Abstract
The influence of vancomycin and flavophospholipol (FPL) on the transfer rate of conjugative plasmids harboring the vancomycin resistance operon vanA was determined in several clinical and animal isolates of Enterococcus faecium. FPL significantly inhibited the frequency of transfer of conjugative VanA plasmids up to 70-fold. Vancomycin had no significant effect on the transfer rate of VanA plasmids.
The emergence and spread of glycopeptide resistance among enterococci have caused great concern (12, 22, 30). Acquired glycopeptide resistance among enterococci is most commonly associated with the vanA determinant. This gene cluster is frequently located on conjugative plasmids (7, 17, 18; G. Werner, I. Klare, and W. Witte, Letter, J. Clin. Microbiol. 37:2383–2384, 1999). VanA resistance has been detected in strains of human, animal, and environmental origin (1, 6, 13, 26). Based on these observations, it has been proposed that in addition to imprudent use of glycopeptides in human medicine, the feeding of the glycopeptide antibiotic avoparcin in animal husbandry has contributed to the emergence and spread of vancomycin-resistant enterococci, especially in Europe (26, 29).
Besides the direct selective pressure of glycopeptides exerted on bacteria in different habitats, less is known about additional factors contributing to the spread of VanA plasmids in humans and animals. Therefore, in this study, we investigated the impact of vancomycin and flavophospholipol (FPL) on the gene transfer of VanA plasmids in Enterococcus faecium. FPL (synonymous with flavomycin, bambermycin, and moenomycin) is a phosphoglycolipid antibiotic used as a growth promoter in animal husbandry. We chose vancomycin and FPL because both antibiotics induce the vanA resistance operon (3, 9, 10, 15). Moreover, there is some evidence that FPL can decrease the frequency of transfer of certain resistance plasmids in gram-negative organisms (8, 16). Here we examined whether there is a link between the induction of vancomycin resistance genes and the transfer rate of conjugative VanA plasmids.
The E. faecium donor strains used in this study carry the VanA resistance determinant on large plasmids of 50 to 175 kbp (28; Werner et al., Letter; data not shown) and are resistant to vancomycin (MIC, >1024 mg/liter) and erythromycin (MIC, >8 mg/liter). The strains were not clonally related as determined by pulsed-field gel electrophoresis (PFGE) analysis (data not shown). The strains were obtained from sewage (AW2), pigs (2E121198, 2121198, and 9191198), and a hospital (7090 and 6011). E. faecium 64/3 was used as the recipient strain (28), which is resistant to rifampin and fusidic acid. All donors are rifampin and fusidic acid sensitive. The MIC of FPL for strains AW2, 7090, and 6011 was 16 mg/liter, and that for strains 2E121198, 2121198, 9191198, and 64/3 was >128 mg/liter. The genetic transfer of VanA plasmids was studied in vitro by filter mating as described elsewhere (5). The donor/recipient ratio used was 1:1, and the filters were placed on brain heart infusion (BHI) agar containing different concentrations of FPL (0.05, 0.25, 1, 8, and 16 mg/liter) and vancomycin (0.05, 0.25, 1, 4, and 8 mg/liter). One filter each was incubated on BHI agar plates without antibiotics. Transconjugants were selected on BHI agar containing rifampin (30 mg/liter), fusidic acid (20 mg/liter), and vancomycin (50 mg/liter). The frequency of transfer was determined by calculation of the quotient of the number of transconjugant colonies/the number of recipient colonies. The genomic structure of representatively chosen transconjugants was verified by macrorestriction analysis. In addition, the transfer of the vanA gene was verified by Southern hybridization as described elsewhere (23). The transfer frequencies were determined in six independent test series and statistically analyzed by the Mann-Whitney U test.
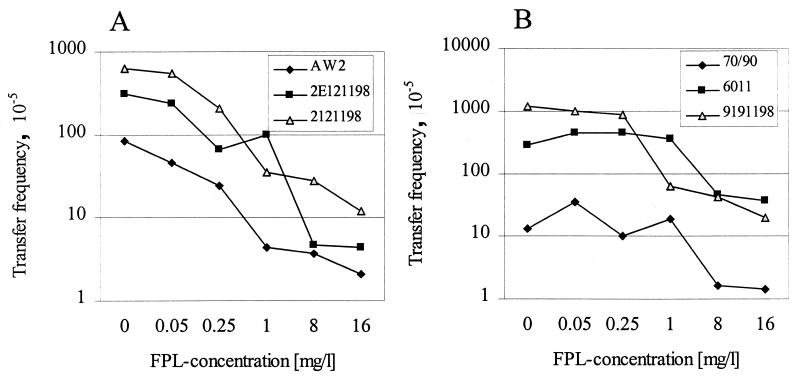
The effect of FPL on the frequency of transfer of the VanA plasmids was determined by using the FPL concentrations for which an induction of the vanA operon was observed (10, 15), and the frequency of transfer of certain resistance plasmids in gram-negative strains was decreased (8). In our study, FPL at concentrations in excess of 1 mg/liter significantly reduced the transfer rate of all six donor strains tested (Fig. 1). While the animal isolates showed a marked inhibition of the transfer rate at 1 mg/liter, concentrations of 8 and 16 mg of FPL per liter were needed to achieve a six- to ninefold reduction of vanA transfer of the clinical isolates (Fig. 1). Concentrations of less than 1 mg/liter significantly inhibited the transfer of the VanA determinant in strains AW2, 2E121198, and 2121198 (Fig. 1A). The strongest inhibition of the conjugative transfer was observed at concentrations of 8 and 16 mg/liter (Fig. 1). The genetic transfer in strain 2E121198, for example, was inhibited up to 70-fold, that in strain 9191198 was inhibited up to 60-fold, and that in strain 2121198 was inhibited up to 52-fold in the presence of FPL during the conjugation process. In contrast, the transfer of the VanA determinant was inhibited in the clinical isolates 7090 and 6011 only at high FPL concentrations up to ninefold in strain 7090 and up to eightfold in strain 6011. The results show that VanA gene transfer is clearly inhibited in the presence of FPL, and furthermore, this effect seems to be more pronounced in animal isolates than in clinical isolates of E. faecium.
FIG. 1.
Effect of different concentrations of FPL on the transfer frequency of the VanA plasmids in donor strains AW2, 2E121198, and 2121198 (A) and 7090, 6011, and 9191198 (B).
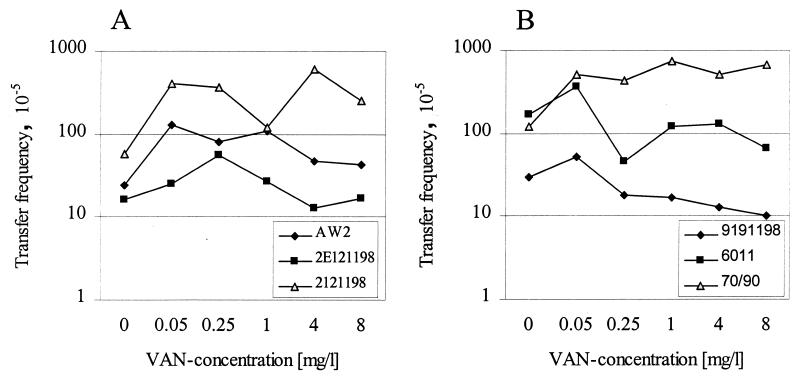
Furthermore, the effect of vancomycin on the frequency of transfer of VanA plasmids was determined by conjugation of the strains in the presence of vancomycin at concentrations for which an induction of the vanA operon was observed previously (3, 9). In our study, vancomycin had no significant effect on the transfer rate of VanA plasmids (Fig. 2). Even at high concentrations of the antibiotic above the MIC for the recipient strain, the frequency of transfer was not significantly altered (Fig. 2). At these concentrations, a decline in the number of recipient cells was observed; however, this was accompanied by a decline in the number of transconjugants. This suggests that mating occurs with relative high frequencies also at vancomycin concentrations above the MIC of the recipient. In strains 7090 and 2121198, a marginal, though not significant, increase in the transfer rate was detected under the influence of vancomycin (Fig. 2).
FIG. 2.
Effect of different concentrations of vancomycin (VAN) on the transfer frequency of the VanA plasmid in the donor strains AW2, 2E121198, and 2121198 (A) and 9191198, 6011, and 7090 (B).
The mechanism for the inhibition of the genetic transfer by FPL is not known yet. One possible explanation is that FPL inhibits lytic transglycosylases encoded by conjugative plasmids. Lytic transglycosylases catalyze the cleavage of the β-1,4-glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine of bacterial peptidoglycan and have been proposed to facilitate the passage of plasmid DNA through the peptidoglycan layer during conjugation (4, 14). Since the mode of action of FPL is the inhibition of bacterial transglycosylases which catalyze the incorporation of disaccharide pentapeptide subunits into nascent peptidoglycan during cell wall synthesis, it would be interesting to investigate whether FPL also interacts with lytic transglycosylases encoded on conjugative plasmids (11, 21). FPL is a phosphoglycolipid antibiotic that acts primarily against gram-positive bacteria, whereas gram-negative bacteria are considered to be inherently resistant due to the permeability barrier of the outer membrane. But, interestingly, FPL also strongly inhibited transglycosylases of gram-negative bacteria, as was shown in a cell-free in vitro system (27). Our observation that FPL inhibits plasmid transfer in FPL-resistant E. faecium as strongly as in FPL-sensitive strains and also in naturally FPL-resistant gram-negative strains supports the speculation that FPL does not simply destabilize the conjugation apparatus by interfering with cell wall synthesis, but specifically interacts with a component of the conjugation system. Further evidence for this assumption comes from our observations that vancomycin did not suppress VanA plasmid transfer. Vancomycin blocks, just like FPL, the transglycosylation step during cell wall synthesis; however, it does so by a specific binding of the antibiotic to the carboxy-terminal d-alanine residues of peptidoglycan precursors and not by direct interaction with transglycosylases (2). Moreover, lytic transglycosylases were found on conjugative plasmids of Escherichia coli for which an inhibition of transfer by FPL was described (8, 19). However, the interaction of FPL with lytic transglycosylases has not been investigated yet; moreover, the functional structure of the transfer apparatus encoded by VanA plasmids of E. faecium remains to be elucidated.
Another possible mechanism for the inhibition of gene transfer by FPL could be due to the membrane activity of the compound. To investigate this further, we tested whether a membrane active cationic peptide (nisin) and a nonionic detergent (Nonidet P-40) below the MIC also influence the conjugational transfer of the VanA plasmids. While nisin had no effect on the transfer efficiency of these plasmids, Nonidet P-40 inhibited the conjugational transfer in two of four strains tested (data not shown). Thus, dysintegrity of the bacterial membrane could also influence the transfer of VanA plasmids, at least in some strains. However, more experimental work is needed, including the characterization of the transfer apparatus of VanA plasmids, to assess whether or not the impact of FPL on the conjugation process is a specific inhibition of lytic transglycosylases encoded by the plasmids or rather is unspecific by physical interaction of the compound with the bacterial membrane.
An important reason for the selection of FPL and vancomycin in our experiments was to investigate whether there is a link between induction of vancomycin resistance and the transfer rate of VanA plasmids. This is of particular interest since FPL is used as a growth promoter in animal husbandry and vancomycin is a major reserve antibiotic in human medicine to combat severe infections caused by gram-positive pathogens. First, evidence for a stimulatory impact of subinhibitory concentrations of antibiotics on gene transfer functions of resistance determinants has been provided by reports dealing with regulation of transfer of conjugative transposons in Bacteroides. It was found that low levels of tetracycline stimulate the transfer of the tetracycline resistance determinant tetQ by at least 1,000-fold (25). This stimulation is probably due to transcriptional activation of the tetracycline resistance operon containing regulatory genes which control the transfer of the transposon (20, 24).
The vancomycin resistance operon vanA is induced not only by low concentrations of glycopeptides, but also by other cell wall active antibiotics, including FPL (3, 9, 10, 15). However, the activation of vancomycin resistance genes seems not to induce transfer functions of conjugative VanA plasmids. We could show that at concentrations of vancomycin and FPL which induce the vanA operon, the rate of VanA plasmid transfer was not affected by vancomycin and was even drastically decreased by FPL. These results suggest that there is no functional link between induction of VanA-type vancomycin resistance and the transfer rate of conjugative VanA plasmids. In vivo trials have to be conducted to assess further the relevance of the inhibitory effect of FPL on transfer of resistance determinants in vivo.
Acknowledgments
We thank Peter Schmid (Intervet International GmbH, Frankfurt am Main, Germany) for gifts of strains and Ute Hentschel for critical reading of the manuscript.
The work in Würzburg was supported by Intervet International GmbH, Wiesbaden, Germany, and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Aarestrup F M. Occurrence of glycopeptide resistance among Enterococcus faecium isolates from ecological and conventional poultry farms. Microb Drug Resist. 1995;1:255–257. doi: 10.1089/mdr.1995.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Arthur M, Reynolds P, Courvalin P. Glycopeptide resistance in enterococci. Trends Microbiol. 1996;10:401–407. doi: 10.1016/0966-842X(96)10063-9. [DOI] [PubMed] [Google Scholar]
- 3.Baptista M, Depardieu F, Courvalin P, Arthur M. Specificity of induction of glycopeptide resistance genes in Enterococcus faecalis. Antimicrob Agents Chemother. 1996;40:2291–2295. doi: 10.1128/aac.40.10.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer M, Eferl R, Zellnig G, Teferle K, Dijkstra A, Koraimann G, Högenauer G. Gene 19 of plasmid R1 is required for both efficient conjugative DNA transfer and bacteriophage R17 infection. J Bacteriol. 1995;177:4279–4288. doi: 10.1128/jb.177.15.4279-4288.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clewell D B, An F Y, White B A, Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918) J Bacteriol. 1985;162:1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coque T M, Tomayko J F, Ricke S C, Okhyusen P C, Murray B E. Vancomycin-resistant enterococci from nosocomial, community, and animal sources in the United States. Antimicrob Agents Chemother. 1996;40:2605–2609. doi: 10.1128/aac.40.11.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evers S, Quintiliani R, Jr, Courvalin P. Genetics of glycopeptide resistance in enterococci. Microb Drug Resist. 1996;2:219–223. doi: 10.1089/mdr.1996.2.219. [DOI] [PubMed] [Google Scholar]
- 8.George B A, Fagerberg D J. Effect of bambermycins, in vitro, on plasmid-mediated antimicrobial resistance. Am J Vet Res. 1984;45:2336–2341. [PubMed] [Google Scholar]
- 9.Grissom-Arnold J, Alborn W E, Nicas T I, Jaskunas S R. Induction of VanA vancomycin resistance genes in Enterococcus faecalis: use of a promoter fusion to evaluate glycopeptide and nonglycopeptide induction signals. Microb Drug Resist. 1997;3:53–64. doi: 10.1089/mdr.1997.3.53. [DOI] [PubMed] [Google Scholar]
- 10.Handwerger S, Kolokathis A. Induction of vancomycin resistance in Enterococcus faecium by inhibition of transglycosylation. FEMS Microbiol Lett. 1990;58:167–170. doi: 10.1111/j.1574-6968.1990.tb13972.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoskins J, Matsushima P, Mullen D L, Tang J, Zhao G, Meier T I, Nicas T I, Jaskunas S R. Gene disruption studies of penicillin-binding proteins 1a, 1b, and 2a in Streptococcus pneumoniae. J Bacteriol. 1999;181:6552–6555. doi: 10.1128/jb.181.20.6552-6555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huycke M M, Sahm D F, Gilmore M. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4:239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klare I, Heier H, Claus H, Reissbrodt R, Witte W. VanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol Lett. 1995;125:165–171. doi: 10.1111/j.1574-6968.1995.tb07353.x. [DOI] [PubMed] [Google Scholar]
- 14.Koonin E V, Rudd K E. A conserved domain in putative bacterial and bacteriophage transglycosylases. Trends Biochem Sci. 1994;19:106–107. doi: 10.1016/0968-0004(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 15.Lai M H, Kirsch D R. Induction signals for vancomycin resistance encoded by the vanA gene cluster in Enterococcus faecium. Antimicrob Agents Chemother. 1996;40:1645–1648. doi: 10.1128/aac.40.7.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebek G. Effect of flavomycin on episomal resistant bacteria. Zentbl Vet Med B. 1972;19:532–539. [PubMed] [Google Scholar]
- 17.Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319:157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 18.Leclercq R, Derlot E, Weber M, Duval J, Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989;33:10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehnherr H, Hansen A M, Ilyina T. Penetration of the bacterial cell wall: a family of lytic transglycosylases in bacteriophages and conjugative plasmids. Mol Microbiol. 1998;30:454–457. doi: 10.1046/j.1365-2958.1998.01069.x. [DOI] [PubMed] [Google Scholar]
- 20.Li L-Y, Shoemaker N B, Salyers A A. Location and characteristics of the transfer region of a Bacteroides conjugative transposon and regulation of transfer genes. J Bacteriol. 1995;177:4992–4999. doi: 10.1128/jb.177.17.4992-4999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mani N, Sanchet P, Jiang Z D, McNaney C, DeCenzo M, Knighti B, Stankis M, Kuranda M, Rothenstein D M. Screening systems for detecting inhibitors of cell wall transglycosylation in Enterococcus. J Antibiot. 1998;51:471–479. doi: 10.7164/antibiotics.51.471. [DOI] [PubMed] [Google Scholar]
- 22.Murray B E. Drug therapy: vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342:710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- 23.Ohlsen K, Koller K-P, Hacker J. Analysis of expression of the alpha-toxin gene (hla) of Staphylococcus aureus by using a chromosomally encoded hla::lacZ gene fusion. Infect Immun. 1997;65:3606–3614. doi: 10.1128/iai.65.9.3606-3614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens A M, Sanders J M, Shoemaker N B, Salyers A A. Genes involved in production of plasmidlike forms by a Bacteroides conjugal chromosomal element share significant amino acid homology with two-component regulatory systems. J Bacteriol. 1992;174:2935–2942. doi: 10.1128/jb.174.9.2935-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens A M, Shoemaker N B, Salyers A A. The region of a Bacteroides conjugal chromosomal tetracycline resistance element which is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J Bacteriol. 1990;172:4271–4279. doi: 10.1128/jb.172.8.4271-4279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Bogaard A E, Jensen L B, Stobberingh E E. Vancomycin-resistant enterococci in turkeys and farmers. N Engl J Med. 1997;337:1558–1559. doi: 10.1056/NEJM199711203372117. [DOI] [PubMed] [Google Scholar]
- 27.Van Heijenoort Y, Derrien M, Van Heijenoort J. Polymerization by transglycosylation in the biosynthesis of the peptidoglycan of Escherichia coli K 12 and its inhibition by antibiotics. FEBS Lett. 1978;89:141–144. doi: 10.1016/0014-5793(78)80540-7. [DOI] [PubMed] [Google Scholar]
- 28.Werner G, Klare I, Witte W. Arrangement of the vanA gene cluster in enterococci of different ecological origin. FEMS Microbiol Lett. 1997;155:55–61. doi: 10.1111/j.1574-6968.1997.tb12685.x. [DOI] [PubMed] [Google Scholar]
- 29.Witte W. Medical consequences of antibiotic use in agriculture. Science. 1998;279:996–997. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]
- 30.Woodford N. Glycopeptide-resistant enterococci: a decade of experience. J Med Microbiol. 1998;47:849–862. doi: 10.1099/00222615-47-10-849. [DOI] [PubMed] [Google Scholar]