fungal infections in normal and immunocompromised hosts, including aspergillosis, blastomycosis, coccidioidomycosis, cryptococcosis, histoplasmosis, paracoccidioidomycosis, sporotrichosis, and disseminated Penicillium marneffei infections (3, 8, 13–15). Orally administered itraconazole has shown efficacy equivalent to that of fluconazole in the treatment of oral Candida infections in AIDS (10, 20), and the oral agent has been used by some authors for the treatment of candidemia (4). However, for management of candidemia and other opportunistic mycoses, the absence of an intravenous (i.v.) formulation of itraconazole has been a limitation, since many neutropenic and other immunocompromised patients have difficulty swallowing the oral capsule formulation of the drug (4, 13).
A novel, i.v. formulation of itraconazole solubilized in hydroxypropyl-β-cyclodextrin (HPBCD) has successfully undergone clinical evaluation (11, 19, 21) and has recently been approved for clinical use. i.v. administration of itraconazole should facilitate the establishment of high and dependable levels of the compound in plasma, an objective some authors regard as desirable for optimum antifungal prophylaxis and therapy (5, 7).
This study is a first evaluation of the efficacy of i.v. itraconazole-cyclodextrin in experimental disseminated Aspergillus fumigatus, Candida albicans, and Cryptococcus neoformans infections. The animal host chosen is the guinea pig, since efficaceous oral doses of itraconazole in this host are similar to those used in human patients. Studies of oral itraconazole in mice have rarely indicated activity for this agent. Although 100% survival was reported in experimental murine paracoccidioidomycosis treated with itraconazole at 10 mg/kg of body weight (9), three studies of murine aspergillosis found no activity even at daily doses of 100 mg/kg (1, 2, 12). By contrast with its poor efficacy in mice, itraconazole has consistently shown high activity when given orally or intraperitoneally (i.p.) to guinea pigs infected with many different fungal pathogens (16, 17). Use of guinea pigs necessitated implantation of a venous catheter for repeated i.v. infusions of itraconazole, the same mode of administration used for human patients, as opposed to bolus injection, which would be necessary with mice and which was used in previous experiments with itraconazole-cyclodextrin given i.p. to guinea pigs (17).
Specific-pathogen-free, DH guinea pigs, weighing 350 to 450 g (Charles River Associates, Brussels, Belgium), were housed in individual cages and provided with food and water ad libitum. Conditions were approved by the Animal Welfare Committee of the Janssen Research Foundation. Animals were anesthetized with pentobarbital sodium (Nembutal), given i.p. at 15 mg/kg, and Thalamonal, given intramuscularly at 0.9 ml/kg, and their necks were shaved and disinfected with isobetadine antiseptic. An incision was made at the site of the jugular vein, and a liver biopsy needle was pushed under the skin to emerge at a central position in the back. Through the bore of the biopsy needle, a fine, sterile catheter (PhysioCath small-animal vascular catheter, 6 by 10 in; Baxter Health Care Corporation, Deerfield, Ill.) was passed through to the opening in the neck. The biopsy needle was now removed. The jugular vein was dissected carefully from surrounding tissue, two surgical threads were placed under it, and the distal thread was used to tie off the vein close to the jaw of the animal. The vein was now opened with a fine, bent needle, and the catheter was pushed into the vein to a distance of approximately 3 cm and tied in place with the proximal thread. The opening was sutured and disinfected with antiseptic powder. A proprietary elasticated jacket was placed around the animal to fix the dorsal exit site of the catheter against the movements of the animal. The catheter passed through a hollow spring to a swivel mechanism that allowed the animal full movement within its cage, and the catheter was coupled to an infusion pump (Graseby syringe pump 3200) which was used to provide a continuous infusion of pyrogen-free physiological saline. The animals were kept warm under infrared lamps until they had recovered from the operation anesthesia and were then placed in special cages designed to hold the catheter swivel mechanism centrally.
The i.v. itraconazole-cyclodextrin solution (Janssen Research Foundation, Beerse, Belgium) contained itraconazole at 10 mg/ml in 40% HPBCD; a 40% HPBCD solution was used as a placebo. The i.v. line was flushed with 0.5 ml of 15% HPBCD, and then the i.v. itraconazole solution or the placebo was infused via the pump over a 30- to 60-min period. After each infusion the line was flushed with a 15% HPBCD solution over 30 min to minimize precipitation of itraconazole at the catheter-vein junction. At autopsy examination, approximately 25% of animals with indwelling catheters were found to have small accumulations of fluid in the thorax, presumably the consequence of prolonged irritation from the catheter and the infusions given. In a minority of animals (details are given below), the irritation led to a sterile pneumonia. All treatments were given twice a day (b.i.d.) at dose levels of the same order as the 200 mg b.i.d. recommended for initial human therapy with i.v. itraconazole.
A fumigatus infections. A. fumigatus B19119 was grown on potato-dextrose agar (Difco Laboratories, Detroit, Mich.) at 35°C for 6 days. Conidia for inoculation were suspended in sterile physiological saline containing 0.05% (vol/vol) Tween 80, and concentrations were adjusted by hemocytometer counts. Groups of 3 or 6 animals were infected with 4,000 CFU/g via the indwelling catheter.
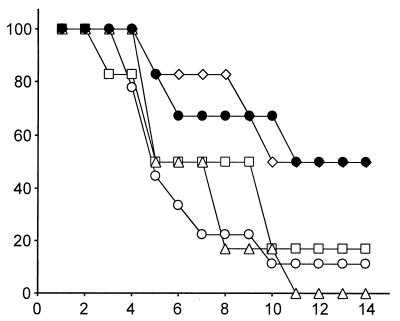
Under these conditions, placebo-treated animals died at an average of 5.8 days after infection (Fig. 1; Table 1). Mean survival times were slightly prolonged for animals treated i.v. with itraconazole at total daily doses of 2.5 and 5 mg/kg (Table 1), but the difference was not statistically significant, and it is evident from the survival curves (Fig. 1) that neither of these doses given i.v. affected overall survival. For animals treated for 13 days with 10 mg/kg, mean survival time was significantly prolonged (Table 1). This dose also effected a significant increase in mean survival time over that observed with placebo treatment in animals that had been immunosuppressed with mechlorethamine hydrochloride given i.p. at 0.25 mg/kg 5 days and 4 days before challenge (Table 1). At all treatment doses, the percentages of homogenized organs in which A. fumigatus was undetectable by postmortem culture were higher than those in placebo-treated animals (Table 1). The results obtained with this model are comparable with effects previously achieved with oral (16) and i.p. (17) itraconazole doses half as great as those used in the present study.
FIG. 1.
Mortality curves for guinea pigs with disseminated aspergillosis that were treated from day 0 to day 13 with itraconazole-cyclodextrin given i.v. Open circles, placebo treatment; squares, 1.25 mg/kg b.i.d.; triangles, 2.5 mg/kg b.i.d.; diamonds, 5 mg/kg b.i.d.; filled circles, immunosuppression with mechlorethamine hydrochloride and treatment with i.v. itraconazole at 10 mg/kg b.i.d.
TABLE 1.
Results of i.v. itraconazole treatment in guinea pigs with disseminated fungal infections
| Fungus infection | Itraconazole treatment (mg/kg b.i.d.) | First day of treatment | Last day of treatment | Day on which expt ended | n | MSTa ± SD (days) | Organs sampledb | % Organs with no detectable fungus (90% CI)c |
|---|---|---|---|---|---|---|---|---|
| A. fumigatus | Placebo | 0 | 13 | 14 | 9e | 5.8 ± 1.9 | K, L, S | 3.7 (2.3–9.7) |
| A. fumigatus | 1.25 | 0 | 13 | 14 | 6 | 7.8 ± 4.2 | K, L, S | 38.9 (20.0–57.8) |
| A. fumigatus | 2.5 | 0 | 13 | 14 | 6 | 7.0 ± 2.4 | K, L, S | 55.6 (36.3–74.8) |
| A. fumigatus | 5 | 0 | 13 | 14 | 6 | 11.0 ± 3.7* | K, L, S | 88.9 (76.7–100) |
| A. fumigatus | 5d | 0 | 13 | 14 | 6 | 9.8 ± 3.9* | K, L, S | 72.2 (54.9–89.6) |
| C. albicans | Placebo | 0 | 9 | 10 | 11e | 4.9 ± 1.4 | B, K, L, S, Sk | 1.8 (1.1–4.8) |
| C. albicans | 1.25 | 0 | 9 | 10 | 6 | 5.5 ± 2.4 | B, K, L, S, Sk | 33.3 (19.2–47.5) |
| C. albicans | 2.5 | 0 | 9 | 10 | 6 | 8.8 ± 2.9* | B, K, L, S, Sk | 80.0 (68.0–92.0) |
| C. albicans | 2.5 | 1 | 13 | 17 | 5 | 17 ± 0.0* | B, K, L, S, Sk | 100.0 |
| C. albicans | 5 | 1 | 13 | 17 | 6 | 17 ± 0.0* | B, K, L, S, Sk | 100.0 |
| C. albicans | 2.5 | 3 | 13 | 17 | 6 | 9.7 ± 6.0 | B, K, L, S, Sk | 40.0 (25.3–54.7) |
| C. neoformans | Placebo | 0 | 9 | 10 | 3 | NA | B, Ly, M, Sk | 33.3 (10.9–55.7) |
| C. neoformans | 2.5 | 0 | 9 | 10 | 6 | NA | B, Ly, M, Sk | 95.8 (89.1–100) |
| C. neoformans | 5 | 0 | 9 | 10 | 5 | NA | B, Ly, M, Sk | 100.0 |
| C. neoformans | Placebo | 0 | 29 | 30 | 3 | NA | B, Ly, M, Sk | 25.0 (10.5–39.5) |
| C. neoformans | 2.5 | 0 | 29 | 30 | 4 | NA | B, Ly, M, Sk | 100.0 |
| C. neoformans | 5 | 0 | 29 | 30 | 5 | NA | B, Ly, M, Sk | 100.0 |
| C. neoformans | 2.5 | 10 | 29 | 30 | 6 | NA | B, Ly, M, Sk | 20.8 (7.2–34.5) |
| C. neoformans | 5 | 10 | 29 | 30 | 8e | NA | B, Ly, M, Sk | 62.5 (48.4–76.6) |
MST, mean survival time. Asterisks indicate significant prolongation of survival over that of the placebo-treated group (Mann-Whitney U test, P < 0.05). NA, not applicable; no fatalities.
B, brain; K, left kidney; L, liver; Ly, lymph; M, muscle; S, spleen; Sk, skin.
CI, confidence interval.
Animals immunosuppressed by pretreatment with mechlorethamine hydrochloride.
Data pooled from two experiments.
C. albicans infections. C. albicans B2630 was grown in Sabouraud broth (Oxoid, Basingstoke, United Kingdom) for 24 h at 30°C with constant rotation and then diluted into sterile physiological saline, and the concentration was adjusted by spectrophotometry. Groups of three or six guinea pigs were infected via the i.v. catheter with 104.2 to 104.6 CFU/g.
When i.v. itraconazole treatment was begun 1 h after infection and continued for 9 days, daily doses of 2.5 and 5 mg/kg resulted in a dose-related prolongation of survival of animals relative to those in the placebo-treated group that was statistically significant at the higher dosage (Table 1). The percentage of organs in which C. albicans was undetectable at the time of death also rose in a dose-dependent manner (Table 1). These results are similar to those obtained by 14 days of oral itraconazole treatment at 1.25 and 2.5 mg/kg in a comparable model (16).
When i.v. treatment was initiated 1 day after infection and continued through day 13, animals treated with a daily dose of 5 or 10 mg of itraconazole per kg all survived until day 17 (Table 1). For these groups, no C. albicans could be detected in any of five organs per animal sampled by culture at autopsy. When initiation of treatment at doses of 2.5 mg/kg b.i.d. was delayed until 3 days after C. albicans challenge, the survival of the animals was prolonged and the percentages of culture-negative organs increased relative to those of controls, but not to the level of statistical significance. Previous studies with oral and i.p. treatment in this guinea pig C. albicans model have reported only results obtained with treatment started on or before the day of infection (16, 17).
Sterile pneumonias were seen postmortem in one placebo-treated animal and in one animal treated with 2.5 mg/kg b.i.d. starting on day 1. Results from these animals were not included in the analysis in Table 1.
C. neoformans infections. C. neoformans B42419 was grown on Sabouraud agar at 37°C for 2 days, resuspended in physiological saline, and injected i.v. at 200 CFU/g of body weight to give a chronic infection with no fatalities by 10 days, even among placebo-treated animals. Early treatment with i.v. itraconazole, initiated on the day of infection, resulted in a dose-related increase in the percentage of tissues with no C. neoformans detectable in culture 10 and 30 days after infection, by comparison with placebo-treated animals (Table 1). When initiation of therapy was delayed to 10 days after infection, a daily dose of 10 mg/kg was required to reduce the detectability of the fungus in tissues below control levels (Table 1). Oral and i.p. treatment with itraconazole in the same animal model begun 3 days after challenge previously showed results, dose for dose, that were intermediate between those achieved with therapy starting on day 0 and on day 10 in the present study (16, 17). In the cryptococcosis experiments, 5 of the 45 animals had extensive thoracic fluid accumulations at autopsy, and results from these animals were not included in the analysis.
Plasma itraconazole concentrations. Three noninfected guinea pigs with implanted i.v. catheters were infused with itraconazole-cyclodextrin i.v. at a dose of 2.5 mg/kg. Measurement of itraconazole and hydroxy-itraconazole concentrations by high-performance liquid chromatography (HPLC) (18, 21) on plasma pooled from the three animals showed itraconazole levels of 1,040, 516, and 443 ng/ml and hydroxy-itraconazole levels of 0, 21, and 37 ng/ml at 0, 2, and 4 h postinfusion, respectively. The HPLC assay has a within-assay precision of 1.2 to 1.5% and an interday precision of 3.6 to 4.8% (18). In humans given 200-mg i.v. itraconazole infusions twice daily, doses equivalent to 3 to 4 mg/kg in a person weighing 50 to 70 kg, trough levels of the two compounds combined were on the order of 1 μg/ml (19). These data suggest that itraconazole is metabolized more rapidly by guinea pigs than by humans, a difference that needs to be taken into consideration in comparing the present animal studies with the potential effects of itraconazole in humans.
Throughout our experiments, direct comparison of i.v. with oral or i.p. itraconazole treatment in matched groups of animals was not attempted in view of the high cost and complexity of establishing and maintaining i.v. catheterization in the guinea pigs. The same limitation also restricted the numbers of animals in each experimental group. Without the catheterization procedure, it would have been technically impossible to administer repeated infusions of i.v. itraconazole-cyclodextrin.
The animals undoubtedly sustained a stress burden from the permanently implanted i.v. catheter that would be expected to reduce the robustness of innate host defenses against the experimental infections and therefore lower the apparent antifungal efficacy of itraconazole treatment. Despite this, the agent unequivocally showed efficacy under various conditions across all three experimental models, confirming its inherent potency in vivo, which has been confirmed for oral therapy in objective clinical trials (3, 14, 15). The availability of an i.v. itraconazole formulation should extend the advantages of its use to patients who are unconscious or otherwise unable to ingest oral medications.
We conclude that the high efficacy of i.v. itraconazole in vivo supports the continuing investigation of the antifungal efficacy of this formulation in life-threatening Candida, Aspergillus, and Cryptococcus infections in humans.
Acknowledgments
We are grateful to Louis Stoffels for extensive assistance in setting up the i.v. catheterization procedure, to Ludi Van Beijsterveldt for the itraconazole HPLC measurements, and to Peter De Backker, Steve Clijmans, and Bert Evers for technical assistance.
This study was supported in part by a grant from Ortho-Biotech.
REFERENCES
- 1.Abruzzo G K, Flattery A M, Gill C J, Kong L, Smith J G, Krupa D, Pikounis V B, Kropp H, Bartizal K. Evaluation of water-soluble pneumocandin analogs L-733560, L-705589, and L-731373 with mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1995;39:1077–1081. doi: 10.1128/aac.39.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark J M, Whitney R R, Olsen S J, George R J, Swerdel M R, Kunselman L, Bonner D P. Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob Agents Chemother. 1991;35:615–621. doi: 10.1128/aac.35.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denning D W, Lee J Y, Hostetler J S, Pappas P, Kauffman C A, Dewsnup D H, Galgiani J N, Graybill J R, Sugar A M, Catanzaro A, et al. NIAID Mycoses Study Group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994;97:135–144. doi: 10.1016/0002-9343(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 4.Edwards J E, Jr, Bodey G P, Bowden R A, Büchner T, de Pauw B E, Filler S G, Ghannoum M A, Glauser M, Herbrecht R, Kaufmann C A, et al. International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin Infect Dis. 1997;25:43–59. doi: 10.1086/514504. [DOI] [PubMed] [Google Scholar]
- 5.Glasmacher A, Molitor E, Hahn C, Bomba K, Ewig S, Leutner C, Wardelmann E, Schmidt-Wolf I G, Mezger J, Marklein G, Sauerbruch T. Antifungal prophylaxis with itraconazole in neutropenic patients with acute leukaemia. Leukemia. 1998;12:1338–1343. doi: 10.1038/sj.leu.2401137. [DOI] [PubMed] [Google Scholar]
- 6.Graybill, J. R., J. Vazquez, R. O. Darouiche, R. Morhart, D. Greenspan, C. Tuazon, L. J. Wheat, J. Carey, I. Leviton, R. G. Hewitt, et al. Randomized trial of itraconazole oral solution for oropharyngeal candidiasis in HIV/AIDS patients. Am. J. Med. 104:33–39. [DOI] [PubMed]
- 7.Lamy T, Bernard M, Courtois A, Jacquelinet C, Chevrier S, Dauriac C, Grulois I, Guiguen C, Le Prise P Y. Prophylactic use of itraconazole for the prevention of invasive pulmonary aspergillosis in high risk neutropenic patients. Leuk Lymphoma. 1998;30:163–174. doi: 10.3109/10428199809050939. [DOI] [PubMed] [Google Scholar]
- 8.Lortholary O, Denning D W, Dupont B. Endemic mycoses: a treatment update. J Antimicrob Chemother. 1999;43:321–331. doi: 10.1093/jac/43.3.321. [DOI] [PubMed] [Google Scholar]
- 9.McEwen J G, Peters G R, Blaschke T F, Brummer E, Perlman A M, Restrepo A, Stevens D A. Treatment of paracoccidioidomycosis with itraconazole in a murine model. J Trop Med Hyg. 1985;88:295–299. [PubMed] [Google Scholar]
- 10.Phillips P, De Beule K, Frechette G, Tchamouroff S, Vandercam B, Weitner L, Hoepelman A, Stingl G, Clotet B. A double-blind comparison of itraconazole oral solution and fluconazole capsules for the treatment of oropharyngeal candidiasis in patients with AIDS. Clin Infect Dis. 1998;26:1368–1373. doi: 10.1086/516342. [DOI] [PubMed] [Google Scholar]
- 11.Prentice H G, Caillot D, Dupont B, Menichetti F, Schuler U. Oral and intravenous itraconazole for systemic fungal infections in neutropenic haematological patients: meeting report. London, United Kingdom, 20 June 1998. Acta Haematol. 1999;101:56–62. doi: 10.1159/000040923. [DOI] [PubMed] [Google Scholar]
- 12.Schaffner A, Bohler A. Amphotericin B-refractory aspergillosis after itraconazole—evidence for significant antagonism. Mycoses. 1993;36:421–424. doi: 10.1111/j.1439-0507.1993.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan D J, Hitchcock C A, Sibley C M. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiku H, Horiuchi A, Kuramoto A, Harada M, Niho Y. Clinical study of itraconazole for systemic fungal infections complicated with hematological malignancies—multicenter cooperative study. West-Japan Systemic Fungal Infection Study Group. J Jpn Assoc Infect Dis. 1998;72:1208–1218. doi: 10.11150/kansenshogakuzasshi1970.72.1208. [DOI] [PubMed] [Google Scholar]
- 15.Sirisanthana T, Supparatpinyo K, Perriens J, Nelson K E. Amphotericin B and itraconazole for treatment of disseminated Penicillium marneffei infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26:1107–1110. doi: 10.1086/520280. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem J. Oral and parenteral treatment with itraconazole in various superficial and systemic experimental fungal infections. Comparisons with other antifungals and combination therapy. Br J Clin Pract Suppl. 1990;71:32–40. [PubMed] [Google Scholar]
- 17.Van Cutsem J. In vitro antifungal spectrum of itraconazole and treatment of systemic mycoses with old and new antimycotic agents. Chemotherapy. 1992;38(Suppl. 1):3–11. doi: 10.1159/000239046. [DOI] [PubMed] [Google Scholar]
- 18.Van de Velde V J S, Van Peer A P, Heykants J J P, Woestenborghs R J H, Van Rooy P, De Beule K L, Cauwenbergh G F M L. Effect of food on the pharmacodynamics of a new hydroxypropyl-β-cyclodextrin formulation of itraconazole. Pharmacotherapy. 1996;16:424–428. [PubMed] [Google Scholar]
- 19.Vandewoude K, Vogelaers D, Decruyenaere J, Jaqmin P, De Beule K, Van Peer A, Woestenborghs R, Groen K, Colardyn F. Concentrations in plasma and safety of 7 days of intravenous itraconazole followed by 2 weeks of oral itraconazole solution in patients in intensive care units. Antimicrob Agents Chemother. 1997;41:2714–2718. doi: 10.1128/aac.41.12.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez J A. Options for the management of mucosal candidiasis in patients with AIDS and HIV infection. Pharmacotherapy. 1999;19:76–87. doi: 10.1592/phco.19.1.76.30509. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Goldman M, Wu J, Woestenborghs R, Hassell A E, Lee P, Baruch A, Pesco-Koplowitz L, Borum J, Wheat L J. A pharmacokinetic study of intravenous itraconazole followed by oral administration of itraconazole capsules in patients with advanced human immunodeficiency virus infection. J Clin Pharmacol. 1998;38:593–602. doi: 10.1002/j.1552-4604.1998.tb04465.x. [DOI] [PubMed] [Google Scholar]