Abstract
Background:
In malnourished patients with colorectal cancer, hypoalbuminemia is common and was proposed to determine the postoperative outcome of colorectal surgery. Mounting articles published but have not been evaluated. We aim to assess the predictive value of preoperative hypoalbuminemia in patients undergoing colorectal surgery.
Methods:
We performed a literature search from PubMed, Euro PMC, and Cochrane with the terms serum albumin, hypoalbuminemia, prognosis, outcome, colorectal cancer, and neoplasm. We also hand-searched and included any relevant papers. Hypoalbuminemia is defined as plasma albumin level < 3.5 mg/dL. We restricted the included studies to English language and adults undergoing colectomy, laparotomy, laparoscopy, or abdominoperineal resection. Any types of articles were included, except an abstract-only publication and those that did not report the key exposure or outcome of interest. The key exposures were mortality, hospitalization time, and morbid conditions (thrombosis, surgical site infection, sepsis, and wound events). We pooled the odds ratio from each included literature as effect size. The Newcastle Ottawa scale and GRADE were used to determine the quality of each included study.
Results:
Hereof 7 observational studies (236,480 individuals) were included. Our meta-analysis found that preoperative hypoalbuminemia can predict the postoperative outcome in colorectal cancer patients. Individuals with hypoalbuminemia were not associated with 30-day mortality (risk ratio [RR] 2.05 [0.72, 5.86], P = .18, I2 = 99%) but were associated with morbidity (RR 2.28 [1.78, 2.93], P < .00001, I2 = 87.5%), surgical complication (RR 1.69 [1.34, 2.13], P < .00001, I2 = 98%), and hospitalization (RR 2.21 [1.93, 2.52], P < .00001, I2 = 0%). According to newcastle ottawa scale, the included studies are of moderate to sound quality.
Conclusions:
The current systematic review and meta-analysis showed that preoperative hypoalbuminemia was significantly associated with morbidity, length of stay, and surgical complication but not mortality.
Keywords: colorectal cancer, hypoalbuminemia, outcome, postoperative
1. Introduction
The third leading cause of cancer-related deaths worldwide is colorectal cancer (CRC).[1] The incidence and death of CRC show significant geographic variation, with relatively similar regional trends in men and women.[1,2] The best curative choice for patients with localized CRC is generally accepted to be aggressive surgery, despite significant regional variations in screening programs and treatment methods. Even though new treatments have increased survival, over 45% of CRC cases will pass away due to the tumor.[2]
Regional differences in the prognosis of CRC patients have been reported frequently; however, neither the tumor-node-metastasis categorization nor the currently accepted prognostic variables can fully account for these disparities. To increase our understanding of the issue and raise the standard of care in CRC, we must have a greater understanding of these aspects and how they interact, including how they relate to patients, healthcare professionals, treatments, or institutions.[3] It is becoming more common to link hematologic, immunological, and nutritional measures with cancer prognosis. Serum albumin is a valuable biomarker for many illnesses. It has been linked to several acute, chronic, and neoplastic disorders, as well as healthy populations, as a significant prognostic indicator.[4–8]
Many predictive models use baseline serum albumin (BSA) to define or improve therapies in particular contexts. In the context of CRC, BSA has been characterized as a prognostic factor linked with survival and a predictor of surgical morbidity and death.[6,9,10]
BSA measurement is simple, affordable, accurate, and trustworthy, frequently used to determine patients general state with many types of medical conditions. Consequently, we aimed to evaluate the predictive value of preoperative hypoalbuminemia in patients undergoing colorectal surgery.
2. Methods
The authors performed a literature search from PubMed, Euro PMC, and Cochrane with the terms serum albumin, hypoalbuminemia, prognosis, outcome, colorectal cancer, and neoplasm. A detailed search strategy can be seen in Table 1. An initial search and title and abstract screening for pertinent papers were carried out independently by the 2 authors. We settled disagreements through discussion. After eliminating duplicates, we applied inclusion and exclusion criteria to the prospective full-texts for evaluation. We also hand-searched and included any relevant papers. All authors completed the literature search on December 20, 2022. This systematic review was conducted according to the preferred reporting items for systematic reviews and meta-analyses guideline.[11] We registered our systematic review to the PROSPERO (CRD42023388595).
Table 1.
Search query used at different search engine.
| Scientific database | Search terms |
|---|---|
| PubMed | (“albumin s”[All Fields] OR “albumine”[All Fields] OR “albumines”[All Fields] OR “albumins”[MeSH Terms] OR “albumins”[All Fields] OR “albumin”[All Fields] OR (“serum albumin”[MeSH Terms] OR (“serum”[All Fields] AND “albumin”[All Fields]) OR “serum albumin”[All Fields]) OR (“hypoalbuminaemia”[All Fields] OR “hypoalbuminemia”[MeSH Terms] OR “hypoalbuminemia”[All Fields])) AND (“prognosis”[MeSH Terms] OR “prognosis”[All Fields] OR “prognoses”[All Fields] OR (“mortality”[MeSH Subheading] OR “mortality”[All Fields] OR “survival”[All Fields] OR “survival”[MeSH Terms] OR “survivability”[All Fields] OR “survivable”[All Fields] OR “survivals”[All Fields] OR “survive”[All Fields] OR “survived”[All Fields] OR “survives”[All Fields] OR “surviving”[All Fields]) OR (“outcome”[All Fields] OR “outcomes”[All Fields]) OR (“prognostic”[All Fields] OR “prognostical”[All Fields] OR “prognostically”[All Fields] OR “prognosticate”[All Fields] OR “prognosticated”[All Fields] OR “prognosticates”[All Fields] OR “prognosticating”[All Fields] OR “prognostication”[All Fields] OR “prognostications”[All Fields] OR “prognosticator”[All Fields] OR “prognosticators”[All Fields] OR “prognostics”[All Fields])) AND (“colorectal neoplasms”[MeSH Terms] OR (“colorectal”[All Fields] AND “neoplasms”[All Fields]) OR “colorectal neoplasms”[All Fields] OR (“colorectal”[All Fields] AND “cancer”[All Fields]) OR “colorectal cancer”[All Fields] OR (“colonic neoplasms”[MeSH Terms] OR (“colonic”[All Fields] AND “neoplasms”[All Fields]) OR “colonic neoplasms”[All Fields] OR (“colon”[All Fields] AND “cancer”[All Fields]) OR “colon cancer”[All Fields]) OR (“rectal neoplasms”[MeSH Terms] OR (“rectal”[All Fields] AND “neoplasms”[All Fields]) OR “rectal neoplasms”[All Fields] OR (“rectal”[All Fields] AND “cancer”[All Fields]) OR “rectal cancer”[All Fields]) OR (“neoplasm s”[All Fields] OR “neoplasms”[MeSH Terms] OR “neoplasms”[All Fields] OR “neoplasm”[All Fields])) |
| Europe PMC | albumin OR serum albumin OR hypoalbuminemia AND prognosis OR survival OR outcome OR prognostic AND colorectal cancer OR colon cancer OR rectal cancer OR neoplasm |
| Cochrane | albumin OR serum albumin OR hypoalbuminemia AND prognosis OR survival OR outcome OR prognostic AND colorectal cancer OR colon cancer OR rectal cancer OR neoplasm |
We restricted the included studies to English language and adults undergoing colectomy, laparotomy, laparoscopy, or abdominoperineal resection. Any types of articles were included, except an abstract-only publication and those that did not report the key exposure or outcome of interest.
The authors (N.M. and J.H.) separately extracted data from each included study on the information: Author, year, research design, age, gender, albumin level, mortality, morbidity, length of stay, surgical complications, such as surgical site infection, thrombosis event, sepsis, and wound event, and other outcomes of interests. We recorded all data on standardized forms. We defined hypoalbuminemia as plasma albumin level < 3.5 mg/dL. We described the mortality rate as deaths from all causes within 30 days or during the hospitalization for the index procedure.
The primary outcome of the current study is mortality. Secondary outcomes were hospitalization time, thrombosis, surgical site infection, sepsis, and wound events.
The possibility of bias in each included publication was evaluated using the Newcastle Ottawa scale (NOS), which is used to rate the quality of non-randomized research in meta-analyses. It has been suggested that the NOS be used while reviewing observational studies. For cohort studies, the NOS has 3 domains – selection, comparability, and outcome – and 8 elements. A study with a score of 7 to 9 is considered high quality; studies with scores of 4 to 6 have a significant risk of bias, and studies with scores of 0 to 3 have an extremely high risk of bias. The maximum possible score is 9.
We used Review Manager 5.4 (Cochrane Collaboration) to conduct the meta-analysis. We calculated the risk ratios (RRs) for dichotomous variables using the Mantel-Haenszel algorithm, and their 95% confidence intervals are provided. Despite the heterogeneity, We utilized a random-effects model for the calculation. In this investigation, the threshold for statistical significance was set at .05, and all P values were 2-tailed. The likelihood of publication bias was assessed using an inverted funnel-plot approach.
3. Results
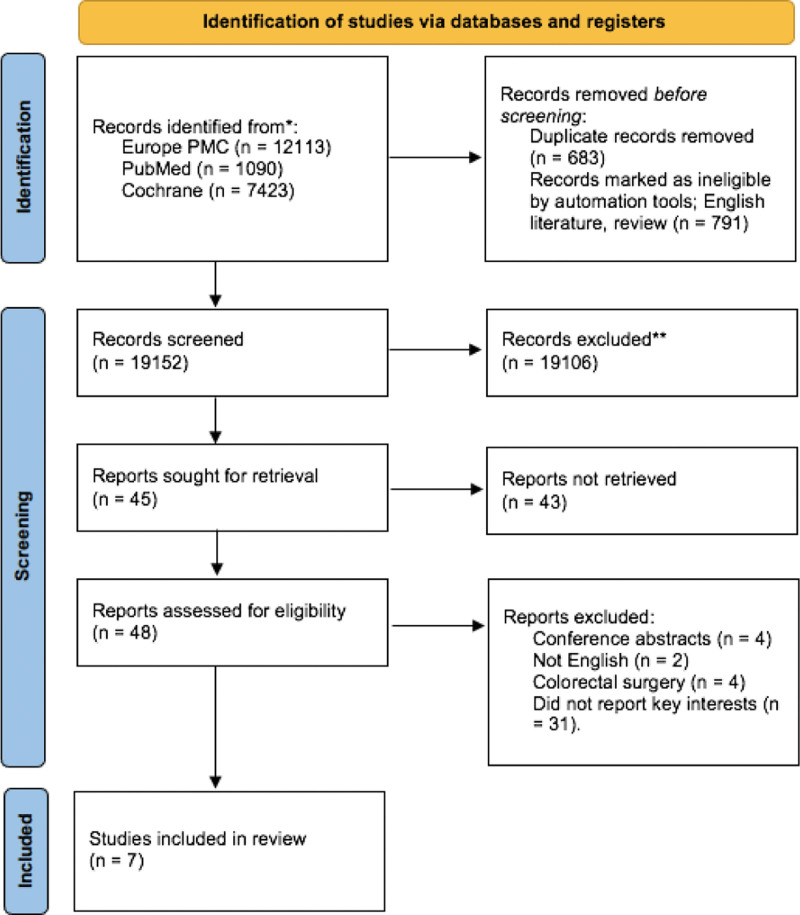
From 3 unique medical electronic databases, we extracted a total of 20,626 studies. After omitting duplicates and using a filter from each database, we were left with 19,152 studies. After screening the titles and abstracts, we excluded a total of 19,106 literature. After evaluating 48 full-text articles for eligibility, we excluded 41 of them because conference abstracts (n = 4), not English literature (n = 2), colorectal surgeries, but not colorectal carcinoma (n = 4), and did not report key interest (n = 31). A detailed literature saturation process can be seen in Figure 1. Overall, there were 236,460 patients from 7 studies.[12–18] The characteristics of the included studies are displayed in Table 2
Figure 1.
PRISMA flow diagram of current systematic review.
Table 2.
Characteristics of each cohort from eligible studies (n = 236,460).
| Study ID, NOS, country details | Age (yr, mean ± SD) | Male (n) | Tumor location (n) | Albumin level (mg/dL) | 30-d mortality rate (n) | SSI | Sepsis | Thrombotic events (n) | Wound events (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Others | No | HA | No | HA | No | HA | No | HA | No | HA | ||||
| Lai 2010, 7, China | 62.5 ± 13.6 | 1968 | 1449 | 2283 | - | - | 17 | 28 | 57 | 33 | ||||||
| Hu 2019, 7, US | 66.2 ± 13.8 | 13,897 | - | - | - | - | 248 | 196 | 1713 | 543 | 715 | 245 | 474 | 253 | 259 | 85 |
| Egenvall 2018, 8, Sweden | 68.8 ± 10.9 | 229 | 121 | 80 | 180 | - | - | - | - | - | - | - | - | - | - | - |
| González-Trejo 2017, 8, US | 59.1 ± 14.9 | 782 | 96 | 203 | 1166 | 3.42 ± 0.59 | - | - | - | - | - | - | - | - | - | - |
| Haskins 2017, 7, US | 68 ± 2.7 | 2585 | - | - | - | 3.95 ± 0.51 | 35 | 19 | - | - | 127 | 36 | 63 | 17 | 381 | 89 |
| Chiang 2015, 8, China | - | - | - | - | - | - | ||||||||||
| Larson 2020, 7, US | - | 40,278 | - | - | - | - | 711 | 310 | 780 | 7209 | 2882 | 503 | 1063 | 200 | 91 | 723 |
HA = hypoalbuminemia, No = normal, NOS = newcastle ottawa scale, SD = standard deviation, SSI = surgical site infection, US = United States.
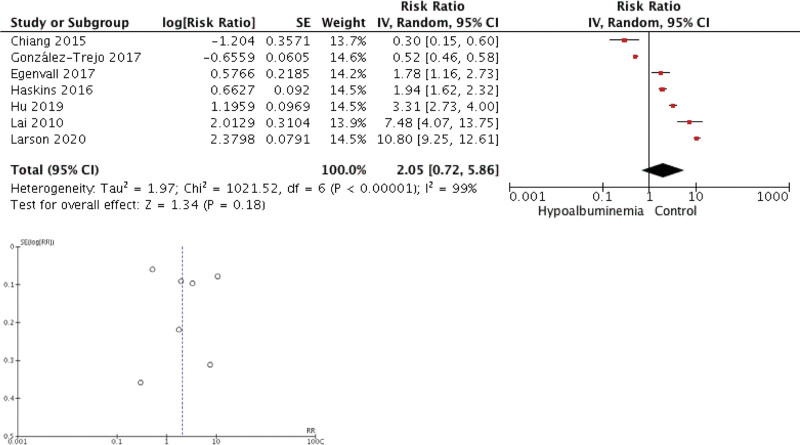
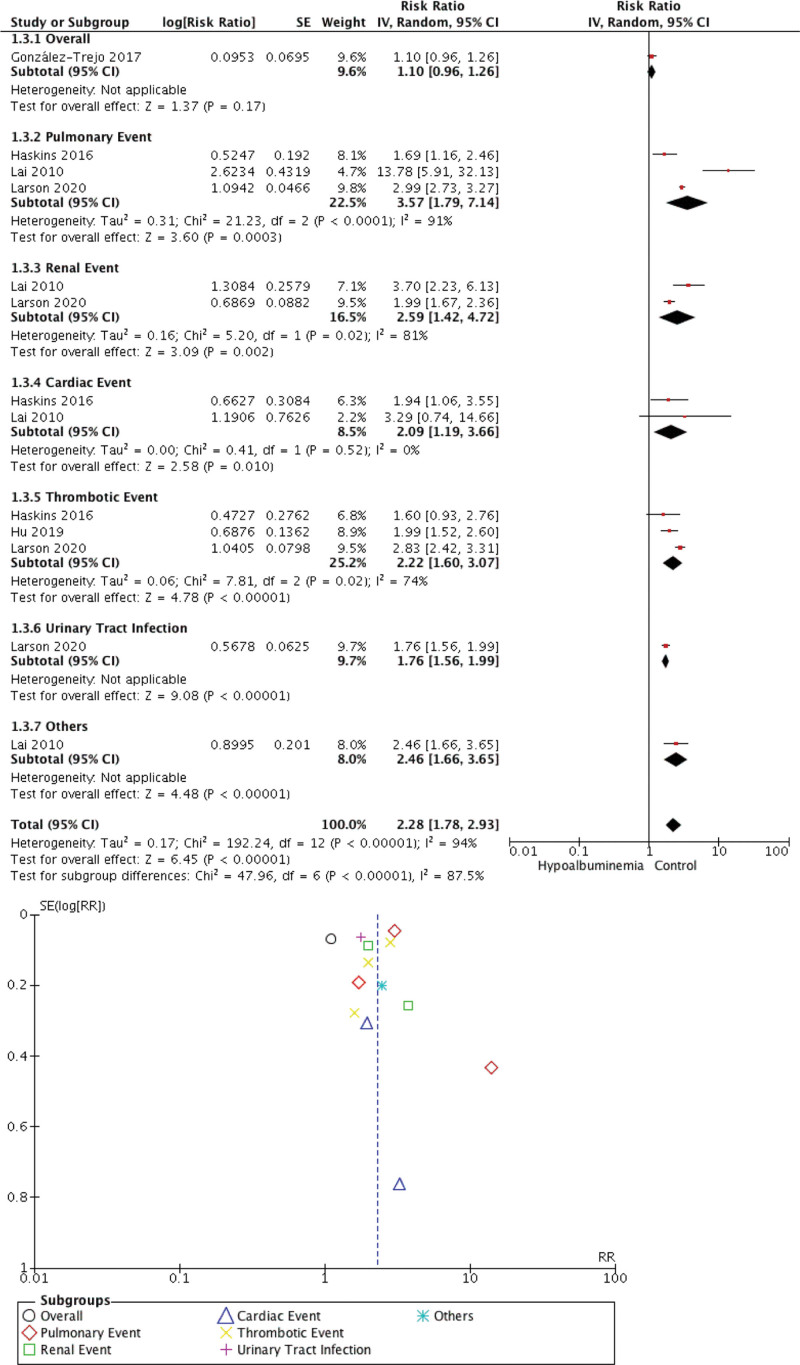
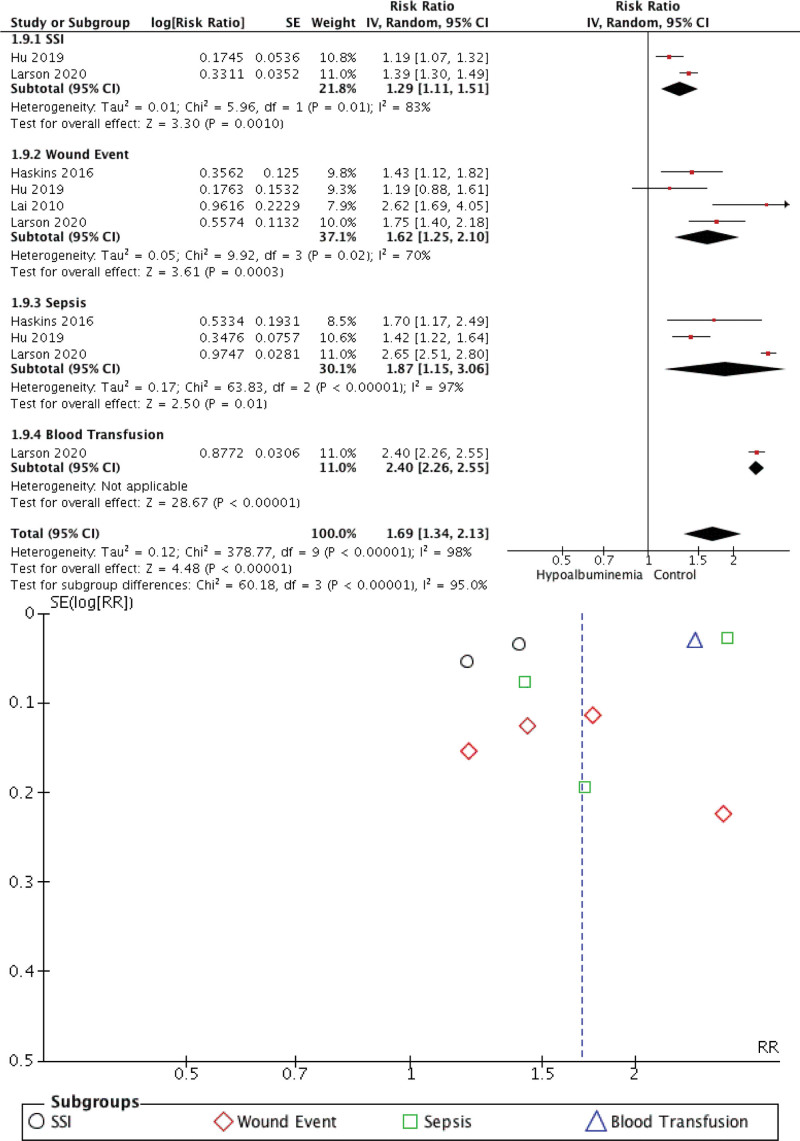
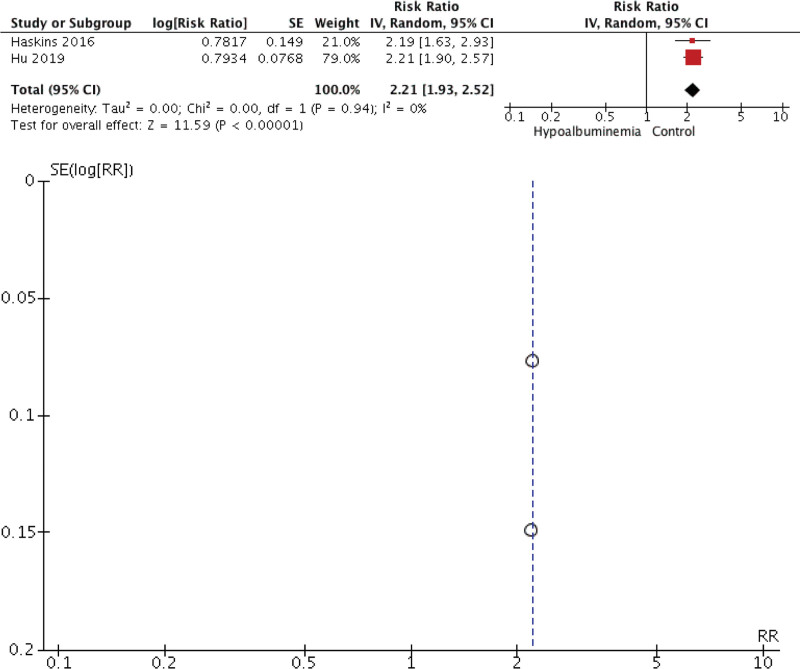
Our meta-analysis found that preoperative hypoalbuminemia can predict the postoperative outcome in CRC patients. Individuals with hypoalbuminemia were not associated with 30-day mortality (RR 2.05 [0.72, 5.86], P = .18, I2 = 99%) but were associated with morbidity (RR 2.28 [1.78, 2.93], P < .00001, I2 = 87.5%), surgical complication (RR 1.69 [1.34, 2.13], P < .00001, I2 = 98%), and hospitalization (RR 2.21 [1.93, 2.52], P < .00001, I2 = 0%). The forest plot and funnel plot showing the correlation between hypoalbuminemia and mortality, morbidity, surgical complication, and length of stay were shown in Figures 2, 3, 4, and 5, respectively.
Figure 2.
Meta-analysis showing correlation between hypoalbuminemia and mortality.
Figure 3.
Meta-analysis showing correlation between hypoalbuminemia and morbidity.
Figure 4.
Meta-analysis showing correlation between hypoalbuminemia and surgical complication.
Figure 5.
Meta-analysis showing correlation between hypoalbuminemia and length of stay.
The inverted funnel plot demonstrated a qualitatively asymmetrical shape for mortality and surgical complication but not for morbidity and hospital stay duration. According to NOS, all studies were of good quality.
4. Discussions
Patients with cancer, including those with colon cancer, frequently struggle with malnutrition, which renders them helpless. Malnutrition in colon cancer patients can be brought on by increased metabolic rates brought on by the disease, decreased food intake, problems with hepatic protein synthesis, blood loss, etc.[19,20] Serum albumin level is frequently utilized, even though it cannot comprehensively represent the nutritional state of individuals.[20,21] It is commonly acknowledged that hypoalbuminemia is a reliable sign of malnutrition.[22] The current study showed that preoperative hypoalbuminemia was significantly associated with poor postoperative outcomes.
In several published studies, it has been discussed how comorbidities and hypoalbuminemia are correlated. Hospitalized patients with mild hypoalbuminemia (albumin 25–35 g/L) had lower body mass indices, were older and had higher rates of hypertension, congestive heart failure, and chronic renal failure than patients with normal albumin levels.[23] Acute renal failure was strongly associated with severe hypoalbuminemia, which was found in 4% of chronic obstructive pulmonary disease patients.[24] In individuals with chronic heart failure, the prevalence of hypoalbuminemia ranged from 20% to 25% to 90% in frail, elderly patients with acute heart failure.[25] Here, we found that hypoalbuminemia was correlated with a wide range of comorbidities in patients with colorectal cancer. Early detection of malnutrition in individuals with multiple comorbidities is advised because even minor hypoalbuminemia carries a higher postoperative risk.
4.1. Key results and interpretation
There were some notable findings from the current study. Firstly, we wanted to stress that in the subset analysis of morbidity, we found that hypoalbuminemia is associated with thromboembolic events. Folsom et al[26] found that low serum albumin was a minor predictor of an elevated risk of venous thromboembolism. In the data from their 2 cohorts, the adjusted hazard ratio for albumin below the fifth percentile was 1.28 and 1.8. In colon and rectal surgery, deep vein thrombosis has been linked to hypoalbuminemia (serum albumin level 35 mg/L).[27] A comparable outcome in cancer patients was also seen. It is unclear how hypoalbuminemia and VTE are related, but hyperinflammatory or hypercoagulable conditions may cause them.
Preoperative hypoalbuminemia (serum albumin 30 g/L) was an independent predictor of the development of superficial and deep surgical site infections and an extended hospital stay after gastrointestinal surgery in a multi-institutional study.[28] Other operational methods also reported the associations.[29,30] According to the meta-analysis, a slight drop in serum albumin substantially impacted the length of the hospital stay, surgical site infection, and other comorbidities.[8,31] Low serum albumin was a good indicator of malnutrition, linked to slow infection and wound healing. Albumin has an immunomodulatory function, and hypoalbuminemia mice had abnormal macrophage activation and granuloma development.[32,33] A negative acute-phase protein during an inflammatory response to bacterial infection is serum albumin.
4.2. Implications in daily practice
Serum albumin is a negative acute-phase protein that expresses less and loses more when there is inflammation or when a person is underweight. Therefore, hypoalbuminemia in both acutely and chronically ill people is a sign of malnutrition.[34,35] According to several research, hypoalbuminemia doesn’t become clinically important until levels lower than 25 g/L. The influence of preoperative hypoalbuminemia on subsequent colon cancer metastasis is unknown, though. As a result, we needed to conduct additional research.
4.3. Limitations
The potential for publication bias, as is illustrated by the asymmetrical funnel plot, is the limitation of this systematic review and meta-analysis. Most of this research used an observational, retrospective methodology. Last, the current systematic review and meta-analysis needed to match better. The present systematic review and meta-analysis included 8 studies, which is insufficient to conduct meta-regression. As a result, the outcome could be affected by several confounders.
5. Conclusions
The current systematic review and meta-analysis showed that preoperative hypoalbuminemia was significantly associated with morbidity, length of stay, and surgical complication but not mortality. More studies still need to be conducted to see any possible confounding variables affecting hypoalbuminemia in predicting the outcome in patients with colorectal cancer.
Corrections
This article was originally published with incorrect affiliations for Teddy Tjahyanto, Jason Gunawan Lie, Daniel Octavianus, Johanes Andrew, Yusuf Damar Jatinugroho, and Christian Shiady. Their affiliations have been changed to Department of Medicine, Universitas Tarumanagara, Jakarta, Indonesia in the online version. These authors also originally had incorrect degrees listed. Their degrees have been changed from MD to BSc in the online version.
Author contribution
Conceptualization: Natalia Maria Christina, Daniel Octavianus, Jeremiah Hilkiah Wijaya, Jason Gunawan Lie, Hans Albertus.
Data curation: Natalia Maria Christina, Teddy Tjahyanto, Hans Albertus, Daniel Octavianus, Johanes Andrew, Yusuf Damar Jatinugroho, Jeremiah Hilkiah Wijaya.
Formal analysis: Natalia Maria Christina, Tiffanie Almas Santoso, Hans Albertus, Daniel Octavianus, Johanes Andrew, Yusuf Damar Jatinugroho, Jeremiah Hilkiah Wijaya.
Funding acquisition: Natalia Maria Christina, Teddy Tjahyanto, Hans Albertus, Jeremiah Hilkiah Wijaya, Jason Gunawan Lie.
Investigation: Natalia Maria Christina, Teddy Tjahyanto, Hans Albertus, Daniel Octavianus, Jeremiah Hilkiah Wijaya.
Methodology: Natalia Maria Christina, Tiffanie Almas Santoso, Daniel Octavianus, Johanes Andrew, Yusuf Damar Jatinugroho, Christian Shiady, Jeremiah Hilkiah Wijaya.
Project administration: Natalia Maria Christina, Christian Shiady, Jeremiah Hilkiah Wijaya.
Resources: Natalia Maria Christina, Teddy Tjahyanto, Daniel Octavianus, Johanes Andrew, Yusuf Damar Jatinugroho, Christian Shiady.
Software: Natalia Maria Christina, Hans Albertus, Daniel Octavianus, Johanes Andrew, Yusuf Damar Jatinugroho, Christian Shiady, Jeremiah Hilkiah Wijaya.
Supervision: Natalia Maria Christina, Hans Albertus, Derby Ayudhia Utami Iskandar Putri, Johanes Andrew, Jeremiah Hilkiah Wijaya.
Validation: Natalia Maria Christina, Teddy Tjahyanto, Daniel Octavianus, Derby Ayudhia Utami Iskandar Putri, Jeremiah Hilkiah Wijaya.
Visualization: Natalia Maria Christina, Johanes Andrew, Yusuf Damar Jatinugroho, Christian Shiady, Jeremiah Hilkiah Wijaya.
Writing – original draft: Natalia Maria Christina, Hans Albertus, Jeremiah Hilkiah Wijaya, Jason Gunawan Lie, Derby Ayudhia Utami Iskandar Putri.
Writing – review & editing: Natalia Maria Christina, Derby Ayudhia Utami Iskandar Putri, Jeremiah Hilkiah Wijaya.
Abbreviations:
- BSA
- basal serum albumin
- CRC
- colorectal cancer
- NOS
- newcastle ottawa scale
- RR
- risk ratio
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
How to cite this article: Christina NM, Tjahyanto T, Lie JG, Santoso TA, Albertus H, Octavianus D, Putri DAUI, Andrew J, Jatinugroho YD, Shiady C, Wijaya JH. Hypoalbuminemia and colorectal cancer patients: Any correlation?: A systematic review and meta-analysis. Medicine 2023;102:8(e32938).
References
- [1].Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- [2].Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019;11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baidoun F, Elshiwy K, Elkeraie Y, et al. Colorectal cancer epidemiology: recent trends and impact on outcomes. Curr Drug Targets. 2021;22:998–1009. [DOI] [PubMed] [Google Scholar]
- [4].Kitsios GD, Mascari P, Ettunsi R, et al. Co-administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: a meta-analysis. J Crit Care. 2014;29:253–9. [DOI] [PubMed] [Google Scholar]
- [5].Tan Y, Jiang L, Liu H, et al. The effect of preoperative hypoalbuminemia on complications after primary hip arthroplasty. J Orthop Surg Res. 2021;16:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gelfand Y, De la Garza Ramos R, Nakhla JP, et al. Predictive value of hypoalbuminemia and severe hypoalbuminemia in oncologic spine surgery. Clin Neurol Neurosurg. 2021;210:107009. [DOI] [PubMed] [Google Scholar]
- [7].Choi JW, Park J-S, Lee CH. () Genetically determined hypoalbuminemia as a risk factor for hypertension: instrumental variable analysis. Sci Rep. 2021;11:11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhao D-W, Zhao F-C, Zhang X-Y, et al. Association between postoperative hypoalbuminemia and postoperative pulmonary imaging abnormalities patients undergoing craniotomy for brain tumors: a retrospective cohort study. Sci Rep. 2022;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fan W, Yin W, Zhou F, et al. The correlation between paclitaxel chemotoxicity and the plasma albumin level in cancer patients. J Clin Pharm Ther. 2022;47:2237–44. [DOI] [PubMed] [Google Scholar]
- [10].Chen WS, Huang YS, Xu LB, et al. Effects of sarcopenia, hypoalbuminemia, and laparoscopic surgery on postoperative complications in elderly patients with colorectal cancer: A prospective study. Neoplasma. 2020;67:922–32. [DOI] [PubMed] [Google Scholar]
- [11].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Larson DW, Abd El Aziz MA, Perry W, et al. Additional value of preoperative albumin for surgical risk stratification among colorectal cancer patients. Ann Nutr Metab. 2020;76:422–30. [DOI] [PubMed] [Google Scholar]
- [13].Chiang JM, Chang CJ, Jiang SF, et al. Pre-operative serum albumin level substantially predicts post-operative morbidity and mortality among patients with colorectal cancer who undergo elective colectomy. Eur J Cancer Care (Engl). 2017;26. [DOI] [PubMed] [Google Scholar]
- [14].Haskins IN, Baginsky M, Amdur RL, et al. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr. 2017;36:1333–8. [DOI] [PubMed] [Google Scholar]
- [15].González-Trejo S, Carrillo JF, Carmona-Herrera DD, et al. Baseline serum albumin and other common clinical markers are prognostic factors in colorectal carcinoma: a retrospective cohort study. Medicine (Baltim). 2017;96:e6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Egenvall M, Mörner M, Martling A, et al. Prediction of outcome after curative surgery for colorectal cancer: preoperative haemoglobin, C-reactive protein and albumin. Colorectal Dis. 2018;20:26–34. [DOI] [PubMed] [Google Scholar]
- [17].Hu W-H, Eisenstein S, Parry L, et al. Preoperative malnutrition with mild hypoalbuminemia associated with postoperative mortality and morbidity of colorectal cancer: a propensity score matching study. Nutr J. 2019;18:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lai C-C, You J-F, Yeh C-Y, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis. 2011;26:473–81. [DOI] [PubMed] [Google Scholar]
- [19].Eckart A, Struja T, Kutz A, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020;133:713–22.e7. [DOI] [PubMed] [Google Scholar]
- [20].Li S, Zhang J, Zheng H, et al. Prognostic role of serum albumin, total lymphocyte count, and mini nutritional assessment on outcomes after geriatric hip fracture surgery: a meta-analysis and systematic review. J Arthroplasty. 2019;34:1287–96. [DOI] [PubMed] [Google Scholar]
- [21].Loftus TJ, Brown MP, Slish JH, et al. Serum levels of prealbumin and albumin for preoperative risk stratification. Nutr Clin Pract. 2019;34:340–8. [DOI] [PubMed] [Google Scholar]
- [22].Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7:193–9. [DOI] [PubMed] [Google Scholar]
- [23].Akirov A, Masri-Iraqi H, Atamna A, et al. Low albumin levels are associated with mortality risk in hospitalized patients. Am J Med. 2017;130:1465.e11–9. [DOI] [PubMed] [Google Scholar]
- [24].Chen C-W, Chen Y-Y, Lu C-L, et al. Severe hypoalbuminemia is a strong independent risk factor for acute respiratory failure in COPD: a nationwide cohort study. Int J Chron Obstruct Pulmon Dis. 2015;10:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. [DOI] [PubMed] [Google Scholar]
- [26].Folsom AR, Lutsey PL, Heckbert SR, et al. Serum albumin and risk of venous thromboembolism. Thromb Haemost. 2010;104:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Königsbrügge O, Posch F, Riedl J, et al. Association between decreased serum albumin with risk of venous thromboembolism and mortality in cancer patients. Oncologist. 2016;21:252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hennessey DB, Burke JP, Ni-Dhonochu T, et al. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252:325–9. [DOI] [PubMed] [Google Scholar]
- [29].Bohl DD, Shen MR, Kayupov E, et al. Hypoalbuminemia independently predicts surgical site infection, pneumonia, length of stay, and readmission after total joint arthroplasty. J Arthroplasty. 2016;31:15–21. [DOI] [PubMed] [Google Scholar]
- [30].Sullivan SA, Van Le L, Liberty AL, et al. Association between hypoalbuminemia and surgical site infection in vulvar cancers. Gynecol Oncol. 2016;142:435–9. [DOI] [PubMed] [Google Scholar]
- [31].Schaible UE, Kaufmann SHE. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Butragueño Laiseca L, Oikonomopoulou N, Miranda Herrero MC, et al. Neurological complications after gamma-knife radiosurgery for hypothalamic hamartoma. Eur J Paediatr Neurol. 2016;20:745–9. [DOI] [PubMed] [Google Scholar]
- [33].Charlie-Silva I, Klein A, Gomes JMM, et al. Acute-phase proteins during inflammatory reaction by bacterial infection: Fish-model. Sci Rep. 2019;9:4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kose E, Wakabayashi H, Yasuno N. Polypharmacy and Malnutrition management of elderly perioperative patients with cancer: a systematic review. Nutrients. 2021;13:1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gupta A, Gupta E, Hilsden R, et al. Preoperative malnutrition in patients with colorectal cancer. Can J Surg. 2021;64:E621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]