Abstract
Many chemotherapeutic drugs cause adverse pulmonary reactions leading to severe pulmonary disease. Though methotrexate (MTX) is used for the treatment of cancer and other diseases, it is highly toxic with multiple adverse effects including pulmonary toxicity. Essential oils represent an open frontier for pharmaceutical sciences due to their wide range of pharmacological properties. Pumpkin seeds oil (PSO) was used to investigate its ability to alleviate methotrexate-induced lung toxicity in rats. Lung tissue from MTX-treated group revealed a decrease in malondialdehyde, glutathione, and nitric oxide accompanied by a marked inhibition in cholinesterase activity, and enhanced catalase activity, tumor necrosis factor-α, interleukin-6 and vascular endothelial growth factor levels. Analysis of PSO revealed that the oil was rich in hexadecanoic acid, decane methyl esters, squalene, polydecane, docosane, and other derivatives. Administration of PSO ameliorated the oxidant/antioxidant and proinflammatory changes induced by MTX in the lung tissue. Histological examinations confirmed the potency of PSO in reducing the histopathological alterations induced by MTX. Immunohistochemical analysis showed decreased nuclear factor-kappa B and caspase 3 expression after PSO. The present data indicated the protective efficiency of PSO against MTX-induced lung injury by decreasing oxidative damage, inflammation and apoptosis and could thus be recommended as an adjuvant therapy.
Subject terms: Biochemistry, Medical research
Introduction
Chemotherapeutic agents are used extensively in solid and hematologic malignancies. Pulmonary diseases induced by chemotherapy represent particular challenges for pulmonary and critical care practitioners1. Many cancer chemotherapeutic drugs can cause interstitial pneumonitis/fibrosis which is the most common clinical manifestation associated with drug-induced pulmonary damage2.
Methotrexate (MTX) is a folic acid antagonist that competitively inhibits dihydrofolate reductase (DHFR) disrupting the conversion of dihydrofolate to tetrahydrofolate which is the primary carbon donor for purine and pyrimidine synthesis3. Methotrexate is used as an anti-neoplastic agent for the treatment of different types of solid organ malignancies due to its therapeutic effects. High doses of MTX were used to treat certain types of cancer but since 1990 it has been used at much lower doses to treat rheumatic diseases4. MTX has shown efficacy in treating several other diseases as psoriasis, psoriatic arthritis, inflammatory bowel disease, and small-vessel vasculitis5,6.
However, MTX was reported to be highly cytotoxic and induce multiple potential adverse effects. This limited the use of MTX due to the high incidence of associated toxicities like nephrotoxicity7, hepatotoxicity8, pulmonary toxicity9, cardiovascular toxicity10, and gastrointestinal mucositis11. Hypersensitivity pneumonitis is the most frequent type of pulmonary toxicity related to MTX12. MTX sub-acute pneumonitis is manifested by dyspnea, nonproductive cough, fever, and crackles with tachypnea on physical examination, leading to pulmonary fibrosis observed in approximately 10% of MTX Crohn disease patients13.
Pumpkin (Cucurbita spp.) from the Cucurbitaceae family is an annual climber plant and a traditional food known in Europe since the sixteenth century. Several studies have highlighted the protective efficiency of pumpkin seed oil (PSO) against many diseases. Its anticancer14, antidiabetic15, antioxidant16, antiinflammatory17, cytoprotective18, and anti-mutagenic19 potencies have been reported. Al-Okbi et al.17 reported that PSO significantly inhibited the elevated plasma tumor necrosis factor-alpha (TNF-α) and malondialdehyde (MDA) levels, thereby reducing the severity of inflammation in arthritic rat model and producing significant improvements in inflammatory and oxidative stress biomarkers.
Therefore, the present study was designed to investigate the role of PSO in alleviating the adverse effects of MTX on the levels of oxidant (malondialdehyde and nitric oxide) and antioxidant (glutathione, catalase and superoxide dismutase) parameters, and the proinflammatory cytokines (interleukin-6 and TNF-α) in the lung tissue of adult male rat. Moreover, the activity of cholinesterase enzyme and the levels of vascular endothelial growth factor responsible for angiogenesis were estimated. The analysis of PSO was performed to identify its active components. The histopathological investigation of the lung tissue in addition to the immunohistochemical examination of both nuclear factor kappa-B and caspase 3 were also carried out.
Materials and methods
Experimental animals
Forty adult male albino rats, weighing 160–200 g, were purchased from a local supplier. All animals were housed and kept in cages in the animal house of the Zoology Department, Faculty of Science, Cairo University, under standard conditions of light and temperature (12 h of light/dark). Rats were fed on a standard pelted chow and water was provided ad libitum. Animals were kept under observation for about 4 days for adaptation before the onset of the experiment. All experimental procedures were undertaken according to the approval of the Institutional Animal Care and Use Committee of Cairo University (CU-IACUC) No. CU/I/F/72/19. The study was performed in compliance with the ARRIVE guidelines. All methods were carried out in accordance with the relevant guidelines and regulations.
Chemicals
Methotrexate was supplied by Mylan Company (France). PSO was purchased from Elhawag Company for Natural oils and Cosmetics (Egypt) licensed under no. 159, 2,150 in 2009. Glutathione, acetylthiocholine iodide, 5,5'-dithiobis-2-nitrobenzoic acid (DTNB), and phosphate buffers were obtained from Sigma Aldrich.
Experimental design
Forty rats were used in the present experiment and were divided into four groups (ten rats in each group); control group (Cont), pumpkin seed oil (PSO) group, methotrexate (MTX) group and MTX + PSO group. Control group received a single intraperitoneal (i.p.) injection of saline solution (0.9% NaCl) followed by an oral administration of saline for 7 consecutive days. The second group was the PSO group in which rats were injected daily with pumpkin seeds oil (PSO) using an oral stomach gavage at a dose of 1 ml/kg20 for 7 consecutive days. The third group represented the MTX group in which animals were treated with a single i.p. injection of MTX at a dose of 20 mg/kg according to Saygin et al.21 followed by an oral administration of saline for 7 consecutive days. The fourth group was the MTX + PSO group in which animals were treated with a single i.p. injection of MTX (20 mg/kg) followed by PSO (1 ml/kg) for 7 consecutive days.
Handling of tissue samples
The rats were sacrificed by sudden decapitation without anesthesia. After decapitation, the lung tissue of each animal was carefully removed, washed in ice-cold saline, blotted with filter papers, then the right half of the lung was cut on an ice-cold glass plate, and frozen for further analysis. 5 mg of each frozen lung tissue was homogenized in 5 ml 20 mM phosphate buffer (pH 7.4). The homogenates were centrifuged at 3000 r.p.m. for 10 min at 4 °C, and the obtained supernatants were stored at − 25 °C until further biochemical investigations. The other half of the lung from all treated rats was prepared for histopathology and immunohistochemical investigations.
Methods
Gas chromatography mass spectrometry (GC/MS) analysis of PSO
The GC/MS analysis of PSO was performed using a Thermo Scientific, Trace GC Ultra/ISQ Single Quadrupole MS, TG-5MS fused silica capillary column (30 m, 0.251 mm, 0.1 mm film thickness). For GC/MS detection, an electron ionization system with ionization energy of 70 eV was used. Helium was used as the carrier gas at a constant flow rate of 1 ml/min. The injector and MS transfer line temperature was set at 280 °C. The oven temperature was programmed at an initial temperature of 40 °C (hold 3 min) to 280 °C as a final temperature at an increasing rate of 5 °C/min (hold 5 min).
The quantification of all the identified components was investigated using a percent relative peak area. A tentative identification of the compounds was performed based on the comparison of their relative retention times and mass spectra with those of the NIST, WILLY 9 library data of the GC/MS system.
Biochemical analysis
Determination of malondialdehyde (MDA) content
Malondialdhyde (MDA; the end product of lipid peroxidation) was determined in rat lung tissue homogenates according to the method of Ohkawa et al.22 using kit No. MD 2529 (Biodiagnostic, Egypt). The reaction of thiobarbituric acid (TBA) with MDA takes place in an acidic medium at a temperature of 95 °C for 30 min to form thiobarbituric acid reactive substances (TBARS). The absorbance of the samples and standard was read against blank at 534 nm in a Helios Alpha Thermospectronic (UVA 111615, England) spectrophotometer. Malondialdhyde content in the lung tissue homogenate (sample) was measured in nmol/g tissue.
Determination of glutathione (GSH) content
The concentration of GSH was measured using Kit No. GR 2511 (Bio diagnostic, Egypt) which is based on the method described by Beutler et al.23. This method is based on the reduction of 5,5' dithiobis (2-nitrobenzoic acid) (DTNB) with glutathione to produce a yellow compound. The reduced chromogen was directly proportional to reduced glutathione concentration and its absorbance was measured spectrophotometrically at 405 nm. GSH concentration was expressed in mmol/g tissue.
Determination of nitric oxide content
Nitric Oxide (NO) is synthesized in the biological system by the enzyme nitric oxide synthase (NOS).The final products of NO in vivo are nitrite (NO2-) and nitrate (NO3-). Determination of NO content was carried out according to the method of Montgomery and Dymock24 which depends on the addition of Griess reagent in acidic medium. Griess reagent converts nitrite into a deep purple azo compound. The formed nitrous acid diazotizes sulphanilamide and the product is coupled with N-(1-naphthyl) ethylene-diamine. Absorbance of the formed reddish-purple azo dye was measured spectrophotometrically at 540 nm in a Helios Alpha Thermospectronic (UVA 111615, England) spectrophotometer.
Determination of catalase (CAT) activity
Catalase activity in rat lung tissue homogenate was determined by the method of Aebi25 using Kit No. CA 2517 (Biodiagnostic, Egypt). The method is based on the reaction of CAT with a known quantity of H2O2, as each unit of CAT decomposes 1 µM of H2O2 per min at 25 °C, at pH 7.0. The reaction is stopped after exactly 1 min with catalase inhibitor. In the presence of horse radish peroxidase (HRP), the remaining H2O2 reacts with 3,5 dichloro-2-hydroxybenzene sulfonic acid (DHBS) and 4-amino-phenazone (AAP) to form a quinone imine dye whose color intensity is inversely proportional to the amount of catalase in the sample. The decomposition of H2O2 was followed directly by the decrease in absorbance at 240 nm. The difference in the absorbance per unit time is a measure of the CAT activity.
Determination of superoxide dismutase (SOD) activity
Superoxide dismutase (SOD) activity in lung tissue homogenate was assayed according to the procedure of Nishikimi et al.26. The assay depends on the ability of SOD to inhibit phenazine methosulphate-mediated reaction of nitrobluetetrazolium dye. SOD catalyzes the dismutation of the superoxide anion to molecular oxygen and hydrogen peroxide. The increase in absorbance was measured at 560 nm for 5 min at 25 °C for blank and sample.
Determination of cholinesterase (ChE) activity
The present study used a modification of the method of Ellman et al.27 as described by Gorun et al.28. The method measures the rate of production of thiocholine as acetylthiocholine hydrolysis proceeds. ChE is incubated with acetylthiocholine for a specific time interval and the reaction is then stopped with a reagent containing the color indicator DTNB. The color was read immediately at 412 nm and ChE activity was determined as µmol SH/g tissue/min (SH sulfhydryl group).
Determination of inflammatory cytokines
Tumor necrosis factor alpha (TNF- α)
Tumor necrosis factor-alpha (TNF-α) was measured using rat TNF-α Elisa kit Catalog No. SG-20127 which was obtained from SinoGeneClon Biotech Co., Ltd. (Hangzhou, China). The developed color was read at 450 nm using a microtiter plate reader. The concentration was then calculated from a standard curve.
Interleukin 6 (IL-6)
Interleukin-6 (IL-6) was measured using rat interleukin-6 Elisa kit Catalog No. SG-2026 which was supplied by SinoGeneClon Biotech Co., Ltd. (Hangzhou, China). The color change was measured at a wavelength of 450 nm using a microtiter plate reader. The concentration of IL-6 in the samples was then determined from a standard curve.
Determination of vascular endothelial growth factor (VEGF)
Vascular endothelial cell growth factor (VEGF) was measured using rat vascular endothelial growth factor Elisa kit Catalog No. SG-20402 which was purchased from SinoGeneClon Biotech Co., Ltd. (Hangzhou, China). The color change was measured at wavelength of 450 nm. The concentration was then calculated by comparing the optical density of the samples to the standard curve.
Histopathology
Lung sections were collected from different groups and preserved in 10% neutral buffered formalin for histopathological and immunohistochemical evaluation. After alcohol dehydration and paraffin embedding steps, tissues were cut at 5 µm thickness on glass slides. Hematoxylin and eosin stains (H&E) were used to stain the tissue sections. The lung sections were examined using bright field microscope (Olympus BX43) and digital images were captured using fixed digital camera (Olympus DP27). The lung score system was performed as previously mentioned29.
Immunohistochemical analyses
Lung Sects. (5 μm) were cut from paraffin blocks into positive charged glass slides. Ordinary steps of deparaffinization and rehydration were performed via a series of graded alcohol. Heat-induced epitope retrieval was conducted. The lung sections were washed then protein blocking using bovine serum albumin (BSA) and hydrogen peroxide was applied. After that, primary antibodies (mouse monoclonal Anti-NF-κB p65 (sc-8008) were incubated with caspase-3 (sc-65497, Santa Cruz Biotechnology Inc., Dallas, TX, USA) at a dilution of 1:150 overnight at 4 °C. After that, tissue sections were incubated with horseradish-peroxidase (HRP) labeled goat anti-mouse secondary antibody (Abcam, Cambridge, UK) for 2 h then 3,3′-diaminobenzidine (DAB) Substrate detection kit (Thermo Scientific) was applied. Counterstaining was done using Mayer's hematoxylin, dehydration followed by xylene clearance and mounting in dibutylphthalate polystyrene xylene (DPX). Area % of positive reaction was quantified using digital software (Cell Sens dimensions, Olympus software). Negative control step was achieved via omitting the primary antibody incubation step.
Statistical analysis
All data were expressed as mean ± standard error of mean (S.E.M.). Significance was determined at p < 0.05. Comparisons between the means of different groups of animals and those of control animals were carried out using one-way analysis of variance (ANOVA) for each time interval. All statistical analyses were done using SPSS (Statistical Package for Social Sciences, version 14). When statistically significant, ANOVA test was followed by Duncan as post hoc test for multiple comparisons.
Ethics approval and consent to participate
All experimental procedures were undertaken according to the approval of the Institutional Animal Care and Use Committee of Cairo University (IACUC) No. CU/I/F/72/19.
Results
Identification of pumpkin seed oil (PSO) components using GC/MS
Identification of pumpkin seed oil (PSO) components was performed based on the comparison of their relative retention time and mass spectra with those of the NIST, Willy 9 library data of the GC/MS system. The spectrum of the unknown components was compared with the spectrum of the known components stored in the Willy 9 library. The retention time, name, molecular weight and structure of the components of the test materials were ascertained. The GC/MS analysis of PSO has shown its phytochemical constituents and their peaks (Fig. 1), which contribute to the medicinal activity of the plant. The identification of the active components of PSO with their retention time and percentage composition revealed that PSO used in this study was rich in hexadecanoic acid (42.06%), decane methyl esters (25.9%), squalene (11.16%), polydecane (5.56%), docosane (2.56%), and other derivatives (Table 1).
Figure 1.
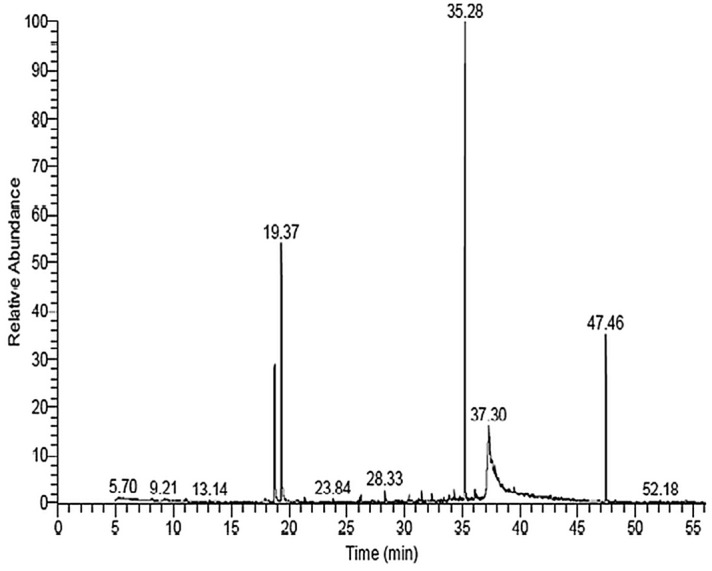
The Chromatogram and Spectrum of Identified Compounds From Pumpkin Seed Oil (PSO) Using Trace GC Ultra / ISQ Single Quadrupole MS, TG- 5MS Fused Silica Capillary Column.
Table 1.
Identification of Pumpkin Seed Oil (PSO) Components Using GC/MS.
| Compound name | RT/min | Area % | MW | MF |
|---|---|---|---|---|
| 1. N, N Dimethyl vinyl amine | 5.14 | 0.28 | 71 | C4H9N |
| 2. 3 (Prop2enoyloxy) tetra decane | 9.21 | 0.31 | 268 | C17H32O2 |
| 3. OCimene | 11.06 | 0.27 | 134 | C10H14 |
| 4. Methyl 3 Methyl 4 ( trimethysilyl) 2butenoate | 13.61 | 0.20 | 186 | C9H18O2Si |
| 5. 2, 4 Decadien-1-ol | 17.96 | 0.35 | 154 | C10H18O |
| 6. 2,4Decadienal | 19.37 | 15.85 | 152 | C10H16O |
| 7. Hentriacontane | 21.41 | 0.22 | 436 | C31H64 |
| 8. Docosane | 23.85 | 0.32 | 310 | C22H46 |
| 9. 2,3 Dihydro5,10,15,20te traphenyl22 H, 24Hprophyrin | 25.09 | 0.19 | 616 | C44H32N4 |
| 10. Pentadecane | 26.15 | 0.33 | 212 | C15H32 |
| 11. Farnesol | 26.26 | 0.51 | 222 | C15H26O |
| 12. 3,4,5,6Tetrakis(pchlorophenoxy) 1,2dicyanobenzene | 27.22 | 0.22 | 632 | C32H16Cl4N2O4 |
| 13. Pentadecane | 28.33 | 0.83 | 212 | C15H32 |
| 14. Methyl DELTA.meso(Methylthio)methyl)mesopyropheophorbide A | 28.46 | 0.27 | 671 | C36H40CuN4O3S |
| 15. MethylE7hexadecene | 29.01 | 0.19 | 238 | C17H34 |
| 16. Heptadecane,2,6,10,14tetramethyl | 30.41 | 0.41 | 296 | C21H44 |
| 17. Dodecanal | 31.25 | 0.30 | 184 | C12H24O |
| 18. Tetradecanoic acid, Trimethylsilyl ester | 31.50 | 0.76 | 300 | C17H36O2Si |
| 19. Pentadecane | 32.40 | 0.52 | 296 | C21H44 |
| 20. Decanoic acid, methyl Ester | 33.02 | 0.23 | 186 | C11H22O2 |
| 21. 1-Hexacosanol | 33.19 | 0.34 | 140 | C10H20 |
| 22. 1-H. Indole2carboxylic acid,1(trimethylsilyl)5[(trimethylsilyl)oxy],trimethylsilyl ester | 33.41 | 0.30 | 393 | C18H31NO3Si3 |
| 23. n-Hexadecanoic Acid | 33.90 | 0.75 | 256 | C16H32O2 |
| 24. Dodecane, 2,6,10trimethyl | 34.29 | 0.75 | 212 | C15H32 |
| 25. Hexadecanoic acid, trimethylsilyl ester | 35.27 | 42.06 | 328 | C19H40O2Si |
| 26. Docosane | 35.61 | 0.27 | 310 | C22H46 |
| 27. Isochiapin B | 35.88 | 0.76 | 346 | C19H22O6 |
| 28. Octadecane | 36.12 | 1.03 | 254 | C18H38 |
| 29. 9-Octadecenoic acid, methyl ester | 36.25 | 0.54 | 296 | C19H36O2 |
| 30. Hexadecen,1-ol, 3,7,11,15tetramethyl, [R[ R*,R*(E)]] | 36.35 | 0.21 | 296 | C20H40O |
| 31. Docosane | 36.78 | 0.19 | 490 | C35H70 |
| 32. 13, Oxabicyclo[10.1.0-Tridecane] | 37.30 | 6.73 | 182 | C12H22O |
| 33. 9-Octadecenoic acid (Z) hexadecyl ester | 37.74 | 0.21 | 506 | C34H66O2 |
| 34. Docosane | 37.85 | 0.54 | 310 | C22H46 |
| 35. Linoleic acid ethyl ester | 38.18 | 0.25 | 308 | C20H36O2 |
| 36. 7, Pentatriacontene | 38.48 | 0.29 | 490 | C35H70 |
| 37. 31, dodecaene | 39.18 | 0.29 | 662 | C39H42N4O6 |
| 38. Docosane | 39.51 | 0.60 | 310 | C22H46 |
| 39. Octadecane | 39.63 | 0.24 | 296 | C20H40O |
| 40. Dodecachloro3,4benzo-phenanthren | 40.63 | 0.24 | 636 | C18Cl12 |
| 41. Docosane | 41.10 | 0.19 | 310 | C22H46 |
| 42. Docosane | 41.67 | 0.25 | 310 | C22H46 |
| 43-Docosane | 42.64 | 0.20 | 310 | C22H46 |
| 44. Octan2one, 3,6dimethy | 43.18 | 0.24 | 156 | C10H20O |
| 45. Octadecane | 43.58 | 0.26 | 254 | C18H38 |
| 46. Squalene | 47.46 | 11.16 | 410 | C30H50 |
| 47. Cholesta8,24dien3ol,4methy | 54.40 | 0.30 | 398 | C28H46O |
Biochemical results
The oxidant/antioxidant parameters
Malondialdehyde (MDA) levels
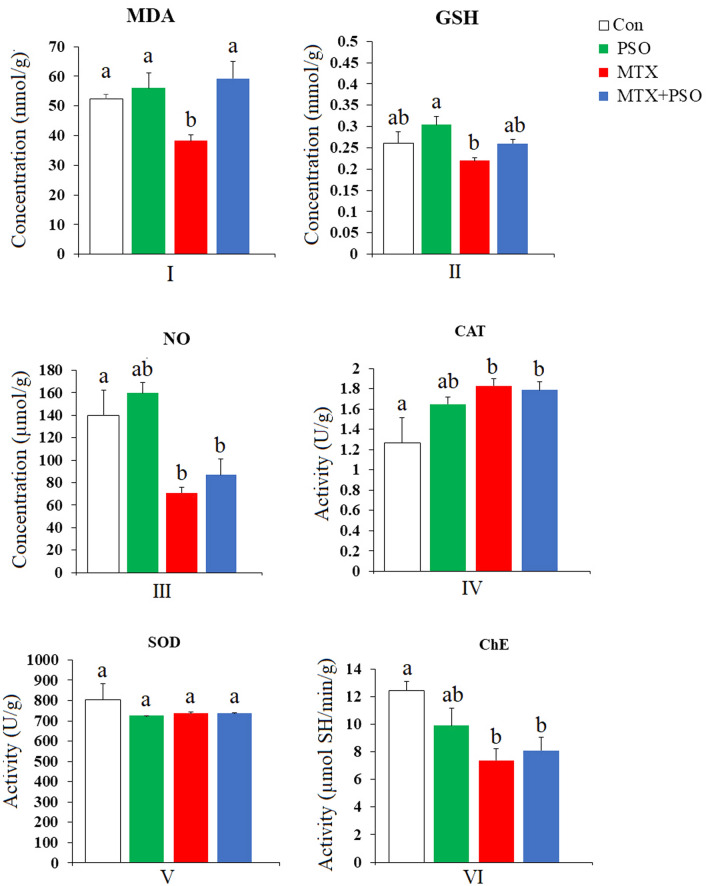
A single i.p. injection of MTX caused a marked decrease in MDA levels in the lung of adult male rats after eight days of treatment (Fig. 2). The observed decrease in MDA level was significant at p value ˂ 0.05 compared to the control group, recording a percentage difference of − 26.72%. The oral administration of PSO for eight consecutive days induced a slight non significant increase in MDA levels in the lungs of rats as compared to control group. Treatment of MTX-injected rats with PSO prevented the change in the MDA level.
Figure 2.
The effect of oral administration of pumpkin seed oil (PSO) on malondialdehyde (MDA) (I), glutathione (GSH) (II), nitric oxide (NO) (III) levels and catalase (CAT) (IV), superoxide dismutase (SOD) (V), and cholinesterase (ChE) (VI) activities in methotroxate-intoxicated rat lungs. Different letters indicate significantly different means (p value ˂ 0.05). Same letters indicate non significant changes.
Glutathione (GSH) level
The intraperitoneal injection of MTX provoked a non significant decrease in GSH levels in the lungs of MTX-treated rats as compared to control group and MTX group treated with PSO (Fig. 2). However, the daily oral administration of PSO induced a non significant increase in GSH content in the lung tissue of MTX + PSO-treated rats as compared to control value.
Nitric Oxide (NO) level
The data represented in Fig. 2 revealed that a single MTX injection to adult male rats induced a marked decrease in NO levels in the lung recording − 49.13% below the control levels. The oral administration of PSO slightly improved NO levels from − 49.13 to − 37.68% in rat lung tissue compared to control values.
Catalase (CAT) activity
The present data demonstrated that rats injected with MTX showed a significant increase in CAT activity, being 44.3% above the control values. Treatment of MTX-intoxicated rats with PSO induced an increase in CAT activity with a percentage difference of 41.1% as compared to control group (Fig. 2).
Superoxide dismutase (SOD) activity
Analysis of data concerning the activity of SOD in lung tissue from the investigated groups yielded non significant changes with a slight decrease in the enzyme activity after PSO and MTX treatments alone or combined with each other (Fig. 2).
Cholinesterase (ChE) activity
A single MTX injection was found to induce a marked inhibition in cholinesterase enzymatic activity recording 40.5% below the control values (Fig. 2). When MTX-intoxicated rats were treated daily with PSO for eight days, an improvement in the enzyme activity was obtained, being − 34.8% but still significantly lower than control group.
Pro-inflammatory cytokines
Tumor necrosis factor-alpha (TNF-α) Level:
A single i.p. injection of MTX induced a significant elevation in TNF-α level in rats’ lungs recording a percentage difference of 19.10% versus control values (Fig. 3). Treatment of MTX-intoxicated rats daily with PSO for eight days ameliorated the increase in TNF-α in lung tissue lowering the percentage difference to − 5.11% compared to the control group.
Figure 3.
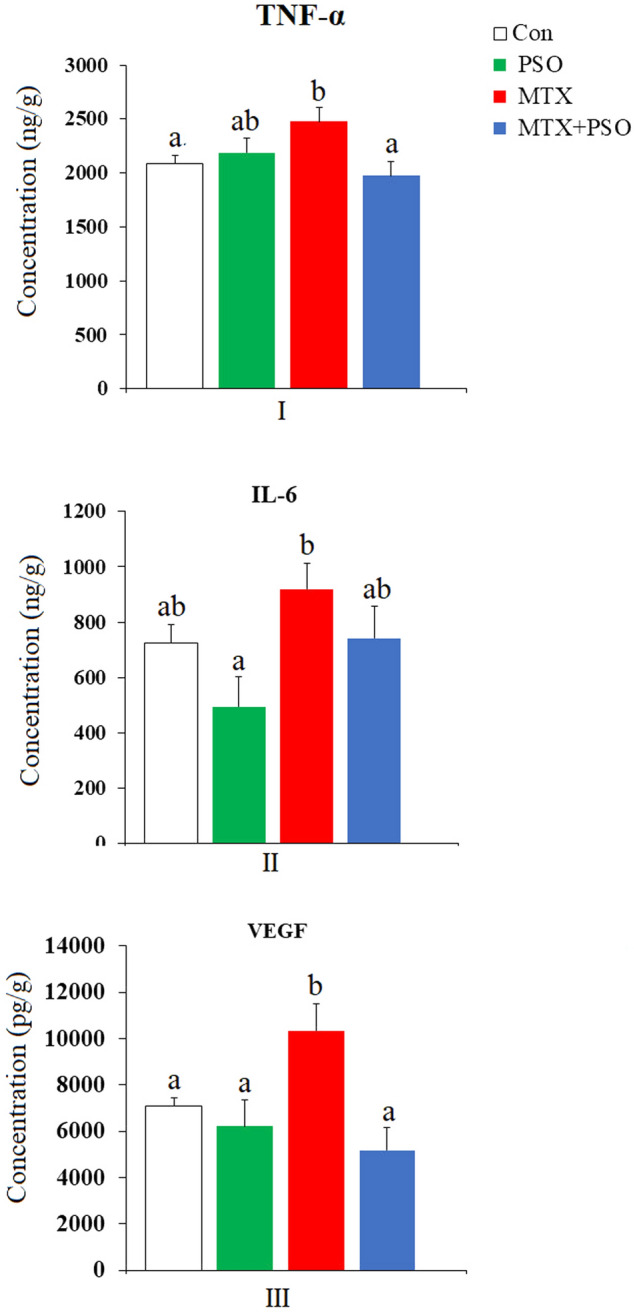
The effect of oral administration of pumpkin seed oil (PSO) on tumor necrosis factor-alpha (TNF-α) (I), interleukin-6 (IL-6) (II) and vascular endothelial growth factor (VEGF) (III) levels in methotrexate-intoxicated rat lungs. Different letters indicate significantly different means (p value ˂ 0.05). Same letters indicate non significant changes.
Interleukin-6 (IL-6) level
The levels of interleukin-6 (IL-6) in lung tissue of PSO-treated rats showed a non significant decrease (− 31.77%) compared to control group (Fig. 3). On the contrary, MTX injection evoked a non significant increase in IL-6 recording 26.93% above the control group. The daily oral administration of PSO to MTX-injected rats for eight days attenuated the increase in IL-6 resulting from MTX injection reducing the percentage difference from 26.93 to 2.32%.
Vascular endothelial growth factor (VEGF) Level
A marked increase in VEGF levels was recorded in lung tissue of MTX-injected rats (Fig. 3). The observed increase was significant at p value ˂ 0.05 versus the control and PSO groups and recorded 45.98% compared to the control values. Treatment of MTX-injected rats with PSO induced a non significant decrease in VEGF levels after 8 days of treatment, the percentage difference being reduced from 45.98% after MTX to − 26.89% after PSO.
Histopathological and immunohistochemical examinations
Histopathology
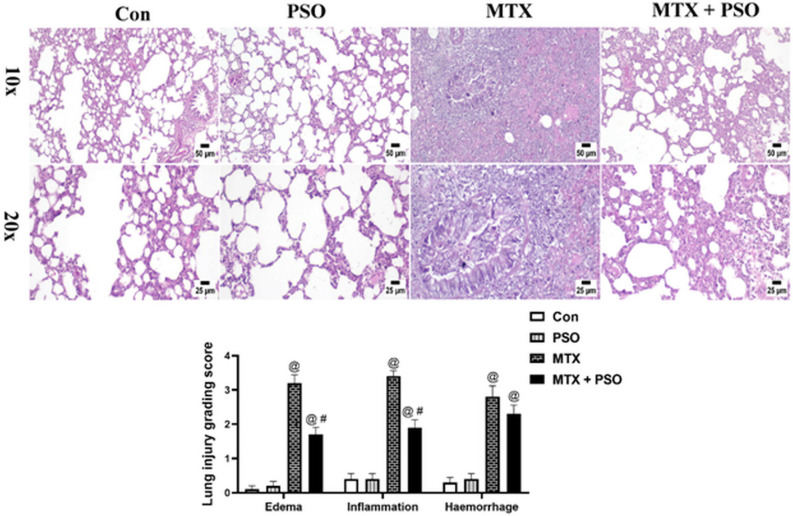
Lung sections from control and PSO groups showed normal histological structure of lung tissue without any detectable alterations. Adversely, examination of MTX group revealed severe lung injury. The interstitial tissue was markedly expanded with numerous mononuclear inflammatory cells infiltration associated with abundant edema and variable hemorrhagic areas. The bronchi and bronchioles displayed mucus exudates in their lumens mixed with sloughed epithelial cells, inflammatory cells and tissue debris. Administration of PSO to MTX-intoxicated rats induced marked protection of the lung tissue that was characterized by fewer numbers of inflammatory cells infiltration, mild edema and hemorrhages occupying the lung alveoli and inter-alveolar septa. The lung scoring system revealed a significant increase in MTX group when compared to control or PSO groups in all evaluated parameters. However, MTX + PSO group showed a significant decrease in comparison with MTX group concerning edema and inflammation (Fig. 4).
Figure 4.
Effect of PSO on recovery of lung tissue from MTX-induced lung injury (H&E). Control and PSO groups showed normal histological structure of lung alveoli, bronchi and interstitial tissue. MTX group showed severe interstitial pneumonia with abundant edema and heavy mononuclear inflammatory cells infiltration, the higher magnification (20x) showed that the bronchiolar lumen was occluded with mucous exudate and inflammatory cells with desquamated epithelial cells. MTX + PSO group showed marked reduction of inflammatory reaction in the lung parenchyma. Chart represents the lung score injury of different groups. Values are expressed as means ± SE. @ significant from Con, # significant from MTX. Significant difference is considered at p < 0.05.
Immunohistochemistry
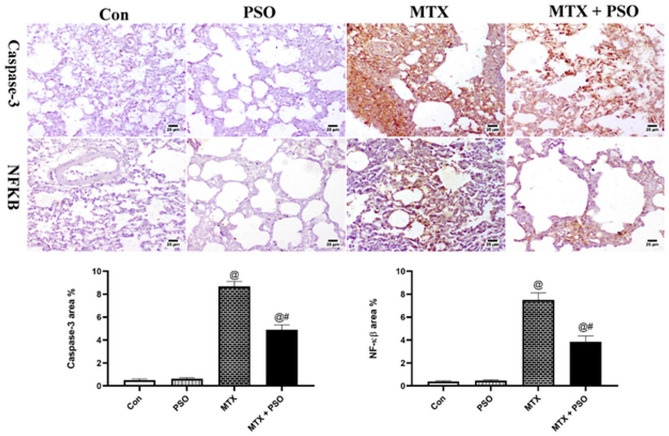
Evaluation of caspase-3 and NF-kβ was performed in lung sections of different groups. Concerning caspase-3 immune staining, control and PSO showed weak to negative expression in the alveolar lining and in the interstitial tissue. However, strong positive expression of caspase-3 was detected in the interstitial tissue, alveoli and lining epithelium of bronchi and bronchioles of MTX group. Moderate expression was detected in MTX + PSO group. Similar results were obtained in NF-kβ immune staining. The statistical analysis of area % expression showed a significant increase in MTX group when compared to other groups regarding caspase-3 and NF-kβ staining (Fig. 5).
Figure 5.
Immunohistochemical staining of caspase-3 and NF-kβ in different groups. Control and PSO groups showed limited to negative expression in both markers. MTX showed strong positive expression in the inflamed lung sections concerning caspase-3 and NF-kβ. Remarkable decrease in immune staining is detected in MTX + PSO group of both caspase-3 and NF-kβ. Charts represent area % expression of immune markers in different groups. Values are expressed as means ± SE. @ significant from Con, # significant from MTX. Significant difference is considered at p < 0.05.
Discussion
Lung damage may result from drug-induced pulmonary toxicity or autoimmune-mediated inflammation12. The therapeutic use of MTX is limited by its toxicity21 which leads to alteration of the dosage regimen, or complete drug withdrawal12.
The increased levels of inflammatory markers, TNF-α, IL-6 and VEGF, in the present study, clearly demonstrate the cytotoxic pro-inflammatory effects of MTX on lung tissue. The increase in pro-inflammatory cytokines may be explained by the ability of MTX to up regulate the expression and secretion of these cytokines which may further enhance the production of ROS and the subsequent lung tissue damage.
Compared to other organs, lungs are highly vulnerable as they are directly exposed to high levels of atmospheric oxygen. Hence, the respiratory epithelium is a major target for oxidative stress. Therefore, the enzymatic and non-enzymatic antioxidant defense systems in the lung are rich and highly efficient to protect it from oxidant-induced damage30.
The present study revealed that a single MTX injection caused a significant decrease in the content of the oxidative markers MDA and NO, and a nonsignificant decrease in GSH levels and SOD activity in rat lungs. However, an elevation in CAT activity was recorded in the lung tissue of MTX-injected rats.
Oxidative stress induces radical mediated damage to cellular bio-membranes causing lipid peroxidation, which leads to the generation of oxidized products capable of modifying DNA, protein, and other macromolecules31. Reactive oxygen species (ROS) target the major cellular macromolecules leading to their damage and subsequently cell death in response to oxidative insult. This triggers the onset and progression of tissue damage and oxygen may increase active free radical exposure32. The present inflammation induced by MTX suggests that the production of ROS may be in progress. The increase in CAT enzyme supports this notion.
The histopathological examination results of the present study indicated severe lung injury in the MTX-treated group, which revealed marked expanded interstitial tissue with numerous mononuclear inflammatory cells, infiltration, abundant edema and variable hemorrhagic areas with mucus exudates in the lumen of bronchi and bronchioles mixed with sloughed epithelial cells, inflammatory cells and tissue debris. Similarly, it has been shown that MTX–induced pulmonary toxicity was correlated with hypersensitivity pneumonitis33 characterized by interstitial lymphocyte infiltration with hyperplasia, formation of small granulomatous areas, and sometimes eosinophilic infiltration, other patterns showing obstructive pneumonitis, acute interstitial pneumonia, pulmonary fibrosis, and pleural effusion34.
The present findings do not agree with the reported increase in MDA level and decrease in CAT and SOD activities in rat lung after MTX35. This may be explained by the short time interval applied in the present study. Hence, the activity of SOD and the level of GSH were not changed as compared to the control. However, the present increase in CAT activity after MTX suggests that this enzyme is the first antioxidant to respond to the generation of free radicals in lung tissue.
The histopathological alterations observed in the present study indicate severe lung injury resulting from MTX. The progression of tissue damage will eventually lead to the decrease in MDA levels due to the cellular death. Several histopathological studies revealed that MTX-induced lung injury caused significant lymphocytic inflammation and congestion with pneumocyte hyperplasia, epithelial degeneration and fibrous tissue deposition36. Injury of the lung epithelial cells which line the airspaces increases mucus secretion, enhances neutrophils influx into the lungs, and activates transcription factors and gene expression of pro-inflammatory mediators37. This explains the presence of mucus exudates in the lumen of bronchi and bronchioles among various cells and tissue debris in the lung sections of MTX-treated rats.
Catalase catalyzes the dismutation of hydrogen peroxide to water and oxygen38. In the lung, CAT is localized in alveolar type II pneumocytes and macrophages39. It is considered to be the most important antioxidant enzyme consuming exogenous hydrogen peroxide in type II pneumocytes, which are the most resistant cell types in the lung40. The significant increase in CAT activity, in the present study, in lung tissue after MTX injection emphasizes the importance of catalase as a major antioxidant that confers protection to the lung under conditions of increased oxidative stress.
There are three isoforms of NOS in the human respiratory system. Neuronal NOS (nNOS) was detected in nonadrenergic non-cholinergic nervous system, airway nerves and epithelium. Endothelial NOS (eNOS) is present mainly in endothelial cells of the lung vasculature while inducive NOS (iNOS) has been associated with the pro- and anti-inflammatory responses. All isoforms transform the L-arginine into L-citrulline and release nitric oxide41. Any change of their activity may lead to alteration in NO level and contribute to the pathogenesis of various respiratory diseases42.
It has been reported that eNOS is activated by TNF-α and other inflammatory stimuli43. It may be suggested that the activation of eNOS by the elevated inflammatory cytokines induced by MTX may lead to the generation of NO which interacts with superoxide radical producing peroxynitrite44. This is a strong oxidant and can precipitate cellular damage either directly by nitration of tyrosine leading to irreversible dysfunction of important proteins, or indirectly by initiating the production of other reactive molecules with cellular toxicity42,45. NO was reported to exert vasoprotective effects on the endothelium keeping blood vessels dilated, and also controlling blood pressure46.The reduction in NO may interfere with its vasoprotective effects thus promoting MTX toxicity in the lung tissue.
Several studies pointed at the promising therapeutic effects of PSO14–19. The present results revealed that the ability of PSO to attenuate MTX-induced biochemical changes in rat lung was evident after PSO administration for 8 consecutive days. PSO maintained MDA and GSH near control levels and slightly improved NO levels in rat lung tissue compared to control values. Treatment of MTX-injected rats with PSO also maintained the increased activity of CAT in the lung tissue.
Analysis of PSO using GC/MS, in the present study, showed its richness in hexadecanoic acid, decane methyl esters, squalene, polydecane, docosane, and other derivatives. Hexadecanoic and octadecanoic acids were documented as antioxidant47, anticancer48, antiapoptotic49 and anti-inflammatory47 agents. The present results concerning analysis of the active constituents of PSO were in line with other studies reporting high amounts of free fatty acids (oleic, linoleic, palmitic, and stearic acids) in pumpkin oil50,51. Furthermore, results of the present study also agree with Omar and Sarhan52 who attributed the improvement in acid-aspiration pneumonia model after pumpkin seed oil treatment to its composition.
Squalene is a lipophilic isoprenoidtriterpene that contributes to the antioxidant effect of pumpkin oil as a guard against membrane lipid peroxidation53. Squalene oxidation was reported to occur earlier than other lipids, blocking the peroxidative reactions as it breaks lipid peroxidation chains thus stabilizing and protecting cell membranes54. Moreover, emerging evidence supported the involvement of squalene in the regulation of glutathione peroxidase, CAT, SOD and glutathione S-transferase expression and activation, prevention of SOD and CAT alterations and reconstitution of GSH55.
In addition, PSO was reported to decrease GST activity that catalyzes GSH conjugation and GR activity but elevated GSH level indicating lower turnover of GSH, an essential regulator of the cellular redox state56. The present elevated CAT activity reflected increased concentrations of hydrogen peroxide in rats’ lung tissue exposed to MTX, and the persistence of PSO in reducing the high influx of hydrogen peroxide.
It can be suggested that the ability of PSO to maintain the increased levels of CAT induced by MTX, in the present study, was due to the potent antioxidant constituents of PSO. The elevated CAT activity may indicate increased production of free radicals as hydrogen peroxide and supports the role of CAT as one of the important antioxidants in lung tissue. Several reports confirmed the potency of different constituents of PSO. Eraslan et al.57 suggested that pumpkin seed oil may have caused physiological alterations in the activities of lung SOD, brain CAT and liver GSH-Px after subacute aflatoxin poisoning in mice due to its potential of eliminating free radicals generated under normal biological conditions. Similarly, it has also been reported that pumpkin seed oil/extract/isolate alters the same antioxidant enzyme activities58. Recently, it has been reported that treatment of diabetic mice with 2% squalene increased the activity of liver catalase and glutathione peroxidase, while a dose of 600 mg of squalene increased catalase and superoxide dismutase activities and reduced hydrogen peroxide levels59.
In line with the present findings, Shaban et al.60 suggested that the antioxidant activities of pomegranate extracts could be related to their constituents, including phenolic components, fatty acids (such as punicic acid and conjugated α-linolenic acids), phytosterols, and triterpenoids. Moreover, Habashy et al.61 found that the improvement of the antioxidant defense system by Vitis vinifera polyphenols fraction (VVPF) in the lung tissue was higher than in other studied organs and suggested that this may be due to the potent reducing power of VVPF and its ability in quenching ABTS radical. This potency was related to the phenolic content of VVPF, including vanillic, gallic, caffeic, p-coumaric, syringic, ferulic, salicylic, and ellagic acids, along with the flavonoids and resveratrol and its efficiency in scavenging peroxide, superoxide, and hydroxide radicals62.
Omar and Sarhan52 found that pumpkin oil partially attenuated the histological and ultra structural alterations and reduced inducible NO synthase immune-expression in lung tissue of experimentally-induced acid aspiration pneumonia model.
Thus, the failure of PSO to restore the present decrease in NO levels may be due to the reduced iNOS expression which affects NO production in the lung tissue of MTX-treated rats. This may be beneficial in reducing the nitrosative stress and peroxynitrite formation in these animals.
In the present study, MTX injection resulted in a marked reduction in cholinesterase (ChE) activity in the lung tissue. Besides its classical role in hydrolyzing acetylcholine (ACh) in the central and peripheral nervous systems, acetylcholinesterase (AChE) was also associated with stress responses and inflammation63. Abnormal expression and structural alterations of AChE were observed in different tumors, as reduced activity may contribute to lung cancer growth64. On the other hand, AChE was found to be involved in tumor suppression by its participation in apoptosis indicating its potential role as a marker and regulator of apoptosis and tumor development65.
The present reduction in ChE activity in the lung tissue of MTX-intoxicated rats may be due to the state of inflammation and subsequent ROS generation that developed in the lung tissue. The study of Tsakiris et al.66 revealed that AChE is very sensitive to free radicals and that hydroxyl radicals participate in AChE inhibition. The decrease in the enzyme activity may also be related to the mechanism of action of MTX due to the involvement of the enzyme in tumourgenesis and proliferation.
Pioneer reports highlighted the potential role of oleic acid in the cholinergic system67, and showed a choline acetyltransferase (ChAT)-like activity responsible for increased production of ACh by isolated rat brain synaptosomes bound to neuronal plasma membranes. In addition, it has been reported that oleic acid enhanced choline uptake68 and affected the expression of ChAT, which probably promotes ACh production69. Furthermore, Abed et al.70 reported that squalene and palmitic acid exhibited different rates of inhibition against AChE.
The inability of PSO to restore ChE activity in MTX-intoxicated may be explained by two mechanisms. First, PSO constituents enhance choline uptake and choline acetyltransferase activity and inhibit ChE activity and hence promote cholinergic transmission. Second, the decrease in ChE may be a compensatory mechanism that aims to increase ACh since neuronal or non-neuronal sources of ACh could decrease inflammation by their local action on macrophages, through the alpha-7 nicotinic ACh receptor thus reducing nuclear translocation of the transcription factor NF-κB, and macrophage production of the pro-inflammatory cytokine TNF-α71.
Increased levels of TNF-alpha and IL-6, in the present study, indicate that MTX has pro-inflammatory effects on lung tissue.
TNF-alpha is a potent pro-inflammatory cytokine responsible for the pathogenesis of chronic inflammatory diseases and oxidative stress. Its actions are achieved by regulating growth, proliferation, differentiation, and viability of activated leukocytes, inducing cellular release of other cytokines and chemokines72 and ensuring the death of damaged cells by apoptosis17. The binding of TNF-α to its receptor triggered and propagated the signaling cascades leading to the development of local or systemic inflammation which activates NF-κB forming a positive feedback mechanism which enhanced the inflammatory process17,72.
IL-6 is a pleiotropic cytokine having both pro- and anti-inflammatory properties. It is produced by different immune cells and synergizes with TNF-alpha and IL-1 to promote systemic inflammatory response73.
Studies documented increased TNF-alpha, interleukin-1 (IL-1), interleukin-8 (IL-8), and monocyte chemotactic protein-1 in acute MTX-induced pulmonary toxicity74,75. Kurt et al.76 attributed the high levels of TNF-alpha, MDA, myeloperoxidase (a component of the antioxidant defense system), and endothelial tissue-1 (a potent vasoconstrictor) to inhibition of tissue macrophage infiltration in lung of MTX-intoxicated rats which affects the synthesis of interleukins. The present increase in both TNF-alpha and IL-6 confirms a pro-inflammatory role of these cytokines in MTX-induced toxicity.
It has been suggested that the action of MTX on pro-inflammatory cytokines is mediated by NF-kB. MTX injection, in the present study, showed strong positive expression of NF-kB in the inflamed lung sections. The activation of NF-kB can trigger cascades involved in the expression of iNOS and initiation of inflammation, cell proliferation, immune response and apoptosis77. It is activated by numerous stimuli as pro-inflammatory cytokines (TNF-alpha or IL-1β)78. Thus, the present increased expression of NF-kB may be mediated by the activation of TNF-α and IL-6 in the rat lung tissue by MTX injection.
The present results highlighted the inhibition of pro-inflammatory cytokines as one of the mechanisms by which PSO overcomes MTX toxicity.
Pumpkin oil was reported to contain polyphenols and other bioactive phytochemicals with anti-inflammatory effects79. Lai et al.80 indicated that the reduction of the inflammatory cellular infiltrate and the area percent of collagenous fibers after pumpkin oil administration in the lung of pumpkin oil and acid aspiration group were attributed to the unsaturated free fatty acid constituents in pumpkin oil.
Al-Okbi et al.17 attributed the bioactivity of PSO to linoleic acid which represented one-third of the total fatty acid. Omega-3 fatty acids can regulate the synthesis of lipid mediators, release of cytokines, and activate white blood cells and endothelial cells, thereby regulating the body’s excessive inflammatory response to reduce lung inflammation47,81.
Results in the present study concerning vascular endothelial growth factor (VEGF) level indicated a significant elevation of VEGF level in rat lung tissue of MTX-treated group.
The predominant source of VEGF in the lung is the alveolar epithelium, in addition to smooth muscle cells, macrophages, and endothelial cells which express VEGF. High levels of VEGF persist in the lungs in adulthood82 suggesting its role in normal lung maintenance and in the pathogenesis of acute respiratory distress syndrome (ARDS)83.
Thus, the increase in lung VEGF levels, in the present study, after MTX could be secondary to the increase in cytokine production as TNF-α and IL-6 have been reported to up regulate VEGF production in rheumatoid arthritis (RA)84.
It has been reported that the application of omega-3 fatty acids rapidly reduced the inflammatory reaction of lung tissue, by transforming leukotriene B4, which aggravates inflammatory reaction, into leukotriene B5 series (less active), therefore reducing pulmonary edema and improving pulmonary vascular permeability85. Huang et al.86 concluded that omega-3 acids can improve respiratory function and respiratory status of acute lung injury (ALI) patients and suggested that omega-3 fatty acid could be a potential effective and safe strategy for ALI treatment. The present restoration in VEGF levels after PSO treatment may be attributed to the potent anti-inflammatory properties of PSO constituents.
Apoptosis is a critical and vital process which occurs during chemically-induced toxicity. The present study showed a marked strong positive expression of the apoptotic marker caspase-3 in the interstitial tissue, alveoli and lining epithelium of bronchi and bronchioles of MTX group by immunohistochemical analysis. Caspase-3 is a key element in the apoptotic process and signals the induction of cell death79. Caspase-3 was considered as a valuable marker of apoptosis87. This may explain the severe lung injury detected in MTX-intoxicated rats.
The present findings are in agreement with the results of Kurt et al.76 who found similar histological damage in the MTX group with high caspase-3 expression. The authors attributed these changes to excessive cytokine levels, endothelial-1 secretion and ROS formation, which activate caspase-3 pathway causing lung damage. Crumbling of peptide chains, electric charge modification with cross-linking of proteins, and oxidation of specific amino acids, due to ROS and increased susceptibility to proteolysis by precise proteases88, may underlie the strong positive expression of caspase-3 and NF-κB detected in the immunohistochemical analysis in the present study in the lung interstitial tissue, alveoli and lining epithelium of bronchi and bronchioles of MTX group.
In the present study, PSO produced its protective effect by reducing inflammation and apoptosis, thereby attenuating and alleviating MTX-induced lung damage as evident in the histopathological examination which showed marked protection of the lung tissue with fewer numbers of inflammatory cells, mild edema and hemorrhage in the MTX + PSO group. Using the immunohistochemical technique, PSO was also shown to exert an antiapoptotic effect by reducing caspace 3 expression in the lung tissue of MTX-injected rats.
According to the results of the present study, it may be concluded that a single injection of MTX induces lung toxicity mediated by the increase in ROS, pro-inflammatory cytokines and transcription factors (NF-kappa). These alterations lead eventually to apoptosis and severe lung damage. A novel finding of the present study was the reduction of cytotoxicity and hence MTX-induced lung injury after PSO. The oral administration of PSO ameliorated most of the changes induced by MTX through the potent antioxidant, anti-inflammatory and anti-apoptotic activity of its constituents. The biochemical findings coincided with the histological examination indicating a marked protection of the lung tissue after PSO. The limitation of the present study was the use of a single dose of MTX and the short experimental period.
Thus, the pulmonary toxicity of MTX must be taken into consideration during its use in chemotherapy and in the treatment of RA and other diseases especially in patients suffering from pulmonary disorders. Patients under MTX therapy must also be monitored regularly to ensure that their lungs are not affected by MTX treatment. The use of natural antioxidants and anti-inflammatory agents is also highly recommended in patients under chemotherapy.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and biochemical analyses were performed by A.M.A., H.S.A.E., S.M.M. The histological and immunohistochemical studies were performed by M.R.M. The first draft of the manuscript was written by A.M.A. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The final revision and approval were carried out by N.A.A. The authors consent to publication.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was supported by Cairo University research funds.
Data availability
All data generated or analyzed during this study are included in this published article. This study was carried out in accordance with ARRIVE guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Limper AH. Chemotherapy-induced lung disease. Clin. Chest Med. 2004;25:53–64. doi: 10.1016/S0272-5231(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 2.Kinniry, P., & Singh, I. Drug-Induced Lung Disease (Critical Care Medicine). https://www.renalandurologynews.com/home/decision-support-in-medicine/critical-care-medicine/drug-induced-lung-Disease (2019).
- 3.Ahmadzadeh A, Zamani N, Hassanian-Moghaddam H, Hadeiy SK, Parhizgar P. Acute versus chronic methotrexate poisoning; a cross-sectional study. BMC Pharmacol. Toxicol. 2019;20:39. doi: 10.1186/s40360-019-0316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedek TG. Methotrexate: From its introduction to non-oncologic therapeutics to anti-TNF-alpha. Clin. Exp. Rheumatol. 2010;28(5 Suppl 61):3–8. [PubMed] [Google Scholar]
- 5.Khan ZA, Tripathi R, Mishra B. Methotrexate: A detailed review on drug delivery and clinical aspects. Exp. Opin. Drug Del. 2012;9:151–169. doi: 10.1517/17425247.2012.642362. [DOI] [PubMed] [Google Scholar]
- 6.Conway R, Low C, Coughlan RJ, O'Donnell MJ, Carey JJ. Methotrexate and lung disease in rheumatoid arthritis: A meta-analysis of randomized controlled trials. Arth. Rheumat. 2014;66:803–812. doi: 10.1002/art.38322. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura M. Methotrexate-induced acute kidney injury in patients with hematological malignancies: Three case reports with literature review. Renal Replac. Ther. 2018;4(1):39. doi: 10.1186/s41100-018-0180-9. [DOI] [Google Scholar]
- 8.Conwar R, Carey JJ. Risk of liver disease in methotrexate treated patients. World J. Hepat. 2017;9:1092–1100. doi: 10.4254/wjh.v9.i26.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakubovic BD, Donovan A, Webster PM, Shear NH. Methotrexate-induced pulmonary toxicity. Can. Resp. J. 2013;20:153–155. doi: 10.1155/2013/527912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Verdia A, Angulo F, Hardwicke FL, Nugent KM. Acute cardiac toxicity associated with high-dose intravenous methotrexate therapy: Case report and review of the literature. Pharmacother. J. Hum. Pharm. Drug Ther. 2005;25:1271–1276. doi: 10.1592/phco.2005.25.9.1271. [DOI] [PubMed] [Google Scholar]
- 11.Boukhettala N, et al. Methotrexate induces intestinal mucositis and alters gut protein metabolism independently of reduced food intake. Am. J. Physiol. 2009;296:E182–E190. doi: 10.1152/ajpendo.90459.2008. [DOI] [PubMed] [Google Scholar]
- 12.Distefano G, et al. HRCT patterns of drug-induced interstitial lung diseases: A review. Diagnostics. 2020;10:244. doi: 10.3390/diagnostics10040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Andrea N, Triolo L, Margagnoni G, Aratari A, Sanguinetti CM. Methotrexate-induced pneumonitis in Crohn's disease. Case report and review of the literature. Multidiscip. Resp. Med. 2010;5:1–8. doi: 10.1186/2049-6958-5-5-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medjakovic S, Hobiger S, Ardjomand-Woelkart K, Bucar F, Jungbauer A. Pumpkin seed extract: Cell growth inhibition of hyperplastic and cancer cells, independent of steroid hormone receptors. Fitoterapia. 2016;110:150–156. doi: 10.1016/j.fitote.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Boaduo NKK, Katerere D, Eloff JN, Naidoo V. Evaluation of six plant species used traditionally in the treatment and control of diabetes mellitus in South Africa using in vitro methods. Pharmaceut. Biol. 2014;52:756–761. doi: 10.3109/13880209.2013.869828. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Huang G. Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 2019;126:743–746. doi: 10.1016/j.ijbiomac.2018.12.261. [DOI] [PubMed] [Google Scholar]
- 17.Al-Okbi SY, et al. Anti-inflammatory activity of two varieties of pumpkin seed oil in an adjuvant arthritis model in rats. Grasas Aceites. 2017;68:e180–e180. doi: 10.3989/gya.0796161. [DOI] [Google Scholar]
- 18.Shayesteh R, et al. Cytoprotective effects of pumpkin (Cucurbita Moschata) fruit extract against oxidative stress and carbonyl stress. Drug Res. 2017;67:576–582. doi: 10.1055/s-0043-110484. [DOI] [PubMed] [Google Scholar]
- 19.Elfiky SA, Elelaimy IA, Hassan AM, Ibrahim HM, Elsayad RI. Protective effect of pumpkin seed oil against genotoxicity induced by azathioprine. J. Basic Appl. Zool. 2012;65:289–298. doi: 10.1016/j.jobaz.2012.10.010. [DOI] [Google Scholar]
- 20.Fawzy EI, El Makawy AI, El-Bamby MM, Elhamalawy HO. Improved effect of pumpkin seed oil against the bisphenol-A adverse effects in male mice. Toxicol. Rep. 2018;5:857–863. doi: 10.1016/j.toxrep.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saygin M, et al. The impact of methotrexate on lung inflammatory and apoptotic pathway biomarkers: The role of gallic acid. Biomed. Pharmacother. 2016;84:1689–1696. doi: 10.1016/j.biopha.2016.10.077. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Beutler E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 24.Montgomery HACD, Dymock JF. Determination of nitrite in water. Analyst. 1961;86:414. [Google Scholar]
- 25.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 26.Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/S0006-291X(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 27.Ellman GL, Burkhalter A, LaDou J. A fluorometric method for the determination of hippuric acid. J. Lab. Clin. Med. 1961;57:813–818. [PubMed] [Google Scholar]
- 28.Gorun V, Proinov I, Băltescu V, Balaban G, Bârzu O. Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Anal. Biochem. 1978;86:324–326. doi: 10.1016/0003-2697(78)90350-0. [DOI] [PubMed] [Google Scholar]
- 29.Fouad GI, Mousa MR. The protective potential of alpha lipoic acid on amiodarone-induced pulmonary fibrosis and hepatic injury in rats. Mol. Cell. Biochem. 2021;476:3433–3448. doi: 10.1007/s11010-021-04173-7. [DOI] [PubMed] [Google Scholar]
- 30.Macnee W. Pathogenesis of chronic obstructive pulmonary disease. Clin. Chest Med. 2007;28:479–513. doi: 10.1016/j.ccm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- 32.Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012;3:645460. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohbayashi M, et al. Induction of pulmonary fibrosis by methotrexate treatment in mice lung in vivo and in vitro. J. Toxicol. Sci. 2010;35:653–661. doi: 10.2131/jts.35.653. [DOI] [PubMed] [Google Scholar]
- 34.Dweik RA. Drug-induced pulmonary disease. In: Baum GL, Crapo JD, Celli BR, Karlinsky JB, editors. Textbook of Pulmonary Diseases. 6. Lippincott Raven; 1988. pp. 477–490. [Google Scholar]
- 35.Kahraman H, et al. Protective effects of erythropoietin and N-acetylcysteine on methotrexate-induced lung injury in rats. Balkan Med. J. 2013;30:99. doi: 10.5152/balkanmedj.2012.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fikry EM, Safar MM, Hasan WA, Fawzy HM, El-Denshary EE. Bone marrow and adipose-derived mesenchymal stem cells alleviate methotrexate-induced pulmonary fibrosis in rat: Comparison with dexamethasone. J. Biochem. Mol. Toxicol. 2015;29:321–329. doi: 10.1002/jbt.21701. [DOI] [PubMed] [Google Scholar]
- 37.Bialas AJ, Sitarek P, Milkowska-Dymanowska J, Piotrowski WJ, Gorski P. The role of mitochondria and oxidative/antioxidative imbalance in pathobiology of chronic obstructive pulmonary disease. Oxid. Med. Cell Longev. 2016;2016:7808576. doi: 10.1155/2016/7808576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridovich I, Freeman B. Antioxidant defenses in the lung. Ann. Rev. Physiol. 1986;48:693–702. doi: 10.1146/annurev.ph.48.030186.003401. [DOI] [PubMed] [Google Scholar]
- 39.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am. J. Resp. Crit. Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 40.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur. J. Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 41.Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antosova M, Plevkova J, Strapkova A, Buday T. Nitric oxide-important messenger in human body. Open. J. Mol. Integr. Physiol. 2012;2:98–106. doi: 10.4236/ojmip.2012.23014. [DOI] [Google Scholar]
- 43.De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: A novel pathway relevant to the pathophysiology of endothelium. Arterioscler. Thromb. Vasc. Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 44.Aizawa H. Role of nitric oxide in airway inflammation and hyperresponsiveness in bronchial asthma. Allergol. Int. 1999;48:25–30. doi: 10.1046/j.1440-1592.1999.00113.x. [DOI] [Google Scholar]
- 45.Kleniewska P, Gorąca A. Influence of endothelin 1 receptor blockers and a nitric oxide synthase inhibitor on reactive oxygen species formation in rat lungs. Physiol. Res. 2016;65:789–798. doi: 10.33549/physiolres.933263. [DOI] [PubMed] [Google Scholar]
- 46.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 47.Asif M, Saleem M, Saadullah M, Yaseen HS, Al Zarzour R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology. 2020;28:1153–1161. doi: 10.1007/s10787-020-00744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee HJ, et al. Role of omega-3 polyunsaturated fatty acids in preventing gastrointestinal cancers: Current status and future perspectives. Expert Rev. Anticancer Ther. 2018;18:1189–1203. doi: 10.1080/14737140.2018.1524299. [DOI] [PubMed] [Google Scholar]
- 49.Abdalla AN, et al. Proapoptotic activity of Achillea membranacea essential oil and its major constituent 1, 8-cineole against A2780 ovarian cancer cells. Molecules. 2020;25:1582. doi: 10.3390/molecules25071582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sener B, et al. Antimicrobial and antiviral activities of two seed oil samples of Cucurbitapepo L. and their fatty acid analysis. Nat. Prod. Commun. 2007;2:1934578X0700200. [Google Scholar]
- 51.Badr SEA, et al. Chemical composition and Biological activity of ripe pumpkin fruits (Curcubitapepo L.) cultivated in Egypttian habitats. Nat. Prod. Res. 2011;25:1524–1539. doi: 10.1080/14786410903312991. [DOI] [PubMed] [Google Scholar]
- 52.Omar NM, Sarhan NR. The possible protective role of pumpkin seed oil in an animal model of acid aspiration pneumonia: Light and electron microscopic study. Acta Histochem. 2017;119:161–171. doi: 10.1016/j.acthis.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Ryan E, Galvin K, O’Connor TP, Maguire AR, O’Brien NM. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 2007;62:85–91. doi: 10.1007/s11130-007-0046-8. [DOI] [PubMed] [Google Scholar]
- 54.Micera M, Botto A, Geddo F, Antoniotti S, Bertea CM, Levi R, Querio G. Squalene: More than a step toward sterols. Antioxidants. 2020;9:688. doi: 10.3390/antiox9080688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ravi Kumar S, Narayan B, Sawada Y, Hosokawa M, Miyashita K. Combined effect of astaxanthin and squalene on oxidative stress in vivo. Mol. Cell. Biochem. 2016;417:57–65. doi: 10.1007/s11010-016-2713-2. [DOI] [PubMed] [Google Scholar]
- 56.Radić I, et al. Protective effects of pumpkin (Cucurbita pepo L.) seed oil on rat liver damage induced by chronic alcohol consumption. Arch. Biol. Sci. 2021;73:8. doi: 10.2298/ABS201205008R. [DOI] [Google Scholar]
- 57.Eraslan G, Kanbur M, Aslan Ö, Karabacak M. The antioxidant effects of pumpkin seed oil on subacute aflatoxin poisoning in mice. Environ. Toxicol. 2013;28:681–688. doi: 10.1002/tox.20763. [DOI] [PubMed] [Google Scholar]
- 58.Nkosi CZ, Opoku AR, Terblanche SE. Antioxidative effects of pumpkin seed (Cucurbita pepo) protein isolate in CCl4-induced liver injury in low-protein fed rats. Phytother. Res. 2006;20:935–940. doi: 10.1002/ptr.1977. [DOI] [PubMed] [Google Scholar]
- 59.Martirosyan D, Ashoori MR, Serani A, Zhang K, Mirmiranpour H. Assessment of squalene effect on antioxidant enzymes and free radicals in patients with type 2 diabetes mellitus. Bioact. Comp. Health Dis. 2022;5:236–250. [Google Scholar]
- 60.Shaban NZ, Mohammed AS, Abu-Serie MM, Maher AM, Habashy NH. Inhibition of oxidative stress, IL-13, and WNT/β-catenin in ovalbumin-sensitized rats by a novel organogel of Punica granatum seed oil saponifiable fraction. Biomed. Pharmacother. 2022;154:113667. doi: 10.1016/j.biopha.2022.113667. [DOI] [PubMed] [Google Scholar]
- 61.Habashy NH, Kodous AS, Abu-Serie MM. Targeting ROS/NF-κB sigaling pathway by the seedless black Vitis vinifera polyphenols in CCl4-intoxicated kidney, lung, brain, and spleen in rats. Sci. Rep. 2021;11:16575. doi: 10.1038/s41598-021-96008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abu-Serie MM, Habashy NH. Vitis vinifera polyphenols from seedless black fruit act synergistically to suppress hepatotoxicity by targeting necroptosis and pro-fibrotic mediators. Sci. Rep. 2020;10:2452. doi: 10.1038/s41598-020-59489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang XJ, Greenberg DS. Acetylcholinesterase involvement in apoptosis. Front. Mol. Neurosci. 2012;5:40. doi: 10.3389/fnmol.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaked I, Meerson A, Wolf Y. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009;31:965–973. doi: 10.1016/j.immuni.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y, Wang X, Wang T. Acetylcholinesterase, a key prognostic predictor for hepatocellular carcinoma, suppresses cell growth and induces chemosensitization. Hepatology. 2011;53:493–503. doi: 10.1002/hep.24079. [DOI] [PubMed] [Google Scholar]
- 66.Tsakiris S, Angelogianni P, Schulpis KH, Stavridis JC. Protective effect of L-phenylalanine on rat brain acetylcholinesterase inhibition induced by free radicals. Clin. Biochem. 2000;33:103–106. doi: 10.1016/S0009-9120(99)00090-9. [DOI] [PubMed] [Google Scholar]
- 67.Massarelli R, Ferret B, Sorrentino G, Hattori H, Kanfer JN. Choline acetyltransferase-like activity bound to neuronal plasma membranes. Neurochem. Res. 1988;13:1193–1198. doi: 10.1007/BF00971638. [DOI] [PubMed] [Google Scholar]
- 68.Schenkel LC, Bakovic M. Palmitic acid and oleic acid differentially regulate choline transporter-like 1 levels and glycerolipid metabolism in skeletal muscle cells. Lipids. 2014;49:731–744. doi: 10.1007/s11745-014-3925-4. [DOI] [PubMed] [Google Scholar]
- 69.Granda B, Tabernero A, Tello V, Medina JM. Oleic acid induces GAP-43 expression through a protein kinase C-mediated mechanism that is independent of NGF but synergistic with NT-3 and NT-4/5. Brain Res. 2003;988:1–8. doi: 10.1016/S0006-8993(03)03253-0. [DOI] [PubMed] [Google Scholar]
- 70.Abed SA, Sirat HM, Taher M. Tyrosinase inhibition, anti-acetylcholinesterase, and antimicrobial activities of the phytochemicals from Gynotroches axillaris Blume. Pak. J. Pharm. Sci. 2016;29:2071–2078. [PubMed] [Google Scholar]
- 71.Cox MA, Bassi C, Saunders ME. Beyond neurotransmission: Acetylcholine in immunity and inflammation. J. Intern. Med. 2020;287:120–133. doi: 10.1111/joim.13006. [DOI] [PubMed] [Google Scholar]
- 72.Sonis ST. The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit. Rev. Oral Biol. Med. 2002;13:380–389. doi: 10.1177/154411130201300502. [DOI] [PubMed] [Google Scholar]
- 73.Mateen S, Zafar A, Moin S, Khan AQ, Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta. 2016;455:161–171. doi: 10.1016/j.cca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 74.Hamada K, Kakigawa N, Sekine S, Shitara Y, Horie T. Disruption of ZO-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis. Cancer Chemother. Pharmacol. 2013;72:757–765. doi: 10.1007/s00280-013-2238-2. [DOI] [PubMed] [Google Scholar]
- 75.Yamauchi Y, et al. Methotrexate induces interleukin-8 production by human bronchial and alveolar epithelial cells. Clin. Sci. 2004;106:619–625. doi: 10.1042/CS20030262. [DOI] [PubMed] [Google Scholar]
- 76.Kurt A, et al. Protective effects of infliximab on lung injury induced by methotrexate. Arch. Bronconeumol. 2015;51:551–557. doi: 10.1016/j.arbres.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 77.Nijkamp FP, Folkerts G. Nitric oxide: Initiator and modulator. Clin. Exp. Allergy. 1997;27:347–350. doi: 10.1111/j.1365-2222.1997.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 78.Hinz M, Arslan SÇ, Scheidereit C. It takes two to tango: IκBs, the multifunctional partners of NF-κB. Immunol. Rev. 2012;246:59–76. doi: 10.1111/j.1600-065X.2012.01102.x. [DOI] [PubMed] [Google Scholar]
- 79.Tang CC, Lin WL, Lee YJ, Tang YC, Wang CJ. Polyphenol-rich extract of Nelumbonucifera leaves inhibits alcohol-induced steatohepatitis via reducing hepatic lipid accumulation and anti-inflammation in C57BL/6J mice. Food Funct. 2014;5:678–687. doi: 10.1039/c3fo60478k. [DOI] [PubMed] [Google Scholar]
- 80.Lai CC, Liu WL, Chen CM. Glutamine attenuates acute lung injury caused by acid aspiration. Nutrients. 2014;6:3101–3116. doi: 10.3390/nu6083101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scorletti E, Byrne CD. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Asp. Med. 2018;64:135–146. doi: 10.1016/j.mam.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Calfee CS, et al. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Resp. Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barratt S, Medford AR, Millar AB. Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respiration. 2014;87:329–342. doi: 10.1159/000356034. [DOI] [PubMed] [Google Scholar]
- 84.Nakahara H, et al. Anti–interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arth. Rheum. 2003;48:1521–1529. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 85.Gutierrez-Delgado RI, et al. Effect of omega-3 fatty acids supplementation during pregnancy on lung function in preschoolers: A clinical trial. J. Asthma. 2019;56:296–302. doi: 10.1080/02770903.2018.1452934. [DOI] [PubMed] [Google Scholar]
- 86.Huang Z, et al. The effects and safety of omega-3 fatty for acute lung injury: A systematic review and meta-analysis. World J. Surg. Oncol. 2020;18:1–8. doi: 10.1186/s12957-020-01916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Golbs A, Heck N, Luhmann HJ. A new technique for real-time analysis of caspase-3 dependent neuronal cell death. J. Neurosci. Methods. 2007;161:234–243. doi: 10.1016/j.jneumeth.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 88.Bathri R, Bose P, Gujar VS, Kumar L. The role of ROS in COPD progression and therapeutic strategies. React. Oxy. Spec. 2017;4:237–250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. This study was carried out in accordance with ARRIVE guidelines.