Abstract
Background and Objectives
SARS-CoV-2 infection has been associated with a syndrome of long-term neurologic sequelae that is poorly characterized. We aimed to describe and characterize in-depth features of neurologic postacute sequelae of SARS-CoV-2 infection (neuro-PASC).
Methods
Between October 2020 and April 2021, 12 participants were seen at the NIH Clinical Center under an observational study to characterize ongoing neurologic abnormalities after SARS-CoV-2 infection. Autonomic function and CSF immunophenotypic analysis were compared with healthy volunteers (HVs) without prior SARS-CoV-2 infection tested using the same methodology.
Results
Participants were mostly female (83%), with a mean age of 45 ± 11 years. The median time of evaluation was 9 months after COVID-19 (range 3–12 months), and most (11/12, 92%) had a history of only a mild infection. The most common neuro-PASC symptoms were cognitive difficulties and fatigue, and there was evidence for mild cognitive impairment in half of the patients (MoCA score <26). The majority (83%) had a very disabling disease, with Karnofsky Performance Status ≤80. Smell testing demonstrated different degrees of microsmia in 8 participants (66%). Brain MRI scans were normal, except 1 patient with bilateral olfactory bulb hypoplasia that was likely congenital. CSF analysis showed evidence of unique intrathecal oligoclonal bands in 3 cases (25%). Immunophenotyping of CSF compared with HVs showed that patients with neuro-PASC had lower frequencies of effector memory phenotype both for CD4+ T cells (p < 0.0001) and for CD8+ T cells (p = 0.002), an increased frequency of antibody-secreting B cells (p = 0.009), and increased frequency of cells expressing immune checkpoint molecules. On autonomic testing, there was evidence for decreased baroreflex-cardiovagal gain (p = 0.009) and an increased peripheral resistance during tilt-table testing (p < 0.0001) compared with HVs, without excessive plasma catecholamine responses.
Discussion
CSF immune dysregulation and neurocirculatory abnormalities after SARS-CoV-2 infection in the setting of disabling neuro-PASC call for further evaluation to confirm these changes and explore immunomodulatory treatments in the context of clinical trials.
Severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2), the pathogen responsible for coronavirus disease 2019 (COVID-19), has caused morbidity and mortality at an unprecedented scale.1 Survivors of previous coronavirus infections, including the SARS epidemic in 2003, have demonstrated a constellation of persistent symptoms,2 and clinical evidence has emerged on the long-term consequences of COVID-19 that can similarly affect multiple organ systems.3
Often referred to as long COVID or long-haul COVID,4 these symptoms, which include fatigue, dyspnea, cognitive and mental disorders, pain, smell and taste dysfunction, and gastrointestinal issues,5 may persist for months after onset of symptoms. Taken together, these symptoms are termed postacute sequelae of SARS-CoV-2 infection (PASC), and although clear diagnostic criteria are yet to be established, PASC is being commonly defined as persistent symptoms beyond 4–12 weeks from onset of acute symptoms.6 Follow-up studies of patients after COVID-19 have reported that 30%–80% develop symptoms lasting 1–6 months.7,8 This syndrome can occur in various populations, including children9 and young adults and those who had only mild COVID-19.10
Reported neurologic and psychiatric symptoms of PASC (neuro-PASC) range from headaches11 and loss of taste and smell12 to sleep disturbances13 and cognitive impairment with difficulties in concentration, language and executive function,14 and clinically significant depression and anxiety.15 There have also been suggestions of autonomic neurocirculatory abnormalities,16 including orthostatic intolerance syndromes.17
The main proposed mechanisms of neuropathology in acute COVID-19 are systemic inflammation, neuroinflammation, and microvascular injury with leakage of blood products into the parenchyma and microthrombosis,14,18 with only rare reports suggesting direct viral invasion of the nervous system.19,20 Several studies in patients with subacute neuro-COVID noted activation of innate immune responses,21,22 including autopsy studies supporting this as a potential mechanism.18,23 For neuro-PASC, some reports suggest possible localization of autonomic symptoms16 to brainstem dysfunction or vascular injury.24 A postviral immune-mediated process appears to be the most likely cause in this context,25 and some immune dysregulation features were reported in long-COVID cohorts.26-28 Overall, however, the pathophysiology of neuro-PASC remains unclear.29
The goal of this report is to describe and characterize the clinical and laboratory features of neuro-PASC in patients who had persistent neurologic symptoms following SARS-CoV-2 infection and who underwent an intensive investigation at the NIH Clinical Center, including clinical examination, questionnaires and brain imaging, extensive analysis of blood and CSF samples, and autonomic testing.
Methods
Study Participants
All participants were first recruited and screened through a post–COVID-19 convalescence study at the NIH (NCT04573062), from which those with neurologic symptoms were referred to an observational study specifically designed to characterize ongoing neurologic abnormalities (NCT04564287). Participants at least 18 years of age were eligible for participation if they met the following inclusion criteria: positive SARS-CoV-2 laboratory result at least 6 weeks before enrollment, persistent neurologic symptoms, no contraindication to a lumbar puncture (e.g., anticoagulation), MRI (e.g., implanted metal), or autonomic testing (e.g., symptomatic coronary artery disease), and no condition before the diagnosis of COVID-19 that would confound interpretation of the tests (e.g., prior diagnosis of orthostatic hypotension).
Autonomic function and CSF immunophenotypic analysis obtained in this study were compared with data collected before the SARS-CoV-2 pandemic from healthy volunteers (HVs) tested under other NIH studies NCT02669212 or NCT01875588. The autonomic testing and CSF analysis pipelines were identical in methodology to allow for direct comparisons of the neuro-PASC and HV groups.
Standard Protocol Approvals, Registrations, and Patient Consents
The studies mentioned above were approved by the Institutional Review Board at the NIH. Written informed consent was obtained from all participants.
Demographic and Clinical Variables
All participants underwent comprehensive clinical evaluation including a detailed history and a neurologic examination by a study neurologist, with a standardized inventory on COVID-19 history and recovery and the neurologic symptoms before, during, and after COVID-19. Neurologic assessment also included determination of the Karnofsky Performance Status (KPS) and completion of the Montreal Cognitive Assessment (MoCA) and the University of Pennsylvania Smell Identification Test (UPSIT) as well as 6 Patient-Reported Outcome Measurement Information System (PROMIS) questionnaires30 (Pain Intensity, Fatigue, Cognitive Function Abilities, Anxiety, Depression, and Sleep Disturbance).
Biofluids
Blood tests included SARS-CoV-2 nucleocapsid antibody (Elecsys, Roche Diagnostics GmBH, Germany), vitamin B12 level, thyroid-stimulating hormone, serum protein electrophoresis, antiphospholipid panel, d-dimer, erythrocyte sedimentation rate (ESR), and plasma catechols. A lumbar puncture was performed to obtain CSF for research assays including white blood cell count, protein, glucose, IgG index, oligoclonal bands (OCBs), catechols, and immunophenotyping using multicolor flow cytometry.31 Serum and CSF samples were also analyzed by an electrochemiluminescence multiplex immunoassay (Meso Scale Discovery) to determine levels of antibodies directed against 3 different SARS-CoV-2 antigens (spike protein, nucleocapsid protein, and receptor-binding domain [RBD]).
Brain MRI
All participants completed a brain MRI on a 3 T Philips Achieva scanner (Philips Medical Systems, Best, The Netherlands) with a 32-channel head coil, using a standardized protocol that included the following whole-brain sequences: pre- and post-contrast (Gadavist [gadobutrol], 0.1 mmol/kg), 3D fluid-attenuated inversion recovery (3D-FLAIR) (repetition time [TR]/effective echo time [TE]/inversion time [TI] 4,800/279/1,650 ms), sagittal pre- and post-contrast T1-weighted (TR/TE: 600/28.33), axial diffusion-weighted (TR/TE: 8,618/65, diffusion b-value: 1,000), and axial double-echo T2-proton density weighted (TR/TEs: 3,532/100,15.4). Additional coronal short tau inversion recovery images through the olfactory bulbs (TR/TE/TI: 4,000/54/200) were obtained. All scans were read by a neuroradiologist specializing in imaging of infectious diseases (D.A.H.).
Autonomic Function Testing
Participants underwent continuous physiologic monitoring during the Valsalva maneuver and tilt-table testing. Setup involved placing an IV catheter in an antecubital vein for blood drawing and placement of several noninvasive monitoring devices including a brachial automated cuff for measurement of forearm blood flow (FBF) by impedance plethysmography (D. E. Hokanson, Inc., Bellevue, WA), metal plates on fingers for tracking skin electrical conductance (SEC, a measure of sweating), a thermistor for finger temperature, a brachial cuff for manual blood pressure measurement, and a finger cuff for continuous tracking of blood pressure (Nexfin system, BMEYE, Amsterdam, the Netherlands; or Nova system, Finapres Medical Systems, Amsterdam, The Netherlands). Cardiac stroke volume (SV) in mL was calculated and displayed from software bundled with the Nexfin and Nova systems.
Brachial blood pressure was obtained during supine rest and tilting. After ≥15 minutes with the participant supine, baseline heart rate variability (HRV) data were obtained over at least 3 minutes, and a blood sample was drawn through the IV for assay of plasma concentrations of catechols. Then, each participant performed a Valsalva maneuver (30 mm Hg, 12 seconds) at least 3 times until a technically adequate tracing was obtained.32 The patient was then tilted head-up at 70° from horizontal. Blood pressure (BP) and heart rate (HR) were recorded, and blood samples drawn through the IV every 4 minutes during the tilting, for up to 40 minutes, or until the testing was stopped due to sudden hypotension or presyncopal symptoms. Data analysis included additional variables: (1) Baroreflex-cardiovagal gain, quantified from the slope of the linear relationship between cardiac interbeat interval (in ms) and the systolic BP (in mm Hg) during Phase II of the Valsalva maneuver, as described previously. (2) Baroreflex-sympathoneural function, using the baroreflex areas method for Phases II and III/IV of the Valsalva maneuver.33 (3) Pressure recovery time (in seconds) after release of the Valsalva maneuver. (4) HRV in the time and frequency domains assessed using the HRV module bundled with the PowerLab recording device (LabChart 8.1.13, ADInstruments, Colorado Springs, CO). Low-frequency and high-frequency (HF) power of HRV in ms2 were measured from the continuously recorded electrocardiogram. In the time domain, the standard deviation of interbeat intervals for normal beats (SDNN) and coefficient of variation of interbeat intervals for normal beats (CVNN) were calculated. (5) Cardiac output (CO) was calculated from the SV times the simultaneously measured HR. (6) Forearm vascular resistance (in units of mm Hg·min/dL) was calculated from the mean arterial pressure (MAP) divided by FBF. (7) Total peripheral resistance (TPR, in units of mm Hg·min/L) was calculated from the MAP (−6 mm Hg for assumed central venous pressure), the quantity divided by the CO. Plasma and CSF catechols (including catecholamines) were assayed by batch alumina extraction followed by liquid chromatography with series electrochemical detection, as described previously.34
Statistical Analyses
Descriptive statistics were used to present frequencies of clinical, laboratory, and imaging abnormalities, further divided into categories for any findings that could be graded. Data from tilt-table testing and flow cytometry were also compared with historical groups of HVs who underwent the same procedures. Differences in variables between groups were examined using the Fisher exact test for categorical variables, 2-sample t tests for continuous variables with normal distributions, and the Wilcoxon 2-sample test for continuous variables with non-normal distributions. For repeated measures data during head-up tilt table testing, between-within (2-way) analyses of variance were used, with calculation of p values for the time effect, the group effect, and the time-group interaction effect. When appropriate, a mixed-effects analysis using a restricted maximum likelihood model was applied.
An exploratory correlation analysis was performed to test for associations among clinical and laboratory parameters of the neuro-PASC cohort, using the Fisher exact test for associations between 2 categorical variables, Point-Biserial correlation for a continuous variable and a categorical variable, and Pearson correlation coefficients for 2 continuous variables.
Statistical analyses were performed using SAS version 9.4. A significance level (α) of 0.05 was used for all statistical tests.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Results
Clinical Characteristics
Between October 2020 and March 2021, 12 participants were recruited from a cohort of 173 individuals (see Figure 1A for flowchart of inclusion in the study). Demographics and clinical characteristics of these patients are detailed in Table 1. Participants were all White and mostly female (83%), with a mean age of 45 ± 11 years. The majority of participants had a history of depression/anxiety before COVID-19 (n = 7, 58%). A third of the patients (n = 4) had a prior history of resolved long-term disability after an infectious disease (infectious mononucleosis, Lyme disease, amoebiasis due to Entamoeba histolytica, and severe sepsis due to group A Streptococcus).
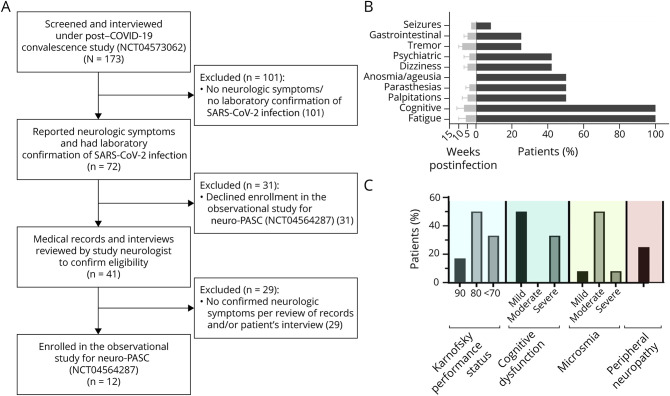
Figure 1. Flowchart for Inclusion in the Study and Symptoms and Signs of Neurologic Postacute Sequelae of SARS-CoV-2 Infection (Neuro-PASC).
(A) Flowchart describing the process of including/excluding participants for this observational study. (B) Symptoms of neuro-PASC; bars on the right represent the frequency of symptoms in the cohort (%), whereas the bars on the left represent the time of onset after SARS-CoV-2 infection (mean ± SD). (C) Neurologic findings in neuro-PASC; bars represent the frequency of signs in the cohort (%). COVID-19 = coronavirus disease 2019; NCT = National Clinical Trial.
Table 1.
Clinical Characteristics of Patients With Neurologic Postacute Sequelae of SARS-CoV-2 (Neuro-PASC)

COVID-19 was diagnosed between March and December 2020 by PCR in 10 of 12 cases and serologic testing in the other 2 cases. COVID-19 symptoms were mostly mild (11/12, 92%), with 1 patient meeting the criteria for moderate disease due to transient documented hypoxemia that did not require hospitalization. Symptoms of acute COVID-19 are detailed in Table 1. Overall, symptoms were typical, with cough being the most common (10/12, 83%). Three patients described onset of paresthesias during the acute illness, and 1 patient had acute onset of severe depression that required hospitalization.
Patients were seen at a median of 9 months after acute COVID-19 (range 3–12 months). Only 1 participant had already received a SARS-CoV-2 vaccine before being evaluated. Frequencies of persistent symptoms are described in Figure 1B. The most common symptoms were fatigue and cognitive difficulties. Some patients described symptoms suggesting an autonomic disorder such as palpitations (n = 6, 50%), orthostatic dizziness (n = 5, 42%), and gastrointestinal symptoms (n = 3, 25%). Dysgeusia/anosmia was reported in 6 patients. Of interest, 5 participants (42%) had worsening of anxiety/depression requiring new or adjusted psychiatric drug regimen. One participant, a 51-year-old woman, had new-onset brief episodes of disorientation and was found to have right temporal epileptiform activity on electroencephalography, leading to initiation of antiepileptic treatment.
Neurologic examination showed signs consistent with mild peripheral neuropathy in 3 participants (25%). Per the cognitive assessment, there was evidence for mild cognitive impairment in half (n = 6) of the patients (MoCA score <26). The most commonly affected domain on MoCA was short-term memory, for which all participants had some degree of impairment, and 4 (33%) had evidence for severe dysfunction (scored 0–1 of 5).
As determined by the KPS, 4 participants (33%) had a score of 70, indicating inability to carry on normal activity. Six participants (50%) had a score of 80 indicating normal activity with effort, and 2 (17%) had only minor signs or symptoms (score of 90). Smell testing using UPSIT demonstrated different degrees of microsmia in 8 participants (66%): mild (n = 1), moderate (n = 6), and severe (n = 1). Figure 1C summarizes the findings on these neurologic evaluations. On PROMIS questionnaires, the most prominent findings were high T-scores for reported fatigue (65 ± 9, range 44–72) and low T-scores for reported cognitive function abilities (35 ± 9, range 26–59). Results from depression, anxiety, and sleep disturbance questionnaires were only slightly increased (T-scores of 54 ± 6, 53 ± 7, and 53 ± 12, respectively), and pain intensity T-scores were not increased in the group (48 ± 7, range 31–61).
Auxiliary Testing
Brain MRI scans showed that 1 patient had small caliber of the olfactory bulbs consistent with bilateral olfactory bulb hypoplasia (Figure 2). This 34-year-old woman reported phantom burning smells as part of her neuro-PASC and had moderate microsmia per UPSIT. This finding was considered to be congenital in view of bilaterally shallow olfactory fossae and small olfactory sulci. Additional incidental findings included mild chronic small vessel ischemic changes in a 58-year-old woman, a pituitary hyperintense lesion (hemorrhagic adenoma vs proteinaceous Rathke cleft cyst) in a 51-year-old woman, and an enlarged perivascular space in the basal ganglia with adjacent gliosis in a 48-year-old woman.
Figure 2. Bilateral Hypoplastic Olfactory Bulbs in a Patient With Neuro-PASC.
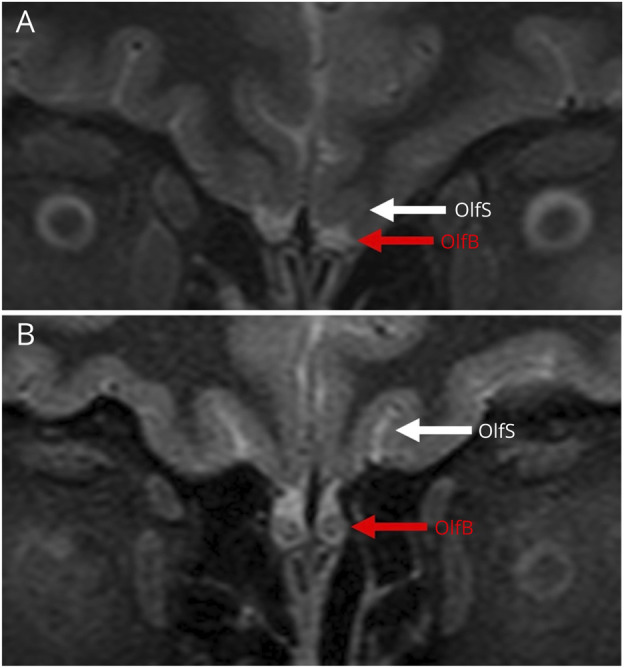
These T2 images from brain scans of study participants show that although most participants (B) had a normal appearance of the olfactory bulbs (OlfB, red arrow) and olfactory sulcus (OlfS, white arrow), 1 participant, a 34-year-old woman with reported parosmia and documented moderate microsmia, had evidence for bilateral hypoplastic olfactory bulbs (A). The presence of shallow olfactory fossae and small olfactory sulci in this patient suggests that this is most likely congenital.
Clinical blood tests were overall unremarkable, specifically with no evidence for increase in d-dimer levels. The ESR was within normal limits in all participants except 1 with mild elevation (53 mm/h). Antibodies to the nucleocapsid protein of SARS-CoV-2 were positive in 9 cases (75%) per the clinical assay, but on the multiplex assay, all the participants had positive serum antibodies directed against the nucleocapsid protein. Eleven of 12 (92%) also had evidence for serum antibodies directed against both the spike protein and RBD.
CSF analysis showed no evidence for pleocytosis in any of the cases (eTable 1, links.lww.com/NXI/A857). One case had mildly increased CSF protein (52 mg/dL) and CSF-serum IgG-index (0.64) but with no evidence for unique intrathecal OCBs. Three cases (25%) had unique intrathecal OCBs, of which 1 case (the previously mentioned 51-year-old woman with new-onset seizures) also had a high CSF-serum IgG index (1.1). These 3 cases did not have any previous history of a disabling systemic infection. CSF antibodies directed against SARS-CoV-2 proteins were positive in a subset of participants, with only the 1 vaccinated participant being positive for all 3 antibodies tested. Among the other participants, 3 were positive for 2 antibodies (nucleocapsid + spike n = 2; RBD + spike n = 1), and 3 participants were positive for a single antibody (spike n = 2; nucleocapsid n = 1). eTable 2 summarizes the antibody titers in the participants.
The main findings of immunophenotyping of CSF are presented in Figure 3. Due to technical issues, data were available from 11 participants with neuro-PASC. Compared with HVs, patients with neuro-PASC had lower frequencies of effector memory phenotype (CD45RA−CD27−) both for CD4+ T cells (11.8 ± 5 vs 22.2 ± 7, p < 0.0001) and for CD8+ T cells (5.3 ± 3 vs 10.7 ± 6, p = 0.002) and an increased frequency of B cells (CD3−CD19+: 1.9 ± 1.7 vs 0.8 ± 0.7, p = 0.02) and specifically increased antibody-secreting B cells (IgD−CD27high: 7.6 ± 15 vs 0 ± 0, p = 0.009). In addition, patients with neuro-PASC had an increased frequency of NK cells (CD3−CD56+: 5.8 ± 3 vs 3.2 ± 2, p = 0.002) and an increased CD56 bright-dim ratio among these cells (0.49 ± 0.3 vs 0.22 ± 0.2, p = 0.009). There was also an increase in cells expressing with T-cell immunoglobulin and ITIM domains (TIGIT) on CD8+ T cells (67.3 ± 10 vs 53.7 ± 12, p = 0.006) and of programmed death ligand 1 (PD-L1) on monocytes (CD3−CD14+: 35.9 ± 21 vs 15.6 ± 9, p = 0.02).
Figure 3. Main Differences Found by Immunophenotyping of Spinal Fluid Between Patients With PASC and HVs.
Each dot represents 1 participant; bars represent SD from mean (top edge of rectangle). Numbers on top crossing line are p values based on the Wilcoxon 2-sample test. HVs = healthy volunteers; NK = natural killer; PASC = postacute sequelae of SARS-CoV-2; PD-L1 = programmed death ligand 1 TIGIT = T-cell immunoglobulin and ITIM domains.
Immunophenotyping of peripheral blood mononuclear cells (PBMCs) confirmed similar lower rates of effector memory T-cells in the neuro-PASC group and higher expression of PD-L1 on monocytes, but the changes in B-cell and NK populations and the increased frequency of TIGIT+ in CD8+ T cells were unique to the CSF (eTable 3, links.lww.com/NXI/A857).
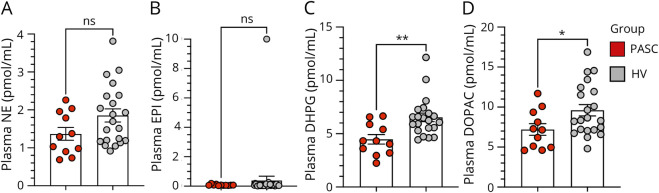
Autonomic testing with measurement of catecholamine levels was completed in 11 patients with neuro-PASC (1 patient failed to complete all parts of autonomic testing due to logistical issues). When compared with data from 22 HVs, CSF levels of catechols did not differ between the groups. The neuro-PASC group had lower mean plasma levels of 3,4-dihydroxyphenylglycol (DHPG) and 3,4-dihydroxyphenylacetic acid (DOPAC) (Figure 4, C and D), whereas levels of other catechols did not differ (Figure 4, A and B and eFigure 1, links.lww.com/NXI/A857). DHPG was still decreased (p = 0.03) after excluding data from participants with neuro-PASC who were on a drug known to interfere with neuronal uptake of catecholamines (tricyclic antidepressant, duloxetine).
Figure 4. Plasma Levels of Catecholamines in Patients With Postacute Sequelae of SARS-CoV-2 Infection (PASC, Red) and Healthy Volunteers (HVs, Gray).
Each dot represents 1 participant; bars represent standard errors of the mean (top edge of rectangle). *p < 0.05 and **p < 0.01 by independent means t tests. (A) Plasma levels of norepinephrine (NE); (B) Plasma levels of epinephrine (EPI); (C) Plasma levels of 3,4-dihydroxyphenylglycol (DHPG); (D) Plasma levels of 3,4-dihydroxyphenylacetic acid (DOPAC). ns = not significant.
For physiologic data obtained during supine rest, data from 1 participant with neuro-PASC who was on a beta-adrenoceptor blocker were excluded from the analysis. Comparison to HVs showed that the neuro-PASC group had a higher mean HR and systolic BP (Figure 5, A and B), lower mean values for indices of HRV in the time domain (SDNN and CVNN) (eFigure 2, C and D, links.lww.com/NXI/A857), lower mean baroreflex-cardiovagal gain (Figure 5C), and lower HF power of HRV (Figure 5D). One participant with PASC had physiologic evidence for baroreflex-sympathoneural failure (outlier in eFigure 2G).
Figure 5. Differences in Physiologic Variables in Patients With Postacute Sequelae of SARS-CoV-2 Infection (PASC, Red) Compared With Healthy Volunteers (HVs, Gray).

Each dot represents 1 participant; bars represent standard errors of the mean (top edge of rectangle). p Values are for independent means t tests. (A) Heart rate (HR); (B) finger systolic blood pressure (BPs); (C) baroreflex-cardiovagal gain; (D) high-frequency (HF) power of heart rate variability; ns = not significant.
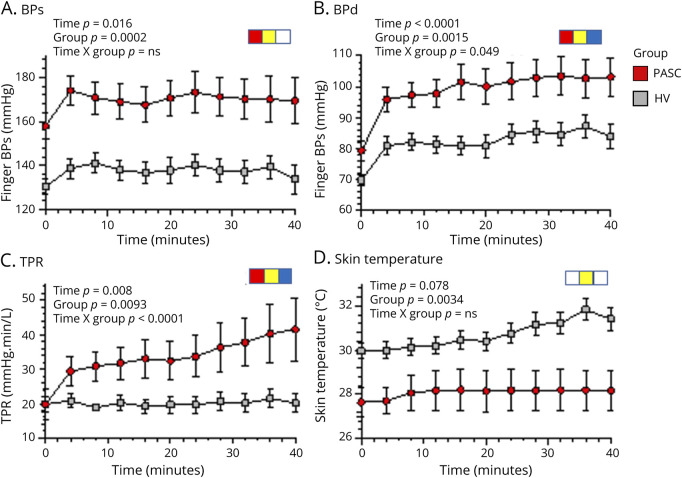
During head-up tilt-table testing, the neuro-PASC group had higher mean values for systolic and diastolic BP (Figure 6, A and B), MAP, and HR (eFigure 3, links.lww.com/NXI/A857) than did HVs. Calculated TPR increased during tilting, with the increase in TPR larger in the neuro-PASC group (significant time-group interaction effect; Figure 6C). Mean skin temperature was lower in the PASC group during supine rest and throughout the period of tilting (Figure 6D). Head-up tilting was associated with progressively increasing plasma norepinephrine and epinephrine levels, without a group difference in mean levels of either catecholamine at baseline or during tilting (eFigure 3). Two of the 11 participants with PASC and 7 of the 22 HVs developed tilt-evoked sudden hypotension, resulting in cessation of the tilt table testing for safety reasons. Removal of data from these participants did not affect obtained group differences in the physiologic measures (eFigure 4).
Figure 6. Differences in Physiologic Variables During Head-Up Tilt Table Testing in Patients With Postacute Sequelae of SARS-CoV-2 Infection (PASC, Red) Compared With Healthy Volunteers (HVs, Gray).
Data are from all participants. Data at each time point are presented as mean (dot) and standard error of the mean (bars). (A) Finger systolic blood pressure (BPs); (B) finger diastolic blood pressure (BPd); (C) total peripheral resistance (TPR); (D) skin temperature; red symbols indicate significant time effect, yellow symbols significant group effect, and blue symbols significant time-group interaction effect in repeated-measures analyses of variance. The PASC group had higher BPs, BPd, and TPR and lower skin temperature than did the HV group. The PASC group had larger increases in BPd and TPR during head-up tilt.
On correlation analysis, several CSF immunophenotyping markers were associated with a lower score on the PROMIS Cognitive-Function Abilities questionnaire including increased frequencies of cells expressing with the checkpoint molecule PD-1 on both CD4+ T cells (r = −0.73, p = 0.01) and CD8+ T cells (r = −0.77, p = 0.005), an increased CD56 bright-dim ratio among NK cells (r = −0.63, p = 0.04), increased frequency of CD4+ T cells (r = −0.7, p = 0.01), and specifically of effector-memory phenotype (r = −0.66, p = 0.03), which specifically also correlated with a higher T-score on the PROMIS-Fatigue questionnaire (r = 0.79, p = 0.004). There were no significant associations among all other clinical and laboratory measures, including the titer of SARS-CoV-2 antibodies in the CSF.
Discussion
This comprehensive analysis of a small group of patients with neuro-PASC revealed a disabling but difficult to characterize syndrome that develops even after relatively mild COVID-19. A common feature is memory impairment. Microsmia was common, although none of our patients had anosmia. CSF analysis showed immunological abnormalities. The strengths of our study are the research-based evaluation of a relatively homogeneous cohort, including advanced spinal fluid analysis, imaging, and autonomic testing.
Clinically, our cohort presented with symptoms similar to those described in reports from larger cohorts and surveys,35 with fatigue and cognitive impairment being the most common and debilitating symptoms, with a high rate of psychological symptoms36 and substantial adverse effect on quality of life.8 Also consistent with other reports, the majority of the participants were young female adults,37 and all our participants were White,37 which could represent an enrollment bias based on access to research and care. During the acute phase of COVID-19, our participants mostly experienced a typical mild disease, except for a relatively HF of neurologic or psychiatric symptoms in our cohort, including multiple participants with symptoms of peripheral neuropathy and 1 case with an acute mood disorder. Neurologic manifestations of acute COVID-19 have previously been reported mainly in patients with severe disease.38 This characteristic of our cohort could represent a bias resulting from recruitment to a neurologically focused study, but still raises the possibility of acute direct or indirect involvement of the nervous system that increases the risk or contributes to the pathogenesis of long-term neurologic manifestations.
As previous studies suggested the possibility of neuro-PASC being a result of brain inflammation associated with neurotropism of SARS-CoV-2 from the olfactory bulb39 and affecting the brainstem,24 we designed an MRI protocol for imaging of the brainstem and olfactory pathways. We could not identify any structural abnormalities in either region that could be correlated with PASC. One case had bilateral hypoplastic olfactory bulbs, which correlated with parosmia and microsmia in this patient, but other imaging features, specifically shallow olfactory fossae and small olfactory sulci, suggested that this probably was a congenital abnormality.40 Nevertheless, this finding calls for further evaluation of olfactory bulb abnormalities, as pathologic changes have been reported previously in patients with COVID-19,18 and 1 study showed that these abnormalities are also correlated with anosmia.41 In addition, hypometabolism of the olfactory gyrus and connected limbic and brainstem structures has been demonstrated and correlated with symptoms in patients with neuro-PASC.42
Immunophenotyping of CSF was performed to identify possible biomarkers of this challenging syndrome. The data support the possibility of immune dysregulation in at least some patients with neuro-PASC. When compared with HVs, the neuro-PASC group had changes in subtypes of immune cells, including a decrease in effector memory T cells with an increase in B cells, antibody-secreting B cells, and activated NK cells. In addition, there was some increase in immune checkpoint molecules like TIGIT on CD8+ T cells and PD-L1 on monocytes, suggesting the possibility of immune exhaustion.43 The persistence of these immune abnormalities several months after a mild infection suggests the possibility of either a persistent infection or an aberrant immune response to the infection. We failed to detect consistent antibody responses in the CSF to the viral antigens. Further studies are needed to determine the target antigens of the oligoclonal bands.
As previously mentioned, the major caveat in these findings is the comparison to historical groups of nonconvalescent healthy controls, as we did not have CSF available from patients who fully recovered from COVID-19. However, because most healthy individuals are exposed to a wide variety of respiratory pathogens during their lifetime, this is still a valid control population. Indeed, some similar features, including T-cell exhaustion44 and B-cell activation, were identified in previous studies of peripheral blood cells of presumably fully recovered patients, sometimes even 10 months after a mild COVID-19 infection.45 Other reports showed that the subgroup with PASC had a more distinct and significant pattern of T-cell exhaustion.26
We found some correlations between reported cognitive abilities and abnormal immunologic markers, consistent with a previous report suggesting that the severity of cognitive deficits or quality of life measures in patients with neuro-PASC were associated with reduced effector molecule expression in memory T cells.27 This, together with the broad and significant findings we found in different cell types, suggests the possibility that immune dysregulation contributes to or at least correlates with some of the symptomatology of neuro-PASC.
Comprehensive autonomic function testing performed in the same patients with neuro-PASC revealed a pattern of neurocirculatory abnormalities compared with HVs. The main positive findings were decreased baroreflex-cardiovagal function, increased BP, HR, and calculated TPR, decreased skin temperature, and a progressive increase in TPR during head-up tilt table testing. On the other hand, the PASC group did not differ from the HV group in terms of baroreflex-sympathoneural function or BP, HR, or plasma catecholamine responses to head-up tilting. The common features here are therefore (1) decreased baroreflex-cardiovagal function and (2) vasoconstriction that seems to be independent of sympathetic noradrenergic activation. Baroreflex-cardiovagal functions are highly sensitive to sensed global threats to homeostasis, whether physical as in heart failure46 or psychological as in posttraumatic stress disorder.47 Regarding the evidence for generalized vasoconstriction based on finger cuff data, it should be noted that local vasoconstriction could result in overestimation of systemic BP and consequently overestimation of TPR. Because there was no evidence of augmented plasma NE responses to tilting in the PASC group, the increases in TPR during tilting in the PASC group may have a mechanism separate from augmented NE release. One such possible mechanism is endothelial dysfunction.48 Both decreased baroreflex-cardiovagal function and vasoconstriction might be related to immune/inflammatory changes.49 Whether the identified group differences are related to symptoms such as brain fog, chronic fatigue, and gravitational deconditioning50 in the PASC group is unknown and cannot be determined from the present data, but previous reports recognized an association of PASC with a dysautonomic component and the possibility of decreased cerebrovascular autoregulation.17
Limitations of this study include primarily the observational nature of the study and the lack of a fully matched control group, specifically the lack of fully recovered COVID-19 convalescent control group to further eliminate potentially confounding variables. As the CSF data were compared with HVs from the pre-COVID era, it is still difficult to associate these changes with the clinical syndrome of neuro-PASC, as these could be changes triggered by the viral infection without any persistent symptoms. The study design did not exclude antecedent autoimmune, inflammatory, or autonomic disorders being exacerbated as a result of COVID-19. However, the correlations between some of these immunologic markers and reported cognitive impairment and the presence of these changes in a group of participants who have only experienced a mild infection, and are far out from this acute infection, could support this association.
Overall, this report describes clinical, laboratory, and imaging findings in a small but comprehensively tested cohort of patients with neuro-PASC. Our results may guide researchers in pursuing further characterization of neuro-PASC, as the natural history and potential subtypes4 of the syndrome are yet to be defined. The preliminary findings suggesting broad immune dysregulation in the CSF call for further investigation and for evaluation of potential immunomodulatory agents in an effort to decrease the huge public health burden of this syndrome.
Acknowledgment
The authors thank the participants and the staff at the NIH Clinical Center.
Glossary
- 3D-FLAIR
3D fluid-attenuated inversion recovery
- BP
blood pressure
- COVID-19
coronavirus disease 2019
- CVNN
coefficient of variation of interbeat intervals for normal beats
- DHPG
3,4-dihydroxyphenylglycol
- DOPAC
3,4-dihydroxyphenylacetic acid
- ESR
erythrocyte sedimentation rate
- FBF
forearm blood flow
- HF
high frequency
- HR
heart rate
- HRV
heart rate variability
- HV
healthy volunteer
- KPS
Karnofsky Performance Status
- MAP
mean arterial pressure
- MoCA
Montreal Cognitive Assessment
- OCBs
oligoclonal bands
- PASC
postacute sequelae of SARS-CoV-2 infection
- PROMIS
Patient-Reported Outcome Measurement Information System
- PRT
pressure recovery time
- RBD
receptor-binding domain
- SDNN
standard deviation of interbeat intervals for normal beats
- SARS-CoV-2
severe acute respiratory distress syndrome coronavirus 2
- SV
stroke volume
- TE
effective echo time
- TI
inversion time
- TR
repetition time
- TPR
total peripheral resistance
- UPSIT
University of Pennsylvania Smell Identification Test
Appendix. Authors
Study Funding
This work was supported by the Intramural Research Programs of the NINDS.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533-534. doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui DS, Joynt GM, Wong KT, et al. . Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401-409. doi: 10.1136/thx.2004.030205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groff D, Sun A, Ssentongo AE, et al. . Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nath A. Long-haul COVID. Neurology. 2020;95(13):559-560. doi: 10.1212/WNL.0000000000010640 [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Huang L, Wang Y, et al. . 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. doi: 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalbandian A, Sehgal K, Gupta A, et al. . Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Borst B, Peters JB, Brink M, et al. . Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2020;73(5):e1089–e1098. doi: 10.1093/cid/ciaa1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021;110(3):914-921. doi: 10.1111/apa.15673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsend L, Dyer AH, Jones K, et al. . Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belvis R. Headaches during COVID-19: my clinical case and review of the literature. Headache. 2020;60(7):1422-1426. doi: 10.1111/head.13841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrigues E, Janvier P, Kherabi Y, et al. . Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4-e6. doi: 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordvig AS, Fong KT, Willey JZ, et al. . Potential neurologic manifestations of COVID-19. Neurol Clin Pract. 2021;11(2):e135-e146. doi: 10.1212/CPJ.0000000000000897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. 2020;12(1):69. doi: 10.1186/s13195-020-00640-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130-140. doi: 10.1016/s2215-0366(20)30462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dani M, Dirksen A, Taraborrelli P, et al. . Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med (Lond) 2021;21(1):e63-e67. doi: 10.7861/clinmed.2020-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak P, Mukerji SS, Alabsi HS, et al. . Multisystem involvement in post-acute sequelae of COVID-19 (PASC). Ann Neurol. 2021;91(3):367-379. doi: 10.1002/ana.26286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M-H, Perl DP, Nair G, et al. . Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2021;384(5):481-483. doi: 10.1056/NEJMc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulfamante G, Chiumello D, Canevini MP, et al. . First ultrastructural autoptic findings of SARS -Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86(6):678-679. doi: 10.23736/s0375-9393.20.14772-2 [DOI] [PubMed] [Google Scholar]
- 20.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. . Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92(7):699-702. doi: 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edén A, Kanberg N, Gostner J, et al. . CSF biomarkers in patients with COVID-19 and neurologic symptoms: a case series. Neurology. 2021;96(2):e294-e300. doi: 10.1212/wnl.0000000000010977 [DOI] [PubMed] [Google Scholar]
- 22.Heming M, Li X, Räuber S, et al. . Neurological manifestations of COVID-19 feature T cell exhaustion and dedifferentiated monocytes in cerebrospinal fluid. Immunity. 2021;54(1):164-175.e6. doi: 10.1016/j.immuni.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matschke J, Lütgehetmann M, Hagel C, et al. . Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919-929. doi: 10.1016/S1474-4422(20)30308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yong SJ. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem Neurosci. 2021;12(4):573-580. doi: 10.1021/acschemneuro.0c00793 [DOI] [PubMed] [Google Scholar]
- 25.Baig AM. Deleterious outcomes in long-hauler COVID-19: the effects of SARS-CoV-2 on the CNS in chronic COVID syndrome. ACS Chem Neurosci. 2020;11(24):4017-4020. doi: 10.1021/acschemneuro.0c00725 [DOI] [PubMed] [Google Scholar]
- 26.Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70(1):61-67. doi: 10.1136/jim-2021-002051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visvabharathy L, Hanson B, Orban Z, et al. . Neuro-COVID long-haulers exhibit broad dysfunction in T cell memory generation and responses to vaccination. medRxiv. 2021:2021. doi: 10.1101/2021.08.08.21261763 [DOI] [Google Scholar]
- 28.Sun B, Tang N, Peluso MJ, et al. . Characterization and biomarker analyses of post-COVID-19 complications and neurological manifestations. Cells. 2021;10(2):386. doi: 10.3390/cells10020386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maltezou HC, Pavli A, Tsakris A. Post-COVID syndrome: an insight on its pathogenesis. Vaccines (Basel). 2021;9(5):497. doi: 10.3390/vaccines9050497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cella D, Riley W, Stone A, et al. . The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enose-Akahata Y, Azodi S, Smith BR, et al. . Immunophenotypic characterization of CSF B cells in virus-associated neuroinflammatory diseases. PLOS Pathog. 2018;14(4):e1007042. doi: 10.1371/journal.ppat.1007042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein DS, Cheshire WP Jr. Beat-to-beat blood pressure and heart rate responses to the Valsalva maneuver. Clin Auton Res. 2017;27(6):361-367. doi: 10.1007/s10286-017-0474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman F, Goldstein DS. Quantitative indices of baroreflex-sympathoneural function: application to patients with chronic autonomic failure. Clin Auton Res. 2014;24(3):103-110. doi: 10.1007/s10286-014-0234-1 [DOI] [PubMed] [Google Scholar]
- 34.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl. 1994;653(2):131-138. doi: 10.1016/0378-4347(93)E0430-X [DOI] [PubMed] [Google Scholar]
- 35.Davis HE, Assaf GS, McCorkell L, et al. . Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong AW, Shah AS, Johnston JC, Carlsten C, Ryerson CJ. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J. 2020;56(5):2003276. doi: 10.1183/13993003.03276-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham EL, Clark JR, Orban ZS, et al. . Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. 2021;8(5):1073-1085. doi: 10.1002/acn3.51350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ftiha F, Shalom M, Jradeh H. Neurological symptoms due to Coronavirus disease 2019. Neurol Int. 2020;12(1):8639. doi: 10.4081/ni.2020.8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998. doi: 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 40.Huart C, Meusel T, Gerber J, Duprez T, Rombaux P, Hummel T. The depth of the olfactory sulcus is an indicator of congenital anosmia. Am J Neuroradiol. 2011;32(10):1911-1914. doi: 10.3174/ajnr.A2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chetrit A, Lechien JR, Ammar A, et al. . Magnetic resonance imaging of COVID-19 anosmic patients reveals abnormalities of the olfactory bulb: preliminary prospective study. J Infect. 2020;81(5):816-846. doi: 10.1016/j.jinf.2020.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guedj E, Campion JY, Dudouet P, et al. . 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48(9):2823-2833. doi: 10.1007/s00259-021-05215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roe K. A role for T-cell exhaustion in Long COVID-19 and severe outcomes for several categories of COVID-19 patients. J Neurosci Res. 2021;99(10):2367-2376. doi: 10.1002/jnr.24917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diao B, Wang C, Tan Y, et al. . Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gil-Manso S, Miguens Blanco I, López-Esteban R, et al. . Comprehensive flow cytometry profiling of the immune system in COVID-19 convalescent individuals. Front Immunol. 2021;12:793142. doi: 10.3389/fimmu.2021.793142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Creager MA, Creager SJ. Arterial baroreflex regulation of blood pressure in patients with congestive heart failure. J Am Coll Cardiol. 1994;23(2):401-405 [DOI] [PubMed] [Google Scholar]
- 47.Hughes JW, Dennis MF, Beckham JC. Baroreceptor sensitivity at rest and during stress in women with posttraumatic stress disorder or major depressive disorder, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S. J Traum Stress. 2007;20(5):667-676. doi: 10.1002/jts.20285 [DOI] [PubMed] [Google Scholar]
- 48.Haffke M, Freitag H, Rudolf G, et al. . Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J Transl Med. 2022;20(1):138. doi: 10.1186/s12967-022-03346-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brognara F, Castania JA, Kanashiro A, Dias DPM, Salgado HC. Physiological sympathetic activation reduces systemic inflammation: role of baroreflex and chemoreflex. Front Immunol. 2021;12:637845. doi: 10.3389/fimmu.2021.637845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart JM. Chronic fatigue syndrome: comments on deconditioning, blood volume and resulting cardiac function. Clin Sci. 2009;118(2):121-123. doi: 10.1042/cs20090327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.