Abstract
Background and Objectives
Muscle microangiopathy due to dysfunction of endothelial cells because of inflammation is a critical hallmark of dermatomyositis (DM); however, its pathomechanism remains unclear. The aim of this study was to evaluate the effect of immunogloblin G (IgG) from patients with idiopathic inflammatory myopathies (IIM) on muscle endothelial cells in vitro.
Methods
Using a high-content imaging system, we analyzed whether IgG purified from sera from patients with IIM (n = 15), disease controls (DCs: n = 7), and healthy controls (HCs: n = 7) can bind to muscle endothelial cells and induce complement-dependent cellular cytotoxicity.
Results
IgGs from Jo-1 antibody myositis could bind to muscle endothelial cells and caused complement-dependent cell cytotoxicity. RNA-seq demonstrated the upregulation of genes associated with tumor necrosis factor (TNF)-α, triggering receptor expressed on myeloid cells-1 (TREM-1), CD25, and mitochondria pathways after exposure to IgG from the Jo-1, signal recognition particle (SRP), and polymyositis (PM) groups. The high-content imaging system showed that TREM-1 expression in the Jo-1, SRP, and PM groups was increased in comparison with DCs and HCs and that the TNF-α expression in the Jo-1 group was higher in comparison with the SRP, PM, DC, and HC groups. The expression of TREM-1 was observed in biopsied capillaries and the muscle membrane from patients with Jo-1 and in biopsied muscle fiber and capillaries from patients with DM and SRP. The depletion of Jo-1 antibodies by IgG of patients with Jo-1 antibody myositis reduced the Jo-1 antibody–induced complement-dependent cellular cytotoxicity in muscle endothelial cells.
Discussion
Jo-1 antibodies from Jo-1 antibody myositis show complement-dependent cellular cytotoxicity in muscle endothelial cells. IgGs from patients with Jo-1, SRP, and DM increase the TREM-1 expression in endothelial cells and muscles.
Idiopathic inflammatory myopathies (IIMs) are classified into 5 subtypes: polymyositis (PM), dermatomyositis (DM), immune-mediated necrotizing myopathy (IMNM), sporadic inclusion body myositis (sIBM), or overlap myositis including antisynthetase syndrome (ASS).1-3 Pathologic findings of skeletal muscle biopsy specimens and the detection of myositis-specific autoantibodies support a diagnosis of IIM and play an important role in the classification of IIM.4,5 Autoantibodies against aminoacyl-tRNA synthetases (ARSs), such as Jo-1 antibodies, were detected in 25%–35% of patients with IIM.6 Both signal recognition particle (SRP) antibodies and 3-hydroxy-3-methylglutaryl coenzyme (HMGCR) antibodies are clinically used for the diagnosis of IMNM.7,8 In addition, other autoantibodies, including transcription intermediary factor (TIF) 1-gamma (p155/140) antibodies, chromodomain helicase DNA binding protein 4 (Mi-2) antibodies, MJ/nuclear matrix protein 2 (NXP2) antibodies, melanoma differentiation-associated gene 5 (MDA5) antibodies, and clinically amyopathic DM p140 antibodies, are detected in patients with DM.2 Anti–Jo-1 antibodies are observed in 20%–30% of patients with IIM and are the most common type of myositis-specific autoantibody in these patients. Anti-Jo-1–positive patients suffer from myositis, interstitial pneumonia, arthritis, Raynaud phenomenon, and mechanic hands, defining the relatively homogeneous disease called ASS.9 However, whether these antibodies have pathogenic effects on the development of IIM remains to be elucidated.
Vasculopathy due to the loss and dysfunction of endothelial cells because of inflammation in the skin and muscle is an important hallmark of juvenile DM and DM.10 Pathologic observation of muscle biopsy specimens showed reduced capillary density, lymphocyte infiltration, and lymphocytic inflammation around the perimysial blood vessels; thickening and occasionally occlusion of the small vessels; and the deposition of the C5b-9 complement membrane attack complex (MAC) on intrafascicular capillaries.11-15 Some reports showed the deposition of immunogloblin G (IgG) around the perimysial blood vessels in a proportion of DM cases.16 Although the underlying mechanism is unknown, these changes suggest that DM may be an antibody-dependent and complement-mediated microangiopathy, giving rise to ischemia and perifascicular atrophy in the muscle.
In this study, we analyzed the contribution of serum IgG from individual patients with myositis (including Jo-1 antibody–positive myositis, DM, PM and SRP antibody–positive IMNM) to endothelial cell dysfunction in endomysial/perimysial vessels using human muscle endothelial cell lines by RNA-seq and high-content imaging. We found that IgG from patients with Jo-1, SRP, and PM induced the upregulation of triggering receptor expressed on myeloid cells-1 (TREM-1) in muscle endothelial cells and that Jo-1 antibodies caused complement-dependent cellular cytotoxicity.
Methods
Standard Protocol Approvals Registrations, and Patient Consents
Written informed consent was obtained from each participant. The ethics committees of the Medical Faculty of Yamaguchi University approved this research (IRB#: H25-089-4).
Patient Samples
Sera were collected from 42 patients with IIM who had been diagnosed at Yamaguchi University Hospital, based on the clinical and pathologic findings (male, n = 15, female, n = 27; mean age, 57.8 years). All patients with IIM had muscle weakness and a high serum creatine kinase level. Muscle biopsy specimens were obtained from all patients with IIM. PM or DM was diagnosed according to muscle biopsy findings of the invasion of non-necrotic muscle fibers by CD8+ T cells (PM) or positive M×A staining in the muscle (DM). IgGs from 6 patients with Jo-1 antibody–positive myositis, 5 patients with SRP antibody–positive IMNM, 4 patients with PM, and 6 patients with DM were included in the analysis. IgGs from 7 healthy controls (HCs: male, n = 3; female, n = 4) and 7 noninflammatory disease controls (DCs: amyotrophic lateral sclerosis, n = 7) were used as controls. Overlap myositis was defined as myositis with overlapping connective disease features, including non–Jo-1 ASS and overlap antibodies.2
Before these experiments, sera were preserved at −80°C and inactivated at 56°C for 30 minutes. Purification of IgG from sera was performed using a Melon Gel IgG Spin Purification Kit (Thermo Fisher Scientific).
Immunohistochemical Staining of TREM-1, CD25, TNF-α, JC-1, and Live-Dead Cell Staining Using a High-Content Imaging Assay
We used TSM15 cells for all experiments.17 TSM15 cells were generated from a human skeletal muscle microvascular endothelial cell line immortalized with temperature-sensitive SV40 T antigen (tsA58) and telomerase. All experiments were performed 2 days after increasing the temperature from 33°C to 37°C to inactivate immortalization. The protocol of the high-content imaging system was previously described.18 Briefly, 5,000 cells per well were plated and maintained on 96-well plates (CELLSTAR, Greiner) and subsequently cultured in MCDB 131 medium diluting IgG (500 μg/mL) from patients with IIM, DCs, or HCs for 24 hours in TREM-1, CD25, tumor necrosis factor (TNF)-α, and JC-1 MitoMP Detection Kit (JC-1) immunostaining.
For TREM-1, CD25, or TNF-α staining or the IgG binding assay, TSM15 cells were (1) fixed with 4% paraformaldehyde for 15 minutes and then incubated with (2) 0.3% Triton X-100 in phosphate-buffered saline (PBS) for 10 minutes, (3) 0.3% Triton X-100 in PBS/5% fetal bovine serum for 12 hours for blocking, (4) each primary Ab (TREM-1 monoclonal antibody [Proteintech], TNF-α monoclonal antibody [Novus] or CD25 monoclonal antibody [Invitrogen]), or IgG from each patient or healthy individual (50 µg/mL), and (5) secondary Abs (Alexa Fluor 488 goat anti-rabbit IgG, Alexa Fluor 488 goat anti-mouse IgG [Thermo Fisher Scientific]).
For JC-1 staining, 1 µM of the JC-1 MitoMP Detection Kit (DOJINDO, Japan) diluted with MCBD media was incubated with living TSM15 cells at 37°C for 45 minutes in the dark. In the JC-1 dye, JC-1 J-monomer remains fluorescent green and J-aggregates turn fluorescent red, reflecting a higher mitochondrial membrane potential. The ratio of green-to-red fluorescence intensity for JC-1 shows the mitochondrial membrane potential.
To evaluate the complement-dependent cytotoxicity, TSM15 cells were incubated with IgG (500 µg/mL) diluted with the MCDB 131 medium from patients with IIM or HCs at 37°C for 24 hours. TSM15 cells were then incubated with Low-Tox-M rabbit complement (final concentration 2%, Cedarlane Laboratories Ltd) at 37°C for 23 hours and were stained using a Live/Dead Cell Staining Kit II (Takara-Bio, Japan). In the assay, calcein-AM stains live cells as green while EthD-III stains dead cells as red.
Plate images were captured using a Bz X800 Fluorescence Microscope (Keyence Corporation, Japan) or an In Cell Analyzer 2000 (GE Healthcare) at ×20 magnification with 4 fields of view per well (equivalent to 800–1,000 cells). The images were then analyzed with the IN Carta image analysis software program (Cytiva), the In Cell Analyzer software program (Cytiva), or the BZ-X800 imaging software program (Keyence Corporation, Osaka, Japan). The data represent the mean values from 6 experiments for IgG binding or 3 experiments for TREM-1, CD25, TNF-α, and JC-1.
Whole-Transcriptome Analysis by RNA-Seq
We incubated IgG (500 µg/mL) with TSM15 cells at 37°C for 12 hours. Samples were obtained from the following individuals: patients with Jo-1 antibody–positive myositis (n = 3), patients with SRP antibody–positive myopathy (n = 3), patients with PM (n = 3), and healthy individuals (n = 3). We used TSM15 cells with a culture medium as controls. The method for RNA-seq was previously described.19,20 Briefly, an RNeasy Mini Kit (Qiagen) was used for extraction and purification of mRNA from TSM15 cells. A NEBNext Ultra II RNA Library Prep kit (New England Biolabs, NEB) and NEBNextplex Oligos for Illumina were applied for creating complementary DNA (cDNA). This method involved fragmentation in NEBNext First Strand Synthesis Reaction Buffer at 94°C for 15 minutes with NEBNext Random Primers, the NEBNext Strand Synthesis Enzyme Mix for the reverse transcription, the concentration of the library fragments, and the insertion of the index sequences during PCR amplification. AMPure XP beads (Beckman Coulter) were used for purification, and an Agilent 2200 TapeStation (D1000, Agilent) was applied to confirm the quality of the library. An Illumina Next-seq DNA sequencer with a 75 bp pair-end cycle sequencing kit (Illumina) was applied to sequence libraries, and the CLC Genomics Workbench software program (ver.8.01; Qiagen) was applied to trim and map the data for the mouse reference genome GRCm38 release-92. The mapped data were normalized by transcripts per million (TPM) and then converted to log2 (TPM + 1). In the volcano plots, p-values obtained by the unpaired Student t test and the fold-change (FC) obtained by subtracting the average values in HCs from those in patients with IIM were applied. An ingenuity pathway analysis (IPA, Qiagen), which included genes with p value <0.05 and with >50% increase or decrease in FC, was performed to analyze the pathways of the detected genes.
Western Blotting of Anti–Jo-1 Autoantibodies
Total protein from TSM15 cells was prepared with radio-immunoprecipitation assay Lysis and Extraction Buffer (Thermo Fisher Scientific). The membrane protein of TSM15 cells was also prepared using a Pierce Cell Surface Protein Isolation Kit (Thermo Fisher Scientific) in accordance with the manufacturer's instructions. The method of Western blotting was previously described. In brief, 15 µg of total protein, 15 µg of membrane protein, or 1 μg of recombinant human Jo-1 protein (Fitzgerald) was transferred by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to polyvinylidene difluoride (PVDF) membranes (Amersham). Anti–Jo-1 antibodies (Novus) or Jo-1 IgG from Jo-1 antibody–positive myositis, as the primary antibody, were incubated on PVDF membranes for 1 hour and then incubated with anti-rabbit or anti-human secondary fluorescent antibodies for 1 hour. The bands were visualized using a chemiluminescence kit (ImmunoStar LD, Japan), and the relative density of bands was calculated using the Quantity One software program (Bio-Rad).
Immunohistochemical Staining of Muscle Specimens From Patients With Jo-1 Antibody–Positive Myositis
We performed immunohistochemical staining of 10-μm-thick section of frozen-fixed muscle specimens from patients with Jo-1 antibody–positive myositis and controls. After sections were deparaffinized, the antigen (TREM-1) was activated by heating (98°C) for 10 minutes. Indirect immunofluorescence was performed with anti–TREM-1 antibodies (dilution 1:50, Proteintech) as the primary antibody and anti-rabbit secondary fluorescent antibodies with Dapi (Alexa Fluor 488 goat anti-rabbit IgG, Invitrogen, dilution 1:200) as the secondary antibody.
Removal of Jo-1 Autoantibodies From Jo-1 IgG by Immunoprecipitation
Jo-1 IgG (500 μg/mL) was incubated with 1 μg of HIS-tagged Jo-1 recombinant protein (Fitzgerald) for 1 hour in 2 tubes. After the Jo-1 autoantibody bound to an antigen (Jo-1) and made immune complexes of Jo-1, 40 μL of EZview Red HIS-Select HC Nickel Affinity Gel (Sigma-Aldrich) was incubated in one of the 2 tubes for 2 hours. After centrifugation, supernatants (Jo-1 IgG with removal of Jo-1 antibodies) were collected for the experiments (eFigure 6, links.lww.com/NXI/A856). The anti–Jo-1 autoantibodies in these supernatants (Jo-1 IgG with/without the removal of Jo-1 antibody) were assayed by Western blotting.
Statistical Analyses
All statistical analyses were performed using the Prism 9 software program (Graph Pad). A paired Student t test (2-sided) was used for single comparisons. For multiple comparisons, a one-way analysis of variance (ANOVA) was performed with the Tukey multiple comparisons test when the data were normally distributed. p Values of *<0.05, **<0.01, and ***<0.001 were considered to indicate statistical significance.
Data Availability
Data not provided within this article are available in anonymized form and can be shared by request from any qualified investigator. Sharing requires approval of a data transfer agreement by Yamaguchi University.
Results
IgGs From Patients With IIM Bound to Muscle Endothelial Cells and Induced Complement-Dependent Cytotoxicity
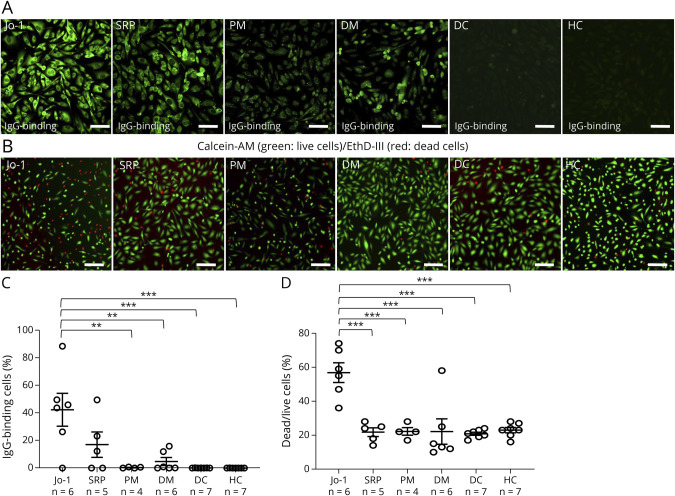
We first screened the binding of IgG from each of the 42 patients with IIM, and IgG binding was observed in 5 of 6 patients with Jo-1 antibody–positive myositis, 1 of 5 patients with SRP antibody–positive myositis, and 2 patients with nonspecific myositis (eFigure 1, links.lww.com/NXI/A856). We next examined the binding of IgG from patients with Jo-1 antibody–positive myositis (Jo-1 group: n = 6), SRP antibody–positive IMNM (SRP group: n = 5), PM (PM group: n = 4), DM (DM group: n = 6), DCs (n = 7), and HCs (n = 7) (Figure 1A). Notably, IgGs from patients in the Jo-1 group significantly bound to TSM15 cells, in comparison with IgGs from the SRP group, PM group, DM group, DCs, and HCs (Figure 1, A–C). The IgGs from the Jo-1 group induced significantly more complement-dependent cytotoxicity in TSM15 cells in comparison with IgGs from the SRP group, PM group, DM group, DCs, and HCs (Figure 1, B and D).
Figure 1. Binding of IgGs From Patients With IIM to Muscle Endothelial Cells and Induced Complement-Dependent Cytotoxicity.
(A) Representative immunostaining showing the binding of IgG (500 µg/mL) from patients with Jo-1 antibody–positive myositis (Jo-1), SRP antibody–positive IMNM (SRP), PM, dermatomyositis (DM), disease controls (DCs), or healthy controls (HCs) to TSM15 (green, human-IgG). Scale bar, 100 µm. Images were captured by an IN Cell Analyzer 2000. (B) The complement-dependent cytotoxicity (ratio of dead/live cells) in TSM15 cells was assayed after exposure to IgGs bound to TSM15 cells from patients with Jo-1, SRP, PM, and DM, DCs, and HCs. (C) Scatter plots of the percentage of binding of IgG from patients with Jo-1 antibody–positive myositis (Jo-1, n = 6), patients with SRP antibody–positive immune-mediated necrotizing myopathy (IMNM) (SRP, n = 5), patients with polymyositis (PM, n = 4), patients with dermatomyositis (DM, n = 6), disease controls (DCs, n = 7), and healthy controls (HCs, n = 7) to TSM15 cells. p Values were determined by a one-way ANOVA, followed by the Tukey multiple comparison test (**p < 0.01, ***p < 0.001 vs the DC or HC group). (D) Scatter plots of the percentage of dead/live cells in TSM15 cells as determined by high-content imaging. Data are shown as mean ± SEM. Statistical significance was determined by a one-way ANOVA, followed by the Tukey multiple comparison test (***p < 0.001 Jo-1 vs SRP, PM, DM, DCs, or HCs). ANOVA = analysis of variance; IIMs = idiopathic inflammatory myopathies; PM = polymyositis; SRP = signal recognition particle.
The Analysis of the Alteration of the Gene Expression in Muscle Endothelial Cells After Incubation With IgG From Patients With IIM
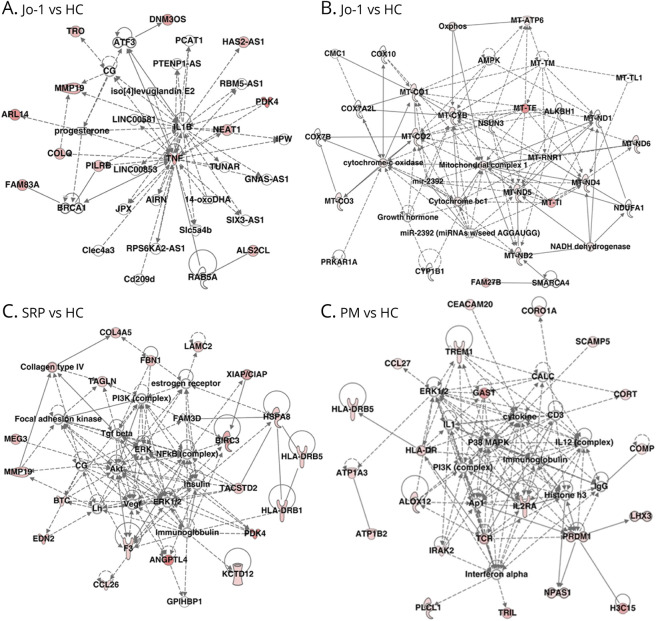
TSM15 cells were incubated with IgGs from each patient in the Jo-1 group (n = 3), SRP group (n = 3), and PM group (n = 3) or HCs (n = 3) for a whole-transcriptome analysis by RNA-seq to identify altered pathways. TSM15 cells were used as controls. Over 55,000 genes were detected from approximately 20 million reads per sample in TSM15 cells. Volcano plots and heat maps revealed the significant differential gene expression in 187 genes (FC > 1.5; p < 0.05; 79 upregulated genes and 108 downregulated genes) between the Jo-1 and HC groups, 194 genes (FC > 1.5; p < 0.05; 127 upregulated genes and 67 downregulated genes) between the SRP and HC groups, and 422 genes (FC > 1.5; p < 0.05; 278 upregulated genes and 144 downregulated genes) between the PM and HC groups. An IPA was performed using the abovementioned upregulated or downregulated genes to identify important pathways of TSM15 cells after incubation with IgGs from the Jo-1, SRP, and PM groups (Figure 2, A–D). Regarding the upregulated genes, TNF-α and mitochondria complex were identified in the center of the network in the Jo-1 group (Figure 2, A and B); nuclear factor-κB was identified in the center of the network in the SRP group (Figure 2C); and TREM-1 and ILR2A (CD25) were observed as upstream molecules of signaling in the PM group (Figure 2D), suggesting that TREM-1 and TNF-α had been upregulated.
Figure 2. Whole-Transcriptome Analysis With RNA-Seq of TSM15 Cells After Exposure to IgG From Patients With Jo-1, SRP, or PM.
TSM15 cells were incubated with IgGs from patients with Jo-1 (n = 3), patients with SRP (n = 3), patients with PM (n = 3), and healthy controls (n = 3). TSM15 cells without exposure to IgGs were also used as a control. (A, B) In the network analysis of the upregulated genes, TNF-α and mitochondria complex were detected in the center of the network analysis in the Jo-1 group. (C) In the network analysis of the upregulated genes, nuclear factor-κB was detected in the center of the network in the SRP group. (D) In the network analysis of the upregulated genes, TREM-1 and ILR2A (CD25) were observed as upstream molecules of signaling in the PM group. PM = polymyositis; SRP = signal recognition particle.
IgGs From the Jo-1, SRP, and PM Groups Induced the Increase of TREM-1
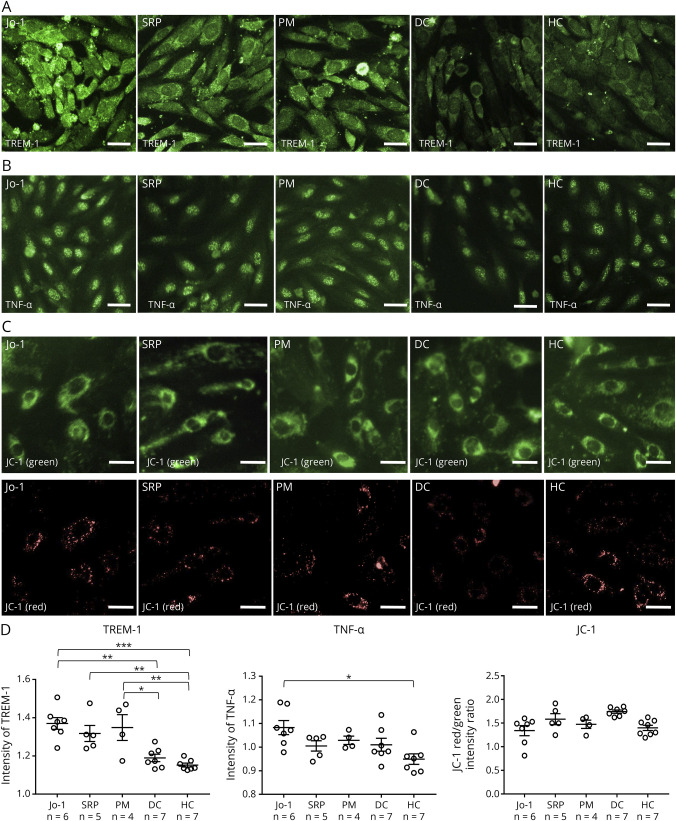
To confirm the data obtained by RNA-seq and the pathway analysis, the change in the protein levels of TREM-1, CD25, and TNF-α after exposure to IgGs from the Jo-1, SRP, PM, and HC groups was examined (Jo-1, n = 7; SRP, n = 5; PM, n = 4; DC amyotrophic lateral sclerosis [patients with ALS], n = 7; HC, n = 6; Figure 3, A–C). The high-content imaging system revealed that the amount of TREM-1 protein in TSM15 cells in the Jo-1, SRP, and PM groups was significantly increased in comparison with the DC and HC groups (Figure 3, A and D) and the amount of TNF-α in TSM15 cells in the Jo-1 group was significantly increased in comparison with the HC group (Figure 3, B and D). The CD25 expression and mitochondrial membrane potential (red/green ratio using the JC-1 dye) was not changed after exposure to IgGs from the Jo-1, SRP, PM, or HC group (eFigure 5, links.lww.com/NXI/A856 and Figure 3, C and D).
Figure 3. Changes of TREM-1 and TNF-α in TSM15 Cells After Exposure to IgGs From Patients With Jo-1, SRP, or PM.
(A, B) Immunostaining of TSM15 cells for TREM-1 and TNF-α (green) after exposure to IgGs (500 µg/mL) from patients with Jo-1, patients with SRP, patients with PM, DCs, or HCs. (C) Immunostaining of TSM15 cells for JC-1 (green/red) after exposure to IgGs (500 µg/mL) from patients with Jo-1, patients with SRP, patients with PM, DCs, or HCs. The JC-1 red/green intensity ratio reflects the mitochondrial potential. Images were captured by an IN Cell Analyzer 2000. Scale bar, 20 μm. (D) Scatter plots of the intensity of TREM-1, TNF-α, or the JC-1 red/green intensity ratio, as determined by high-content imaging after exposure to IgGs from patients with Jo-1 (n = 7), patients with SRP (n = 5), patients with PM (n = 4), DCs (n = 7), and HCs (n = 7). The data were normalized to cultures that had not been exposed to human IgG and were obtained from 3 independent experiments. p Values were determined by a one-way ANOVA, followed by the Tukey multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001 vs the DC or HC group). ANOVA = analysis of variance; DCs = disease controls; DM = dermatomyositis; HCs = healthy controls; PM = polymyositis; SRP = signal recognition particle.
TREM-1 Expression in the Capillaries Around Perimysial Blood Vessels and the Muscle Membrane in Muscle Biopsy Specimens of Patients With Jo-1 Antibody–Positive Myositis
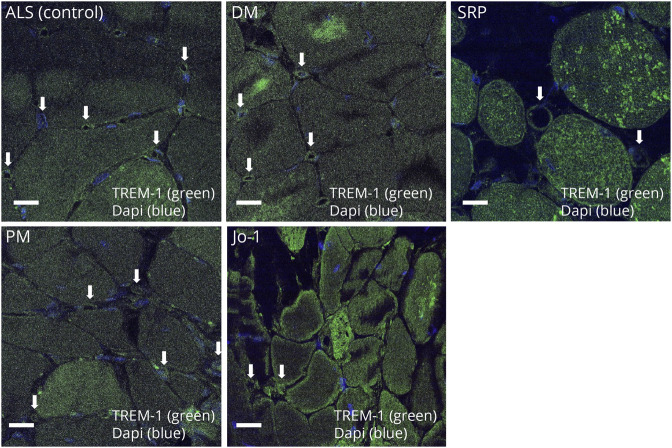
Immunohistochemical staining of muscle biopsy specimens demonstrated the expression of TREM-1 in the capillaries around the perimysial blood vessels and the muscle membrane/fiber in Jo-1 antibody–positive myositis, in the capillaries and muscle fibers in DM and SRP, but not in PM or ALS (as controls) (Figure 4).
Figure 4. TREM-1 Expression in Muscle Biopsy Specimens From Patients With Jo-1 Antibody–Positive Myositis.
Immunohistochemical staining of muscle biopsy specimens demonstrated the expression of TREM-1 in the capillaries around the perimysial blood vessels and the muscle membrane and muscle fiber of patients with Jo-1 antibody–positive myositis, in the capillaries and muscle fibers of those with dermatomyositis (DM) and SRP antibody–positive myositis, but not in specimens from patients with polymyositis (PM) or amyotrophic lateral sclerosis (ALS) (as controls). The arrow indicates the capillaries. SRP = signal recognition particle.
Jo-1 Antibodies From Patients With Jo-1 Antibody–Positive Myositis Bound to Jo-1 in TSM15 Cells
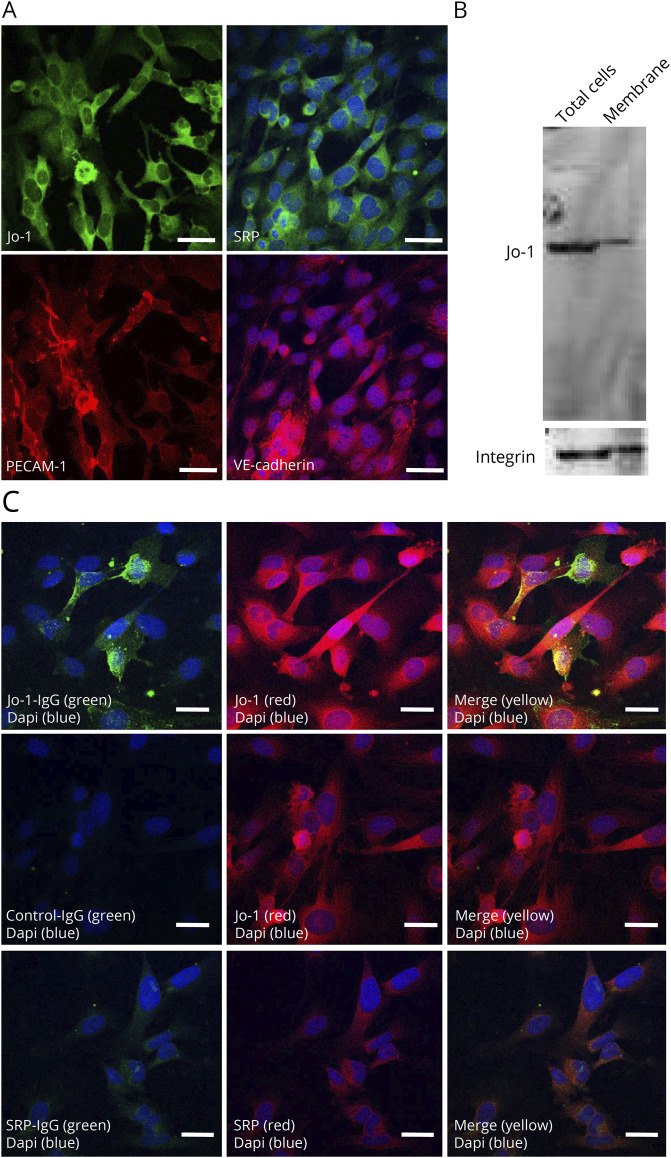
An immunohistochemical analysis showed the expression of Jo-1 and SRP in TSM15 cells (Figure 5, A and B). Western blotting of the cell membrane fraction revealed the band of Jo-1 protein in TSM15 cells (Figure 5B). Double immunostaining with commercial anti-Jo-1 Abs and IgG from Jo-1 antibody–positive myositis demonstrated colocalization on the cell surface and cytoplasm of TSM15 cells; this was not observed with IgGs from SRP and HCs (Figure 5C).
Figure 5. Jo-1 Antibodies Bound to Jo-1 in TSM15 Cells.
(A) Immunohistochemical staining of Jo-1 and SRP in TSM15 cells. Platelet endothelial cell adhesion molecule 1 and vascular endothelia-cadherin are stained in red; nuclei are counterstained with DAPI in blue. (B) In TSM15 cells, Western blotting demonstrated the band of Jo-1 in total cell lysis and cell membrane fraction. (C) Immunofluorescence labeling of TSM15 cells with IgG from patients with Jo-1 antibody–positive myositis (500 μg/mL) (green) or SRP antibody–positive myositis (500 μg/mL) (green) and commercial anti–Jo-1 antibodies (red) or anti-SRP antibodies (red) shows the colocalization of anti–Jo-1 antibodies and Jo-1 (merged in yellow). Scale bar, 50 μm. SRP = signal recognition particle.
Removal of Jo-1 Antibodies From IgGs From Patients With Jo-1 Antibody Myositis Reduces Complement-Dependent Cytotoxicity
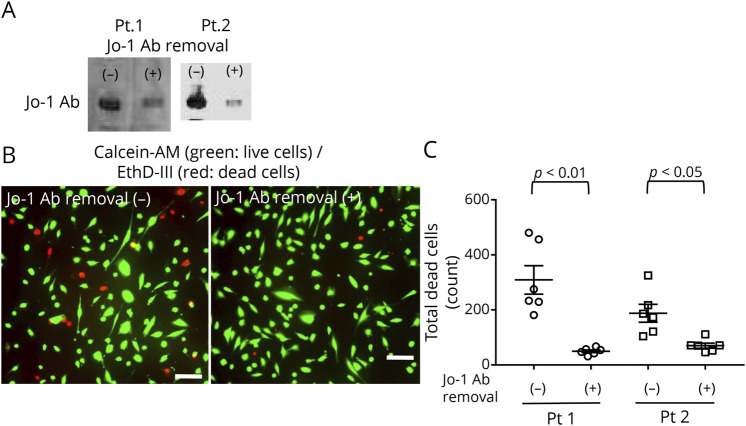
We prepared 2 Jo-1 IgGs (one with Jo-1 antibodies and one without Jo-1 antibodies) from 2 patients from Jo-1 antibody–positive myositis using immunoprecipitation (eFigure 6, links.lww.com/NXI/A856). Western blotting demonstrated the reduction of Jo-1 antibodies (Figure 6A). The removal of Jo-1 antibodies from Jo-1 IgGs from these 2 patients with Jo-1 antibody–positive myositis resulted in a significant reduction in complement-dependent cellular cytotoxicity (Figure 6, B and C).
Figure 6. Effect of the Removal of Jo-1 Antibodies From Jo-1 Antibody Myositis on Complement-Dependent Cytotoxicity in TSM15 Cells.
(A) Western blotting demonstrated the reduction of Jo-1 antibodies. (B) Representative immunostaining to detect complement-dependent cytotoxicity in TSM15 cells before and after the reduction of Jo-1 antibodies. Calcein-AM stains live cells green while EthD-III stains dead cells red. Scale bar, 100 μm. (C) The removal of Jo-1 antibodies from 2 IgGs (500 µg/mL) with Jo-1 antibody myositis significantly decreased the complement-dependent cytotoxicity in TSM15 cells. Data are shown as the mean ± SEM of 6 independent experiments. Statistical significance was determined by an unpaired 2-tailed t-test. Jo-1 Ab removal (−), Jo-1-IgG before the reduction of Jo-1 autoantibodies; Jo-1 Ab (+), Jo-1-IgG after the reduction of Jo-1 autoantibodies.
Discussion
Based on the pathologic observation of muscle biopsy specimens, the pathomechanism of IIM is considered to differ between DM and ASS and between PM and sIBM.18 In DM, the primary target of the immune response is the vascular endothelium of perimysial and perifascicular blood vessels: Inflammatory cells infiltrate at perivascular sites and complement deposition of the terminal C5b-9 MAC around the muscle capillaries leads to endothelial damage.15,16 In PM and sIBM, the primary target of the immune response is the muscle fibers: Active invasion of non-necrotic muscle fibers by autoaggressive cytotoxic CD8+ T cells and macrophages causes muscle damage.21 In IMNM, the accumulation of complement C5b-9 with the deposition of IgG was observed, suggesting the role of a complement-mediated mechanism in muscle necrosis, although this phenomenon may be secondary because complement has been shown to accumulate with fibers with necrosis from any cause, including dystrophy.22 In Jo-1 antibody–positive myositis, whether the primary target of the immune response is the vascular endothelium remains a matter of debate; however, perifascicular necrosis and vascular changes around the muscle capillaries are observed in muscle biopsy specimens of patients with Jo-1 antibody–positive myositis.23 As pathologic examination involves inherent limitations, an in vitro model could be useful for further understanding the molecular mechanism of endothelial dysfunction in IIM.
We recently established a human skeletal muscle microvascular endothelial cell line, TSM15, which harbors tsA58 and telomerase genes. These cells have tight junction proteins (claudin-5, occludin, and ZO-1), transporters, and high transendothelial electrical resistance.17 In this study, we used TSM15 cells and first showed that IgG derived from patients with IIM can bind to and have an effect on TSM15 cells. The IgGs from Jo-1 antibody–positive myositis could bind to TSM cells and induce the complement-dependent cellular cytotoxicity in TSM15 cells in comparison with IgGs from PM, DC, and HC groups. We next examined the results of whole RNA-seq and the pathway analysis and found that the genes associated with TNF-α and TREM-1 were significantly upregulated after exposure to IgG from the Jo-1, SRP, and PM groups in comparison with HCs. The high-content imaging system showed that the amount of TREM-1 in the Jo-1, SRP, and PM groups was increased in comparison with the DC and HC groups and that the amount of TNF-α in the Jo-1 group was higher than that in the SRP, PM, DC, and HC groups. Furthermore, the expression of Jo-1 was observed on the cell surface of TSM15 cells, and double immunostaining with a commercial anti–Jo-1 antibody and IgG from patients with Jo-1 antibody myositis demonstrated colocalization in TSM15 cells, suggesting that IgG from patients with Jo-1 antibody myositis reacted with Jo-1. The depletion of Jo-1 antibody from IgG from patients with Jo-1 antibody myositis reduced TSM15 cell death induced by Jo-1 antibody and complement. These results suggest that IgGs from patients with Jo-1, SRP, and PM increase the TREM-1 expression on TSM15 cells and that Jo-1 antibodies from patients with Jo-1 antibody myositis cause complement-dependent cellular cytotoxicity in TSM15 cells.
TREM-1 is an immune receptor expressed on neutrophils, monocytes/macrophages, and endothelial cells, which exerts proinflammatory effects in both infectious and noninfectious diseases by Toll-like receptor and/or nucleotide-binding and oligomerization domain-like receptor signaling.24-28 TREM-1 plays an important role in several acute and chronic diseases, including ischemia reperfusion, septic shock, pancreatitis, atherosclerosis, inflammatory bowel disease, rheumatic disease, psoriasis, and cancer.29 In addition, the overexpression of TREM-1 is associated with neurodegenerative disorders, such as Alzheimer disease and ischemia/stroke.30 The TREM-1 receptor and its signaling pathways contribute to driving inflammation through the induction of nuclear factor-κB; cyclic adenosine monophosphate-responsive element-binding protein; activator protein 1 and ETS like-1 protein signaling; and the transcription of several proinflammatory cytokines such as TNF-α, interferon type 1, and chemokines.29,31 It is known that DM is generally associated with increased interferon type 1 levels.32 Previous reports suggest the expression of M×A, another type 1 interferon-inducible antiviral protein, on both perifascicular capillaries and muscle fibers in muscle biopsy specimens from patients with DM.15 TREM-1 has 2 forms as a membrane-bound receptor and soluble protein. Membrane TREM-1 features 3 distinct domains: an Ig-like structure (most likely responsible for ligand binding); a transmembrane part; and a cytoplasmic tail, which associates with the adapter molecule DNAX activating protein of 12kDa.31 Soluble TREM-1 (sTREM-1) lacks the transmembrane and intracellular domain and thus has no signal transduction properties.33 The main function of sTREM-1 is the neutralization of TREM-1 inflammatory activity. Increased serum sTREM-1 has been reported in some diseases, including myocardial infarction, inflammatory bowel disease, and rheumatoid arthritis.29,30 In this study, we first observed that IgGs from patients with IIM, including Jo-1, SRP, and PM, induced the upregulation of TREM-1 and its expression in the perimysial blood vessels of patients with DM and the muscle of patients with DM, SRP, and Jo-1, suggesting that TREM-1 triggers inflammation and causes the migration of inflammatory cells through the secretion of proinflammatory cytokines, such as TNF-α and chemokines in patients with IIM with Jo-1, DM, or SRP. The activation of TREM-1 may not be associated with Jo-1 antibodies because this activation was observed not only in patients with Jo-1 but also in patients with DM and SRP.
We first clarified the role of Jo-1 antibodies in the perimysial blood vessels of patients with IIM: Jo-1 antibodies from patients with Jo-1 antibody–positive myositis gave rise to complement-dependent cellular cytotoxicity in TSM15 cells. These results support the pathologic observation demonstrating the deposition of C5b-9 complement MAC and the loss of clustered capillaries on endo/perimysial capillaries in patients with Jo-1 antibody–positive myositis and dermatomyositis.15,22 The binding of Jo-1 antibodies on the cell membrane of Jo-1 in muscle capillaries can induce the deposition of complement and causes lysis of endomysial capillaries and muscle ischemia, resulting in the perifascicular atrophy and necrosis that are observed in Jo-1 antibody–positive myositis. To stop the inhibition of complement-induced cell damage, eculizumab, which targets C5 and inhibits the cleavage of C5 to C5a and C5b-9, may be a promising drug for the treatment of Jo-1 antibody–positive myositis.34 The role of complement on IMNM remains to be elucidated35 because this study also showed that IgGs from IMNM (SRP antibody) did not induce complement-mediated cell death in muscle endothelial cells, which is consistent with the low accumulation of complement in the capillaries of muscle biopsy specimens from patients with IMNM. In addition, a clinical trial of anti-C5 monoclonal antibodies for patients with IMNM failed to show significant clinical effects.35 It is still unclear whether other myositis-specific autoantibodies, including other ARS (PL7, PL12, EJ, OJ, KS), Mi-2, TIF-1γ, NXP2, MDA5, and HMGCR antibodies, affect the complement-dependent cellular cytotoxicity in the muscle capillaries. Further studies will be required to address this question. Two limitations of this study were that it lacked data obtained from primary cultured cells from patients with myositis and that it did not include an in vivo analysis.
In conclusion, our data show that IgG from patients with IIM increases the TREM-1 expression of muscle capillaries and that Jo-1 antibodies from patients with Jo-1 antibody–positive myositis cause complement-dependent endothelial cell damage, thus indicating the potential ability to trigger inflammation in both perifascicular capillaries and muscle fibers in DM and AAS. Further studies to clarify the effect of other myositis-specific autoantibodies on muscle endothelial cells using our in vitro model will be required to understand the pathophysiology of several IIM-specific autoantibodies.
Glossary
- ALS
amyotrophic lateral sclerosis
- ANOVA
analysis of variance
- ARS
aminoacyl-tRNA synthetases
- ASS
anti-synthetase syndrome
- DCs
disease controls
- DM
dermatomyositis
- FC
fold-change
- HCs
healthy controls
- HMGCR
3-hydroxy-3-methylglutaryl coenzyme
- IgG
immunogloblin G
- IIMs
idiopathic inflammatory myopathies
- IMNM
immune-mediated necrotizing myopathy
- IPA
ingenuity pathway analysis
- JC-1
JC-1 MitoMP Detection Kit
- MAC
membrane attack complex
- MDA5
melanoma differentiation-associated gene 5
- NXP2
nuclear matrix protein 2
- PBS
phosphate-buffered saline
- PM
polymyositis
- PVDF
polyvinylidene difluoride
- sIBM
sporadic inclusion body myositis
- SRP
signal recognition particle
- sTREM-1
soluble TREM-1
- TIF
transcription intermediary factor
- TNF-α
tumor necrosis factor-α
- TPM
transcripts per million
- TREM-1
triggering receptor expressed on myeloid cells-1
- tsA58
temperature-sensitive SV40 T antigen
Appendix. Authors
Study Funding
Research grants (Nos. 18K07526 and 20H00529) from the Japan Society for the Promotion of Science, Tokyo, Japan, and Takeda research foundation.
Disclosure
All authors report no financial disclosure relevant to the submitted manuscripts. T. Kanda received speaking fees outside this work from Teijin Phama Limited, Nihon Pharmaceutical Co., Ltd., Kaketsuken, Mitsubishi Tanabe Pharma, Takeda Pharmaceutical company, Novartis, and Biojen. Go to Neurology.org/NN for full disclosures.
References
- 1.Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004; 14(5): 337-345. 10.1016/j.nmd.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 2.Senécal JL, Raynauld JP, Troyanov Y. Editorial: a new classification of adult autoimmune myositis. Arthritis Rheumatol. 2017;69(5):878-884. doi. 10.1002/art.40063 [DOI] [PubMed] [Google Scholar]
- 3.Dalakas MC. Inflammatory myopathies: update on diagnosis, pathogenesis and therapies, and COVID-19-related implications. Acta Myol. 2020;39(4):289-301. doi. 10.36185/2532-1900-032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghirardello A, Doria A. New insights in myositis-specific autoantibodies. Curr Opin Rheumatol. 2018;30(6):614-622. doi. 10.1097/bor.0000000000000548 [DOI] [PubMed] [Google Scholar]
- 5.Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5(2):109-129. doi. 10.3233/jnd-180308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouwer R, Hengstman GJ, Vree Egberts W, et al. Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis. 2001;60(2):116-123. doi. 10.1136/ard.60.2.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allenbach Y, Drouot L, Rigolet A, et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine. 2014;93(3):150-157. doi. 10.1097/md.0000000000000028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengstman GJ, ter Laak HJ, Vree Egberts WT, et al. Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis. 2006;65(12):1635-1638. doi. 10.1136/ard.2006.052191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monti S, Montecucco C, Cavagna L. Clinical spectrum of anti-Jo-1-associated disease. Curr Opin Rheumatol. 2017;29(6):612-617. doi. 10.1097/bor.0000000000000434 [DOI] [PubMed] [Google Scholar]
- 10.De Paepe B. Vascular changes and perifascicular muscle fiber damage in dermatomyositis: another question of the chicken or the egg that is on our mind. Ann Transl Med. 2017;5(1):22. doi. 10.21037/atm.2016.12.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Visser M, Emslie-Smith AM, Engel AG. Early ultrastructural alterations in adult dermatomyositis. J Neurol Sci. 1989;94(1-3):181-192. doi. 10.1016/0022-510x(89)90228-1 [DOI] [PubMed] [Google Scholar]
- 12.Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990;27(4):343-356. doi. 10.1002/ana.410270402 [DOI] [PubMed] [Google Scholar]
- 13.Jain A, Sharma MC, Sarkar C, et al. Detection of the membrane attack complex as a diagnostic tool in dermatomyositis. Acta Neurol Scand. 2011;123(2):122-129. doi. 10.1111/j.1600-0404.2010.01353.x [DOI] [PubMed] [Google Scholar]
- 14.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;372(18):1734-1747. doi. 10.1056/nejmra1402225 [DOI] [PubMed] [Google Scholar]
- 15.Lahoria R, Selcen D, Engel AG. Microvascular alterations and the role of complement in dermatomyositis. Brain. 2016;139(7):1891-1903. doi. 10.1093/brain/aww122 [DOI] [PubMed] [Google Scholar]
- 16.Kissel JT, Mendell JR, Rammohan KW. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med. 1986;314(6):329-334. doi. 10.1056/nejm198602063140601 [DOI] [PubMed] [Google Scholar]
- 17.Sano H, Sano Y, Ishiguchi E, et al. Establishment of a new conditionally immortalized human skeletal muscle microvascular endothelial cell line. J Cell Physiol. 2017;232(12):3286-3295. doi. 10.1002/jcp.25772 [DOI] [PubMed] [Google Scholar]
- 18.Shimizu F, Takeshita Y, Sano Y, et al. GRP78 antibodies damage the blood-brain barrier and relate to cerebellar degeneration in Lambert-Eaton myasthenic syndrome. Brain. 2019;142(8):2253-2264. doi. 10.1093/brain/awz168 [DOI] [PubMed] [Google Scholar]
- 19.Kohno M, Kobayashi S, Yamamoto T, et al. Enhancing calmodulin binding to cardiac ryanodine receptor completely inhibits pressure-overload induced hypertrophic signaling. Commun Biol. 2020;3(1):714. doi. 10.1038/s42003-020-01443-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu F, Ogawa R, Mizukami Y, et al. GRP78 antibodies are associated with blood-brain barrier breakdown in anti-myelin oligodendrocyte glycoprotein antibody-associated disorder. Neurol Neuroimmunol Neuroinflamm. 2021;9(1):e1038. doi. 10.1212/nxi.0000000000001038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971-982. doi. 10.1016/s0140-6736(03)14368-1 [DOI] [PubMed] [Google Scholar]
- 22.Dalakas MC. Myositis: are autoantibodies pathogenic in necrotizing myopathy? Nat Rev Rheumatol. 2018;14(5):251-252. doi. 10.1038/nrrheum.2018.54 [DOI] [PubMed] [Google Scholar]
- 23.De Paepe B, De Bleecker JL. The nonnecrotic invaded muscle fibers of polymyositis and sporadic inclusion body myositis: on the interplay of chemokines and stress proteins. Neurosci Lett. 2013;535:18-23. doi. 10.1016/j.neulet.2012.11.064 [DOI] [PubMed] [Google Scholar]
- 24.Mescam-Mancini L, Allenbach Y, Hervier B, et al. Anti-Jo-1 antibody-positive patients show a characteristic necrotizing perifascicular myositis. Brain. 2015;138(9):2485-2492. doi. 10.1093/brain/awv192 [DOI] [PubMed] [Google Scholar]
- 25.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410(6832):1103-1107. doi. 10.1038/35074114 [DOI] [PubMed] [Google Scholar]
- 26.Matesanz-Isabel J, Sintes J, Llinàs L, de Salort J, Lázaro A, Engel P. New B-cell CD molecules. Immunol Lett. 2011;134(2):104-112. doi. 10.1016/j.imlet.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 27.Rigo I, McMahon L, Dhawan P, et al. Induction of triggering receptor expressed on myeloid cells. (TREM-1) in airway epithelial cells by 1,25(OH)2 vitamin D3. Innate Immun. 2012;18(2):250-257. doi. 10.1177/1753425911399796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jolly L, Carrasco K, Derive M, Lemarie J, Boufenzer A, Gibot S. Targeted endothelial gene deletion of triggering receptor expressed on myeloid cells-1 protects mice during septic shock. Cardiovasc Res. 2018;114(6):907-918. doi. 10.1093/cvr/cvy018 [DOI] [PubMed] [Google Scholar]
- 29.Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM-1 and its potential ligands in non-infectious diseases: from biology to clinical perspectives. Pharmacol Ther. 2017;177:81-95. doi. 10.1016/j.pharmthera.2017.02.043 [DOI] [PubMed] [Google Scholar]
- 30.Natale G, Biagioni F, Busceti CL, Gambardella S, Limanaqi F, Fornai F. TREM receptors connecting bowel inflammation to neurodegenerative disorders. TREM Receptors Connecting Bowel Inflamm Neurodegenerative Disord Cell. 2019;8(10):1124. doi. 10.3390/cells8101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003; 3(6): 445-453. 10.1038/nri1106 [DOI] [PubMed] [Google Scholar]
- 32.Haq SA, Tournadre A. Idiopathic inflammatory myopathies: from immunopathogenesis to new therapeutic targets. Int J Rheum Dis. 2015;18(8):818-825. doi. 10.1111/1756-185x.12736 [DOI] [PubMed] [Google Scholar]
- 33.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7(12):1266-1273. doi. 10.1038/ni1411 [DOI] [PubMed] [Google Scholar]
- 34.Moghadam-Kia S, Oddis CV, Aggarwal R. Modern therapies for idiopathic inflammatory myopathies (IIMs): role of biologics. Clin Rev Allergy Immunol. 2017;52(1):81-87. doi. 10.1007/s12016-016-8530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalakas MC. Complement in autoimmune inflammatory myopathies, the role of myositis-associated antibodies, COVID-19 associations, and muscle amyloid deposits. Expert Rev Clin Immunol. 2022;18(4):413-423. doi. 10.1080/1744666x.2022.2054803 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided within this article are available in anonymized form and can be shared by request from any qualified investigator. Sharing requires approval of a data transfer agreement by Yamaguchi University.