Abstract
Background
The COVID-19 pandemic has put substantial strain on the healthcare system of which the effects are only partly elucidated. This study aimed to investigate the impact on pancreatic cancer care.
Methods
All patients diagnosed with pancreatic cancer between 2017 and 2020 were selected from the Netherlands Cancer Registry. Patients diagnosed and/or treated in 2020 were compared to 2017–2019. Monthly incidence was calculated. Patient, tumor and treatment characteristics were analyzed and compared using Chi-squared tests. Survival data was analyzed using Kaplan–Meier and Log-rank tests.
Results
In total, 11019 patients were assessed. The incidence in quarter (Q)2 of 2020 was comparable with that in Q2 of 2017–2019 (p = 0.804). However, the incidence increased in Q4 of 2020 (p = 0.031), mainly due to a higher incidence of metastatic disease (p = 0.010). Baseline characteristics, surgical resection (15% vs 16%; p = 0.466) and palliative systemic therapy rates (23% vs 24%; p = 0.183) were comparable. In 2020, more surgically treated patients received (neo)adjuvant treatment compared to 2017–2019 (73% vs 67%; p = 0.041). Median overall survival was comparable (3.8 vs 3.8 months; p = 0.065).
Conclusion
This nationwide study found a minor impact of the COVID-19 pandemic on pancreatic cancer care and outcome. The Dutch health care system was apparently able to maintain essential care for patients with pancreatic cancer.
Introduction
The past years we have been confronted with a SARS-CoV-2 pandemic, causing COVID-19 infections in millions of people worldwide.1 The pandemic has negatively impacted people's lives directly and indirectly. During the first COVID-19 wave (March–May 2020), a decrease in the number of cancer diagnoses was observed in the Netherlands. This was mainly the result of suspension of screening, individuals with symptoms avoiding medical care or experiencing a delay in seeking medical care.2 , 3 This led to further research on the topic and the restarting of screening programs.4, 5, 6 Medical professionals provided general guidelines for the management of surgical and systemic treatments in cancer care and created opportunities to deviate, well considered, from the usual treatment guidelines.7, 8, 9, 10
In two global cross-sectional surveys among hepato-pancreato-biliary (HPB) surgeons, the majority of respondents reported a reduction in surgery for HPB cancers and pancreatic surgery in general.11 , 12 The most important reasons listed for cancellation of HPB cancer surgery were lack of intensive care beds, national and hospital directives to stop non-emergency operations, and concern around patients contracting COVID-19 in the post-operative period. To promote continuity in treatment when surgery was unattainable (neo-adjuvant) chemotherapy was frequently used for (borderline) resectable pancreatic cancer during the pandemic, particularly in countries with high infection rates of COVID-19. This while resection is the only therapy in pancreatic cancer considered to be potentially curative.13
Although pancreatic cancer is a relatively low volume but highly fatal cancer, it is not yet known to what extent clinical practice of pancreatic cancer was affected by the SARS-CoV-2 pandemic in the Netherlands. The aim of the current population-based study is to evaluate to what extent incidence, treatment, and prognosis of Dutch patients with pancreatic cancer were affected in the first year of the SARS-CoV-2 pandemic, in which hospitals were under great pressure and medical resources and supplies were scarce.
Materials and methods
Study design and database
This retrospective nationwide cohort study used the Netherlands Cancer Registry (NCR), a population-based registry which collects data on all newly diagnosed cancer patients in the Netherlands (in 2020, approximately 17.4 million inhabitants.14 The NCR gets notifications on cancer from the Dutch Nationwide Pathology Databank (PALGA) and the Dutch National Hospital Care Registration (LBZ) containing data about hospital discharges and outpatient visits. Subsequently, trained NCR registrars routinely extract patient, tumor, and treatment information from medical records of all Dutch hospitals. Information on vital status was obtained through annual linkage with the Municipal Administrative Database, in which all deceased or emigrated individuals in the Netherlands are registered. This study was approved by the Privacy Review Board of the NCR and Scientific Committee of the Dutch Pancreatic Cancer Group (DPCG).15 The need for a separate approval from an ethics committee in the Netherlands was waived.
Patient inclusion and data collection
All patients aged 18 years or older with a primary invasive pancreatic (ductal) adenocarcinoma diagnosis based on pathology and/or clinical diagnosis (hereinafter referred to as ‘pancreatic cancer’) between 2017 and 2020 were selected from the NCR (International Classification of Disease for Oncology 3rd edition: C25 excluding C25.4 and sporadic morphology codes, Supplementary Table 1). Variables that have been derived from the NCR database include patient, tumor and diagnostic process characteristics. Registered treatments were surgical resection with or without neo- and/or adjuvant treatment, systemic therapy (chemotherapy, targeted therapy), radiotherapy (including stereotactic body radiation therapy [SBRT]) and ablative therapy).
Definitions
The first COVID-19 wave in the Netherlands occurred from March 2020 when first far-reaching measures were taken to contain the spread of the virus, until May 2020 when measures were reduced.16 Variables of two groups were compared based on incidence year (2017–2019 versus 2020), and quarters [Q] of 2020. Some treatment characteristics (interval resection-start adjuvant treatment, use of neo- and/or adjuvant treatment, type of adjuvant systemic therapy, type of palliative systemic therapy (in patients without tumor resection), number of resections, number of patients receiving (neo- and/or adjuvant) systemic therapy and number of patients receiving radiotherapy) were compared based on date of first treatment (2017–2019 versus 2020). Tumor stage was based on pathological TNM classification (UICC TNM) 8th ed., supplemented with clinical TNM classification.17 Sex was defined based on binary categorization (male/female) as registered in the Netherlands Cancer Registry.
Statistical analysis
Incidence rates were calculated for every month, quarter and year using crude rates (number of diagnoses per 100.000 inhabitants). Absolute incidence numbers of a specific period were divided by the total population in the Netherlands on the first day of that period.18 This number was multiplied by 100.000. Incidence rate ratios (IRR) were calculated by using ‘rateratio function’ of RStudio version 4.0.3 (RStudio, PBC, Boston, MA, USA)) by which monthly incidence of 2020 was compared with the mean in the same period in 2017–2019. Results were reported as numbers and percentages or as median with interquartile range (IQR). To evaluate differences between characteristics of the 2017–2019 and the 2020 group, Chi-squared, Mann–Whitney U and Kruskal–Wallis tests were used as appropriate. To evaluate overall survival (OS), the Kaplan Meier method was used, and the log-rank test was used for comparisons between groups. OS was defined as the time from diagnosis (incidence date) to death from any cause, censored at last follow-up date or until February 1, 2022. Data was analyzed using IBM SPSS Statistics for Windows, version 28.0.0.0 (IBM CORP., Armong, NY USA). P-values of <0.05 were considered to be statistically significant.
Results
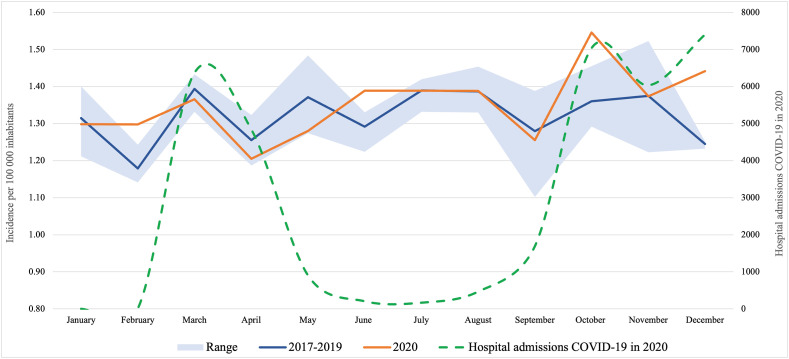
Overall, 11 019 patients with newly diagnosed pancreatic cancer were included: 2650, 2696, 2843 and 2830 in 2017–2020 respectively. The incidence of pancreatic cancer in the Netherlands was 16.2 per 100.000 inhabitants in 2020, compared to 15.7 in 2017–2019 (IRR 1.03; 95% CI: 0.98–1.07; p = 0.245). No significant decrease in incidence of pancreatic cancer was observed during the first COVID-19 wave (March–May 2020) compared to the same months in previous years (Fig. 1 ). However, in Q4-2020 there was a significantly higher incidence rate (4.4 per 100.000) compared to the same period in 2017–2019 (4.0 per 100.000) (IRR 1.10; 95% CI 1.01–1.19; p = 0.031). This higher incidence rate was only found in metastatic disease (2.6 vs 2.2, IRR 1.16; 95% CI 1.04–1.29; p = 0.010, Supplementary Table 2).
Figure 1.
Incidence of pancreatic cancer per 100 000 inhabitants over time
Patient and tumor characteristics
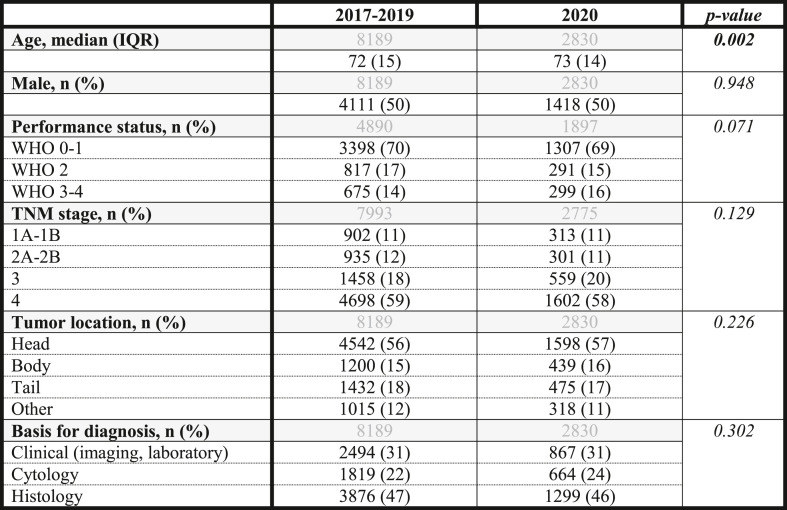
Baseline characteristics of patients in 2020 and 2017–2019 were comparable (Table 1 ), except for a slightly higher median age in 2020 (p = 0.002). When comparing Q2-2020 with the other quarters of 2020, no significant differences were found in patient or tumor characteristics (Supplementary Table 3).
Table 1.
Baseline characteristics of patients diagnosed with pancreatic cancer in 2017-2019 and 2020
In grey the total number of patients included in each analysis per variable.
Missings 2017–2019 and 2020 in percentage (%): Performance status 40% and 33%, TNM stage 2% and 2%.
Diagnostic process
The median time between first hospital consultation for symptomatic pancreatic cancer and diagnosis (incidence date) was shorter for patients in 2020 (5 days) compared to 2017–2019 (6 days) (p < 0.001). For patients who were diagnosed in Q2-2020, this median time interval was 5 days, compared to 4–6 days in the other quarters of 2020 (p = 0.238, Supplementary Table 5).
Between 2020 and 2017–2019 population, there was no difference in the use of cytology or histology for diagnosis, while less diagnostic laparoscopies (2% vs 3%, p = 0.004) and explorative surgeries (3% vs 4%, p = 0.003) were performed in 2020 (Supplementary Table 4). In 2020, significantly more patients were discussed in multidisciplinary team (MDT) meetings with expert centers involved (63%) than in 2017–2019 (60%, p = 0.013).
Treatment process
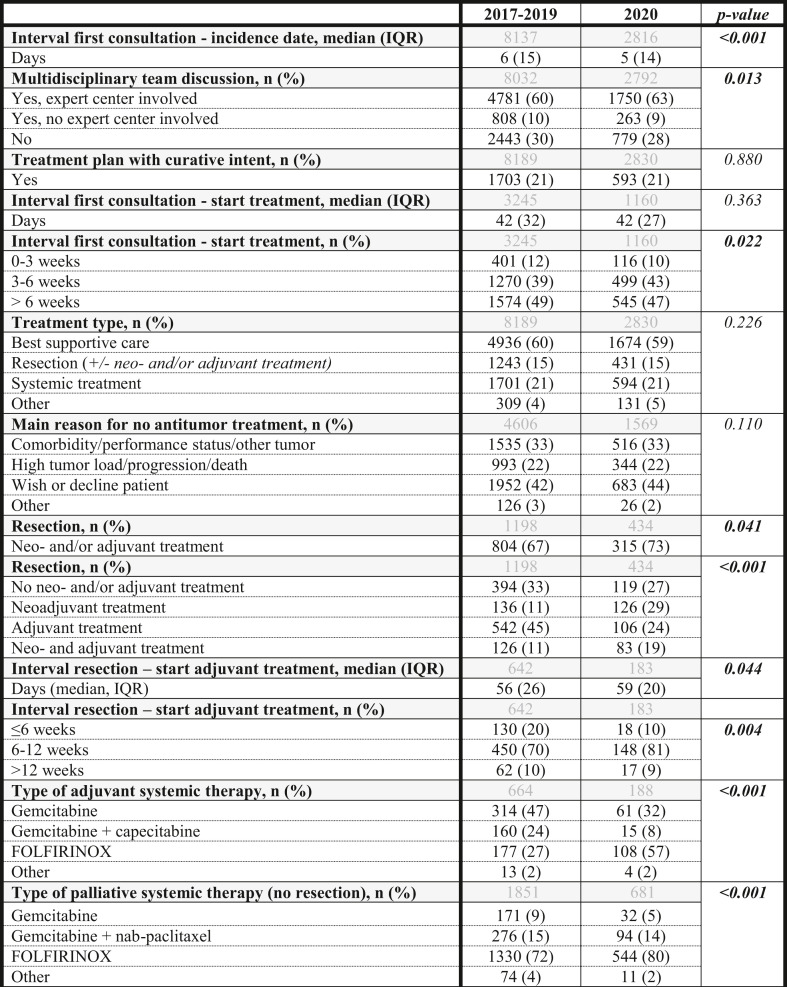
Comparable rates of treatment strategies with curative intent were planned in 2020 and 2017–2019 (21% and 21%, respectively; p = 0.880). Median interval from first hospital consultation to start antitumor treatment was comparable between 2020 (42 days; IQR 27) and 2017–2019 (42 days; IQR 32) (p = 0.363, Table 2 ). In 2020, 10% of patients started treatment within 3 weeks of first consultation compared to 12% in 2017–2019 (p = 0.022).
Table 2.
Diagnostic and treatment process of patients diagnosed with pancreatic cancer in 2017–2019 and 2020
In grey the total number of patients included in each analysis per variable.
Missings 2017–2019 and 2020 in percentage (%): Interval first consultation-start treatment 1% and 0.5%, Multidisciplinary team discussion 2% and 1%, Interval first consultation-start treatment 1% and 1%, Reason no treatment 6% and 5%, Interval resection-start adjuvant therapy 4% and 3%, Type of adjuvant systemic therapy 1% and 1%, Type of palliative systemic therapy (no resection) 0.3% and 0.3%.
Comparable rates of patients underwent tumor resection (16% in 2020, and 15% in 2017–2019, p = 0.466). In 2020, 36% of all patients received systemic therapy, compared to 33% in 2017–2019 (p = 0.018).
In 2020, 73% of patients undergoing resection received neo- and/or adjuvant therapy, compared to 67% in previous years (Table 2). Significantly more patients received neoadjuvant (29%) or neo- and adjuvant treatment (19%) in 2020 compared to 2017–2019 (11% neoadjuvant and 11% neo- and adjuvant treatment in 2017–2019) (<0.001). Although the median interval between resection and start of adjuvant therapy only slightly differed between 2020 (59 days) and previous years (56 days, p = 0.044), in 2020 significantly less patients (10%) started adjuvant therapy within six weeks of surgery compared to previous years (20%, p = 0.004). In 2020, the use of FOLFIRINOX in adjuvant and palliative treatment increased, while gemcitabine-based schedules decreased (p < 0.001, Table 2). Within 2020, only minor non-significant deviations were observed within quarters (Supplementary Table 5) and no increase in the use of capecitabine instead of 5-fluorouracil (in FOLFIRINOX).
Rates of palliative systemic therapy were comparable between 2020 (24%) and 2017–2019 (23%, p = 0.183).
Between 2020 and 2017–2019 no significant difference was observed in the rates of patients who did not receive any antitumor treatment (59% and 60%, respectively; p = 0.252, Supplementary Table 4).
Overall survival
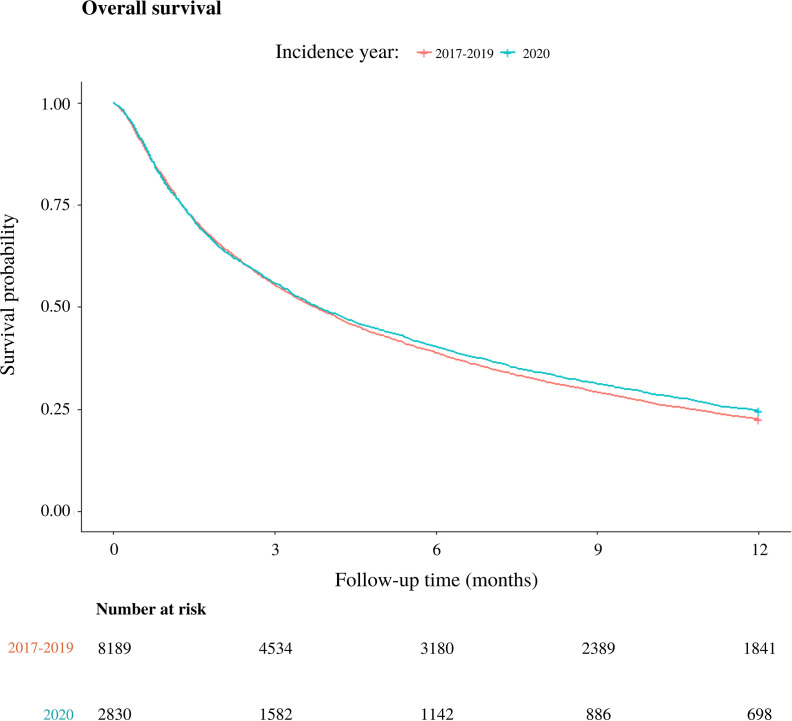
Median OS for patients diagnosed in 2020 was 3.8 (95% confidence interval [95%CI] 3.6–4.2) months versus 3.8 (95%CI: 3.6–3.9) months in 2017–2019 (p = 0.065, Supplementary Fig. 1). In 2020, 1-year OS was 25% compared to 23% in 2017–2019 (p = 0.019).
Discussion
This nationwide population-based cohort study showed that the SARS-CoV-2 pandemic probably had minimal impact on pancreatic cancer care in the Netherlands. A non-significant decrease in incidence was found during Q2-2020 (first COVID-19 wave) compared to Q2 of 2017–2019, followed by, what seemed to be, a catching up moment in Q4 with a significant increase in incidence compared to 2017–2019. This increase was mainly due to a higher incidence of patients presenting with metastatic disease which apparently had no impact on resection rates and overall survival in the total cohort.
Our finding that the nationwide incidence of pancreatic cancer in 2020 seemed hardly affected by the SARS-CoV-2 pandemic was confirmed by a study on all specimen numbers in the Dutch pathology database (PALGA) in 2019 and 2020.19 Furthermore, our observations are in line with incidence of pancreatic cancer in the first COVID-19 wave in France, Italy and Japan.20, 21, 22 A retrospective cohort study of patients diagnosed with metastatic pancreatic cancer in the United States, however, showed an overall decrease of 14% in diagnoses when comparing 2020 to 2019, though this has not been statistically tested.23 Also, the previously conducted studies in France, Italy, Japan, and the United States, unlike this study, had a limited reach, e.g. using data from a single institution or multiple (surgical) centers. In addition, the current study looks at the entire trajectory from diagnosis to treatment and death.
Previous studies on the impact of COVID-19 on prostate, colorectal and breast cancer in the Netherlands showed a more pronounced decrease in incidence during the first COVID-19 wave.2 , 3 , 24, 25, 26, 27 Most studies however declare that these reductions in diagnoses often include early-stage tumors, clarified by the temporary suspension of national screening programs. This could explain the difference in impact on incidence between pancreatic cancer and other cancer sites, as pancreatic cancer is known as an aggressive cancer which is usually diagnosed at an advanced stage.13 Symptoms are frequently more severe and pronounced and thus more easily recognizable as an urgent setting. This may cause patients to seek medical help regardless of their fear of COVID-19 hotbeds in healthcare, as well as urgent referrals to secondary care, even if healthcare systems are already overloaded. This is also supported by the fact that our study demonstrated that there was no stage migration and performance status shift in patients diagnosed with pancreatic cancer in 2020 compared to 2017–2019 or between quarters in 2020. This corresponds with previous mentioned studies in France, Japan, and the United States, which also showed no change in baseline characteristics and/or tumor stage in patients diagnosed with pancreatic cancer during the first COVID-19 wave.20 , 22 , 23
Furthermore, in our study the diagnostic process appeared to be even somewhat faster as compared to previous years and a higher rate of patients were discussed in multidisciplinary expert team meetings compared to 2017–2019. This was in line with the ESMO guidelines issued during the first COVID-19 wave, which stated that stable patients with newly diagnosed resectable pancreatic cancer were high priority patients which needed urgent multidisciplinary team discussion.9 It could also be a result of best practice studies, e.g., PACAP-1 trial.28
Our study showed that in 2020 a comparable rate of patients received anti-tumor treatment, with a corresponding rate of curative intention compared to patients diagnosed in 2017–2019. More patients received neoadjuvant treatment and a lower number of patients received adjuvant treatment, though the use of FOLFIRINOX as an adjuvant treatment increased. This is inconsistent with previous research, demonstrated by a global survey among HPB-surgeons, initiated in the UK, which revealed that the majority of centers reported 40% less pancreatic surgery during the first peak COVID-19 wave.12 , 29 Studies were however consistent on the increase of neoadjuvant treatment. The same study from the UK showed that one-fourth of centers changed treatment pathways for (borderline) resectable patients from upfront surgery to neoadjuvant chemo (radio)therapy.29 In addition, a French study demonstrated an increase of neoadjuvant treatment over upfront surgery.20 These changes in treatment modalities were in accordance with the advice from the ESMO guidelines, and the local guidelines in the Netherlands (Dutch Association of Medical Oncology (NVMO), Dutch Association of Surgery (NVvH)), which assigned a high level of priority to the initiation of the most active neoadjuvant treatment for patients with (borderline) resectable or locally advanced pancreatic cancer.7, 8, 9 Guidelines also advised to postpone adjuvant therapy (with an aim to start within 3 months after surgery), which clarifies the lower rate of patients in 2020 starting adjuvant chemotherapy within 6 weeks of surgery compared to 2017–2019. Also, the additional value of neoadjuvant treatment has been studied over the past years in the PREOPANC-1 and -2 studies, with the latter enrolling patients with (borderline) resectable pancreatic cancer in the time frame reviewed in the current study and experiencing only minor impact during the first wave of the COVID-19 pandemic.30, 31, 32, 33 This may have contributed to the observed increased use of neoadjuvant and decrease of adjuvant treatment.
Despite the pressure on (cancer) care, there was no negative impact of SARS-CoV-2 pandemic on survival of patients with pancreatic cancer in the Netherlands. Thus, patients and care givers were able to maintain life expectancy by playing the hand they were dealt. Moreover, the fact that no major impact on incidence or tumor stage has been found, may also explain why no effect is seen on survival.
For patients with locally advanced or metastatic disease, it was encouraged to carefully weigh benefits of treatment and performance status before suggesting palliative treatment. However, this study illustrated that best supportive care was not chosen more often during the SARS-CoV-2 pandemic than in previous years.
The results of this study should be interpreted in light of some limitations. First, the incidence of pancreatic cancer in the Netherlands has shown an increasing trend over the years.34 The stabilization of the incidence during the first COVID-19 year could therefore be an underestimation. A second possible limitation is the fact that we were only able to analyze survival data up until 1 February 2022, causing a median follow-up of 4.2 months (IQR 10.9). However, in this aggressive cancer with a median OS of 3.8 months (95% CI 3.6–4.2), this only applies to the small cohort of patients that underwent resection of the tumor. Third, the study design does not allow to exclude possible residual confounding. After all, between 2017 and 2019 and 2020, as can be seen in the results, many other medical issues have also improved in pancreatic cancer care in the Netherlands (for example, more use of neoadjuvant treatment, shorter postoperative hospital stay, more endoscopic surgery, etc.). In addition, several studies were conducted during this period that (possibly) have improved pancreatic care nationally (e.g. PORSCH, PREOPANC-1 and -2 and PACAP-1 trials).28 , 30, 31, 32, 33 , 35 Therefore, interpretations of the results must be made with caution. The main strength of this study is the use of nationwide, population-based data from the NCR for all adult patients diagnosed with pancreatic cancer. Therefore, our results accurately reflect daily practice during COVID-19 and the preceding years.
To conclude, pancreatic cancer is known to be an aggressive and fatal disease requiring rapid intervention allowing no delays in diagnosis and treatment. This study demonstrates only minor changes in incidence, tumor characteristics, treatment, and survival during the first SARS-CoV-2 pandemic. The current study displays that, even in health crises and limited resources, the Dutch health care system was apparently able to maintain essential medical services for these patients. We believe that this is mainly due to cooperation of medical specialists in national associations, prioritizing of care based on urgency and (digital) alternatives to substitute for hospital visits in the Dutch healthcare system. Despite modifications in treatment pathways, the SARS-CoV-2 pandemic has not significantly impaired the outcome of patients with pancreatic cancer.
Author contributions
The study was designed and performed under the auspices of the Dutch Pancreatic Cancer Group (DPCG) and on behalf of the collaborators of the COVID and Cancer-NL consortium. MG, LvdG, and JdV contributed to conception and design of the study. MG performed the statistical analysis. MG, LvdG and JdV interpreted the study results and wrote the first draft of the manuscript. IdH, MGB, MJB, JW, VdM and MvV wrote sections of the manuscript. All authors contributed to revision of the manuscript, read, and approved the submitted version.
Funding
This work was supported by the Netherlands Organisation for Health Research and Development ZonMw (project number: 10430022010014). The funding source had no role in the analyses and writing of the manuscript.
Data availability statement
The datasets analyzed for this study are managed by the Netherlands Comprehensive Cancer Organisation (IKNL). Researchers who are interested in accessing the used data, are invited to send a methodologically sound proposal to IKNL [https://iknl.nl/en/ncr/apply-for-data]. Approval by a scientific committee (DPCG) as well as compliance with IKNL objectives and privacy legislation (GDPR) are required. Data will be available for the next 5 years following the date of article publication. The study protocol is available upon request to interested researchers via judith.de.vos@mumc.nl.
Conflict of interest
JdV has served as a consultant for Amgen, AstraZeneca, MSD, Pierre Fabre, and Servier, and has received institutional research funding from Servier. All outside the submitted work. The other authors have no conflicts.
Acknowledgments
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as IKNL staff for scientific advice.
This study was written on behalf of the following collaborators of the COVID and Cancer-NL consortium.
- Prof. Dr. S. Siesling: Dept of research and development, Netherlands Comprehensive Cancer Organisation (IKNL) Utrecht, the Netherlands; Technical Medical Centre, Dept Health Technology and Services Research, University of Twente, Enschede, the Netherlands.
- Dr. J.C. van Hoeve: Dept of research and development, Netherlands Comprehensive Cancer Organisation (IKNL) Utrecht, the Netherlands.
- Prof. Dr. M.A.W. Merkx: Dept of research and development, Netherlands Comprehensive Cancer Organisation (IKNL) Utrecht, the Netherlands; dept of Oral and Maxillofacial Surgery, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
- Prof. Dr. N.J. de Wit: Dept of General Practice, Julius centre for Health Sciences and Primary Care, University Medical centre Utrecht (UMCU), Utrecht University, Utrecht, The Netherlands.
- Dr. C.W. Helsper, department of General Practice, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht (UMCU), Utrecht University, Utrecht, The Netherlands).
- M.Sc. I. Dingemans: Dutch Federation of Cancer Patient Organisations (NFK), Utrecht, The Netherlands.
- Prof. Dr. I.D. Nagtegaal: Dept of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands, on behalf of the Dutch Nationwide Pathology Databank (Pathologisch-Anatomisch Landelijk Geautomatiseerd Archief, PALGA).
- Drs. M. van der Schaaf, department of Insight and Innovation, Dutch Hospital Data (DHD), Utrecht, The Netherlands.
- Prof. Dr. C.H. van Gils: Dept of Epidemiology, Julius centre for Health Sciences and Primary Care, University Medical centre, Utrecht, The Netherlands.
- Prof. Dr. H.C.P.M. van Weert: Dept of General Practice, Amsterdam Public Health, Amsterdam UMC location AMC, Amsterdam, The Netherlands.
- Prof. Dr. M. Verheij: Dept of Radiation Oncology, Radboud University Medical Center, Nijmegen, The Netherlands on behalf of SONCOS (Dutch Multidisciplinary Oncology Foundation).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hpb.2023.04.017.
Contributor Information
for the Dutch Pancreatic Cancer Group (DPCG) and the COVID & Cancer-NL consortium:
S. Siesling, J.C. van Hoeve, M.A.W. Merkx, N.J. de Wit, C.W. Helsper, I. Dingemans, and I.D. Nagtegaal
Dutch Nationwide Pathology Databank (Pathologisch-Anatomisch Landelijk Geautomatiseerd Archief, PALGA):
M. van der Schaaf, C.H. van Gils, H.C.P.M. van Weert, and M. Verheij
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Fig. S1.
References
- 1.WHO . 2022. https://covid19.who.int (WHO coronavirus (COVID-19) dashboard). [Available from: [Google Scholar]
- 2.Eijkelboom A.H., de Munck L., Vrancken Peeters M.-J.T.F.D., Broeders M.J.M., Strobbe L.J.A., Bos M.E.M.M., et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in The Netherlands: a population-based study. J Hematol Oncol. 2021;14:64. doi: 10.1186/s13045-021-01073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toes-Zoutendijk E., Vink G., Nagtegaal I.D., Spaander M.C.W., Dekker E., van Leerdam M.E., et al. Impact of COVID-19 and suspension of colorectal cancer screening on incidence and stage distribution of colorectal cancers in The Netherlands. Eur J Cancer. 2022;161:38–43. doi: 10.1016/j.ejca.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M., et al. Fewer cancer diagnoses during the COVID-19 epidemic in The Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(IKNL) IKN COVID-19 en kanker van de spijsverteringsorganen. https://iknl.nl/monitor/covid-19-en-kanker-van-de-spijsverteringsorganen [Available from:
- 6.(IKNL) IKN. Aantal nieuwe kankerpatiënten in 2020 gedaald door coronacrisis, eerste daling in dertig jaar 03-02-2021 [Available from: https://iknl.nl/persberichten/aantal-nieuwe-kankerpatienten-in-2020-gedaald-door.
- 7.(NVVH) NVvH . 22-03-2020. https://heelkunde.nl/nieuws/nieuwsbericht?newsitemid=23658500 (Handvat voor chirurgische ingrepen tijdens corona-crisis). [Available from: [Google Scholar]
- 8.(NVMO) NVvMO . 28-09-2020. https://www.nvmo.org/dossier-covid-19/handvat-covid-19-oncologie-2-0/ (Handvat COVID-19 oncologie 2.0). [Available from: [Google Scholar]
- 9.Catanese S., Pentheroudakis G., Douillard J.Y., Lordick F. ESMO Management and treatment adapted recommendations in the COVID-19 era: pancreatic Cancer. ESMO Open. 2020;5(Suppl 3) doi: 10.1136/esmoopen-2020-000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel R., Saif M.W. Management of pancreatic cancer during COVID-19 pandemic: to treat or not to treat? Jop. 2020;21:27–28. [PMC free article] [PubMed] [Google Scholar]
- 11.Balakrishnan A., Lesurtel M., Siriwardena A.K., Heinrich S., Serrablo A., Besselink M.G.H., et al. Delivery of hepato-pancreato-biliary surgery during the COVID-19 pandemic: an European-African Hepato-Pancreato-Biliary Association (E-AHPBA) cross-sectional survey. HPB. 2020;22:1128–1134. doi: 10.1016/j.hpb.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oba A., Stoop T.F., Lohr M., Hackert T., Zyromski N., Nealon W.H., et al. Global survey on pancreatic surgery during the COVID-19 pandemic. Ann Surg. 2020;272:e87–e93. doi: 10.1097/SLA.0000000000004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lydia van der geest P.V., Veldhuis Patrick. Integraal Kankercentrum Nederland (IKNL); 2021 november. Alvleesklierkanker in Nederland; kleine stappen vooruit. 2021. [Google Scholar]
- 14.Latenstein A.E.J., Mackay T.M., van der Geest L.G.M., van Eijck C.H.J., de Meijer V.E., Stommel M.W.J., et al. Effect of centralization and regionalization of pancreatic surgery on resection rates and survival. Br J Surg. 2021;108:826–833. doi: 10.1093/bjs/znaa146. [DOI] [PubMed] [Google Scholar]
- 15.Strijker M., Mackay T.M., Bonsing B.A., Bruno M.J., van Eijck C.H.J., de Hingh I., et al. Establishing and coordinating a nationwide multidisciplinary study group: lessons learned by the Dutch pancreatic cancer group. Ann Surg. 2020;271:e102–e104. doi: 10.1097/SLA.0000000000003779. [DOI] [PubMed] [Google Scholar]
- 16.Rijksoverheid. Coronavirus tijdlijn: Ontwikkelingen coronavirus in 2020 [Available from: https://www.rijksoverheid.nl/onderwerpen/coronavirus-tijdlijn/2020.
- 17.Brierley J., Gospodarowicz M.K., Wittekind C. In: TNM classification of malignant tumours. 8th ed. Chichester, West Sussex., editors. John Wiley & Sons, Inc.; UK ; Hoboken, NJ: 2017. [Google Scholar]
- 18.Statline CBvdSC . 2022. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/37230ned/table?fromstatweb (Bevolkingsontwikkeling; regio per maand). [updated 31-02-2022. Available from: [Google Scholar]
- 19.van Velthuysen M.L.F., van Eeden S., le Cessie S., de Boer M., van Boven H., Koomen B.M., et al. Impact of COVID-19 pandemic on diagnostic pathology in The Netherlands. BMC Health Serv Res. 2022;22:166. doi: 10.1186/s12913-022-07546-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brugel M., Letrillart L., Evrard C., Thierry A., Tougeron D., El Amrani M., et al. Impact of the COVID-19 pandemic on disease stage and treatment for patients with pancreatic adenocarcinoma: a French comprehensive multicentre ambispective observational cohort study (CAPANCOVID) Eur J Cancer. 2022;166:8–20. doi: 10.1016/j.ejca.2022.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buscarini E., Benedetti A., Monica F., Pasquale L., Buttitta F., Cameletti M., et al. Changes in digestive cancer diagnosis during the SARS-CoV-2 pandemic in Italy: a nationwide survey. Dig Liver Dis. 2021;53:682–688. doi: 10.1016/j.dld.2021.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Ikemura M., Tomishima K., Ushio M., Takahashi S., Yamagata W., Takasaki Y., et al. Impact of the coronavirus disease-2019 pandemic on pancreaticobiliary disease detection and treatment. J Clin Med. 2021:10. doi: 10.3390/jcm10184177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paluri R., Laursen A., Gaeta J., Wang S., Surinach A., Cockrum P. Impact of the COVID-19 pandemic on management of patients with metastatic pancreatic ductal adenocarcinoma in the United States. Oncol. 2022;27:e518–e523. doi: 10.1093/oncolo/oyac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deukeren D.V., Heesterman B.L., Roelofs L., Kiemeney L.A., Witjes J.A., Smilde T.J., et al. Impact of the COVID-19 outbreak on prostate cancer care in The Netherlands. Cancer Treat Res Commun. 2022;31:100553. doi: 10.1016/j.ctarc.2022.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipe M., de Bock E., Geitenbeek R., Boerma D., Pronk A., Heikens J., et al. Impact of the COVID-19 pandemic on surgical colorectal cancer care in The Netherlands: a multicenter retrospective cohort study. J Gastrointest Surg. 2021;25:2948–2950. doi: 10.1007/s11605-021-04936-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinmohamed A.G., Cellamare M., Visser O., de Munck L., Elferink M.A.G., Westenend P.J., et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in The Netherlands. J Hematol Oncol. 2020;13:147. doi: 10.1186/s13045-020-00984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eijkelboom A.H., de Munck L., Lobbes M.B.I., van Gils C.H., Wesseling J., Westenend P.J., et al. Impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID-19 pandemic. Prev Med. 2021;151:106602. doi: 10.1016/j.ypmed.2021.106602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackay T.M., Smits F.J., Latenstein A.E.J., Bogte A., Bonsing B.A., Bos H., et al. Impact of nationwide enhanced implementation of best practices in pancreatic cancer care (PACAP-1): a multicenter stepped-wedge cluster randomized controlled trial. Trials. 2020;21:334. doi: 10.1186/s13063-020-4180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKay S.C., Pathak S., Wilkin R.J.W., Kamarajah S.K., Wigmore S.J., Rees J., et al. Impact of SARS-CoV-2 pandemic on pancreatic cancer services and treatment pathways: United Kingdom experience. HPB. 2021;23:1656–1665. doi: 10.1016/j.hpb.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Versteijne E., van Eijck C.H., Punt C.J., Suker M., Zwinderman A.H., Dohmen M.A., et al. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials. 2016;17:127. doi: 10.1186/s13063-016-1262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Versteijne E., Suker M., Groothuis K., Akkermans-Vogelaar J.M., Besselink M.G., Bonsing B.A., et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38:1763–1773. doi: 10.1200/JCO.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen Q.P., van Dam J.L., Bonsing B.A., Bos H., Bosscha K.P., Coene P., et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer. 2021;21:300. doi: 10.1186/s12885-021-08031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Group DPC . 2021. https://dpcg.nl/studie/preopanc-2/ (Neoadjuvant FOLFIRINOX versus neoadjuvante chemoradiotherapie en adjuvante chemotherapie voor (borderline) resectabel pancreascarcinoom: de PREOPANC-2 studie february). [Available from: [Google Scholar]
- 34.IKNL. Incidentie HPB tumoren. Available from: https://iknl.nl/kankersoorten/hpb-tumoren/registratie/incidentie.
- 35.Smits F.J., Henry A.C., van Eijck C.H., Besselink M.G., Busch O.R., Arntz M., et al. Care after pancreatic resection according to an algorithm for early detection and minimally invasive management of pancreatic fistula versus current practice (PORSCH-trial): design and rationale of a nationwide stepped-wedge cluster-randomized trial. Trials. 2020;21:389. doi: 10.1186/s13063-020-4167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed for this study are managed by the Netherlands Comprehensive Cancer Organisation (IKNL). Researchers who are interested in accessing the used data, are invited to send a methodologically sound proposal to IKNL [https://iknl.nl/en/ncr/apply-for-data]. Approval by a scientific committee (DPCG) as well as compliance with IKNL objectives and privacy legislation (GDPR) are required. Data will be available for the next 5 years following the date of article publication. The study protocol is available upon request to interested researchers via judith.de.vos@mumc.nl.