Abstract
Objective
To examine differences in mortality and/or severe acute respiratory syndrome between selective serotonin reuptake inhibitor- (SSRI) users and non-SSRI users up to 60 days after a positive SARS-CoV-2 real-time reverse transcription PCR test.
Methods
Retrospective cohort study including all Danish residents above the age of eighteen with a positive SARS-CoV-2 PCR test from 26 February, 2020 to 5 October, 2021. The follow-up period was 60 days. The primary outcome was all-cause mortality, and the secondary outcome was severe acute respiratory syndrome. Exposure of interest was SSRI use. Differences between SSRI users and non-users were examined with Cox regression.
Results
Altogether, 286,447 SARS-CoV-2 positive individuals were identified, and 7113 met the criteria for SSRI use. SSRI users had a mean age of 50.4 years, and 34% were males. Non-SSRI users had a mean age of 41.4 years, and 50% were males. Similar vaccination frequency was observed among the two groups. Sertraline was the most commonly used SSRI, followed by citalopram and escitalopram. We found 255 deaths among SSRI users (3.6%) and 2872 deaths among non-SSRI users (1.0%). SSRI use was significantly associated with increased mortality, with a hazard ratio of 1.32 (95% confidence interval, 1.16 –1.50; p 0.015), even when adjusting for age, sex, vaccination status, and comorbidities.
Discussion
We found significantly higher mortality when comparing SSRI users to non-SSRI users within 60 days after a positive SARS-CoV-2 PCR test. Even when considering possible residual confounding, a positive effect of SSRI intake seems highly unlikely. Our study therefore speaks against the hypothesis of repurposing SSRI drugs for COVID-19 treatment.
Keywords: COVID-19, Drug repurposing, SARS-CoV-2, Selective serotonin receptor inhibitors, SSRI
Introduction
To battle COVID-19, repurposing of existing drugs has drawn substantial interest from research communities [1]. Among others, the ability of selective serotonin reuptake inhibitors (SSRI) to function as a sigma-1-receptor (S1R) agonist has drawn attention. It has been suggested that SSRI binding to S1R could prevent COVID-19-related cytokine storm and reduce organ damage [1,2]. A recent meta-analysis found a significant reduction in mortality and a non-significant trend towards less hospitalization among COVID-19 patients taking SSRIs [3]. However, existing studies are, in many cases, impaired by low sample sizes, lack information on vaccination status, have varying definitions for SSRI use, and introduce possible population bias by only examining specific sub-groups, such as patients seeking hospital examination or closely defined high or low-risk groups [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12]]. In Denmark, extensive nationwide real-time reverse transcription PCR testing for SARS-CoV-2 was conducted during the pandemic, and all results were stored in a national database. Furthermore, through data from national registries, we can account for vaccination status, comorbidities, socio-economic factors, and prescription pickups at pharmacies in a nationwide cohort, including both hospitalized and non-hospitalized subjects. The aim of this study was to examine differences in mortality and severe acute respiratory syndrome between SSRI users and non-SSRI users up to 60 days after a positive SARS-CoV-2 PCR test.
Methods
Study population and design
This was a retrospective cohort study assessing mortality and/or severe acute respiratory syndrome up to 60-days after a positive SARS-CoV-2 test. The study included all Danish residents above 18 years of age with a positive SARS-CoV-2-PCR test from 26 February, 2020, to 5 October, 2021. Exposure of interest was SSRI use. SSRI users were compared with the remaining population. Study participants could only enter the study once, and only the first positive test was used.
Data sources
SARS-CoV-2 test results were obtained from the Danish Microbiology Database (MIBA) [13]. All SARS-CoV-2 test facilities in Denmark are by law obliged to submit test results to the register. Data on medications were obtained from the Danish National Prescription Registry [14]. The register contains all prescriptions made by doctors licensed in Denmark and records of all prescription medication sold in Danish pharmacies [14]. Medications are classified according to the international Anatomical Therapeutic Chemical Classification System (ATC) [14]. Data on hospital contacts and comorbidities were obtained from the Danish National Patient Register (LPR3) [15]. This register holds information on all in- and outpatient hospital contacts and procedures covering public and private sector hospitals [15]. Furthermore, the register has records of acute and chronic diagnoses made by doctors licensed in Denmark, covering private and public sector hospitals as well as private practitioners [15]. Entries covering our study period are classified according to the International Classification of Diseases, 10th revision (ICD-10). Income information was obtained from the Income Statistics Register [16]. Data on educational status was obtained from Danish Education Registers [17]. Remaining data were obtained from The Danish Civil Registration System [18]. All data were made available through the national statistical authority “Statistics Denmark”. Unique and permanent personal identifiers allowed cross linkage between the different registries.
Definition of SSRI use
We included all SSRI products with labelling permission in Denmark in our study; i.e. fluoxetine, citalopram, paroxetine, sertraline, fluvoxamine, and escitalopram (ATC codes N0AB03, N0AB04, N0AB05, N0AB06, N0AB08 and N0AB10). We defined active SSRI use as prescription pickups with sufficient daily dispensing to cover a period from at least two days before and 14 days after the positive SARS-CoV-2 PCR test, using the prescription date, dose, and the number of tablets sold. This method has been described more extensively earlier [19].
Covariates
Comorbidities were identified using predefined lists of ICD-10 and ATC codes and expressed as Charlson Comorbidity Index scores (Charlson index) [20]. ICD-10 and ATC codes used to identify baseline comorbidities are listed in Table S1. The Charlson index was grouped into the following categories; 0, 1–2, and ≥3. Vaccination was defined as at least one vaccination dose with a European Medical Agency (EMA)-approved SARS-CoV-2 vaccine at least 14 days prior to a positive SARS-CoV-19 test. Education was defined as the highest obtained level of education according to the Danish Ministry of Children and Educations definitions. Income levels were defined as equivalent annual household income according to definitions by Statistics Denmark.
Outcome
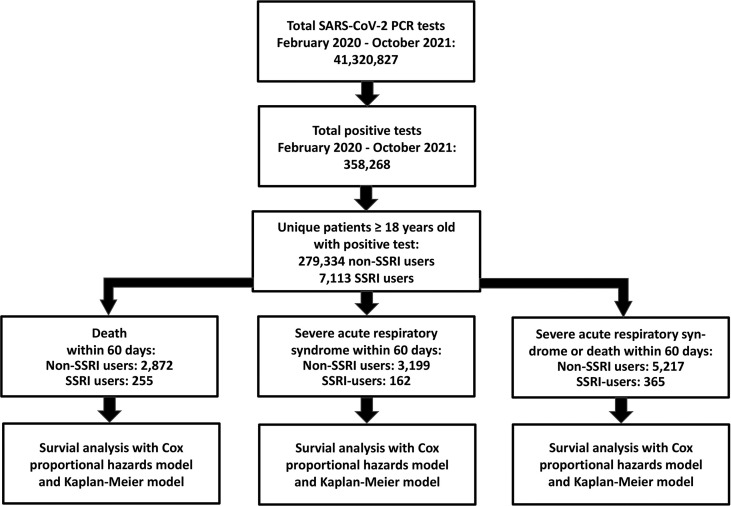
Our outcomes were all-cause mortality and/or severe acute respiratory syndrome with COVID-19. Severe acute respiratory syndrome with COVID-19 was defined as hospitalization for at least 24 h with ICD-10 diagnosis code B972A (severe acute respiratory syndrome due to COVID-19) or intensive care unit (ICU) admission defined by procedure codes “NABE” (observation at ICU), “NABB” (treatment at ICU), or “BGDA0” (respirator treatment). Endpoint analyses were made in three separate analyses; one with mortality, one with severe acute respiratory syndrome, and one with a composite of mortality and severe acute respiratory syndrome (Fig. 1 ).
Fig. 1.
Study design and study population. All data were obtained from Danish National Administrative registries available through Statistics Denmark. Selective serotonin reuptake inhibitor (SSRI) intake was defined as pharmacy pickups with sufficient daily dispensing to cover a period of at least two days before and 14 days after a positive SARS-CoV-2 PCR test. The total number of tests included subjects aged <18 years old. According to Statistics Denmark, the Danish population was 5.82 million at the beginning of the study period and 5.85 million at the end of the study period. Of these individuals, 4.67 million were ≥18 years old. Study participants could only enter the study once, and only the first positive test was used.
Statistical analyses
Baseline characteristics were stratified according to SSRI use. For age, the median and interquartile range was calculated. Differences in baseline data between SSRI users and non-users were examined with a t test and a chi-squared test. In case of missingness, cases were omitted from the analysis. Outcomes were analysed using Kaplan–Meier method and hazard ratios (HR) estimated with Cox regressions. The starting time for risk calculation was the time of a positive PCR test. HR was estimated both unadjusted and adjusted for age, sex, vaccination status, and Charlson index. Charlson index scores were entered as a categorical variable, since it was created and validated as a categorical scoring system. All statistical tests were two-sided, with p < 0.05 considered statistically significant. All calculations were performed in R version 4.2 [21].
Sensitivity analysis
Sensitivity analyses were done by comparing time to event from a positive SARS-CoV-2 PCR test until death and/or severe acute respiratory syndrome, reducing the follow-up period to 30 days, and sub-group analysis according to age, sex, Charlson Index, vaccination status, and dominant virus variant in the society. Furthermore, we tested the effect of applying the Charlson index scores as individual variables in a multivariable analysis. Lastly, we included all comorbidities and socio-economic variables from the baseline table along with age, sex, and vaccination in a multivariable analysis, and performed sensitivity test of missingness by assigning missing values to best and worst case.
Ethics declaration
Data were only available encrypted through Statistics Denmark as deidentified entries. The permission to access and analyse data was granted by The Danish Health Data Authority.
Results
Baseline data
A total of 286,447 eligible study participants with a positive SARS-CoV-2 PCR test were identified (Fig. 1). Of these, 7113 met the criteria for SSRI use. SSRI users were significantly older and had a higher percentage of people of Danish ethnicity, males, and people living alone than non-users (Table 1 ). Sertraline was the most commonly used SSRI, followed by citalopram and escitalopram (Table 2 ). A similar level of education was observed, but a lower annual income was seen for SSRI users (Table 1). Equal vaccination frequency and time from vaccination to a positive test was observed among the two groups (Table 1). SSRI users had fewer SARS-CoV-2 tests done before testing positive than non-SSRI users, but both groups were tested frequently (Table 1).
Table 1.
Baseline data of the study populationa
| Variable | Non-SSRI users (n = 279,334) | SSRI users (n = 7113) | Total (n = 286,447) | pb |
|---|---|---|---|---|
| Male sex (%) | 139,125 (49.8) | 2410 (33.9) | 141,535 (49.4) | <0.001 |
| Age (y), mean (SD) | 41.4 (7.6) | 50.4 (19.1) | 41.6 (17.7) | <0.001 |
| Vaccinated (%) | 27,037 (9.7) | 747 (10.5) | 27,784 (9.7) | 0.0217 |
| Days from vaccination to positive test, mean (SD) | 74.6 (59.8) | 85.1 (67.5) | 74.9 (60) | <0.001 |
| Number of tests until positive, mean (SD) | ||||
| Including first positive test | 21.6 (13.3) | 16.6 (11.8) | 21.4 (13.3) | <0.001 |
| Excluding first positive test | 20.5 (13.1) | 15.7 (11.6) | 20.4 (13.1) | <0.001 |
| Charlson Comorbidity Index score | ||||
| 0 (%) | 258,086 (92.4) | 5858 (82.4) | 263,944 (92.1) | |
| 1–2 (%) | 17,976 (6.4) | 1006 (14.1) | 18,982 (6.6) | |
| ≥3 (%) | 3272 (1.2) | 249 (3.5) | 3521 (1.2) | <0.001 |
| Comorbidities | ||||
| AIDS/HIV (%) | 53 (0.0) | 4 (0.1) | 57 (0.0) | 0.0760 |
| Malignancy (%) | 4650 (1.7) | 228 (3.2) | 4878 (1.7) | <0.001 |
| Liver disease (%) | 716 (0.3) | 53 (0.7) | 769 (0.3) | <0.001 |
| Cerebrovascular disease (%) | 2139 (0.8) | 216 (3.0) | 2355 (0.8) | <0.001 |
| Chronic pulmonary disease (%) | 4910 (1.8) | 323 (4.5) | 5233 (1.8) | <0.001 |
| Dementia (%) | 1214 (0.4) | 221 (3.1) | 1435 (0.5) | <0.001 |
| Heart failure (%) | 1698 (0.6) | 104 (1.5) | 1802 (0.6) | <0.001 |
| Myocardial infarction (%) | 689 (0.2) | 29 (0.4) | 718 (0.3) | 0.0104 |
| Peptic Ulcer disease (%) | 405 (0.1) | 25 (0.4) | 430 (0.2) | <0.001 |
| Peripheral Vascular disease (%) | 1226 (0.4) | 74 (1.0) | 1300 (0.5) | <0.001 |
| Renal disease (%) | 1291 (0.5) | 64 (0.9) | 1355 (0.5) | <0.001 |
| Rheumatic disease (%) | 1955 (0.7) | 92 (1.3) | 2047 (0.7) | <0.001 |
| Diabetes (%) | 13,357 (4.8) | 651 (9.2) | 14,008 (4.9) | <0.001 |
| Hypertension (%) | 18,099 (6.5) | 859 (12.1) | 18,958 (6.6) | <0.001 |
| Schizophrenia, schizotypal, and delusional disorders (%) | 1519 (0.5) | 177 (2.5) | 1696 (0.6) | <0.001 |
| Highest obtained education | ||||
| Basic school (%) | 81,001 (30.1) | 2194 (31.6) | 83,195 (30.1) | |
| High school/vocational (%) | 104,854 (38.9) | 2702 (39.0) | 107,556 (38.9) | |
| Short/medium length higher education (%) | 57,300 (21.3) | 1459 (21.0) | 58,759 (21.3) | |
| Long higher education (%) | 26,096 (9.7) | 581 (8.4) | 26,677 (9.7) | <0.001 |
| Missing (%) | 10,083 (3.6) | 177 (2.5) | 10,260 (3.6) | |
| Level of income, quartiles | ||||
| Lowest (%) | 69,359 (25.3) | 966 (13.6) | 70,325 (25.0) | |
| Mid-lowest (%) | 67,922 (24.8) | 2404 (34.0) | 70,326 (25.0) | |
| Mid-highest (%) | 68,232 (24.9) | 2094 (29.6) | 70,326 (25.0) | |
| Highest (%) | 68,710 (25.1) | 1616 (22.8) | 70,326 (25.0) | <0.001 |
| Missing (%) | 5111 (1.8) | 33 (0.5) | 5144 (1.8) | |
| Ethnicity | ||||
| Native Danish (%) | 200,666 (72.6) | 5635 (79.4) | 206,301 (72.7) | |
| Immigrant (%) | 56,369 (20.4) | 1285 (18.1) | 57,654 (20.3) | |
| Descendant from immigrant (%) | 19,535 (7.1) | 180 (2.5) | 19,715 (6.9) | <0.001 |
| Information missing | 2764 (1.0) | 13 (0.2) | 2777 (1.0) | |
| Living conditions | ||||
| Living in nursing home (%) | 4169 (1.5) | 649 (9.1) | 4818 (1.7) | <0.001 |
| Living alone (%) | 100,691 (36.0) | 3017 (42.4) | 103,708 (36.2) | <0.001 |
SD, standard deviation; SSRI, selective serotonin reuptake inhibitor.
Mean and SD are shown for age, time from vaccination to positive test, and number of tests. The number and percentage of the group are shown for the remaining variables. Missing cases were only observed for ethnicity, income, and education. Number of missing cases is listed in the main table.
p assigns the difference between SSRI users and non-users. Data were obtained from Danish National Administrative registries available through Statistics Denmark.
Table 2.
Hazard ratiosa
| Outcome | Number of events | HR, unadjusted [95% CI] | HR, age- and sex-adjusted [95% CI] | HR, age-, sex-, and Charlson comorbidity index score-adjusted [95% CI] | HR-, age-, sex-, Charlson comorbidity index score-, and vaccination-adjusted [95% CI] |
|---|---|---|---|---|---|
| Death | |||||
| Non-SSRI users (n = 279,334) | 2872 | Reference | Reference | Reference | Reference |
| Total SSRI users (n = 7113) | 255 | 3.53 [3.11–4.02] p < 0.001 | 1.51 [1,33–1,72] p < 0.001 | 1.33 [1.17–1.51] p < 0.001 | 1.32 [1.16–1.50] p 0.015 |
| Sertraline (n = 4092) | 121 | 2.91 [2.42–3.49] p < 0.001 | 1.47 [1.22–1.76] p < 0.001 | 1.27 [1.06–1.53] p < 0.001 | 1.29 [1.08–1.55] p 0.006 |
| Citalopram (n = 1740) | 98 | 5.60 [4.58–6.85] p < 0.001 | 1.64 [1.34–2.00] p < 0.001 | 1.41 [1.15–1.73] p < 0.001 | 1.36 [1.11–1.67] p 0.003 |
| Escitalopram (n = 759) | 28 | 3.64 [2.51–5.29] p < 0.001 | 1.31 [0.91–1.91] p 0.15 | 1.28 [0.88–1.86] p > 0.019 | 1.29 [0.89–1.87] p 0.18 |
| Paroxetine (n = 231) | 7 | 2.98 [1.42–6.26] p 0.0039 | 2.35 [1.12–4.94] p 0.02 | 2.08 [0.99–4.37] p 0.05 | 1.99 [0.95–4.19] p 0.07 |
| Fluoxetine (n = 209) | 1 | 0.33 [0.047–2.37] p 0.27 | 0.43 [0.06–3.04] p > 0.40 | 0.41 [0.06–2.94] p 0.38 | 0.42 [0.06–2.98] p 0.39 |
| Fluvoxamine (n = 1) | 0 | NA | NA | NA | NA |
| Severe acute respiratory syndrome | |||||
| Non-SSRI users (n = 279,334) | 3199 | Reference | Reference | Reference | Reference |
| Total SSRI users (n = 7113) | 162 | 2.0 [1.71–3.1] p < 0.001 | 1.18 [1.01–1.39] p < 0.04 | 1.07 [0,91–1.26] p 0.4 | 1.07 [0.91–1.25] p 0.4 |
| Sertraline (n = 4092] | 67 | 1.43 [1.13–1.83] p 0.004 | 0.99 [0.78–1.26] p 0.94 | 0.89 [0.70–1.14] p 0.35 | 0.90 [0.75–1.15] p 0.39 |
| Citalopram (n = 1740) | 61 | 3.09 [2.40–3.99] p < 0.001 | 1.3 [1.03–1.72] p 0.03 | 1.19 [0.92–1.54] p 0.18 | 1.16 [0.93–1.50] p 0.24 |
| Escitalopram (n = 759) | 24 | 2.79 [1.87–4.17] p < 0.001 | 1.48 [0.99–2.20] p 0.06 | 1.39 [0.93–2.08] p 0.11 | 1.39 [0.93–2.08] p 0.11 |
| Paroxetine (n = 231) | 7 | 2.68 [1.28–5.63] p 0.009 | 1.79 [0.85–3.75] p 0.13 | 1.71 [0.81–3.59] p 0.16 | 1.64 [0.78–3.45] p 0.19 |
| Fluoxetine (n = 209) | 3 | 0.90 [0.29–2.80] p 0.86 | 0.88 [0.28–2.74] p 0.83 | 0.85 [0.27–2.64] p 0.78 | 0.89 [0.29–2.76] p 0.84 |
| Fluvoxamine (n = 1) | 0 | NA | NA | NA | NA |
| Severe acute respiratory syndrome or death | |||||
| Non-SSRI users (n = 279,334) | 5217 | Reference | Reference | Reference | Reference |
| Total SSRI users (n = 7113) | 365 | 2.79 [2.51–3.1] p < 0.001 | 1.40 [1.25–1.55] p < 0.001 | 1.24 [1.12–1.38] p < 0.001 | 1.23 [1.11 –1.37] p < 0.001 |
| Sertraline (n = 4092) | 168 | 2.22 [1.90–2.59] p < 0.001 | 1.31 [1.12–1.53] p < 0.001 | 1.16 [0.99–1.35] p 0.06 | 1.17 [1.00–1.37] p 0.05 |
| Citalopram (n = 1740) | 137 | 4.33 [3.65–5.13] p < 0.001 | 1.52 [1.28–1.80] p < 0.001 | 1.33 [1.12–1.58] p 0.001 | 1.29 [1.09–1.53] p 0.004 |
| Escitalopram (n = 759) | 45 | 3.24 [2.42–4.35] p < 0.001 | 1.43 [1.07–1.92] p 0.02 | 1.36 [1.02–1.83] p 0.04 | 1.36 [1.02–1.83] p 0.04 |
| Paroxetine (n = 231) | 12 | 2.85 [1.62–5.02] p < 0.001 | 1.90 [1.08–3.43] p 0.27 | 1.76 [1.0–3.1] p 0.05 | 1.69 [0.96–2.99] p 0.07 |
| Fluoxetine (n = 209) | 3 | 0.55 [0.18–1.72] p 0.30 | 0.56 [0.18–1.73] p 0.31 | 0.53 [0.17–1.66] p 0.28 | 0.55 [0.18–1.71] p 0.30 |
| Fluvoxamine (n = 1) | 0 | NA | NA | NA | NA |
HR, hazard ratio; SSRI, selective serotonin reuptake inhibitor.
Differences in outcome between SSRI users and non-SSRI users within 60 days after a positive SARS-CoV-2 PCR test. SSRI intake is defined as pharmacy pickups with sufficient daily dispensing to cover a period of at least two days prior to and 14 days after a positive SARS-CoV-2 PCR test. Severe acute respiratory syndrome is defined as ≥24 h of hospitalisation with the ICD–10 diagnosis code B972A or intensive care unit admission. Hazard ratios were estimated with Cox regressions.
Risk assessment
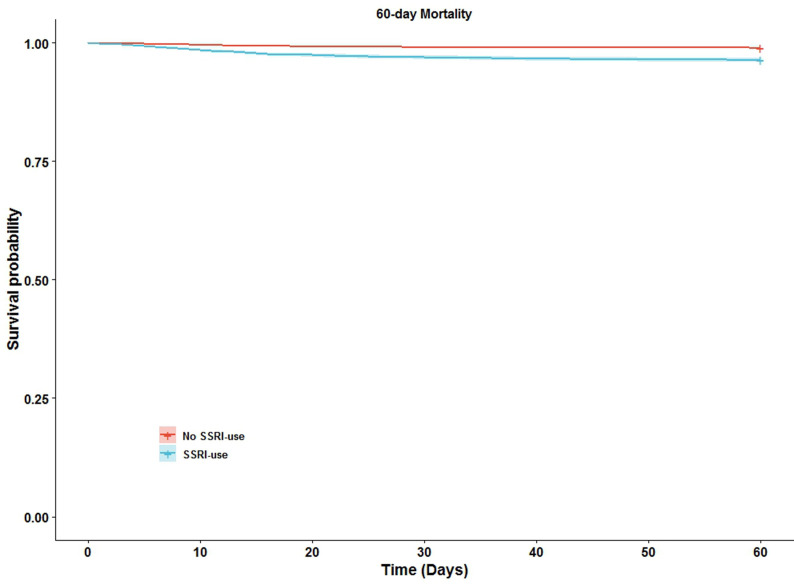
We identified 255 deaths among SSRI users (3.6%) and 2872 deaths among non-SSRI users (1.0%) 60 days after the positive SARS-CoV-2 PCR test. The risk of death and severe acute respiratory syndrome was higher for SSRI users than for non-SSRI users (Table 2, Fig. 2 ). The HR for death remained statistically significant after adjusting for Charlson index, age, and vaccination (HR, 1.32; 95% CI, 1.16–1.50; p 0.015; Table 2). When stratifying SSRIs according to type of drug, only sertraline, citalopram, and paroxetine remained statistically significant (Table 2).
Fig. 2.
60-day mortality of selective serotonin reuptake inhibitor (SSRI) users vs. non-SSRI users. Sixty-day mortality was analysed using the Kaplan–Meier method. Exposed = SSRI users; not exposed = non-SSRI users. Day zero was the date of the positive SARS-CoV-2 PCR test. SSRI use is defined as pharmacy pickups with sufficient daily dispensing to cover a period of at least two days prior to and 14 days after a positive SARS-CoV-2 PCR. The starting time for risk-time calculation was defined as the time of the positive PCR test. Data were obtained from Danish National Administrative registries available through Statistics Denmark; see the method section for details.
Results of sensitivity analysis
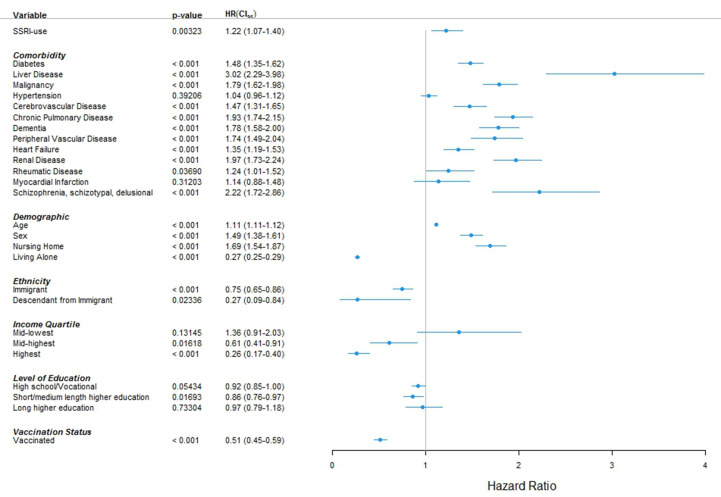
The reduction of follow-up period did not alter the results. No difference was observed in time-to-event between SSRI users and non-SSRI users. A positive effect of SSRI was not seen in any of the sub-group analyses (Table 3 and Table S2). When entering all comorbidities and socio-economic variables of the baseline table along with age, sex, and vaccination status, HR was 1.22 (95% CI, 1.07–1.40; p 0.003) (Fig. 3 ) No major change was seen when entering the Charlson index comorbidities as individual variables, or by applying best- and worst-case scenarios to missing variables (Figs. S1–S3).
Table 3.
Sub-group analysisa
| Sub-group | Non-SSRI users | SSRI users | Deaths, non-SSRI users | Deaths, SSRI users | Hazard ratio (95% CI) | p |
|---|---|---|---|---|---|---|
| Age <39 y | 143,316 | 2246 | 13 | 1 | 4.1 [0.5–31.3] | 0.17 |
| Age ≥39 y | 136,018 | 4867 | 2859 | 254 | 1.3 [1.2–1.5] | <0.001 |
| Females | 140,209 | 4703 | 1279 | 144 | 1.3 [1,1–1.6] | <0.001 |
| Males | 139,125 | 2410 | 1593 | 111 | 1.3 [1.1–1.6] | <0.001 |
| Unvaccinated | 252,297 | 6366 | 2642 | 234 | 1.3 [1.2–1.5] | <0.001 |
| Vaccinated | 27,037 | 747 | 230 | 21 | 1.4 [0.9–2.1] | 0.18 |
| Charlson Comorbidity Index score = 0 | 258,086 | 5858 | 1112 | 84 | 1.6 [1.3–2.0] | <0.001 |
| Charlson Comorbidity Index score = 1–2 | 17,976 | 1006 | 1108 | 107 | 1.2 [1.0–1.5] | 0.04 |
| Charlson Comorbidity Index score ≥3 | 3272 | 249 | 652 | 64 | 1.2 [0.9–1.5] | 0.25 |
SSRI, selective serotonin reuptake inhibitor.
Differences in outcome between SSRI users and non-SSRI users within 60 days after a positive SARS-CoV-2 PCR test for different sub-groups of the study population. The age cut-off was set at 39 years, since 39 was the overall median. Hazard ratios were adjusted for age, sex, vaccination status, and Charlson Comorbidity Index score. See the method section for definitions of individual variables.
Fig. 3.
Multivariable analysis including comorbidities and socio-economic variables. HR, hazard ratio; SSRI, selective serotonin reuptake inhibitor. CI95, 95% confidence interval; s, male sex. The endpoint was all-cause mortality within 60 days after a positive SARS-CoV-2 PCR test. SSRI intake was defined as pharmacy pickups with sufficient daily dispensing to cover a period of at least two days prior to and 14 days after a positive SARS-CoV-2 PCR test. Hazard ratios were estimated with Cox regressions. For ethnicity, “Danish” serves as reference. For income, “lowest quartile” serves as reference. For education, “basic school” serves as reference. In case of missingness, cases were omitted from the analysis. Data were obtained from Danish National Administrative registries available through Statistics Denmark; see the method section for definitions and details.
Discussion
Summary of the principal findings
We found significantly higher mortality when comparing SSRI users to non-SSRI users up to 60 days after a positive SARS-CoV-2 PCR test. The association remained significant, even when adjusting for vaccination, comorbidities, socio-economic factors, age, and sex.
Findings in relation to existing literature
To our knowledge, our study is by far the most extensive study conducted on the effect of SSRIs on COVID-19, and we are the first to show SSRI intake being significantly associated with increased risk of death [1,3]. In previous retrospective studies, Oskostky et al. (3401 SSRI users of 85,584 included) and Hoertel et al. (first study, 195 SSRI users of 7345 included; second study, 388 SSRI users of 41,293 included) showed a significantly reduced mortality risk among SSRI users, while Rauchman et al. (832 SSRI/SNRI users of 9044 included) showed an non-significant trend towards reduced mortality risk [[4], [5], [6],12]. These studies differ markedly from our study by only including hospitalized patients [[4], [5], [6],12]. Denmark has had one of the world's most comprehensive COVID-19 test regimens, with high-quality tests made available to the public free of charge without the need for medical referral. With our nationwide sample size, we can thus close a gap in the current literature. Further we present data on a number of variables not included in previous studies, such as vaccination status, testing frequency and virus variant [[4], [5], [6],12]. While the study by Oskostky et al. and the first study by Hoertel et al. were conducted before EMA approval of vaccinations, the study by Rauchman et al. and the second study of Hoertel et al. were conducted after [4,12]. Uneven distribution of vaccinated study participants could thus bias these studies. We find a high testing frequency among both SSRI users and non-SSRI users but find that SSRI users are tested a bit less. We cannot exclude that differences in testing practice could have identified more asymptomatic cases in the non-SSRI group and affected our risk assessment.
In Denmark, extensive mass screening was carried out. In countries pursuing other testing strategies, other differences in testing practice could arise. Previous studies do not report testing frequency and could thus potentially both under- and overestimate the SSRIs effect on mortality. Previous studies have highlighted fluoxetine and fluvoxamine as superior to other SSRIs, and a recent Cochrane review found that fluvoxamine in addition to standard care might slightly reduce all-cause mortality in SARS-CoV-2-positive patients [1,3,11]. Due to limited use in our study population, we have not been able to examine fluvoxamine. Still, when stratifying SSRI according to drug type, we saw a substantial reduction in HR for death among fluoxetine users (HR, 0.42; 95% CI, 0.06–2.98; p 0.39). With the broad 95% CI, caution is, however, advised, and our results should not be taken as evidence for fluoxetine effect.
Strengths and limitations of the study
The Danish population is a relatively homogenous ethnicity. Furthermore, the registry does not contain information on lifestyle factors and symptom onset. Several lifestyle factors, such as smoking and obesity have been associated with poor COVID-19 outcomes [22]. An uneven distribution of lifestyle factors could thus affect our risk assessment. Regarding symptom onset, it could be noted that we find a similar time-to-event for SSRI users and non-users, indicating that they are tested at the same stage of infection. Mental illness has previously been associated with an increased risk of poor COVID-19 outcomes [23]. We included schizophrenia, schizotypal and delusional disorders in our analysis, but psychiatric diseases not accounted for could affect our risk assessment. As with all study designs, not including direct drug intake observation and study participants' compliance to drug prescriptions is a limitation. We tried to account for this limitation by basing SSRI intake on actual pharmacy pickups. Lastly, it should be noted that the study is retrospective and therefore limited to examining the effect of ongoing use rather than interventions. The strength of our study is the nationwide sample size, the comprehensive nationwide SARS-CoV-2 PCR testing, and the use of central registers. The Danish registries are of high quality, and several studies have confirmed data validity [[14], [15], [16], [17], [18],24,25]. In contrast to previous studies, we used the Charlson index as an adjustment tool. The Charlson index has the benefit of being a well-tested risk assessment tool, with an ability to account for a combined effect of several different combinations of comorbidities [26]. Furthermore, the Charlson index more closely resembles the working conditions of clinical accessing overall health-status comorbidity rather than attempting to isolate the effect of individual comorbidities. On the other hand, the Charlson index is limited by only including a pre-selected number of comorbidities and not accounting for the fact that the pre-selected comorbidities might have different impacts on mortality risk under different conditions.
Understanding possible mechanisms
It is unclear whether the increased HR for death is caused by interactions between SARS-CoV-2 and SSRI or residual confounding. The current dominant hypothesis for the beneficial effects of SSRI intake is anti-inflammatory effects due to SR1 modulation [2,27,28]. Given that a positive effect of SSRI seems highly unlikely with the results of our study, our study speaks against this hypothesis. In the literature, fluoxetine and fluvoxamine are often highlighted due to their strong SR1 affinity [3,27]. It could be noted that the affinity of sertraline for SR1 is higher than that of fluoxetine [29]. Still we found a significantly increased HR for death among sertraline users. One could speculate that a potential anti-inflammatory effect would decrease in long-term users due to restored balances in the immune system over time. Even though it is speculative, we cannot exclude such a mechanism, and it could explain the discrepancy between our study and studies where SSRIs are given to non-users.
Implications for practice or policy
Based on an assumption of SR1 modulation, The U.S. National Institute of Health (NIH) have included the SSRI fluvoxamine as a possible experimental treatment in their guideline for COVID-19 treatment [30]. Our study has not found evidence to support such practice. SSRI can be lifesaving and well indicated to treat depression. Due to the retrospective nature of our study, we can only show an association and not a direct causal relationship. We are therefore not recommending discontinued use of well-indicated SSRI. On the other hand, a positive effect on COVID-19 from SSRI appears unlikely, and the use of SSRI as treatment or prophylaxis for COVID-19 should therefore also be avoided following a do-no-harm principle.
Implications for future research
Previous studies have called for research to investigate the potential anti-viral and anti-inflammatory effects of SSRI intake in COVID-19 patients [[2], [3], [4], [5], [6]]. Our results raise the question of whether such studies would be fruitful. However, in light of our results, it could be interesting to examine mortality and risk of clinical deterioration following an infection in a range of other psychotropic drugs, as they could represent a risk factor to be considered for clinical decision making.
Conclusion
We found significantly higher mortality when comparing SSRI users to non-SSRI users within 60 days after a positive SARS-CoV-2 PCR test. Even when considering possible residual confounding, a positive effect of SSRI intake appears highly unlikely. Our study therefore speaks against the hypothesis of repurposing SSRI drugs for COVID-19 treatment.
Transparency declaration
C. T. P. has previously received research grants from Novo Nordisk and Bayer AG not related to the current study. The remaining authors declare no conflicts of interest.
Funding
The study received no external funding.
Author contributions
Writing -original draft: M. A. S. and D. J. G.; Writing – review and editing: M. A. S., D. J. G., J. T., and C. T. P.; Conceptualization: M. A. S. and J. T.; Methodology: M. A. S., J. T., D. J. G., and C. T. P.; Investigation: D. J. G. and C. T. P.; Formal analysis and software programming: D. J. G. and C. T. P.; Validation: J. T., M. A. S., and C. T. P.; Interpretation: M. A. S., J. T., D. J. G., and C. T. P.; Computing resources and data curation: C. T. P.
Acknowledgement
The authors would like to thank Professor Ove Andersen and data manager Mikkel Porsborg Andersen for their valuable help and discussions regarding the study.
Editor: L. Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2023.04.028.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fico G., Isayeva U., De Prisco M., Oliva V., Solè B., Montejo L., et al. Psychotropic drug repurposing for COVID-19: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2023;66:30–44. doi: 10.1016/j.euroneuro.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto K. Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur Arch Psychiatry Clin Neurosci. 2021;271(2):249–258. doi: 10.1007/s00406-020-01231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firouzabadi D., Kheshti F., Abdollahifard S., Taherifard E., Kheshti M.R. The effect of selective serotonin and norepinephrine reuptake inhibitors on clinical outcome of COVID-19 patients: a systematic review and meta-analysis. Health Sci Rep. 2022;5(6) doi: 10.1002/hsr2.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauchman S.H., Mendelson S.G., Rauchman C., Kasselman L.J., Pinkhasov A., Reiss A.B. Ongoing use of SSRIs does not alter outcome in hospitalized COVID-19 patients: a retrospective analysis. J Clin Med. 2021;11(1):70. doi: 10.3390/jcm11010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoertel N., Sánchez-Rico M., Vernet R., Beeker N., Jannot A.S., Neuraz A., et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;26(9):5199–5212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- 6.Oskotsky T., Maric I., Tang A., Oskotsky B., Wong R.J., Aghaeepour N., et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.33090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reis G., Dos Santos Moreira-Silva E.A., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10(1):e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calusic M., Marcec R., Luksa L., Jurkovic I., Kovac N., Mihaljevic S., et al. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls. Br J Clin Pharmacol. 2022;88(5):2065–2073. doi: 10.1111/bcp.15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seftel D., Boulware D.R. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis. 2021;8(2) doi: 10.1093/ofid/ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyirenda J.L., Sofroniou M., Toews I., Mikolajewska A., Lehane C., Monsef I., et al. Fluvoxamine for the treatment of COVID-19. Cochrane Database Syst Rev. 2022;9(9):CD015391. doi: 10.1002/14651858.CD015391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoertel N., Sánchez-Rico M., Kornhuber J., Gulbins E., Reiersen A.M., Lenze E.J., et al. Antidepressant use and its association with 28-day mortality in inpatients with SARS-CoV-2: support for the FIASMA model against COVID-19. J Clin Med. 2022;11(19):5882. doi: 10.3390/jcm11195882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voldstedlund M., Haarh M., Mølbak K., MiBa Board of Representatives The Danish Microbiology database (MiBa) 2010 to 2013. Euro Surveill. 2014;19(1) doi: 10.2807/1560-7917.es2014.19.1.20667. [DOI] [PubMed] [Google Scholar]
- 14.Kildemoes H.W., Sørensen H.T., Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39:38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M., Schmidt S.A., Sandegaard J.L., Ehrenstein V., Pedersen L., Sørensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baadsgaard M., Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39:103–105. doi: 10.1177/1403494811405098. [DOI] [PubMed] [Google Scholar]
- 17.Jensen V.M., Rasmussen A.W. Danish education registers. Scand J Public Health. 2011;39:91–94. doi: 10.1177/1403494810394715. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen C.B. The Danish Civil registration system. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 19.Fosbøl E.L., Gislason G.H., Jacobsen S., Abildstrom S.Z., Hansen M.L., Schramm T.K., et al. The pattern of use of non-steroidal anti-inflammatory drugs (NSAIDs) from 1997 to 2005: a nationwide study on 4.6 million people. Pharmacoepidemiol Drug Saf. 2008;17(8):822–833. doi: 10.1002/pds.1592. [DOI] [PubMed] [Google Scholar]
- 20.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team . The R Foundation for Statistical Computing; 2017. R: a language and environment for statistical computing.https://www.r-project.org/ Version 3.4.1 “Single Candle” Copyright (C) 2017. [Google Scholar]
- 22.Hamer M., Kivimäki M., Gale C.R., Batty G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vai B., Mazza M.G., Marisa C.D., Beezhold J., Kärkkäinen H., Saunders J., et al. Joint European policy on the COVID-19 risks for people with mental disorders: an umbrella review and evidence- and consensus-based recommendations for mental and public health. Eur Psychiatry. 2022;65(1):e47. doi: 10.1192/j.eurpsy.2022.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt M., Schmidt S.A.J., Adelborg K., Sundbøll J., Laugesen K., Ehrenstein V., et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287:2398–2399. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- 26.Tuty Kuswardhani R.A., Henrina J., Pranata R., Anthonius Lim M., Lawrensia S., Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto Y., Suzuki T., Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psychiatry. 2022;27(4):1898–1907. doi: 10.1038/s41380-021-01432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannestad J., DellaGioia N., Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36(12):2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albayrak Y., Hashimoto K. Sigma-1 receptor agonists and their clinical implications in neuropsychiatric disorders. Adv Exp Med Biol. 2017;964:153–161. doi: 10.1007/978-3-319-50174-1_11. [DOI] [PubMed] [Google Scholar]
- 30.National Institutes of Health – U.S. Government. Update Dec 16 2021. Fluvoxamine|COVID-19 Treatment Guidelines n.d. Available at https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/fluvoxamine/(accessed November 11, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.