Abstract
Background:
Hidradenitis suppurativa (HS) is a chronic, inflammatory, recurrent follicular disorder affecting apocrine gland bearing areas such as axillae, inframammary area and groin. Significant association of HS with metabolic derangements such as hypertension, obesity, hyperlipidemia and hyperinsulinemia has been found. There is dearth of literature on epidemiological and metabolic profile of HS in Indian subjects.
Aim:
The aim of this study is to assess abnormalities in the levels of fasting blood glucose, serum insulin, and lipid profile in patients with HS.
Primary Objective:
To assess the frequency of abnormal levels of fasting blood sugar, serum insulin and lipid profile in patients with HS. Secondary objectives: To assess the frequency of hypertension, raised basal metabolic index, polycystic ovarian syndrome, follicular disorder, erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP) in patients with HS and to assess the severity of of clinical presentation HS using Hurley staging system.
Methodology:
This is a retrospective record based study. Records of clinically diagnosed patients of HS, aged > 18 years fulfilling the inclusion and exclusion criteria were analysed.
Results:
Total 30 patients were recruited with 1:1 male to female ratio. Five (16.67%) cases fulfilled NCEP ATP III criteria for the diagnosis of metabolic syndrome. Statistically significant association was observed between severity of HS, in younger age group (<20 years), moderate to severe BMI, fasting serum insulin, fasting total cholesterol and raised ESR.
Limitations:
This is retrospective, hospital record based study with small sample size.
Conclusion:
Holistic management of HS should be individualized according to need of patient and it should be combined approach including dermatologist, plastic surgeon, psychiatrist and dietician. We recommend an initial screening for derangements in metabolic profile in these patients for more effective management and preventing long term cardiovascular complications.
Keywords: Hidradenitis suppurativa, metabolic profile, treatment
Introduction
Hidradenitis suppurativa (HS) is a chronic, inflammatory, recurrent follicular disorder affecting apocrine gland-bearing areas, such as axillae, inframammary area and groin.[1] The true prevalence of HS is not known. The worldwide prevalence is estimated to be ranging from 0.00033% to as high as 4.1%, whereas period prevalence (2007–2016) in Asians is reported as 0.06%.[12] Clinically, it is characterized by recurrent painful pustules, nodules and abscesses that are complicated by the formation of draining sinuses and widespread scarring leading to functional disability.[2] Multiple sites may be involved; however, inguinal and mammary areas are more commonly affected in women while the anogenital region is more frequently affected in men.[1]
Studies have reported a significant association between HS and a range of metabolic derangements such as hypertension, obesity, hyperlipidaemia and hyperinsulinemia.[3,4,5,6] Syndromes such as metabolic syndrome and polycystic ovarian syndrome are reported to have a significant association with HS, increasing the risk of cardiovascular disorders in these patients.[7,8] Thus, it is important to screen these patients for metabolic derangements and offer timely interventions. Apart from these conditions, additional risk factors such as family history, smoking, frictional occlusion, stress and heat are also seen to be important triggers of HS.[9]
There are insufficient data regarding its prevalence worldwide as well as in India. Its prevalence in western countries is estimated to range between 0.1% and 4% with increased incidence in females.[1,3,4,10] There are no studies available that have estimated its prevalence in India. HS is a rare condition in our country and there is a paucity of studies in the Indian population regarding its epidemiology and association with metabolic abnormalities.
Review of literature
HS is a chronic, recurrent inflammatory disorder leading to scarring over intertriginous areas bearing apocrine glands.[1] Various factors predisposing to HS include family history, smoking, frictional occlusion, stress and heat.[9] Various comorbidities have been reported to be associated with HS, such as metabolic syndrome, dyslipidaemias, hypertension, polycystic ovarian disease (PCOD), insulin resistance, inflammatory bowel disease and spondyloarthropathies.[1,2,3,5,7]
A study by Sabat et al.[7] included 80 patients of HS and 100 age- and sex-matched controls where the prevalence of central obesity, hypertriglyceridemia, hypo-high-density lipoprotein (HDL)-cholesterolaemia and hyperglycaemia was studied. Metabolic syndrome was seen to be significantly more common in patients with HS (40% cases vs. 13% controls). It was hypothesized that metabolic syndrome was occurring as a primary pathology which led to poor microcirculation and bacterial persistence, leading to an outbreak of HS.
In a nationwide study conducted in Korea by Lee et al.,[3] 28,516 patients of HS were evaluated for 10 years for the presence of comorbidities including type 2 diabetes mellitus, hypertension and hyperlipidaemia. Patients with HS were found to have a significantly higher risk of these metabolic derangements. In a French study by Canoui-Poitrine et al.,[4] 302 patients with HS were included and factors associated with disease severity were analysed. It was found that body mass index (BMI) was significantly associated with the severity of the disease.
In a prepubertal case series, Offidani et al.[5] studied the clinical characteristics of eight children with HS. All patients were found to be either overweight or obese. Two patients had hyperandrogenism and one patient had hyperinsulinemia and precocious puberty. Crowley et al.[6] included 154 patients of HS in their study to assess their clinical characteristics. About 38.3% of patients were found to be morbidly obese with an odds ratio of 2.01. About 39.6% of patients had hypertension, 11.7% had hyperlipidaemia and 6.5% had diabetes mellitus. About 35.7% of patients had two cardiovascular risk factors.
In an American population-based study conducted by Garg et al.,[8] 22,990 female patients with HS were included to study the association with PCOD. A significant association was found between HS and PCOS, where 9% of females with HS were found to have PCOS when compared to 2.9% of the general population without HS. Further, the prevalence of PCOS was seen to be significantly higher among HS patients with diabetes mellitus (17.2%) and obesity (11%).
Aim
The aim of this study is to assess abnormalities in the levels of fasting blood glucose, serum insulin, and lipid profile in patients with HS.
Primary objective
The objective of this study is to assess the frequency of abnormal levels of fasting blood glucose, serum insulin, and lipid profile in patients with HS.
Secondary objectives
To assess the frequency of hypertension, raised BMI, and polycystic ovarian disorder in patients with HS.
To assess the severity of clinical presentation using the Hurley staging system.
To assess the frequency of abnormalities in inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
To assess the frequency of other follicular disorders part of the follicular occlusion tetrad in patients with HS.
Materials and Methods
This study is a retrospective record-based study. Patients >18 years of age fulfilling all the three criteria for HS – typical lesions of painful deep-seated nodules, abscesses, draining sinuses, bridged scars and pseudocomedones; affecting typical sites like axillae, groin, buttocks, inframammary and intermammary areas, and perineal and perianal areas; with chronicity and recurrence of lesions, presenting to Dermatology Outpatient Department of a tertiary care hospital during the study period (from January 2018 to October 2021) were recruited. Patients with chronic folliculitis, acute axillary or inguinal lymphadenitis and nodulocystic lesions in flexures of fungal or tubercular aetiology were excluded.
Records of patients clinically diagnosed as HS and registered in the HS clinic during the above study period, fulfilling the inclusion and exclusion criteria, were analysed. The assessment of history, anthropometric measurements, clinical characteristics (types of lesions, number of recurrences etc.) and investigations were done as per the predesigned clinical proforma. SPSS software version 20 was used in the study analysis. A P-value of <0.05 was considered significant.
Results
Clinical parameters
A total of 30 patients were recruited with a 1:1 male-to-female ratio. The mean age of presentation of cases was 29.67 ± 7.98 years with a maximum of 17 (56.7%) cases belonging to the age group 20–30 years followed by 10 (33.3%) cases to the age group 30–40 years. Two (6.7%) cases were of age group <20 years and one (3.3%) case was >40 years age. The mean duration of presentation of the disease was 5.43 ± 5.09 years. Patients in the <20 years age group had severe HS (P-value 0.006) [Figure 1].
Figure 1.
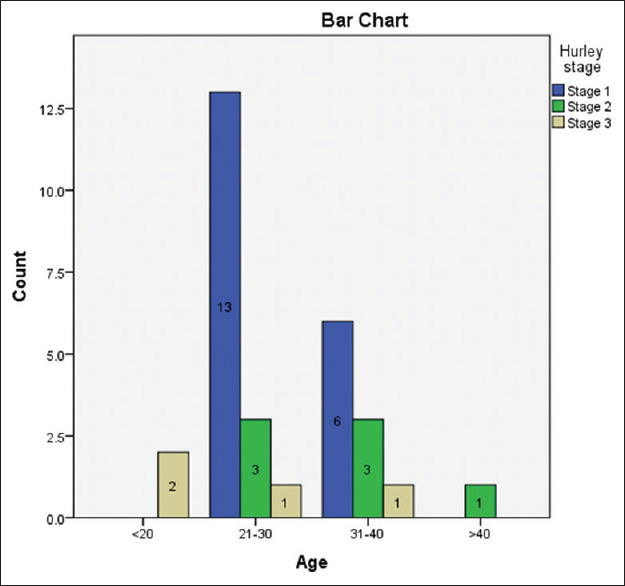
Bar graph showing age distribution of three Hurley stages of hidradenitis suppurativa patients
History of smoking (10–20 pack-years) was present in four (13.3%) patients. Five (16.6%) cases had a positive family history of HS. Most common site of involvement was axilla 19 (63.3%) followed by buttocks 12 (40%), groin 11 (36.7%), perineal/perianal region 8 (26.7%), mammary area 4 (13.3%), and other site such as trunk 3 (10%). Common clinical presentation was pustules, nodules, abscesses, polyporus comedones, sinuses, and bridging scars. Patients presented with symptoms of pain 24 (46.7%), pus discharge 16 (53.3%), itching 14 (46.7%), foul odour 4 (13.3%), and bleeding 2 (6.7%). Nineteen (63.3%) cases belonged to Hurley stage 1, whereas seven (23.3%) and four (13.3%) cases were of the Hurley stages 2 and 3, respectively. Other follicular disorders such as acne, folliculitis and pilonidal sinus were found in 16 (53.3%) cases, 7 (23.3%) cases and 1 (3.3%) case, respectively.
Metabolic parameters
One (3.3%) case had hypertension, 1 (3.3%) had hypothyroidism, 2 (6.7%) had clinical signs of hyperandrogenism, 10 (33.3%) had raised FBS and 2 (6.7%) cases of hyperinsulinemia were seen. Hypertriglyceridemia was present in 13 (43.3%) cases and hypercholesterolaemia in 6 (20%) cases. Five patients (13.3%) had elevated LDL levels. Five (16.67%) cases fulfilled ≥3 out 5 NCEP ATP III criteria for the diagnosis of metabolic syndrome [waist circumference over 40 in (men) or 35 in (women), blood pressure over 130/85 mmHg, fasting triglyceride (TG) level over 150 mg/dl, fasting HDL cholesterol level less than 40 mg/dl (men) or 50 mg/dl (women) and fasting blood sugar over 100 mg/dl]. The mean BMI was 26.77 ± 4.08 kg/m2 with the maximum number of patients; 15 (50%) were pre-obese followed by 10 (33.3%) who were obese [Figure 2]. Two (6.7%) patients were known cases of PCOD. Ten (33.3%) patients had elevated ESR, while three (10%) had raised CRP.
Figure 2.
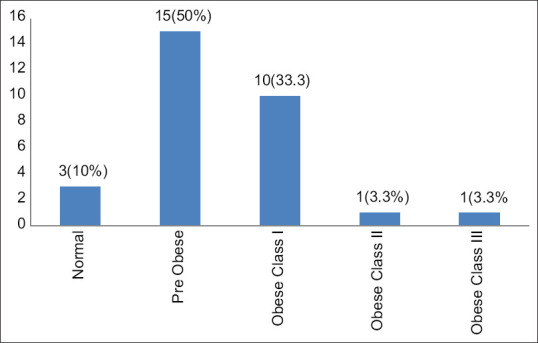
Bar graph showing BMI distribution of hidradenitis suppurativa patients
There was an increased but non-significant association of obesity with the Hurley staging (chai square test 9.348, P-value 0.314) but a significant association of mean BMI with Hurley staging maximum mean BMI in stage 2 [Figure 3 and Table 1].
Figure 3.
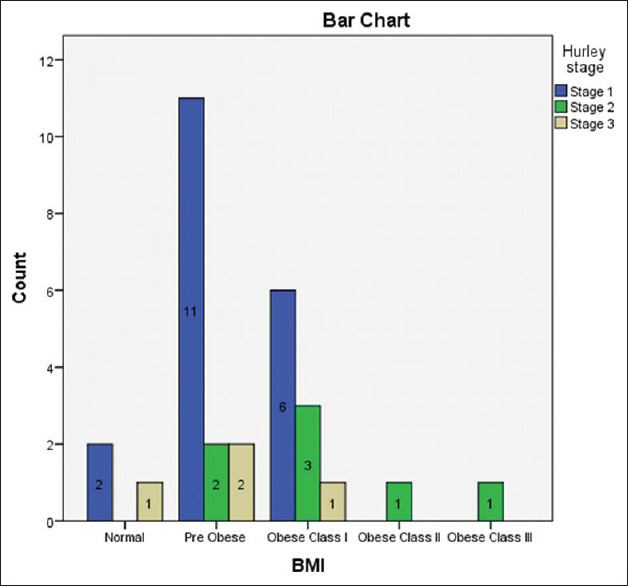
Bar graph showing BMI distribution of three Hurley stages of hidradenitis suppurativa patients
Table 1.
One-way ANOVA for comparing the continuous variables of hidradenitis suppurativa.
| Stage 1 (n=19) mean±SD | Stage 2 (n=7) mean±SD | Stage 3 (n=4) mean±SD | F/Welch statistics | P | Stage 1 vs. Stage 2 difference (P) | Stage 1 vs. Stage 3 difference (P) | Stage 2 vs. Stage 3 difference (P) | |
|---|---|---|---|---|---|---|---|---|
| Age | 29±4.12 | 35.43±12.84 | 22.75±6.24 | 4.131 | 0.027 | −6.43 (0.129) | 6.25 (0.275) | 12.68 (0.025) |
| BMI | 25.91±2.87 | 30.7±5.09 | 23.95±2.82 | 6.339 | 0.006 | −4.79 (0.012) | 1.96 (0.569) | 6.75 (0.012) |
| SBP | 116.74±8.85 | 122.86±15.7 | 130±4.32 | 3.023 | 0.065 | −6.12 (0.394) | −13.26 (0.072) | −7.14 (0.528) |
| DBP | 72.84±7.52 | 74.86±9.3 | 82.5±5.75 | 2.548 | 0.097 | −2.02 (0.829) | −9.66 (0.08) | −7.64 (0.277) |
| ESR | 15.42±7.52 | 21.86±9.08 | 29±8.68 | 5.42 | 0.01 | −6.44 (0.184) | −13.58 (0.013) | −7.14 (0.345) |
| CRP | 3.33±1.78 | 3.91±2.03 | 6.08±5.34 | 0.627 | 0.565 | −0.58 (0.86) | −2.75 (0.131) | −2.17 (0.361) |
| FBS | 96.11±7.87 | 97.29±6.13 | 94.25±9.91 | 0.194 | 0.825 | −1.18 (0.937) | 1.86 (0.902) | 3.04 (0.809) |
| Fasting insulin | 15.95±4.38 | 19.78±7.04 | 23.13±6.41 | 3.624 | 0.04 | −3.83 (0.253) | −7.17 (0.054) | −3.35 (0.582) |
| Total cholesterol | 178.47±18.04 | 203±27.65 | 168±35.89 | 3.858 | 0.034 | −24.53 (0.058) | 10.47 (0.69) | 35 (0.056) |
| Triglycerides | 141.16±34.19 | 169.43±36.91 | 110.75±52.58 | 3.275 | 0.053 | −28.27 (0.218) | 30.41 (0.315) | 58.68 (0.047) |
There was a significant association of ESR with Hurley staging (P-value 0.008) indicating that severe disease has more inflammation [Figure 4 and Table 1]. There was a significant association of hypercholesterolaemia with Hurley staging (P-value 0.013) [Figure 4 and Table 1]. There was a significant association of hyperinsulinemia with the Hurley staging (P-value 0.004) with the highest mean value of serum insulin belonging to the Hurley stage 3 [Table 1].
Figure 4.
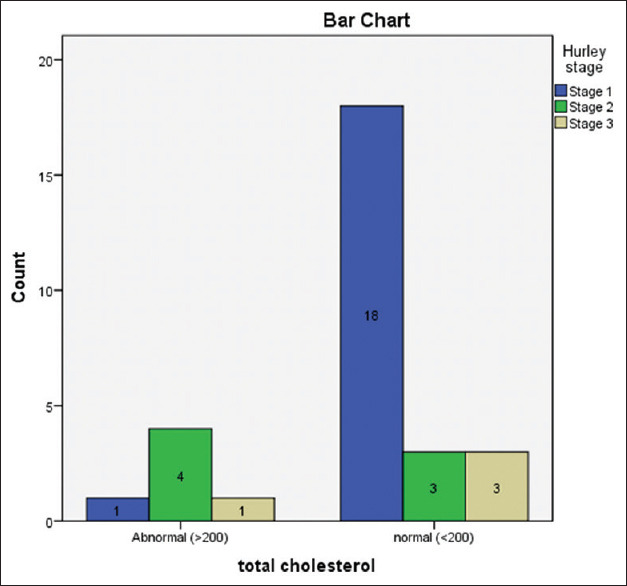
Bar graph showing total cholesterol levels of three Hurley stages of hidradenitis suppurativa patients
Management
Fourteen (46.7%) patients had already taken multiple short courses of antibiotics 14 (46.7). Pus culture sensitivity examination from the purulent discharge of 16 patients from the axilla, groin and perineal region 4 (25%) cases showed methicillin-resistant Staphylococcus aureus; 2 (12.5%), 3 (18.7%), and 1 (6.2%) cases showed Streptococcus epidermidis, Enterobacteriaceae coli, and Pseudomonas, respectively, which were sensitive to linezolid, vancomycin, ceftriaxone, cefuroxime, cotrimoxazole, and doxycycline. No growth was found on the pus culture of six (37.5%) cases. The most common treatment offered to the patients was a combination of topical and systemic antibiotics, that is, clindamycin 1% gel plus doxycycline (100 mg twice daily) or a combination of rifampicin (600 mg) plus clindamycin (300 mg twice daily) or cotrimoxazole Ds with an average duration of 3 months. The next most common treatment given to the patients was isotretinoin (30 mg daily) with/without antibiotics, for an average duration of 6 months. Surgical treatment included wide local excision of nodule and scar in two (6.6%) patients, incision and drainage of an abscess in four (13.3%) patients, and reconstruction of pilonidal sinus in one (3.3%) patient.
Maximum remission in the form of reduction in lesion count, resolution of pus discharge and improved quality of life were observed with combination therapy of antibiotics and isotretinoin. Patients who underwent surgery did not exhibit recurrence, whereas most of the patients who followed up after stopping medical treatment showed recurrence.
Discussion
HS, also known as acne inversa, is a chronic, inflammatory skin disease affecting terminal hair follicles in apocrine-gland-bearing skin. Patients of HS usually present late to tertiary centres. The mean age of presentation was 5.43 years in this study. The most common site of involvement in our study is axilla followed by buttock and groin, whereas it is axilla followed by groin in previous studies.[11] Moderate-to-severe HS has been more commonly associated with comorbidities. In this study, significant association of HS, especially Hurley stages 2 and 3 with young age, elevated ESR, high BMI, high insulin level and high total cholesterol, is found, similar to previous studies [Table 2].
Table 2.
Studies showing metabolic abnormalities in patients of hidradenitis suppurativa.
| Study | Sample size | Type of study | Investigations/Tools | Results |
|---|---|---|---|---|
| 1. Kamat et al.[11] 2021 | 22 cases | Hospital-based retrospective study | BMI, PCOS | High BMI (45.4%) and obesity (18.1%) patients. Patients undergoing procedural plus pharmacotherapy had a better outcome |
| 2. Offidani et al.[5] 2019 | 8 cases | Case series | Body mass index, serum testosterone, and serum insulin | High BMI in all patients, hyperandrogenism in two patients, precocious puberty and hyperinsulinemia in one patient |
| 3. Lee et al.[3]2018 | 28,516 cases | Population-based cross-sectional study | Blood pressure, blood glucose, triglycerides, total cholesterol, low density and high density and low-density lipoprotein | Significantly higher risk of type 2 diabetes (Odds ratio 1.820), hypertension (OR 1.266) and hyperlipidaemia (OR 1.308) |
| 4. Garg et al.[8] 2018 | 22,990 cases (female) | Population-based study | Weight, blood glucose, markers of PCOS | The significant association between HS and PCOS (9% of females with HS had PCOS when compared to 2.9% of the general population without HS). PCOS was significantly higher among HS patients with diabetes mellitus (17.2%) and obesity (11%) |
| 5. Crowley et al.[6] 2014 | 154 cases | Cross-sectional study | Weight, blood pressure, blood glucose, total cholesterol, triglycerides, low-density and high-density lipoprotein | Morbid obesity in 38.3% of patients (odds ratio 2.01) 39.6% of patients with hypertension, 11.7% with hyperlipidaemia, 6.5% with diabetes mellitus and 35.7% of patients had two cardiovascular risk factors |
| 6. Sabat et al.[7] 2012 | 80 cases and 100 controls | Case-control study | Height, weight, blood pressure, waist circumference, blood glucose, triglycerides and high-density lipoprotein | Metabolic syndrome was seen to be significantly more common in patients with HS (40% cases vs. 13% controls) |
| 7. Canoui-Poitrine et al.[4] 2009 | 302 cases | Case control study | Body mass index, blood pressure, blood glucose, total cholesterol, triglycerides, low-density and high-density lipoprotein | Significantly higher BMI in cases compared to controls |
Along with lifestyle modification, that is, weight loss, and abstinence from smoking, the most common treatment given to the patients of HS was systemic antibiotics alone or in combination followed by isotretinoin. Steroids are used to manage acute flare of HS. Holistic management of HS should be individualized according to the need of the patient and it should be a team approach including a dermatologist, plastic surgeon, psychiatrist and dietician.
This is a retrospective, hospital record-based study with small sample size and without a control group. Despite these limitations, this is a pilot study on HS which is still considered uncommon in India. There is a dearth of literature on the epidemiological and metabolic profile of HS in Indian subjects. Based on the preliminary results of our retrospective study on HS, a statistically significant association was observed between the severity of HS, in the younger age group (<20 years), moderate-to-severe BMI, fasting serum insulin, fasting total cholesterol and raised ESR.
We recommend an initial screening for derangements in the metabolic profile in these patients for more effective management and to prevent long-term cardiovascular complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Patil S, Apurwa A, Nadkarni N, Agarwal S, Chaudhari P, Gautam M. Hidradenitis suppurativa: Inside and out. Indian J Dermatol. 2018;63:91–8. doi: 10.4103/ijd.IJD_412_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa) Dermatoendocrinol. 2010;2:9–16. doi: 10.4161/derm.2.1.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Kwon HS, Jung HM, Kim GM, Bae JM. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: A nationwide population-based study. J Eur Acad Dermatol Venereol. 2018;32:1784–90. doi: 10.1111/jdv.15071. [DOI] [PubMed] [Google Scholar]
- 4.Canoui-Poitrine F, Revuz JE, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51–7. doi: 10.1016/j.jaad.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Offidani A, Molinelli E, Sechi A, Brisigotti V, Campanati A, Raone B, et al. Hidradenitis suppurativa in a prepubertal case series: A call for specific guidelines. J Eur Acad Dermatol Venereol. 2019;33(Suppl 6):28–31. doi: 10.1111/jdv.15827. [DOI] [PubMed] [Google Scholar]
- 6.Crowley JJ, Mekkes JR, Zouboulis CC, Scheinfeld N, Kimball A, Sundaram M, et al. Association of hidradenitis suppurativa disease severity with increased risk for systemic comorbidities. Br J Dermatol. 2014;171:1561–5. doi: 10.1111/bjd.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabat R, Chanwangpong A, Schneider-Burrus S, Metternich D, Kokolakis G, Kurek A, et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS One. 2012;7:e31810. doi: 10.1371/journal.pone.0031810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg A, Wertenteil S, Baltz R, Strunk A, Finelt N. Prevalence estimates for hidradenitis suppurativa among children and adolescents in the United States: A gender- and age-adjusted population analysis. J Invest Dermatol. 2018;138:2152–6. doi: 10.1016/j.jid.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Kumari S, Gupta M, Sharma RK, Negi L. Hidradenitis suppurativa: Presenting at atypical sites. J Dermatol Dermatol Surg. 2018;22:79–81. [Google Scholar]
- 10.Prabhu G, Laddha P, Manglani M, Phiske M. Hidradenitis suppurativa in a HIV-infected child. J Postgrad Med. 2012;58:207–9. doi: 10.4103/0022-3859.101403. [DOI] [PubMed] [Google Scholar]
- 11.Kamat D, Gaba S, Kumaran MS. Clinico-Epidemiological characteristics of hidradenitis suppurativa: A retrospective cohort study from a tertiary care centre in Northern India. Indian Dermatol Online J. 2021;12:561–5. doi: 10.4103/idoj.IDOJ_743_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandran NS, Lee JH, Kurokawa I. Hidradenitis suppurativa in South-East Asia and East Asia. Exp Dermatol. 2021;30(Suppl 1):23–6. doi: 10.1111/exd.14340. [DOI] [PubMed] [Google Scholar]


