Abstract
The intracellular penetration and activity of gemifloxacin in human polymorphonuclear leukocytes (PMN) were evaluated. Gemifloxacin reached intracellular concentrations eight times higher than extracellular concentrations. The uptake was rapid, reversible, and nonsaturable and was affected by environmental temperature, cell viability, and membrane stimuli. At therapeutic extracellular concentrations, gemifloxacin showed intracellular activity against Staphylococcus aureus.
Most fluoroquinolones are able to concentrate intracellularly in human phagocytic cells and remain active against facultatively and obligately intracellular pathogens, such as mycobacteria, Legionella spp., Streptococcus pneumoniae, and Staphylococcus aureus (16). Gemifloxacin is a new fluoroquinolone which displays more potent activity than sparfloxacin against gram-positive organisms and anaerobes (5, 7, 10). In addition, it shows excellent in vitro activity against other respiratory pathogens, such as Chlamydia pneumoniae, Haemophilus influenzae, and Mycoplasma pneumoniae (1, 19). The purpose of this study was to evaluate the uptake of gemifloxacin by human polymorphonuclear leukocytes (PMN). Also evaluated were the mechanisms involved in the penetration of this agent into the PMN and its intracellular activity against S. aureus.
PMN uptake of radiolabeled gemifloxacin (64.5 μCi/mg; SB Pharmaceuticals, Harlow, United Kingdom) was determined by means of a velocity gradient centrifugation technique described by Klempner and Styrt (6). PMN were incubated in Hanks balanced salt solution containing different concentrations of gemifloxacin (0.1 to 25 μg/ml). After different incubation periods at 37°C (1 to 60 min), cells were separated from the extracellular solution by centrifugation through a water-impermeable silicone oil barrier. A 10-μl aliquot of the extracellular medium and the entire cell pellet were placed in 3 ml of scintillation fluid (Ready Micro; Beckman Instruments, Inc., Fullerton, Calif.) and counted in a liquid scintillation counter (model LS 1801; Beckman). The accumulation rate of gemifloxacin was calculated and expressed as a cellular-to-extracellular-concentration ratio (C/E ratio) (11). The efflux of cell-associated gemifloxacin was also studied. All assays were performed in duplicate with PMN from four different donors. Data were expressed as means ± standard deviations. Differences among groups were compared by variance analysis, used to assess statistical significance at a P value of ≤0.05.
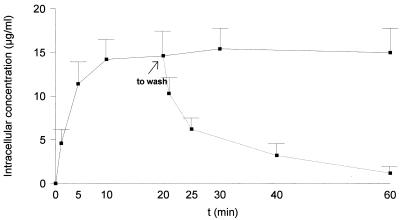
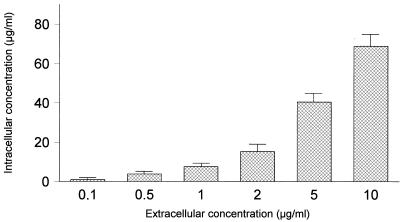
The kinetics of the uptake and efflux of gemifloxacin by PMN are shown in Fig. 1. The uptake of gemifloxacin by the PMN was rapid and high. At extracellular concentrations of 2 μg/ml, the C/E ratios were higher than 7 after 20 min of incubation. This value is similar to those described for ciprofloxacin, ofloxacin, levofloxacin, and sparfloxacin (2, 13, 14) and slightly lower than those described for trovafloxacin and moxifloxacin (17, 18). Reversibility of binding was rapid for gemifloxacin, with 60% of the cell-associated drug being lost after 5 min. The effect of extracellular concentrations of gemifloxacin on PMN uptake is presented in Fig. 2. Cell-associated gemifloxacin was not saturable at concentrations ranging from 0.1 to 25 μg/ml.
FIG. 1.
Gemifloxacin uptake by human PMN and efflux of PMN-associated gemifloxacin after the removal of the extracellular drug (n = 4). The extracellular concentration was 2 μg/ml. Error bars indicate standard deviations.
FIG. 2.
Gemifloxacin uptake by human PMN at different extracellular concentrations (n = 4). Incubations were carried out for 20 min. Error bars indicate standard deviations.
Further studies were performed to elucidate the mechanism of gemifloxacin uptake by PMN (2, 12). The influences of environmental temperature (4 versus 37°C), cell viability, pH (pH 5 to 8), metabolic inhibitors (sodium fluoride at 1.5 × 10−3 M, sodium cyanide at 1.5 × 10−3 M, carbonyl cyanide m-chlorophenylhydrazone at 1.5 × 10−5 M, and 2,4-dinitrophenol at 1 × 10−4 M; Sigma Chemical Co., St. Louis, Mo.), potential competitive substrates (adenosine at 1 mM, d-glucose at 1 mM, and lysine at 1 mM; Sigma), and stimuli of PMN membranes (phorbol myristate acetate [PMA] at 200 nM [Sigma] and either opsonized zymosan [0.9 mg/liter; Sigma] or S. aureus ATCC 25923) were evaluated. The intracellular penetration of gemifloxacin was significantly impaired at 4°C (C/E ratio, 1.0 ± 0.3 versus 8.8 ± 2.3) and significantly increased when dead PMN were used (26.8 ± 7.9 versus 8.8 ± 2.3). This remarkable increase has not been shown by any other fluoroquinolone in formalin-killed PMN. A possible explanation might be that formalin causes structural changes in the PMN which favor nonspecific binding of the gemifloxacin. Gemifloxacin uptake by the PMN was not significantly affected by external pH. Neither the metabolic inhibitors nor the potential competitive substrates (data not shown) affected the intracellular penetration of this quinolone. The penetration of gemifloxacin was unaffected by phagocytosis of opsonized zymosan or by S. aureus. PMA stimulation of PMN membranes significantly increased the uptake of this fluoroquinolone by human PMN (C/E ratio, 58.1 ± 15.9). This controversial effect could be related to the fact that the activation of PMN by opsonized particles differed from that by PMA (8).
The mechanism involved in the intraphagocytic penetration of gemifloxacin is not yet known, and no single model can be postulated in view of our results. Although most data point to a passive mechanism, as has been postulated for many fluoroquinolones, others are typical of an active mechanism. In fact, a passive mechanism (2, 17), a carrier-mediated process (9), and an energy mechanism have been postulated for different fluoroquinolones (3, 12, 18, 20).
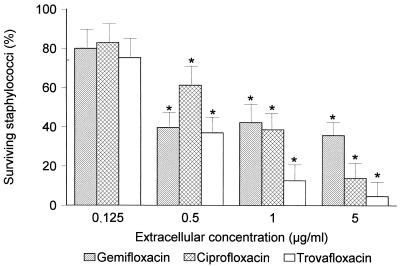
To evaluate the intracellular activities of antimicrobial agents, a previously described method was used (11). The MICs and minimal bactericidal concentrations (MBCs) of ciprofloxacin (Bayer AG, Leverkusen, Germany), trovafloxacin (Pfizer Central Research, Groton, Conn.), and gemifloxacin against S. aureus ATCC 25923 were 0.25, 0.03, and 0.015 μg/ml, respectively. The data were expressed as percentages of surviving staphylococci compared with control levels (without antimicrobial agents) at 3 h. In addition to determining bacterial survival, morphologic studies were also routinely performed at time zero and after 3 h of incubation in order to evaluate the disposition of bacteria (cell associated or extracellular). All assays were performed in duplicate with PMN from four different donors. Data were expressed as means ± standard deviations. Differences among groups were compared by variance analysis, used to assess statistical significance at a P value of ≤0.05. At therapeutic concentrations (0.5, 1, and 5 μg/ml), gemifloxacin showed significant intracellular activity against S. aureus, similar to that observed for ciprofloxacin and lower than that observed for trovafloxacin (Fig. 3). Based on intrinsic activity and C/E values, greater intracellular activity would be expected for gemifloxacin compared to ciprofloxacin. This discrepancy might be related to different degrees of fluoroquinolone activity in the intracellular compartment or to limitations in the method of detecting differences in activity among compounds with very high intracellular activities (4).
FIG. 3.
Intracellular activities of gemifloxacin, ciprofloxacin, and trovafloxacin against S. aureus in human PMN in a 3-h assay (n = 4). Data are expressed as percentages of intracellular surviving staphylococci compared to the level of intracellular surviving staphylococci in controls without antimicrobial agents (taken as 100%). Asterisks, P ≤ 0.05 compared with controls.
In summary, gemifloxacin penetrates into human PMN, reaching high intracellular concentrations and remaining active intracellularly. The high antimicrobial activity of this agent against potential intracellular pathogens enhances its usefulness in clinical settings.
Acknowledgments
We thank Janet Dawson and Patricia Hidalgo for preparation of the manuscript.
This study was partially supported by SB Pharmaceuticals.
REFERENCES
- 1.Erwin M E, Jones R N. Studies to establish quality control ranges for SB-265805 (LB20304) when using National Committee for Clinical Laboratory Standards antimicrobial susceptibility test methods. J Clin Microbiol. 1999;37:279–280. doi: 10.1128/jcm.37.1.279-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García I, Pascual A, Guzmán M C, Perea E J. Uptake and intracellular activity of sparfloxacin in human polymorphonuclear leukocytes and tissue culture cells. Antimicrob Agents Chemother. 1992;36:1053–1056. doi: 10.1128/aac.36.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García I, Pascual A, Perea E J. Intracellular penetration and activity of BAY Y 3118 in human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1994;38:2426–2499. doi: 10.1128/aac.38.10.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hand W L, King-Thompson N L. Contrast between phagocyte antibiotic uptake and subsequent intracellular bactericidal activity. Antimicrob Agents Chemother. 1986;29:135–140. doi: 10.1128/aac.29.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton V H, Goldsmith C E, Ambler J E, Fisher L M. Activity of gemifloxacin against penicillin- and ciprofloxacin-resistant Streptococcus pneumoniae displaying topoisomerase- and efflux-mediated resistance mechanisms. Antimicrob Agents Chemother. 1999;43:2998–3000. doi: 10.1128/aac.43.12.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klempner M S, Styrt B. Clindamycin uptake by human neutrophils. J Infect Dis. 1989;144:472–475. doi: 10.1093/infdis/144.5.472. [DOI] [PubMed] [Google Scholar]
- 7.Marco F, Barrett M S, Jones R N. Antimicrobial activity of LB20304, a fluoronaphthyridone, tested against anaerobic bacteria. J Antimicrob Chemother. 1997;40:605–607. doi: 10.1093/jac/40.4.605. [DOI] [PubMed] [Google Scholar]
- 8.Maridonneau-Parini I, Tringale S M, Tauber A I. Identification of distinct activation pathways of the human neutrophil NADPH-oxidase. J Immunol. 1986;137:2925–2929. [PubMed] [Google Scholar]
- 9.Mémin E, Panteix G, Revol A. Carrier-mediated system for pefloxacin uptake in human monocytes. J Antimicrob Chemother. 1997;40:262–268. doi: 10.1093/jac/40.2.263. [DOI] [PubMed] [Google Scholar]
- 10.Oh J I, Paek K S, Ahn M J, Kim M Y, Kwak J H. In vitro and in vivo evaluations of LB20304, a new fluoronaphthyridone. Antimicrob Agents Chemother. 1996;40:1564–1568. doi: 10.1128/aac.40.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascual A, Tsakayama D, Kovarik J, Gekker G, Peterson P K. Uptake and activity of rifapentine in human peritoneal macrophages and polymorphonuclear leukocytes. Eur J Clin Microbiol. 1987;6:152–157. doi: 10.1007/BF02018197. [DOI] [PubMed] [Google Scholar]
- 12.Pascual A, García I, Perea E J. Fluorometric measurement of ofloxacin uptake by human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1989;33:653–656. doi: 10.1128/aac.33.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascual A, García I, Perea E J. Uptake and intracellular activity of an optically active ofloxacin isomer in human neutrophils and tissue culture cells. Antimicrob Agents Chemother. 1990;34:277–280. doi: 10.1128/aac.34.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pascual A, García I, Conejo M C, Perea E J. Fluorometric and high-performance liquid chromatographic measurement of quinolone uptake by human neutrophils. Eur J Clin Microbiol Infect Dis. 1991;10:969–971. doi: 10.1007/BF02005456. [DOI] [PubMed] [Google Scholar]
- 15.Pascual A, García I, Perea E J. Entry of lomefloxacin and temafloxacin into human neutrophils, peritoneal macrophages, and tissue culture cells. Diagn Microbiol Infect Dis. 1992;15:393–398. doi: 10.1016/0732-8893(92)90079-9. [DOI] [PubMed] [Google Scholar]
- 16.Pascual A. Uptake and intracellular activity of antimicrobial agents in phagocytic cells. Rev Med Microbiol. 1995;6:228–235. [Google Scholar]
- 17.Pascual A, García I, Ballesta S, Perea E J. Uptake and intracellular activity of trovafloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1997;41:274–277. doi: 10.1128/aac.41.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascual A, García I, Ballesta S, Perea E J. Uptake and intracellular activity of moxifloxacin in human neutrophils and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1999;43:12–15. doi: 10.1128/aac.43.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roblin P M, Reznik T, Kutlin A, Hammerschlag M R. In vitro activities of gemifloxacin against recent clinical isolates of Chlamydia pneumoniae. Antimicrob Agents Chemother. 1999;43:2806–2807. doi: 10.1128/aac.43.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walters J D, Zhang F, Nakkula R J. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrob Agents Chemother. 1999;43:2710–2715. doi: 10.1128/aac.43.11.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]