Abstract
Background
The success of immune checkpoint inhibitors has revolutionized cancer treatment options and triggered development of new complementary immunotherapeutic strategies, including T-cell co-stimulatory molecules, such as glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR). BMS-986156 is a fully agonistic human immunoglobulin G subclass 1 monoclonal antibody targeting GITR. We recently presented the clinical data for BMS-986156 with or without nivolumab, which demonstrated no compelling evidence of clinical activity in patients with advanced solid tumors. Here, we further report the pharmacodynamic (PD) biomarker data from this open-label, first-in-human, phase I/IIa study of BMS-986156 ± nivolumab in patients with advanced solid tumors (NCT02598960).
Materials and methods
We analyzed PD changes of circulating immune cell subsets and cytokines in peripheral blood or serum samples collected from a dataset of 292 patients with solid tumors before and during treatment with BMS-986156 ± nivolumab. PD changes in the tumor immune microenvironment were measured by immunohistochemistry and a targeted gene expression panel.
Results
BMS-986156 + nivolumab induced a significant increase in peripheral T-cell and natural killer (NK) cell proliferation and activation, accompanied by production of proinflammatory cytokines. However, no significant changes in expression of CD8A, programmed death-ligand 1, tumor necrosis factor receptor superfamily members, or key genes linked with functional parameters of T and NK cells were observed in tumor tissue upon treatment with BMS-986156.
Conclusions
Despite the robust evidence of peripheral PD activity of BMS-986156, with or without nivolumab, limited evidence of T- or NK cell activation in the tumor microenvironment was observed. The data therefore explain, at least in part, the lack of clinical activity of BMS-986156 with or without nivolumab in unselected populations of cancer patients.
keywords: glucocorticoid-induced tumor necrosis factor receptor–related protein (GITR), nivolumab, immune checkpoint inhibitor, biomarker, pharmacodynamics, BMS-986156
Highlights
-
•
The largest clinical PD dataset to date for a GITR agonist, with or without combination of anti-PD-1, in a broad cancer patient population.
-
•
BMS-986156 + nivolumab induced a significant increase in peripheral T-cell and NK-cell proliferation and activation.
-
•
Limited evidence of T- or NK- cell activation triggered by BMS-986156 + nivolumab in the tumor microenvironment based on paired biopsy assessment.
Introduction
Glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR) is a member of the tumor necrosis factor receptor (TNFR) superfamily.1 GITR is highly expressed on activated T cells and regulatory T cells (Tregs).2 The engagement of GITR by the GITR ligand co-stimulates the T-cell receptor-mediated activation of downstream signaling pathways such as nuclear factor κB and mitogen-activated protein kinase, which promote proliferation and proinflammatory cytokine production, along with increased effector functions and reduced apoptosis in T cells.3,4 In preclinical murine tumor models, GITR agonism demonstrated robust antitumor activity attributable to both Treg inhibition and effector T-cell stimulation.5 Collectively, these data make GITR an attractive therapeutic target in oncology and support the exploration of a human GITR agonist monoclonal antibody (mAb) to enhance antitumor T-cell responses.
BMS-986156 is a fully human agonist mAb of the immunoglobulin G subclass 1 (IgG1) isotype which has preserved immune effector functions such as antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis.6 Several lines of evidence demonstrate that a GITR agonist mAb, such as BMS-986156, could potentially be paired with other therapeutic agents to enhance antitumor immunity, providing the rationale for combination therapies to further enhance antitumor efficacy.7,8 Therefore, BMS-986156 has been evaluated as monotherapy and in combination with checkpoint blockade in early clinical development for the treatment of solid tumors (NCT02598960). Recently, we have reported the clinical dataset of this study indicating that BMS-986156 had a tolerable safety profile and the combination cohort had similar safety profile to that of nivolumab in patients with advanced solid tumors. Both monotherapy and combination therapy exhibited linear pharmacokinetics of BMS-986156. However, the monotherapy failed to generate clinical response, and in the combination cohorts the efficacy was comparable with historical response rates for nivolumab.9 In the current analysis, we aimed to characterize the pharmacodynamic (PD) profile of BMS-986156 in this clinical trial through exploratory biomarkers. To our knowledge, this is the largest clinical PD dataset to date for a GITR agonist, with or without an anti-programmed cell death protein 1 (PD-1) checkpoint inhibitor, in a broad cancer patient population.
Materials and methods
Patients, study design, and treatment
NCT02598960 is an open-label, first-in-human, phase I/IIa trial investigating BMS-986156 as monotherapy and in combination with nivolumab in patients with advanced solid tumors. Generally, BMS-986156 and nivolumab were administered intravenously every 2 weeks (Q2W) in 8-week cycles, for up to three cycles. The escalation monotherapy and combination therapy phases progressed concurrently, wherein cohorts of patients received BMS-986156 monotherapy at doses of 10, 30, 100, 240, or 800 mg Q2W, or combination BMS-986156 at 30, 100, 240, or 800 mg + nivolumab at 240 mg Q2W. The 240 mg and 480 mg doses of BMS-986156 (Q2W) were further investigated in the expansion cohorts. In addition, a dose-expansion cohort evaluating BMS-986156 480 mg every 4 weeks (Q4W) + nivolumab 480 mg Q4W was included as well. Clinical trial details have been disclosed previously.9 All patients provided voluntary written informed consent for study entry and analysis of blood and tumor samples. The study was conducted in compliance with the protocol. The protocol, any amendments, and the patient informed consent form were reviewed and approved by an institutional review board/independent ethics committee before initiation of the study. Patients underwent tumor biopsy at both baseline during screening (within 28 days before day 1 of the study) and day 15 of cycle 1; however, on-treatment tumor biopsy was not mandatory during dose escalation for monotherapy. Additional information on patients, study design, and treatment doses and schedules has been published previously.9
Immunophenotyping
For immunophenotyping flow cytometric analysis, we used a T effector/memory panel that is capable of detecting CD45, CD3, CD4, CD8, CD27, CD45RA, CCR7, HLA-DR, CD38, PD-1, CD152, and Ki67 (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.100784) and an NK panel that detects CD45, CD3, CD56, CD16, NKp46, CD57, CD94, CD27, NKG2D, HLA-DR, Tim-3, and Ki67 (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.100784). Patient blood samples were collected in Cytochex BCT tubes and then managed by the preferred study central lab. After red blood cell lysis, cells were stained using fluorescently labeled antibodies specific for the surface markers listed above. Samples were subsequently fixed, permeabilized, and then stained with an anti-Ki67 antibody. Stained samples were analyzed on a Beckman Coulter Cytoflex S flow cytometer, and the resulting data were analyzed using FlowJo v7 software (FlowJo LLC, Ashland, OR).
Serum cytokines
Cytokine and soluble protein levels in patient serum were measured using Luminex microbead immunoassays that cover 75 analytes in four multiplex panels (catalog numbers: HCYTMAG-60K-PX41; HCP2MAG-62K-PX23; HCYP3MAG-63K-11; HSTCMAG-28SK-17; Millipore Sigma, Billerica, MA). Luminex reagents were purchased from Millipore Sigma, and the profiling assays were carried out following manufacturer’s instructions for sample preparation, incubation times and conditions, and reader settings. The Bristol Myers Squibb in-house established protocol was followed for sample/reagent volume and 384-well assay format.
Immunohistochemistry of patient biopsies
Immunohistochemistry (IHC) for programmed death-ligand 1 (PD-L1) (clone 28-8; Agilent/Dako PharmDx) was carried out on 4-μm-thick formalin-fixed paraffin-embedded (FFPE) sections using a Leica Bond RX (Buffalo Grove, IL) by Mosaic Labs (Lake Forest, CA). From whole slide images, geographic readouts for single/double IHC-positive cells within tumors were generated using Definiens image analysis software (Cambridge, MA). PD-L1 expression was assessed in tumor cell compartment (tumor proportion score); scoring was carried out by a qualified pathologist in samples with a minimum of 100 viable tumor cells. Paired tumor biopsies from screening and cycle 1 day 15 (C1D15) were available from 86 patients representing multiple tumor types, including bladder (n = 12), cervical (n = 19), hepatocellular carcinoma (n = 6), head and neck (n = 16), non-small-cell lung (n = 12), ovarian (n = 18), and other (n = 3).
Gene expression profiling
Gene expression data were generated from 64 subjects with paired FFPE tumor biopsy samples from screening and C1D15 on the targeted gene expression HTG EdgeSeq platform (HTG Molecular, Tucson, AZ) including 3083 probes. Data were transformed into log2 trimmed mean of M-values normalized counts per million before analysis based on manufacturer’s instructions. Gene signature scores were calculated as the arithmetic mean of the expression level of genes included in the signatures including immune signatures for interferon-γ (IFN-γ) pathway activation, inflammation, and cytolytic activity (PRF1, GZMA).10,11
Statistical analysis
Flow cytometry and cytokine data were log2 transformed for analysis. Differences between on-treatment and baseline variables were analyzed by a linear mixed-effects model with Dunnett post hoc test for flow cytometry data and by paired t-test for cytokine data. Changes in IHC and gene expression markers were compared between paired baseline and on-treatment patient samples using a Wilcoxon signed rank test. P < 0.05 was considered statistically significant for all comparisons.
Results
Modulation of peripheral PD biomarkers in patients with advanced solid tumors treated with BMS-986156 in monotherapy or in combination with nivolumab
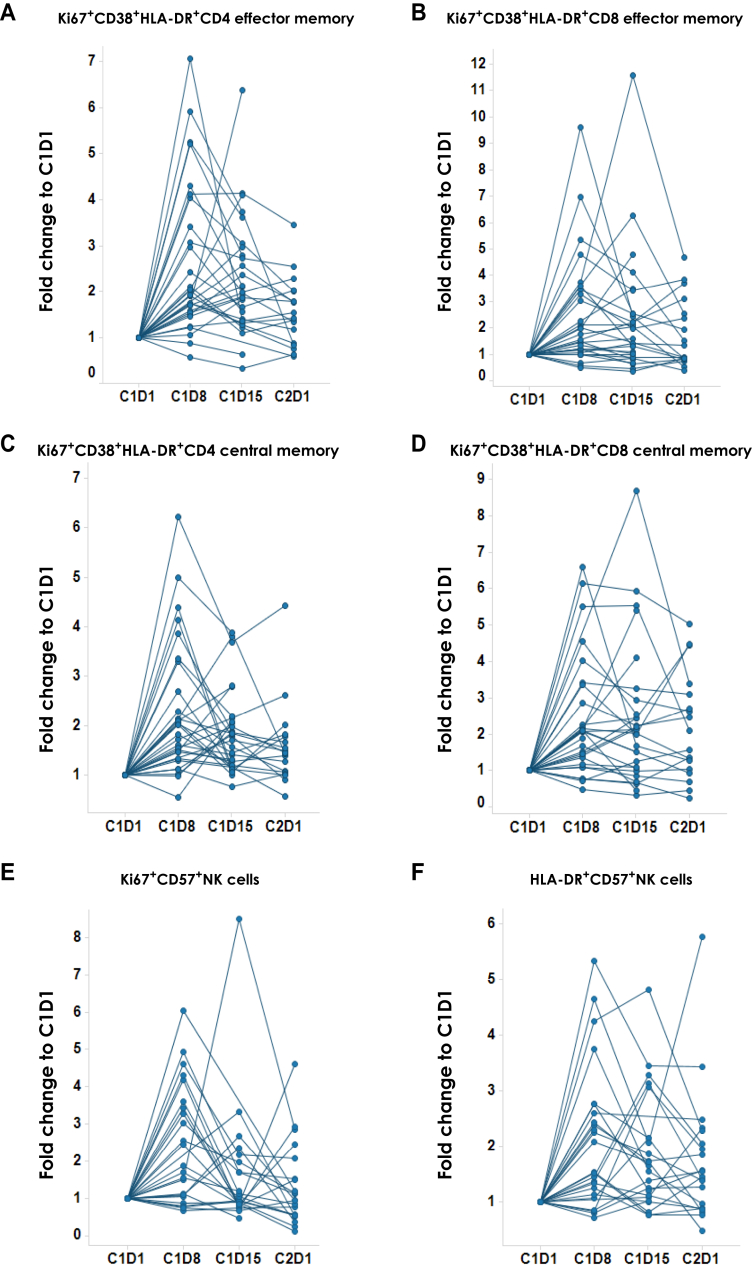
BMS-986156 is a fully agonistic human IgG1 mAb that binds to primary human activated CD4+ and CD8+ T cells with high affinity (EC50: 0.42-0.44 nM).6 We previously reported general enhanced proliferation of T cells and NK cells in the whole blood of patients receiving BMS-986156 monotherapy or BMS-986156 + nivolumab combination in the dose-escalation part of the trial.9 Further analysis by multicolor flow cytometry-based immunophenotyping of peripheral blood from patients enrolled in the expansion cohorts suggested an increased frequency of cycling (Ki67+) and activated (HLA-DR+CD38+) effector and central memory CD4+ and CD8+ T cells (Ki67+ CCR7−CD45RA− and Ki67+ CCR7+CD45RA−, respectively) after treatment with BMS-986156 in combination with nivolumab (n = 30) (Figure 1A-D). In agreement with these data, an increased frequency of HLA-DR+ and Ki67+ within the CD57+ NK cell subset was observed after the combination therapy (n = 26) (Figure 1E and F). In addition, the peak increase in Ki67+ and HLA-DR+ T- and NK cell subpopulations occurred 7 days after infusion (C1D8) (Figure 1).
Figure 1.
Effect of BMS-986156 + nivolumab on proliferating T- and natural killer (NK) cell subpopulations in peripheral blood from patients with advanced solid tumors. Flow cytometric quantification of the frequency of (A) Ki67+CD38+HLA-DR+CD4+ effector memory T cells, (B) Ki67+CD38+HLA-DR+CD8+ effector memory T cells, (C) Ki67+CD38+HLA-DR+CD4+ central memory T cells, (D) Ki67+CD38+HLA-DR+CD8+ central memory T cells, (E) Ki67+CD57+ NK cells, and (F) HLA-DR+CD57+ NK cells in patient peripheral blood at pretreatment (C1D1) and various post-treatment time points. See Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.100784, for summary of P values.
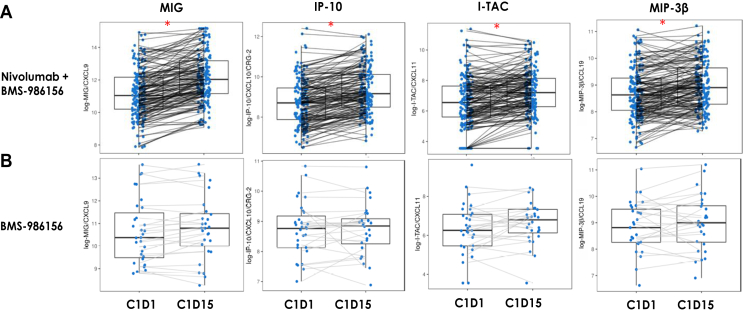
To assess the effect of BMS-986156 with or without nivolumab on cytokine production, we investigated circulating levels of relevant cytokines in patient serum samples at baseline (C1D1) and during treatment (C1D15). Significant increases in serum levels of several proinflammatory cytokines, including IFN-γ-induced chemokines such as MIG (CXCL9), IP-10 (CXCL10), I-TAC (CXCL11), and MIP-3β (CCL19), were observed when BMS-986156 was administered in combination with nivolumab (n = 169) (Figure 2A). BMS-986156 monotherapy induced only a trend toward increased expression of these cytokines (n = 30) (Figure 2B). However, there were no clear dose-dependent effects on cytokine levels or peripheral immune cell proliferation in the dose-escalation study of BMS-986156 (data not shown). Although there was a certain degree of variability associated with kinetics of cytokine modulation, the peak timing for cytokine increase occurred 14 days after infusion at C1D15, and this is consistent between monotherapy and combination therapy (Figure 2).
Figure 2.
Effect of BMS-986156 ± nivolumab on serum cytokine levels. Serum levels in patients treated with (A) BMS-986156 + nivolumab combination or (B) BMS-986156 monotherapy of MIG (CXCL9), IP-10 (CXCL10), I-TAC (CXCL11), and MIP-3β (CCL19) at pre- (C1D1) and on-treatment (C1D15) time points as measured by the Luminex cytokine assay. ∗P < 0.0001 between C1D1 and C1D15.
Effect of BMS-986156 on expression of PD-L1 and immune-related genes in tumor tissue
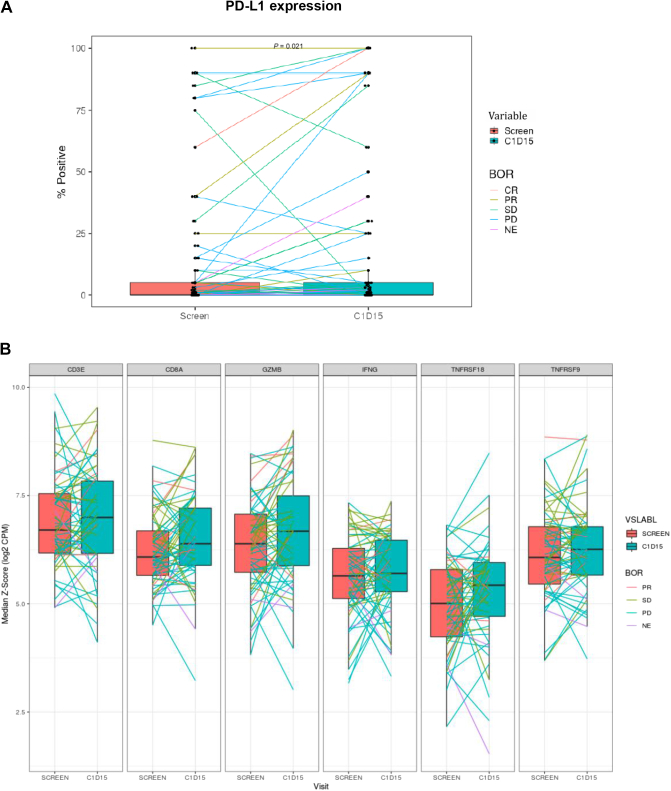
IHC and gene expression analysis was carried out to assess PD changes in PD-L1 and other immune-related markers in tumor tissue. Percent PD-L1-positive tumor cells by IHC were found to be modestly up-regulated upon treatment with combination therapy (Figure 3A, P = 0.02). Through paired tumor biopsy gene expression analysis, we did not observe changes in expression of TNFRSF18 (encoding GITR) as well as other TNFR superfamily members such as TNFRSF9 (encoding CD137). Trend towards increased CD3E and CD8A messenger RNA levels was observed, but statistical significance was not achieved. In addition, genes indicative of T- and NK cell activation and function, such as IFNG and GZMB, did not show significant changes upon treatment with BMS-986156 and nivolumab combination (Figure 3B). Gene expression signatures indicative of tumor-infiltrating immune cells, IFN-γ pathway activation, inflammation, and/or cytolytic activity were also interrogated; however, no major changes during treatment were observed. The data suggest that the combination treatment did not elicit significant immune response in tumor tissue, in contrast to the changes observed in peripheral blood.
Figure 3.
Tumor expression of PD-L1 and immune-related genes during treatment with BMS-986156 + nivolumab. (A) Effect of BMS-986156 + nivolumab on tumor PD-L1 expression assessed by IHC as % of PD-L1-positive tumor cells (TPS, tumor proportion score), P = 0.02. (B) Gene expression levels of CD3E, CD8A, GZMB, IFNG, TNFRSF18 (GITR), and TNFRSF9 (CD137) upon treatment with BMS-986156 + nivolumab combination. BOR, best overall response; CR, complete response; GITR, glucocorticoid-induced tumor necrosis factor receptor-related protein; IHC, immunohistochemistry; NE, not evaluable; PD, progressive disease; PD-L1, programmed death-ligand 1; PR, partial response; SD, stable disease.
Discussion
The success of immune checkpoint inhibitors such as PD-(L)1 and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibodies has revolutionized treatment options for cancer patients.12, 13, 14 Although this class of agents has demonstrated significant antitumor efficacy across a broad range of tumor types, only a subset of patients appear to derive a long-term benefit from these therapies. In addition, cancer patients who progressed on prior immune checkpoint inhibitors have limited treatment options.15, 16, 17 Therefore, development of new immunotherapeutic strategies that can overcome various immune-suppressive and resistance mechanisms represents a high unmet need. It was hypothesized that activation of co-stimulatory molecules on T cells may further potentiate immune responses induced by immune checkpoint blockade, potentially leading to enhanced clinical benefit.6,18 To date, several agonists of co-stimulatory receptors have entered clinical trials, including anti-GITR agonistic antibodies.19, 20, 21, 22
Preclinical studies have demonstrated that GITR activation could synergize with PD-1/PD-L1 blockade and thus lead to potent antitumor immunity.6,7 Results from various murine models suggested a dual mechanism of action (MOA) for GITR agonist therapy attributable to both reduction of regulatory CD4+ T cells and activation of cytotoxic effector CD8+ T cells in the tumor.5 However, to date, there are limited clinical data to confirm whether this MOA would translate from mouse models to patients. Therefore, we recently published our clinical data from the dose-escalation/dose-expansion phase I/IIa study of BMS-986156 with or without nivolumab in 292 patients with advanced solid tumors.8 We did not observe a clear signal of clinical activity of BMS-986156 that would support GITR agonism as an effective therapeutic strategy in a broader cancer patient population.8 Here, we present PD biomarker data from this first-in-human study to expand our understanding of peripheral and tumor PD effects in the context of BMS-986156 therapy.
In patients receiving BMS-986156 as monotherapy in the dose-escalation part of the study, we observed a trend toward an increase in proliferating (Ki67+) CD8 T- and NK cell counts9 accompanied by an enhancement of proinflammatory cytokine production (Figure 2); however, these data were based on a small number of available samples.9 Furthermore, we report a significant increase in frequencies of proliferating and activated effector or central memory T cells, as well as proliferating CD57+ NK cells in the cohorts of patients receiving BMS-986156 + nivolumab (Figure 1). In addition, a robust increase in circulating levels of Th1 cytokines further confirmed treatment-induced peripheral immune cell activation (Figure 2). Nevertheless, historical data indicated similar peripheral immune activation induced by anti-PD-1 therapy.23, 24, 25 Therefore, without a head-to-head comparison to nivolumab monotherapy, dissecting the contribution of BMS-986156 and nivolumab to the PD effects observed in the combination therapy cohorts is not feasible. In addition, there were no clear dose-dependent changes in peripheral PD biomarker levels in patients treated with BMS-986156 alone or combined with nivolumab which could potentially be due to the unselected and/or heterogeneous patient population in the dose-escalation part of the study.
Preclinical studies have suggested that the therapeutic activity of GITR agonists is associated with enhancement of effector CD8+ T-cell responses and decrease in Tregs.5 A recent report has further demonstrated that GITR agonist could reduce circulating and intratumoral Tregs in patients with advanced refractory tumors, although no significant association to clinical response was observed.26 In these analyses, we did not observe a strong evidence of tumor CD8+ T-cell infiltration or Treg depletion upon treatment with BMS-986156 + nivolumab combination in patients with advanced solid tumors.9 While tumor PD-L1 was very modestly up-regulated upon the combination treatment, we did not observe changes in expression of genes associated with the T-cell inflamed phenotype. Expression of TNFR superfamily members, such as GITR or CD137, which would be indicative of T-cell activation, remained largely unchanged during treatment with BMS-986156 + nivolumab combination. The lack of tumor PD effect should be interpreted with caution as these results could also be attributable to intra- and intertumor heterogeneity and/or early tumor biopsy (at day 15 of cycle 1) conducted in this trial. In addition, although the pharmacokinetics of BMS-986156 was linear and exhibited a dose-related increase in exposure that was not affected in combination with nivolumab,9 the operational challenges of obtaining serial on-treatment tumor samples prevented us from confirming the intratumor drug concentration. Therefore, despite clear evidence of biological activity of BMS-986156 in peripheral blood, evidence of immune modulation in the tumor microenvironment is lacking.
In conclusion, in the current work we have summarized peripheral and tumor PD activity data from the phase I/II trial of GITR agonist (BMS-986156) with or without nivolumab. To our knowledge, this is by far the largest clinical biomarker dataset investigating PD activity of GITR agonist therapy, with or without PD-(L)1 blockade. We demonstrate that while BMS-986156 in combination with nivolumab elicited the anticipated PD effects in peripheral blood, there was no effector T-cell activation or Treg depletion in the tumor microenvironment upon treatment with BMS-986156. Similar results of limited clinical activity and inadequate immunological response were also reported from the clinical trials of other GITR agonist antibodies.21,22 The current biomarker data complement our recent publication on the clinical activity of BMS-986156 ± nivolumab,9 and could explain, at least in part, the lack of therapeutic benefit from the GITR agonist in combination with nivolumab in an unselected population of cancer patients.
Acknowledgements
We thank all the patients who made this trial possible and the clinical study teams participating in the trial. We thank Bristol Myers Squibb Clinical Flow Cytometry (Lawrenceville, NJ) and BBRC Clinical Flow Cytometry at Syngene International Limited (Bangalore, India) for conducting the clinical blood immunophenotyping. We would also like to thank Nathan Cheadle, Junchen Gu, Minhua Han, Shih-Min A. Huang, Alan Korman, Penny Phillips, and Mark Selby for their contributions to and support of the BMS-986156 program.
Funding
This study was funded by Bristol Myers Squibb (no grant number).
Disclosure
All authors were employees of Bristol Myers Squibb at the time the study was carried out.
Data sharing
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary data
References
- 1.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronchetti S., Ricci E., Petrillo M.G., et al. Glucocorticoid-induced tumour necrosis factor receptor-related protein: a key marker of functional regulatory T cells. J Immunol Res. 2015;2015 doi: 10.1155/2015/171520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tone M., Tone Y., Adams E., et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci U S A. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esparza E.M., Arch R.H. Glucocorticoid-induced TNF receptor functions as a costimulatory receptor that promotes survival in early phases of T cell activation. J Immunol. 2005;174:7869–7874. doi: 10.4049/jimmunol.174.12.7869. [DOI] [PubMed] [Google Scholar]
- 5.Mahne A.E., Mauze S., Joyce-Shaikh B., et al. Dual roles for regulatory T-cell depletion and costimulatory signaling in agonistic GITR targeting for tumor immunotherapy. Cancer Res. 2017;77:1108–1118. doi: 10.1158/0008-5472.CAN-16-0797. [DOI] [PubMed] [Google Scholar]
- 6.Siu L.L., Steeghs N., Meniawy T., et al. Preliminary results of a phase I/IIa study of BMS-986156 (glucocorticoid-induced tumor necrosis factor receptor–related gene [GITR] agonist), alone and in combination with nivolumab in pts with advanced solid tumors [abstract 104] J Clin Oncol. 2017;35(suppl 15):104. [Google Scholar]
- 7.Wang B., Zhang W., Jankovic V., et al. Combination cancer immunotherapy targeting PD-1 and GITR can rescue CD8(+) T cell dysfunction and maintain memory phenotype. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aat7061. [DOI] [PubMed] [Google Scholar]
- 8.Lu L., Xu X., Zhang B., Zhang R., Ji H., Wang X. Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med. 2014;12:36. doi: 10.1186/1479-5876-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinhuis K., Carlino M., Joerger M., et al. Results from phase 1/2a dose escalation and cohort expansion study for safety, tolerability, and efficacy of GITR agonist (BMS-986156) alone and in combination with nivolumab in patients with advanced solid tumors. JAMA Oncol. 2020;6(1):100–107. doi: 10.1001/jamaoncol.2019.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siemers N.O., Holloway J.L., Chang H., et al. Genome-wide association analysis identifies genetic correlates of immune infiltrates in solid tumors. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0179726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayanan S., Kawaguchi T., Yan L., Peng X., Qi Q., Takabe K. Cytolytic activity score to assess anticancer immunity in colorectal cancer. Ann Surg Oncol. 2018;25(8):2323–2331. doi: 10.1245/s10434-018-6506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris R.L., Blumenschein G., Jr., Fayette J., et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 14.Hodi F.S., O’ Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang L., Chang M., Chang H.M., Chang F. Microsatellite instability: a predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol. 2018;26:e15–e21. doi: 10.1097/PAI.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 16.Smith K.M., Desai J. Nivolumab for the treatment of colorectal cancer. Expert Rev Anticancer Ther. 2018;18:611–618. doi: 10.1080/14737140.2018.1480942. [DOI] [PubMed] [Google Scholar]
- 17.Teng F., Meng X., Kong L., Yu J. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett. 2018;414:166–173. doi: 10.1016/j.canlet.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Linch S.N., McNamara M.J., Redmond W.L. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34. doi: 10.3389/fonc.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denlinger C.S., Infante J.R., Aljumaily R., et al. A phase I study of MEDI1873, a novel GITR agonist, in advanced solid tumors. Ann Oncol. 2018;29(suppl 8) mdy288-027. [Google Scholar]
- 20.Geva R., Voskoboynik M., Beebe A.M., et al. First-in-human phase 1 study of MK-1248, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) monoclonal antibody, as monotherapy or in combination with pembrolizumab in patients with advanced solid tumors. J Clin Oncol. 2018;36(suppl 15):3029. doi: 10.1002/cncr.33133. [DOI] [PubMed] [Google Scholar]
- 21.Koon H.B., Shepard D.R., Merghoub T., Schaer D.A., Sirard C.A., Wolchok J.D. First-in-human phase 1 single-dose study of TRX-518, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) monoclonal antibody in adults with advanced solid tumors. J Clin Oncol. 2016;34(suppl 15):3017. [Google Scholar]
- 22.Tran B., Carvajal R.D., Marabelle A., et al. Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor-related protein agonist AMG 228 in patients with advanced solid tumors. J Immunother Cancer. 2018;6:93. doi: 10.1186/s40425-018-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamphorst A.O., Pillai R.N., Yang S., et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114:4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choueiri T.K., Fishman M.N., Escudier B., et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res. 2016;22:5461–5471. doi: 10.1158/1078-0432.CCR-15-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benson D.M., Jr., Bakan C.E., Mishra A., et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zappasodi R., Sirard C., Li Y., et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med. 2019;25(5):759–766. doi: 10.1038/s41591-019-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.