Abstract
Air pollution and poor air quality is impacting human health globally and is a major cause of respiratory and cardiovascular disease and damage to human organ systems. Automated air quality monitoring stations continuously record airborne pollutant concentrations, but are restricted in number, costly to maintain and cannot document all spatial variability of airborne pollutants. Biomonitors, such as lichens, are commonly used as an inexpensive alternative to assess the degree of pollution and monitor air quality. However, only a few studies combined lichen carbon, nitrogen and sulfur contents, with their stable-isotope-ratio signatures (δ13C, δ15N and δ34S values) to assess spatial variability of air quality and to ‘fingerprint’ potential pollution sources. In this study, a high-spatial resolution lichen biomonitoring approach (using Xanthoria parietina and Physcia spp.) was applied to the City of Manchester (UK), the centre of the urban conurbation Greater Manchester, including considerations of its urban characteristics (e.g., building heights and traffic statistics), to investigate finer spatial detail urban air quality. Lichen wt% N and δ15N signatures, combined with lichen nitrate (NO3−) and ammonium (NH4+) concentrations, suggest a complex mixture of airborne NOx and NHx compounds across Manchester. In contrast, lichen S wt%, combined with δ34S strongly suggest anthropogenic sulfur sources, whereas C wt% and δ13C signatures were not considered reliable indicators of atmospheric carbon emissions. Manchester’s urban attributes were found to influence lichen pollutant loadings, suggesting deteriorated air quality in proximity to highly trafficked roads and densely built-up areas. Lichen elemental contents and stable-isotope-ratio signatures can be used to identify areas of poor air quality, particularly at locations not covered by automated air quality measurement stations. Therefore, lichen biomonitoring approaches provide a beneficial method to supplement automated monitoring stations and also to assess finer spatial variability of urban air quality.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-023-26652-8.
Keywords: Urban environment, Lichen biomonitoring, Source apportionment, Manchester (UK), Xanthoria parietina, Physcia spp
Introduction
Urban air pollution and poor air quality have become an increasing worldwide concern, with urban populations increasingly exposed to a large number of airborne pollutants (Academy of Science of South Africa et al. 2019). Globally, 7 million premature deaths are linked to air pollution each year, with about 40,000 such deaths within the UK (The Royal College of Physicians 2016; WHO 2018). Atmospheric pollutants, such as carbon monoxide/dioxide (CO and CO2), sulfur dioxide (SO2) and nitrogen oxides (NOx; combining nitric oxide (NO) and nitrogen dioxide (NO2)) as well as reduced nitrogen (NHx), are linked to deleterious human health impacts, including respiratory and cardiovascular disease and damage to the human organ system (Academy of Science of South Africa et al. 2019; Berner and Felix 2020; Gu et al. 2021; Schraufnagel et al. 2019). Negative health impacts of CO include replacement of oxygen in haemoglobin, and damage to the nervous and cardiovascular systems (Kampa and Castanas 2008; NAEI 2018a). Sulfur dioxide (SO2) has long been a pollutant of interest due to its role in forming winter-smog and acid rain, with impacts on the human respiratory system; e.g., coughing, nose and throat irritation, bronchoconstriction and dyspnoea (Kampa and Castanas 2008; NAEI 2018b; WHO 2018). Short- or long-term exposure to nitrogen dioxide (NO2) can have a variety of health impacts, of which asthma, respiratory disorder, reduced lung function, bronchitis and cancer are of major concern; there also is evidence that high NO2 levels can impair neurodevelopment in children (Moldanová et al. 2011; The Royal College of Physicians 2016; WHO 2013). Comparably reduced nitrogen compounds (e.g. nitrate—NO3 and ammonia/ammonium – NH3/NH4) reportedly impact on the cardiovascular and respiratory system (Kim et al. 2012; Son et al. 2012; WHO 2013).
Atmospheric pollution by carbon and sulfur compounds is linked closely to fossil fuel combustion (EPA 2008; WHO 2018). Nitrogen oxides (NOx) are released into urban environments from combustion processes, heating, energy production and road traffic (Boltersdorf et al. 2014; DEFRA and DfT 2017). In particular, diesel vehicles are responsible for emissions of primary NO2, especially when moving slowly (Abdull et al. 2020). According to EU/UK legislation (Air quality Directive 2008/50/EC; EU 2008) local authorities are obliged to monitor airborne pollutants, e.g., CO, NO2 and SO2, and comply with set regulatory limit values to limit negative human health impacts (EU 2008). Within the UK, automated air-quality monitoring stations (Automatic Urban and Rural Network – AURN; DEFRA 2020) generate continuous records of air quality. However, these are high-cost infrastructures, restricted in number, only record localised air quality, and are not identifying wider scale areas of poor(er) air quality. Urban environments and associated air quality are complex systems with various influencing factors, including building heights and density, road traffic and localised meteorological conditions, these all influencing spatial variability of pollutant distributions (Salmond and McKendy 2009). This urban environment complexity highlights the necessity to apply additional monitoring methods to achieve finer spatial detail of urban air quality, with implications for understanding deleterious impacts on human health and thereafter informing air quality improvement strategies.
Passive biomonitors are organisms that are already part of a natural ecosystem and active biomonitors are organisms that can be easily transferred into an area under investigation. Both types of biomonitors have been used extensively in environmental pollution studies; e.g., use of tree bark, tree leaves and needles, mosses and lichens (Boltersdorf et al. 2014; Forbes 2015). These natural materials are commonly used where costly technical apparatus cannot be afforded (Forbes et al. 2015). Lichens have been shown to be excellent organisms to monitor atmospheric pollution and air quality, because they can be used to obtain qualitative and quantitative information about environmental conditions (Forbes et al. 2015). Lichens are organisms comprising a self-supporting association between at least a fungal (mycobiont) and a photosynthetic (photobiont) partner, the latter can be either green-algae and/or cyanobacteria (Kirschbaum and Wirth 2010; Nash III 2008; Van der Wat and Forbes 2015). Due to their morphology, lacking roots and cuticle, lichens take up nutrients and airborne pollutants directly from the surrounding atmosphere, by dry and/or wet deposition (Forbes 2015; Forbes et al. 2015).
Only a few previous studies utilised lichen carbon (C) and/or sulfur (S) contents in lichen biomonitoring studies (e.g., Beck and Mayr 2012; Carreras and Pignata 2002; Vingiani et al. 2004). However, numerous studies have used lichens as monitors for nitrogen (N) compounds in (urban) environments, including assessment of the effects of nitrogen pollution on lichen species abundance and distribution patterns (e.g., Bermejo-Orduna et al. 2014; Boltersdorf and Werner 2013, 2014; Gerdol et al. 2014; Pinho et al. 2017). Moreover, lichen nitrogen contents can reflect airborne nitrogen loads, including from anthropogenic impacts (Boltersdorf et al. 2014; Frati et al. 2006; Gadsdon et al. 2010), indicating their suitability for urban air quality studies. In contrast, lichen stable-isotope compositions have not been used to the same extent in air pollution studies, compared to soil, groundwater, precipitation and moss samples (Cape et al. 2004; Widory 2007). A small number of prior studies have investigated the use of lichen stable-isotope-ratio signatures to characterise anthropogenic sources of airborne carbon, nitrogen and sulfur compounds in urban (i.e., Ruhr-Area Germany, Mexico City, Mexico, and Sydney, Australia) and remote/rural (i.e., Antarctic, Germany and China) environments (Batts et al. 2004; Boltersdorf et al. 2014; Boltersdorf and Werner 2013; Lee et al. 2009; López-Veneroni 2009; Wadleigh 2003; Wadleigh and Blake 1999; Wiseman and Wadleigh 2002; Xu et al. 2021).
This study is a first application of a high-spatial resolution lichen passive biomonitoring approach, using lichen species Xanthoria parietina and Physcia spp. in the City of Manchester (UK). The primary aim was: (i) to assess spatial variability of air quality within a large-scale urban environment, Manchester (UK), using lichen carbon, nitrogen and sulfur contents (wt%), combined with their stable-isotope-ratio signatures (expressed as δ13C, δ15N and δ34S values). The latter were used to: (ii) potentially identify major occurrences, and assess potential sources, of airborne pollution across the centre of Manchester. To further pinpoint potential pollution sources: (iii) lichen wt% N and δ15N were combined with previously published lichen nitrate (NO3−) and ammonium (NH4+) contents. Lichen samples collected from a rural setting also have been used to compare contrasting environments experiencing different levels and types of airborne pollution.
Materials and methods
Study area – the City of Manchester (UK)
The urban conurbation of Greater Manchester, located in northwest England (53.4808°N, 2.2426°W), is the third largest UK urban agglomeration (ONS 2021). The City of Manchester (hereafter Manchester), situated at the centre of this conurbation, covering an area ca. 11,500 hectares, with a population of about 566,000 in 2018 (Manchester City Council 2019). It is located in the northwest of England, where climatic conditions can vary greatly and include the wettest and coldest place in England (Met Office 2015). The annual average temperatures in Greater Manchester for the period 1991–2020 (recorded at Rochdale climate station; Lat.: 53.6, Long: -2.183; Met Office 2022) were 6.09 °C (minimum.) and 13.13 °C (max.), with February being the coldest months (average min. temperature 1.41 °C) and July being the warmest (average max. temperature 20.01 °C). The annual average sunshine (hours) and rainfall (mm) were 1265.48 h and 1197.22 mm, respectively (Met Office 2022). Climatic data for the sampling period of this study, between 2016 and 2018, are displayed in Fig. S1.
Public health problems in Manchester that are closely linked to poor air quality particularly relate to premature deaths due to cardiovascular diseases, cancer and respiratory diseases, these being the highest in England compared to the English national average (Manchester City Council 2019; Regan 2018). For instance, childhood hospital admissions for asthma (1st rank in England) and emergency Chronic Obstructive Pulmonary Disease (COPD) hospital admissions over twice the UK national rate illustrate major public health issues within Manchester (Regan 2018). According to EU/UK legislation, the City of Manchester is recording atmospheric pollutants within its central parts, using two automated air-quality monitoring stations, situated at Piccadilly Gardens (Latitude: 53.481520, Longitude: -2.237881, urban centre location; Fig. 1) and on Oxford Road (Latitude 53.472077, Longitude -2.239001, urban traffic location; Fig. 1). However, exceedances of the EU/UK NO2 regulatory value (annual average of 40 µg m−3) are recorded at both monitoring stations, with Oxford Road air quality monitoring station notably exceeding the annual limit value between January 2010 and January 2019 (Fig. S2 a and b). In particular, Manchester Oxford Road is one of the busiest bus routes in Europe and the highest NO2 distributions within Manchester are mainly associated with arterial roads leading into the city centre (Manchester City Council 2016; Martin et al. 2011; Regan 2018). Comparably, Niepsch et al. (2021a) reported elevated NO2 concentrations (measured by passive diffusion tubes) across Manchester’s city centre and along its major road network. Although providing continuous data, the two automated air quality monitoring stations do not provide information on the wider spatial distribution of airborne pollutants. This key limitation requires alternative measurement options to evaluate air quality in greater spatial detail; i.e., the application of a lichen biomonitoring approach to provide further insights into atmospheric pollution variability across the larger urban environment.
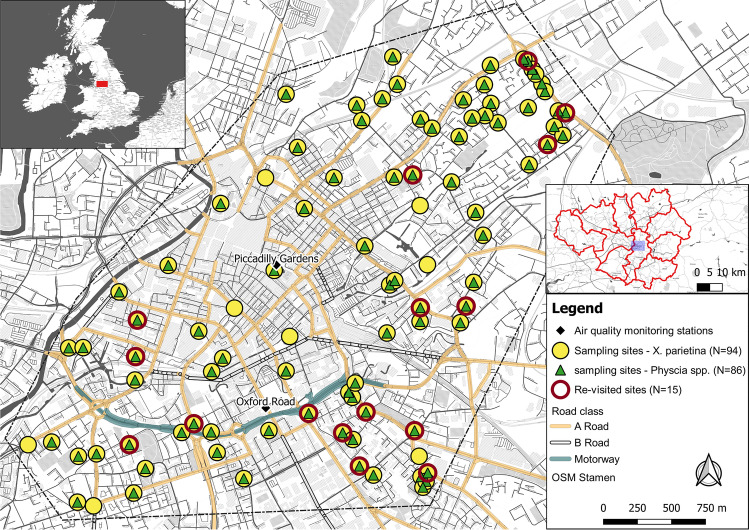
Fig. 1.
Lichen sampling sites (X. parietina: yellow circle; Physcia spp.: green triangle; re-sampled sites in 2018: red outline) distributed across Manchester city centre (research area displayed as dotted line; NE-SW transect). Two automated air-quality monitoring stations (labelled black diamonds) and major road classes also are shown
The research area represents a SW–NE transect across central Manchester (Fig. 1) and includes different land-use types; e.g., town centre and retail, residential and industrial areas, as well as open green spaces (parks). The city centre is characterised by high buildings, high density and slow traffic, including public transport (buses, trams and trains) particularly during peak hours (AM and PM peak; Highway Forecasting and Analytical Services 2015). These characteristics suggest vehicular emissions as a major cause of poor air quality and air pollution, which may subsequently impact on the Manchester population health.
Lichen sampling and preparation
The epiphytic, i.e. growing on plant surfaces, lichen species Xanthoria parietina and two Physcia spp. (Physcia adscendens and Physcia tenella) were used in this study; the latter was combined into a single sample (per location), because both look similar, making it difficult to differentiate younger specimens and were both used because they were often found together or are grown into each other; Dobson 2011; Kirschbaum and Wirth 2010). The lichen species used are considered ‘nitrophytes’; being able to tolerate and withstand high concentrations of nitrogenous airborne pollutants. For this reason they are abundant in different environments, e.g. urban and rural areas, making these lichens suitable for large spatial scale air quality biomonitoring studies (Dobson 2011; Kirschbaum and Wirth 2010).
Urban X. parietina (N = 94; Fig. 1) and Physcia spp. (N = 86; Fig. 1) lichens were sampled from street trees (e.g., Acer spp., Tilia spp. and Fraxinus spp.) situated across Manchester (Fig. 1), between the end of June 2016 and the end of October 2017. Lichen material was obtained from small twigs and branches using a tree pruner (at heights between 2–4 m) from the cardinal direction facing the major road. Vertical NH3 profiles vary with height in urban environments (Zhang et al. 2018), which may impact on lichen stable isotope ratio signatures. However, the profile is more pronounced during night-time compared to daytime (Erisman et al. 1988), when lichens were sampled in this study (i.e. during dry days). Further, vertical pollutant gradients were out of scope for this study.
Depending on lichen cover (on twigs and small branches), one or more cardinal direction (clockwise rotation) of a street tree was sampled and merged into a single sample. Twigs and small branches were targeted for collection to facilitate sampling of younger lichen samples for assessment of more recent air quality. It was not possible to sample only one tree species, due to the low tree cover within the city centre (< 10%; City of Trees 2018), tree availability (i.e., estimated 9.7% street trees in city tree cover, Red Rose Forest 2008) and accessibility and the diversity of ornamental and planted (younger) trees across Manchester (City of Trees 2018). However, tree species with similar bark substrate acidity (Kirschbaum and Wirth 2010) were sampled for lichens. It needs to be stated that environmental factors (i.e. light availability and illumination, precipitation, humidity, bark age, corrugation and acidity) are important factors for lichen community development (Broad 1989; Forbes et al. 2015; Shukla et al. 2014). However, less acidic conditions are generally found in the upper, younger parts of a tree (Broad 1989) where the presence of nitrophytic lichen species on twigs and branches (e.g. X. parietina and Physcia spp.) suggests the presence of atmospheric nitrogen compounds and similar environmental conditions (e.g., eutrophication) across Manchester. Hence, impacting on lichen colonisation and succession favouring the growth of nitrogen-preferring species such as X. parietina (Larsen Vilsholm et al. 2009).
Collected twigs with lichens were stored in paper bags and returned to the laboratory; lichen material then was carefully scraped off the bark using a stainless-steel scalpel and non-lichen detritus was removed under an illuminated magnifying glass. Dry lichen material was ground and homogenised into powder using an agate pestle and mortar and stored in glass vials (away from chemicals and in the dark at room temperature [20°]) until chemical analyses. A second phase of urban lichen sampling was undertaken in 2018 (revisiting the same trees; N = 15; red circles in Fig. 1) to assess the scale of any temporal variability of carbon, nitrogen and sulfur (CNS) contents (%wt) in urban lichen samples, since evidence for temporal variability in lichen chemistry will impact the analysis and interpretation of spatial variability of air quality.
To evaluate whether urban lichen %wt CNS and stable-isotope-ratio signatures are diagnostic for urban environments, X. parietina lichens also were sampled from around a poultry farm in a rural setting (N = 12; Fig. S3). Rural lichens were sampled in close proximity (between 50 to 500 m) and on a general south-west transect away from the farm (between 1 and 3 km), influenced by site accessibility, i.e. fenced and hedged agricultural fields and private property. These lichens were sampled from oak (Quercus spp.) and hawthorn (Crataegus spp.) trees, using the same procedure as undertaken in the urban environment. In contrast to the urban environment, X. parietina was sampled from trees with potentially different physico-chemical bark properties. However, Quercus and Crataegus spp. was primarily sampled in close proximity to the poultry farm, being generally completely covered with lichens, suggesting a surplus of atmospheric nitrogen. For instance, ammonia (NH3) emissions in rural environments are primarily derived from animal waste and chemical fertiliser, whereas NH3 (and NOx) in urban environments is predominantly emitted by vehicular sources (Bishop and Stedman 2015; Cape et al. 2004; NAEI 2016; Stritzke et al. 2015; Sun et al. 2017). The lichen X. parietina flourishes in such nitrogen-rich habitats, e.g. areas high in NOx and NHx compounds, and is found ubiquitously in urban environments and in proximity to domestic livestock due to increases atmospheric nitrogen compounds (Gaio-Oliveira et al. 2004; Pinho et al. 2009; Sparrius 2007; Van Herk 2003; Van Herk et al. 2003), subsequently allowing for robust comparison of lichen chemistry between rural and urban environments.
Lichen carbon, nitrogen and sulfur contents
Lichen total carbon and total nitrogen contents, expressed as percentage by weight (wt%), were determined using a Leco TruSpec®’ CN analyser. Lichen sulfur contents (wt% S) were simultaneously determined with stable-isotope-ratios, following the procedure described in “Lichen stable-isotope-ratio ratios (δ13C, δ15N and δ34S values)”.
About 0.15 g of each sample was weighed into a tin foil and CN analyser, and calibration was undertaken using the standard Ethylenediaminetetra-acetic acid (LECO®; EDTA 502–092). Rice flour (LECO® reference material; N = 32) and Certified Reference Material (CRM) No. 482 (BCR – Trace elements in lichen Pseudevernia furfuracea, sample identification No. 594; N = 31) were used to quantify accuracy and precision of measurements, as well as assess batch-to-batch variability and any experimental bias between the five different sets of samples. Analytical accuracy (± precision, as coefficient of variation—%CV; Table S1) for the lichen CRM was 102% (± 2.19%CV) for wt% N and 99% (± 1.25%CV) for wt% C (Quevauviller et al. 1996). LECO Rice Flour accuracy (± precision, as %CV) was at 88% (± 3.85%CV) for wt% N and 92% (± 1.00%CV) wt% C. Batch-to-batch corrections were not undertaken, because of good repeatability (< 5%CV) for wt% C and wt% N between each analytical batch. Lichen CRM wt% S (by IRMS; “Lichen stable-isotope-ratio ratios (δ13C, δ15N and δ34S values)”) accuracy was 85% (± 13.5%CV). Sulfur content in the lichen CRM is reported as an indicative value (2166 ± 292 mg kg−1; Quevauviller et al. 1996) a possible explanation for the less accurate IRMS analyses; batch-to-batch correction also were not undertaken for wt% S.
Lichen stable-isotope-ratio ratios (δ13C, δ15N and δ34S values)
Ca. 7 mg lichen samples were weighed into tin capsules (6 × 4 mm), handled and closed with stainless steel tweezers and stored in 1.5 mL centrifuge tubes until analysis. Lichen stable-isotope-ratios were analysed by Continuous-Flow Isotope Ratio Mass Spectrometry (CF-IRMS) using an Elementar Pyrocube elemental analyser (EA) interfaced with a VisION isotope ratio mass spectrometer (IRMS) at the Scottish Universities Environmental Research Centre (SUERC), East Kilbride.
Stable-isotope ratios are expressed as ‘delta’ (δ) values, denoting measurement of isotope ratio relative to an accepted international reference frame (Eq. 1; Fry 2006; Sharp 2017), where:
| 1 |
Delta (δ) values are small numbers and are reported in per mille (‰), or parts per thousand (10–3; Coplen 2011), with y being the heavy isotope of element X (e.g., 13C) and R the ratio of abundance of the heavy to light isotope, for the sample (Rsample) and the standard (Rstandard). In this study R is either 13C/12C (δ13C value), 15N/14N (δ15N value) or 34S/32S (δ34S value). A positive δ-value means that the ratio of the heavy to light isotope is higher in the sample compared to the standard, and the opposite for negative δ-values.
Recognised international reference standards were analysed alongside the lichen samples: USGS40 (glutamic acid; N = 8) for δ13C and δ15N, and silver sulphides (IAEA S2, N = 11, and S3, N = 11) for δ34S. Internal laboratory standards, MSAG2 (methanesulfonamide/gelatin; N = 121), M2 (methionine, gelatine, glycine and 15N-enriched alanine; N = 78) were used to monitor drift in δ15N, δ13C and δ34S values through time for each experimental batch (N = 3). IRMS accuracy varied between 98 and 102% for analysed reference standards (Table S2) and analytical precision for reference and internal standards was < 5%CV. Isotopic composition data are not available for lichen CRM BCR No. 482, but this reference material was measured throughout each analytical batch (N = 3) to check for potential analytical biases. Analytical precision (%CV) of repeated lichen CRM measurements (N = 43) was 2.4% (δ13C), 2.9% (δ15N) and 11% (δ34S), respectively. Batch-to-batch correction for lichen δ-values was not undertaken due to good repeatability (< 5%CV) for reference and internal standards across all measurements.
Statistical and geospatial data analysis
Statistical analysis was completed using GraphPad Prism 7 and Minitab 17 statistical software (GraphPad Software Inc. 2018; Minitab 2019). Data visualisation was undertaken using Origin 2019 (OriginLab 2018) and R Studio with “ggplot2” package (RStudio Team 2021; Wickham 2016). Graphical visualisation was undertaken using QGIS 3.22.3 (QGIS Development Team 2022). Shapiro–Wilk test statistics were used to assess whether lichen CNS contents (wt%) and stable-isotope-ratios (δ-values) were normally distributed and inform subsequent statistical analysis, due to its strong statistical power regardless of data distribution and sample size (Razali and Wah 2011). Mann–Whitney tests (non-parametric; wt% C, δ13C and δ34S) or unpaired t-tests (wt% N, wt% S, δ15N) then were used to compare lichen species (X. parietina and Physcia spp.) and wt% CNS and stable-isotope ratio datasets.
To investigate relationships between lichen-derived datasets and potential urban influencing factors, i.e. distance to major road, major road class, traffic counts, surrounding building heights and distance to green spaces, correlation statistics Pearson’s r (parametric) or Spearman ρ (non-parametric) were used, depending on the outcome of normal distribution of the dataset. Data sources for urban influencing factors and classification/grouping justifications are displayed in Table S3. These urban characteristics were used because they might impact on lichen pollutant loadings, by influencing dispersion, dilution and distribution of atmospheric pollutants. Additionally, urban influencing factors were mapped using an open-source machine learning interpolation method, Smart-Map plugin for QGIS (Pereira et al. 2022). The support vector machine (SVM) method can be used for regression and classification of small and large volumes of data (Karamizadeh et al. 2014; Pereira et al. 2022; Zhou et al. 2016). Urban factors (Table S3) were used as covariates for lichen chemical data in the SVM model, as well as X and Y-coordinates, (British National Grid), the value of the lichen data itself (i.e. CNS %wt and stable-isotope ratio signatures). Lichen values for CNS %wt and δ13C, δ15N and δ34S were and interpolated using Inverse Distance Weighting (IDW) withing the outlined research area (Fig. 2). Default IDW parameters were used, as described in Pereira et al. (2022), with a weighing value (p) as 1.00 for all covariables (i.e. urban factors), search radius equalling maximum distance between sampled points and number of neighbours set to 16. Moran’s Index (Moran’s I) was used for spatial correlation of regionalized variables, with univariate Moran’s I measuring the autocorrelation of the variable to be interpolated (i.e. lichen CNS wt% and stable isotope ratio signatures) and bivariate Moran’s I to measure spatial autocorrelation between covariates, i.e. Manchester’s urban factors. If the value is close or equal to zero, it means that the variable does not show spatial autocorrelation (Pereira et al. 2022). Moran’s I values closer to -1 or + 1 indicate greater spatial correlation of the variable (Pereira et al. 2022).
Fig. 2.
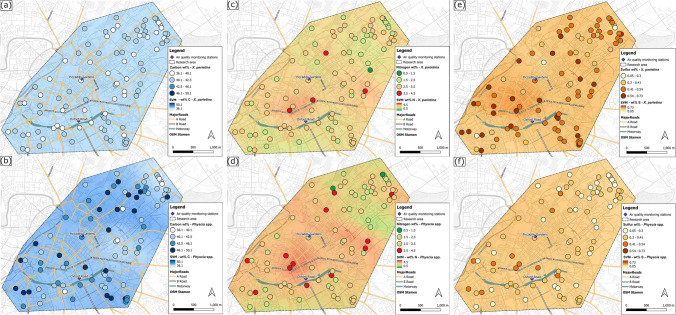
Lichen carbon [(a) and (b)], nitrogen [(c) and (d)] and sulfur contents [(e) and (f)] (wt%) in X. parietina and Physcia spp. colour-coded (low to high) across Manchester; displayed with automated air quality monitoring stations and major road network (A- and B-roads and motorway); and SVM interpolated values (same colouring and range) including Manchester’s urban factors
Results and discussion
Temporal variability of lichen CNS contents and impacts on assessment of spatial variability of lichen chemistry
Only carbon contents (wt% C) in repeat samples of X. parietina lichens (N = 18) were found to be significantly (p < 0.01) different, with elevated contents for samples collected in 2018, compared to 2016/17, whereas wt% N (p = 0.13) and wt% S (p = 0.53) were not significantly different (Fig. S4). The lack of any statistically significant differences for wt% N and wt% S suggest that lichen nitrogen and sulfur contents can be assumed to be reliable indicators of spatial variability of atmospheric nitrogen and sulfur compound concentrations, e.g., NO2 and SO2 did not vary significantly over this study’s period, and lichen wt% N and wt% S can reflect “localised” air quality. Albeit, elevated levels of NO2, including exceedances of the regulatory limit value (40 µg m−3), were recorded at both Manchester air quality monitoring stations, particularly during colder months (e.g. November 2016 to March 2017, Fig. S2; DEFRA 2018a, 2018b), such seasonal variation has not (significantly) impacted on lichen wt N% in this study. In contrast, consistent SO2 concentrations between 1.0–3.1 µg m−3 were recorded at Piccadilly Gardens between 2016 and 2018 (DEFRA 2018b). Indeed, SO2 concentrations in the UK have decreased by 96% since 1990 (NAEI 2018b) and no limit value exceedances, i.e. the WHO recommended daily mean value of 20 µg m−3 (WHO 2005, 2000) or EU limit value of 125 µg m−3 (daily mean; EU 2008), were recorded for Manchester (GMCA 2018). Therefore, lichen wt% S suggest localised impacts by atmospheric sulfur compounds, e.g. from domestic combustion, industrial manufacturing and energy production and transformation (DEFRA 2022) in Manchester.
On the contrary, no data for carbon compounds (e.g., CO and CO2) is available for Manchester’s automated air quality stations (for this study’s period). However, carbon (i.e., elemental and organic) is a major component of particulate matter (i.e. PM10) in urban centres across the UK, derived from incomplete combustion of organic material and vehicle tyre wear (Harrison et al. 2004; Jones and Harrison 2005; Rogge et al. 1993). In this study, lichen samples were not washed prior to analysis and increased wt% C could be due to increased number of entrapped particulates on the lichen surface, when re-sampling was undertaken in 2018. However, differences in lichen wt% C also could be related to microclimatic conditions, bark pH and lichen species-specific compounds, such as genus-specific sugar alcohols that are used by the mycobiont (Beck and Mayr 2012; Gaio-Oliveira et al. 2005a; Hauck 2010; Máguas et al. 2013). Hence, increased wt% C in 2018 could be linked to fungal metabolism, induced by higher availability of nutrients, in particular nitrogen and sulfur (Gaio-Oliveira et al. 2005a). Investigating lichen chlorophyll contents, amino acids and secondary lichen products (of fungal origin; Ranković and Kosanić, 2019) could provide further insights into the causes of increased carbon contents and potentially explain better the observed temporal variability (Gaio-Oliveira et al. 2005a; Hauck 2010).
The lichen wt% N and wt% S content results suggest a potential ‘equilibrium’ (i.e. balance) state of X. parietina with its close surrounding environment (Paoli et al. 2018); i.e. reflecting the local atmospheric conditions at the sampled locations. Nonetheless, seasonal variability of atmospheric pollutants has been reported for urban environments in Europe and the UK (Hernández-Paniagua et al. 2015; Masiol et al. 2017; Vardoulakis et al. 2011), which is also recorded by automated air quality measurements in Manchester (Fig. S2). The results of this study suggest that lichen carbon content at individual locations can vary over multiple-year timescales, in response to temporally variable air quality, and hence cannot be linked to atmospheric carbon composition, and hence air quality, exclusively. In contrast, wt% N and wt% S do not vary over such timescales, due to likely non-significant changes in pollutant concentrations (at least in Manchester) and thus can be considered robust measurements to assess spatial variability in urban air quality.
Spatial variability of carbon, nitrogen and sulfur contents in urban lichens (X. parietina and Physcia spp.) to evaluate potential pollution sources
Lichen CNS contents were found spatially variable across Manchester (Fig. 2), with higher variability in wt % N (Fig. 2 c and d) and S wt% (Fig. 2 e and f), compared to wt% C (Fig. 2 a and b), in both lichen species. Individual lichen data for sampling locations are displayed in the supplementary material (Table S4 and Fig. S5).
Besides spatial variability, species-specific differences in recorded CNS wt% (Fig. S6) were recorded, suggesting potential different suitability of X. parietina (N = 94) and Physcia spp. (N = 87) lichens for (urban) biomonitoring approaches. Such species-specific differences between X. parietina and Physcia spp. (Fig. S6) could be related to different assimilation abilities of nutrients, e.g. N and S compounds, and subsequent differences in biomass increases and net CO2 gain (Dahlman et al. 2003; Johansson et al. 2010). However, 31% of CO2 in Manchester is emitted by road traffic (TfGM and GMCA 2016), which could be related to increased wt% C in Physcia spp. at some locations (Fig. 2 b). In contrast, temporal variability was recorded in X. parietina (“Temporal variability of lichen CNS contents and impacts on assessment of spatial variability of lichen chemistry”), suggesting a potential bias on lichen wt% C variability. Consequently, lichen wt% C data do not provide a beneficial tool for assessment of spatial variability of airborne carbon compounds in an urban environment.
Urban environments are complex systems, comprising rough surfaces that influences the aerodynamic properties of the atmosphere, which are critical for air pollutant deposition and dispersion (Cariolet et al. 2018; Grimmond and Oke 1999). Lichen CNS contents (and stable-isotope ratio signatures; “Spatial variability of lichen stable-isotope-ratio signatures and assessment of pollution source apportionment in the Manchester urban environment”) were considered in relation to different potential urban influencing factors that may impact on dispersion and distribution of pollutants (i.e., major road distances and surrounding building heights), and thus allowing investigation of air quality at the sampling location in finer detail. Correlation statistics for lichen data and potential urban influences (as described in Table S3) are displayed in Table 1. Table 2 illustrates the spatial autocorrelation (Moran’s I) between the lichen dataset and potential urban influencing factors across Manchester.
Table 1.
Correlation statistics (Pearson's r and Spearman ρ) used to investigate relationships between urban influencing factors and lichen-derived CNS contents (wt%) and stable-isotope-ratio signatures (δ13C, δ15N and δ34S). Underlined values represent Spearman ρ, all other values are presented as Pearson’s r, significant values are presented in bold (significance level is indicated by asterisk; *significant at the level p < 0.05 and **significant at the level p < 0.01); MR = distance to major road, RdCl = road class, TC = traffic counts, BH = surrounding building height, GS = distance to greenspace
| MR | RdCl | TC | BH | GS | |
|---|---|---|---|---|---|
| X. parietina | |||||
| wt% C | 0.11 | 0.01 | -0.12 | -0.17 | -0.07 |
| wt% N | -0.21* | 0.19 | 0.07 | 0.30** | 0.17 |
| wt% S | -0.13 | 0.12 | 0.11 | 0.30** | 0.12 |
| δ13C | 0.22* | -0.26* | -0.07 | -0.20 | -0.21* |
| δ15N | -0.28** | -0.34** | 0.22* | 0.25* | 0.20 |
| δ34S | 0.15 | -0.01 | -0.14 | -0.21* | -0.08 |
| Physcia spp. | |||||
| C wt% | -0.26 | 0.00 | -0.11 | -0.02 | 0.00 |
| N wt% | -0.14 | 0.07 | 0.24* | 0.16 | 0.16 |
| S wt% | -0.19 | 0.07 | 0.25* | 0.16 | 0.16 |
| δ13C | 0.20 | -0.11 | -0.13 | -0.08 | -0.15 |
| δ15N | -0.26* | 0.21 | -0.26* | 0.09 | 0.27* |
| δ34S | 0.22* | 0.04 | -0.29** | -0.21 | -0.10 |
Table 2.
Moran’s I (ranging from -1 ≤ to ≤ + 1) to evaluate spatial autocorrelation (i.e. how similar objects are to others surrounding it) between urban influencing factors and lichen-derived CNS contents (wt%) and stable-isotope-ratio signatures (δ13C, δ15N and δ34S); significant values are presented in bold (significance level is indicated by asterisk: *significant at the level p < 0.05); MR = distance to major road, RdCl = road class, TC = traffic counts, BH = surrounding building height, GS = distance to greenspace
| MR | RdCl | TC | BH | GS | |
|---|---|---|---|---|---|
| X. parietina | |||||
| wt% C | 0.07 | -0.07 | -0.09* | -0.08* | -0.10* |
| wt% N | -0.12* | 0.08 | 0.09* | 0.22* | 0.15* |
| wt% S | -0.07 | 0.05 | 0.10* | 0.22* | 0.10* |
| δ13C | 0.09 | -0.06 | -0.13* | -0.17* | -0.16* |
| δ15N | -0.11* | 0.09* | 0.19* | 0.18* | 0.18* |
| δ34S | 0.09 | -0.05 | -0.11* | -0.20* | -0.68 |
| Physcia spp. | |||||
| C wt% | 0.06 | -0.35 | -0.02 | -0.02 | -0.02 |
| N wt% | -0.10* | 0.05 | 0.17* | 0.14* | 0.16* |
| S wt% | -0.10* | 0.05 | 0.17* | 0.16* | 0.16* |
| δ13C | 0.04 | 0.04 | -0.10* | -0.16* | -0.13* |
| δ15N | -0.11* | 0.07 | 0.20* | 0.06 | 0.22* |
| δ34S | 0.13* | -0.08 | -0.17* | -0.23* | -0.08 |
Recorded X. parietina and Physcia spp. carbon contents from the Manchester urban environment are comparable to results presented for Naples, Italy (Vingiani et al. 2004) and interestingly, also a remote Antarctic location (King George Island; Lee et al. 2009). In this study, elevated wt% C were recorded for Physcia spp. within the city centre area and along the major road network (Fig. 2 b), whereas such a pattern was not visible for X. parietina (Fig. 2 a). Carbon wt% in X. parietina and Physcia spp. showed no significant correlation (Spearman ρ, p > 0.05) with Manchester’s urban factors (Table 1), whereas significant spatial correlation (p < 0.05) in X. parietina wt% C was recorded with urban factors (Table 2), indicating spatially dispersed data with potential site-specific influences, e.g. from traffic counts and surrounding building heights and aforementioned sampling location characteristics (i.e. tree bark).
The variability of nitrogen contents in X. parietina sampled from across Manchester was primarily influenced by the sampling locations distance to a major road (A-, B- roads and motorway; p < 0.05; Table 1, Fig. S7) and surrounding building height (p < 0.01; Table 1, Fig. S7). In contrast, no significant relationships for these variables were found for Physcia spp., whereas traffic counts were positively correlated (p < 0.05) with wt% N. Consequently, elevated traffic at sampling locations suggests impacts by atmospheric nitrogen compounds, e.g. from vehicular sources (NOx and NHx). For instance, within Manchester 80% of NOx emissions are related to vehicular emissions, which have previously been reported to impact on lichen wt% N (Bermejo-Orduna et al. 2014; Gombert et al. 2003; Regan 2018). Moreover, Niepsch et al. (2021a) reported the results of a detailed diffusion-tube monitoring study and elevated NO2 concentrations within Manchester’s city centre and along its major road network. Measured diffusion tube NO2 concentrations were significantly positive correlated with wt% N (Pearson’s r = 0.34, p < 0.05), with higher wt% N where atmospheric NO2 concentrations where elevated.
Major roads, e.g. motorways and A roads, are often the main arteries within urban environments and usually have high traffic flows (DfT 2017) and it is well known that pollutants, such as vehicular NO2, decrease rapidly with distance from a major road/source and that densely built-up urban centres with larger and wider buildings impact on pollutant distribution and dispersion (Cyrys et al. 2012; Kubota et al. 2008; Kurppa et al. 2018). In contrast, vegetated green spaces and sparsely built-up areas can have a positive impact on urban air quality (Britter and Hanna 2003; Janhäll 2015; Salmond et al. 2013) that could explain some of the recorded differences in lichen wt% N across Manchester.
Moreover, lichen wt% N showed significant (p < 0.05, Table 2) spatial correlation with urban factors, i.e. distance to highly-trafficked roads (MR and TC), distance to greenspace (GS) and surrounding building heights (BH). Hence, displaying clusters (e.g. the city centre area; Fig. 2) of elevated N wt% being likely influenced by local sources (e.g. vehicular emissions) and poor dispersion of air pollutants within the densely built-up city centre area. Comparable results were reported for lichen biomonitoring studies (incorporating X. parietina and/or Physcia spp.) undertaken in urban, agricultural and industrial regions of Europe; e.g., in the Ruhr-Area and South Germany (Beck and Mayr 2012; Boltersdorf and Werner 2013; Franzen-Reuter 2004), Grenoble, France (Gombert et al. 2003) and the Alentejo Litoral region, Portugal (Pinho et al. 2017).
Nonetheless, urban airborne nitrogen compounds comprise different nitrogen compounds (e.g., nitrate, ammonia and ammonium – NOx and NHx) that presumably can affect nitrogen deposition (wet and dry; Van Herk 2003) and consequently lichen wt% N. Albeit, lichen total wt% N can reflect airborne nitrogen loads, such an approach cannot distinguish between different nitrogen compounds, i.e. nitrate (NO3−) and ammonium (NH4+). Accordingly, different nitrate (NO3−) and ammonium (NH4+) concentrations have been reported in X. parietina samples across Manchester (Niepsch et al. 2021b), suggesting site-specific and diverse sources; e.g., from vehicular, domestic and industrial emissions that potentially impacted on lichen wt% N recorded by this study (further discussed in “Lichen nitrogen stable-isotope ratios (δ15N)”; Fig. 8). Nonetheless, lichen nitrogen contents can provide beneficial information on atmospheric nitrogen compounds across an urban environment, and thus identify potential hotspots of poor air quality and associated deleterious negative health impacts, certainly not distinguishing between potential sources.
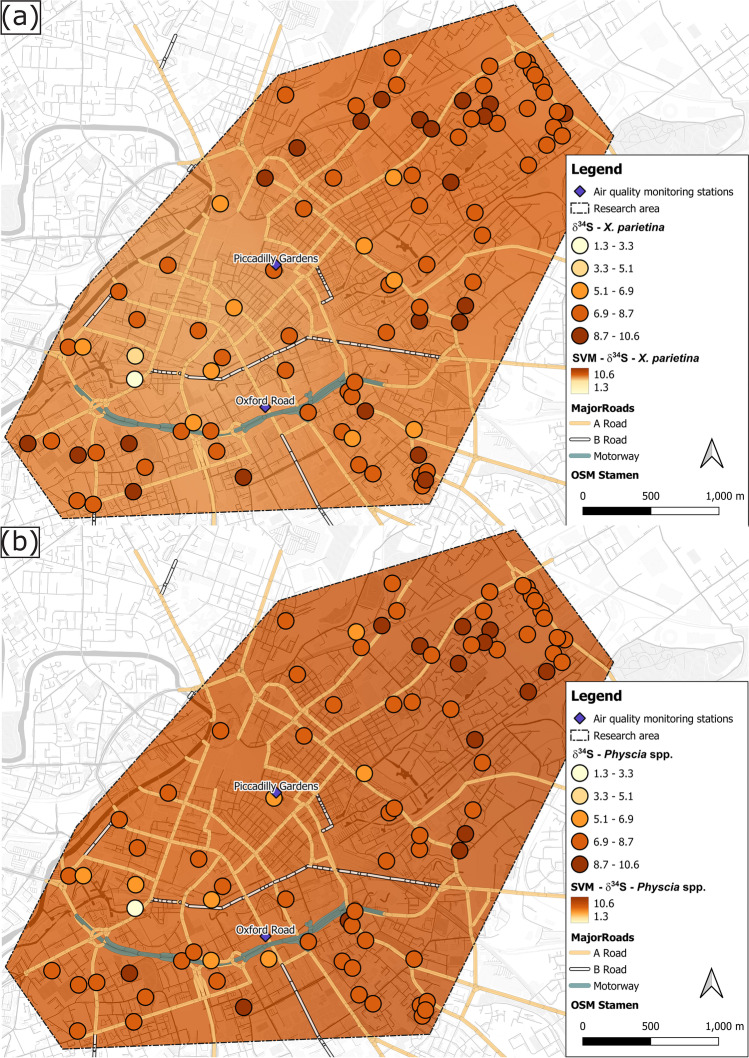
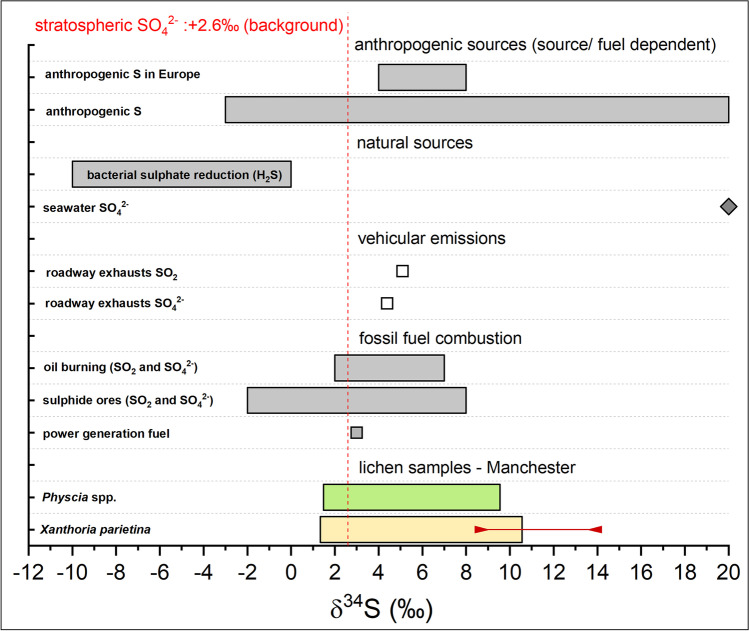
Fig. 8.
δ34S values (‰) of (a) X. parietina and (b) Physcia spp. colour-coded by 34S enrichment (more positive values) across the City of Manchester; displayed with automated monitoring stations and major road network (A- and B-road and motorway); displayed with SVM interpolated values (same colouring and range) including Manchester’s urban factors
For both lichen species, wt% S was elevated within the city centre, whereas lower contents were recorded in more residential areas (northeast and southwest of the research area; Fig. 2 d and e). However, wt% S in both lichen species was spatially correlated with urban factors (TC, BH and GS; Table 2) illustrating the clustering of (similar) data. For example, elevated wt% S within the city centre area (in X. parietina, Fig. 2 e) could be related to sulphate-containing particulates entrapped on the lichen surface, which derive from domestic sources (e.g. wood and coal fires and stoves) and vehicular emissions, e.g. diesel-powered vehicles (AQEG 2012; Clean Air Greater Manchester 2021; Zereini and Wiseman 2011). Moreover, SO2 is an important precursor for PM2.5 formation (AQEG 2012) and elevated wt% S in lichens could indicate areas with elevated PM2.5 concentrations, and thus poor air quality and potential human health impacts (e.g. cardiovascular and respiratory diseases; Regan 2018). Investigating the sulfur content of potential sulfur sources (e.g. petroleum coke used in the UK, NAEI 2018b) and particulate matter (e.g. PM10/2.5) would further refine understanding of the potential influences on elevated lichen wt% S.
Recorded sulfur contents in lichen species sampled across Manchester were higher compared to previous urban lichen wt% S studies, that used transplanted Usnea amblyoclada and Ramalina ecklonii from Cordoba City, Argentina (Carreras and Pignata 2002; González et al. 1996) and Alectoria sarmentosa from Newfoundland, Canada (Wadleigh 2003; Wadleigh and Blake 1999; Wiseman and Wadleigh 2002). Such differences are most likely linked to the different study designs (i.e. active and passive biomonitoring [this study]), the utilised lichen species (e.g. fruticose and foliose [this study]) and subsequent species-specific sensitivity towards atmospheric pollutants, with fruticose lichens generally being more sensitive to atmospheric pollution, compared to foliose (and crustose) lichens (Shukla et al. 2014). Moreover, sulfur is an essential lichen nutrient (Wiseman and Wadleigh 2002), such that differences observed between the X. parietina and Physcia spp. utilised in this study could be related to species-specific sensitivity to high concentrations of sulfur compounds. For instance, X. parietina is reportedly less affected by elevated ambient pollutant concentrations (Dobson 2011; Kirschbaum and Wirth 2010). Further, the geographic region and specific elevated regional pollution patterns could explain higher wt% S recorded for Manchester. For instance, SO2 pollution in Manchester is primarily linked to anthropogenic sources, i.e. industrial processes and combustion from energy industry (Manchester City Council 2018), which is comparable to results presented by lichen transplant studies, i.e. local industrial sources (Carreras and Pignata 2002; Wadleigh 2003; Wadleigh and Blake 1999).
Supplementary datasets, e.g. traffic counts on minor roads, pollutant dispersion and distribution from large point sources and interception by urban vegetation (e.g. uptake of gaseous, aerosol and particulate pollutants; Freer-Smith et al. 1997; Gaston et al. 2010) are scarce. Data classification used in this study were based on available (limited) datasets and grouping justification was based on urban air quality and pollution studies reporting the decline of pollutants (e.g. NO2 within 200 m of highly-trafficked roads: Bermejo-Orduna et al. 2014; Gombert et al. 2003; Laffray et al. 2010) and impact of urban density on dispersion and distribution of pollutants (Table S3). Nonetheless, they were considered useful to investigate the distribution (e.g. from major roads and road traffic), dispersion (e.g. building heights) and spatial variability of pollutants within the urban environment of Manchester and used for geospatial interpolation (Fig. 2). However, due to the complexity of urban environments and additional meteorological influences, such as temperature and rainfall, atmospheric processes and other atmospheric pollutants as well as sampling location-specific influences (e.g. bark pH), lichen wt% CNS also could be related to those additional factors and not just to vehicle exhaust emissions. These factors should be included in future studies to assess spatial variability of air quality in more depth and to also potentially to help pinpoint pollution sources. Overall, lichen wt% N and wt% S have been shown to have been impacted by their site-specific surroundings using an IDW-based machine learning algorithm (SVM, Pereira et al. 2022; Table 2 and Fig. 2).
In conclusion, elevated lichen wt% N and wt% S can be used to document spatial variability (i) and to identify locations with deteriorated air quality (for N and S compounds) and thus identify areas of human health concern that require ameliorative actions. Although, these data alone cannot be used to identify potential airborne pollution sources, whereas lichen stable-isotope-ratio signatures (δ13C, δ15N and δ34S values) have potential utility for source apportionment. It is further suggested to utilise the same lichen species across a sampled area, specifically when the aim is to compare (different) environments, because of the species-specific differences in lichen CNS contents identified by this study (that used foliose lichen X. parietina and Physcia spp.; Fig. S6 a-c). For instance, X. parietina is a good choice for biomonitoring or urban air quality, because of its wide distribution and ability to thrive in nitrogen-rich environments (Olsen et al. 2010; Pinho et al. 2008).
Spatial variability of lichen stable-isotope-ratio signatures and assessment of pollution source apportionment in the Manchester urban environment
Lichen carbon stable-isotope ratios (δ13C)
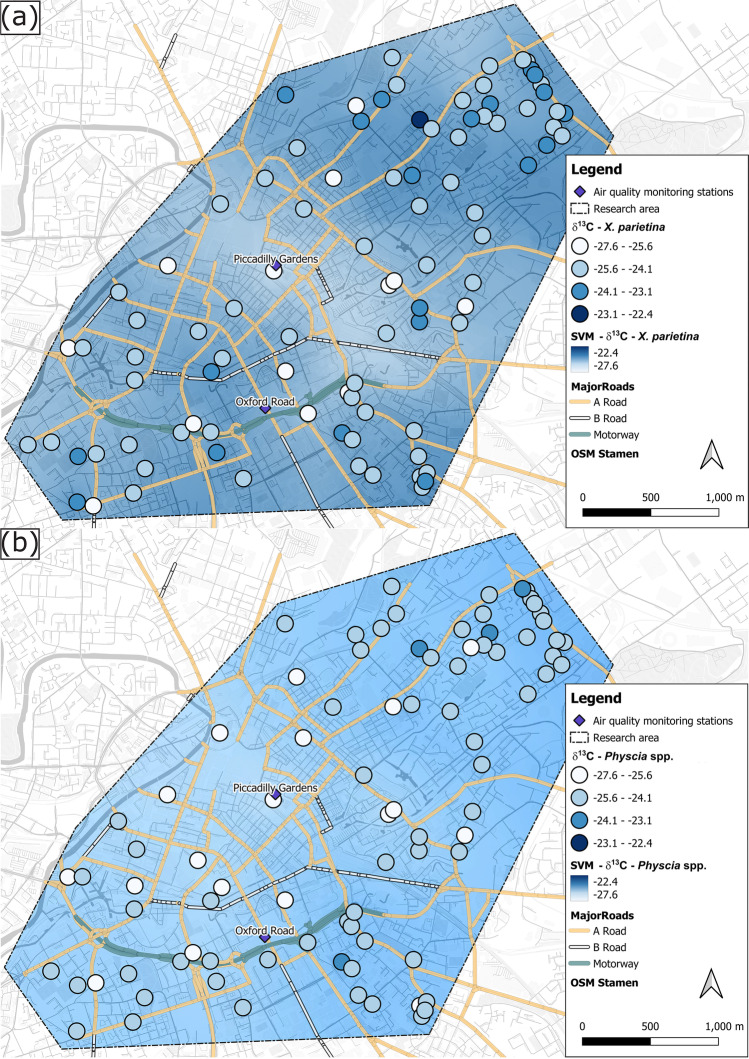
Lichen stable carbon-isotope ratio signatures were found to be variable across Manchester, with more variable δ13C-values in X. parietina (-26.7 to -22.4‰; Fig. 3 a) compared to Physcia spp. (-27.6 to -23.6‰; Fig. 3 b). A significant (p < 0.05) negative relationship between wt% C and δ13C for Physcia spp. (Fig. 1; Table S6) was recorded, whereas no significant relationship was found for X. parietina. Spatial clustering of lichen δ13C-values and urban factors (TC, BH and GS, p < 0.05; Fig. 3), was recorded, with enriched 13C contents in more residential surroundings and along the major road network (Fig. 3), suggesting localised influences. However, green-algal lichens, including X. parietina and Physcia spp., follow the C3 photosynthetic pathway and generally have δ13C values of -28‰ following fractionation of carbon isotopes during uptake of atmospheric CO2 (Batts et al. 2004). Cities are centres of concentrated CO2 emissions (Mitchell et al. 2018) and seasonal variations of CO2 and δ13C-CO2 have been reported in European urban environments; e.g. London (UK), Krakow (Poland), Wrocław (Poland) and Bern (Switzerland) (Górka and Lewicka-Szczebak 2013; Hernández-Paniagua et al. 2015; Pazdur et al. 2013; Sturm et al. 2006; Zimnoch et al. 2004). In this study, temporal variability was recorded for lichen wt% C whereby spatial variability of lichen δ13C values also might have been impacted by temporal variations in urban CO2 concentrations and its δ13C signature.
Fig. 3.
δ13C values (‰) of (a) X. parietina and (b) Physcia spp. colour-coded by 13C-enrichment (more positive values) across Manchester; displayed with automated monitoring stations and major road network (A, and B-road and motorway); displayed with SVM interpolated values (same colouring and range) including Manchester’s urban factors
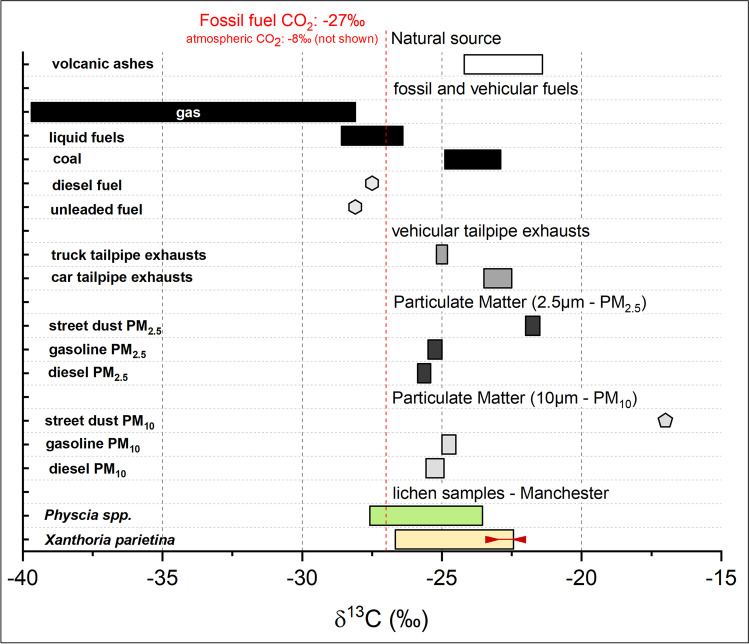
Figure 4 shows the stable-isotope compositions for different potential natural and anthropogenic carbon airborne pollution sources, against which comparisons can be made when attempting source apportionment.
Fig. 4.
δ13C values of potential atmospheric carbon pollutants and pollution sources reported in the literature (Górka et al. 2020; López-Veneroni 2009; Widory 2007, 2006) to undertake lichen δ13C value source apportionment; displayed with δ-values of lichens (X. parietina and Physcia spp.) sampled across Manchester (rural X. parietina are displayed as red solid line on bar for comparison)
Manchester lichen δ13C values are comparable to prior air quality studies in the urban area of Sydney (Australia) and Mexico City (Mexico), that reported street dust PM10, diesel soot and gasoline vehicle exhausts impacting on lichen δ13C values (Batts et al. 2004; López-Veneroni 2009). For instance, Górka et al., (2020) reported PM10-δ13C in ranges of -24.3 to -26.8‰ at an urban site in Poland. Lichen lichen-δ13C span δ13C-PM ranges (Fig. 4), suggesting a potential impact of deposited particulates on the lichen surface, because lichens were not washed prior to analysis. However, because PM-δ13C and other anthropogenic sources (Fig. 4) also span different δ13C values (Fig. 4), a clear distinction of solely anthropogenic sources is not possible using lichen stable carbon-isotope signatures.
Alternatively, minor variation recorded in δ13C values (and carbon contents; “Spatial variability of carbon, nitrogen and sulfur contents in urban lichens (X. parietina and Physcia spp.) to evaluate potential pollution sources”) for both Manchester lichens are potentially linked to different types of sources emitting atmospheric carbon, related δ13C ranges and meteorological parameters (e.g. water and light availability), physiological and/or biochemical processes (e.g. assimilation, metabolism and biosynthesis; Batts et al. 2004; Beck and Mayr 2012; Biazrov 2012; Ciężka et al. 2022; Galimov 2000; Hoefs 2009; Lee et al. 2009), as well as other atmospheric pollutants. For instance, Batts et al. (2004) reported decreasing δ13C with increasing pollution levels, i.e. when NOx (and SO2) increases. In this study, increased atmospheric nitrogen compounds, as suggested by elevated lichen wt% N (“Spatial variability of carbon, nitrogen and sulfur contents in urban lichens (X. parietina and Physcia spp.) to evaluate potential pollution sources”), potentially could have had a similar impact. To evaluate such impacts, lichen wt% C and wt% N, as well as stable carbon and nitrogen isotope ratios were correlated with one another (Table S6). No statistically significant differences between wt% C and wt% N were recorded for X. parietina, whereas δ15N and δ13C were significantly negatively (p < 0.05) correlated (Table S6), i.e. δ13C (Fig. 3) was more negative at sampling locations in proximity to major roads, which also showed an enrichment in 15N (Fig. 5). Moreover, Niepsch et al. (2021a) recorded elevated NO2 concentrations (by passive sampling devices) at locations with high wt% N. Hence, a potential impact on lichen δ13C by elevated nitrogen (and potentially sulfur) concentrations are suggested that require further investigation. However, statistically significant differences between lichen isotope-ratio signatures also be linked to differences in ambient air chemistry in the investigated environments. Investigating isotopic signatures of carbon compounds (among others, e.g. NOx, NHx and SOx) and particulate matter (PM2.5/10) will aid to fully understand influences on lichen carbon-isotope-ratio signatures.
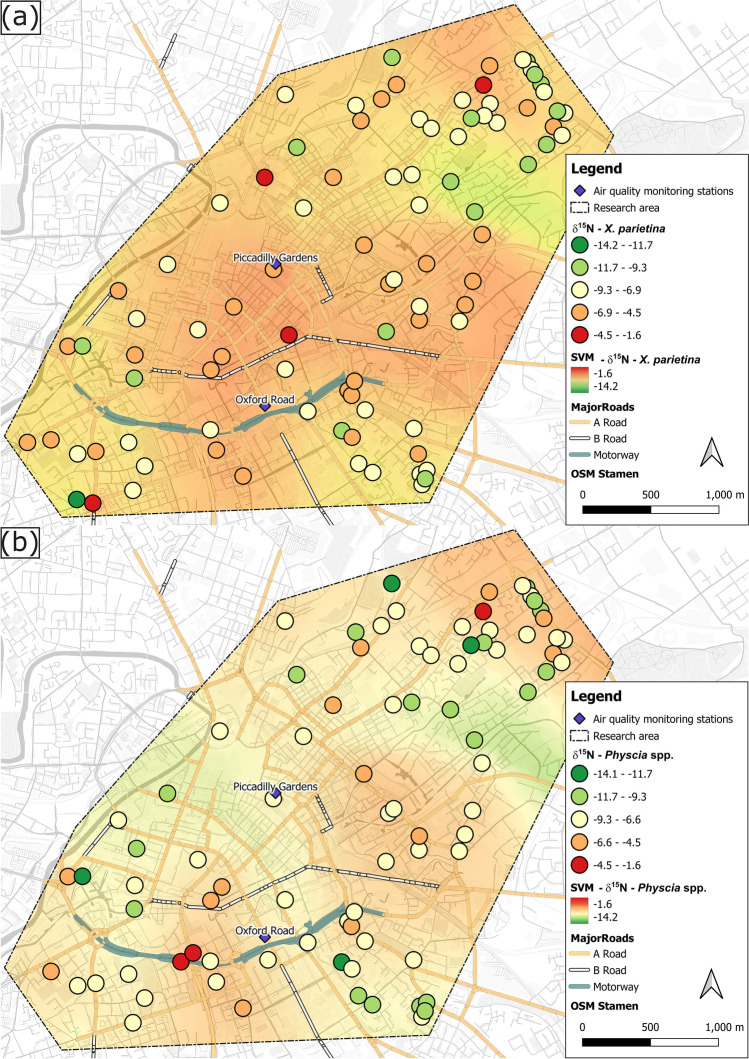
Fig. 5.
δ15N values (‰) of (a) X. parietina and (b) Physcia spp. colour-coded by 15N-enrichment (more positive values) across the City of Manchester; displayed with automated monitoring stations and major road network (A- and B-road and motorway); displayed with SVM interpolated values (same colouring and range) including Manchester’s urban factors
In summary, Manchester lichen δ13C values, as well as wt% C, are not considered reliable indicators of sources of different airborne pollutant carbon compounds in the urban environment (ii), an outcome in accordance with Bermejo-Orduna et al. (2014) who studied the lichen Letharia vulpina along a highly-trafficked motorway in Nevada (USA).
Lichen nitrogen stable-isotope ratios (δ15N)
Spatial variability of δ15N values was recorded for both lichen species (Fig. 5), ranging between -13.6 and -1.6‰ (X. parietina) and -14.2 and -2.0‰ (Physcia spp.) across Manchester. For both lichen species, an increase in wt% N was significantly (p < 0.01) accompanied by an enrichment in 15N (Fig. S5; Table S6). Comparably, statistically significant correlation of lichen-δ15N with urban influencing factors, as well as spatial correlation (Table 2, Fig. 5) with these factors suggest localised sources of pollution in Manchester.
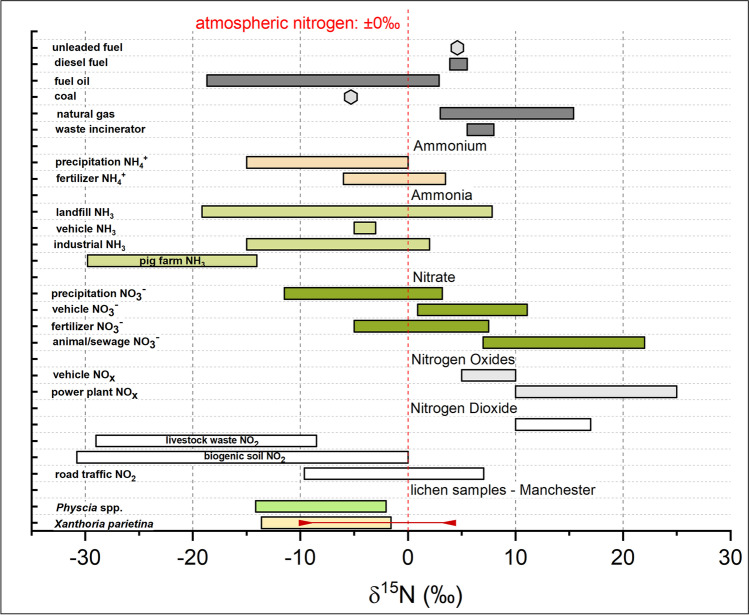
Epiphytic lichens can exhibit a wide range of δ15N values, due to assimilation of nitrogen compounds from different sources (e.g., NOx and NHx; Beck and Mayr 2012). For instance, lichen δ15N values have been reported to be more positive where airborne NOx concentration is high, such as in urban areas, and more negative in rural areas, the latter primarily due to a greater contribution of reduced nitrogen (NHx) from agricultural sources (Boltersdorf et al. 2014; Pearson et al. 2000; Sutton et al. 2004). Figure 6 summarises δ15N ranges of anthropogenically-emitted nitrogen compounds, with this study’s X. parietina and Physcia spp. lichen δ15N values spanning a variety of potential sources, including different atmospheric NHx and NOx compounds.
Fig. 6.
δ15N values of potential atmospheric pollutants and pollution sources reported in the literature (Berner and Felix 2020; Elliott et al. 2007; Felix et al. 2013, 2012; Felix and Elliott 2014; Heaton 1986; Heaton et al. 1997; Liu et al. 2016) to undertake lichen δ15N value source apportionment; displayed with δ-values of lichens (X. parietina and Physcia spp.) sampled across Manchester (rural X. parietina are displayed as red solid line on bar for comparison)
Albeit covering a variety of potential sources (Fig. 6), Manchester X. parietina and Physcia spp. δ15N values (as well as wt% N for both lichen species: Fig. 2) were more positive along highly-trafficked major road network (Table 1, Fig. 5) and suggesting a primary NOx influence on nitrogen isotope signatures. Indeed, lichen enrichment in 15N was recorded at sampling locations in proximity to the highly-trafficked Mancunian Way with approximately 30,000 vehicles passing daily (DfT 2017). Comparably, δ15N was positively correlated with passively-derived NO2 concentrations by Niepsch et al. (2021a) in X. parietina (Pearson’s r = 0.54, p < 0.01) and Physcia spp. (Pearson’s r = 0.37, p < 0.01; Table S6), consequently suggesting a major NOx-related influence in Manchester. Such findings have been reported by Bermejo-Orduna et al. (2014) who analysed the lichen Letharia vulpina δ15N along a major highway (I 80) in California (USA) and by Laffray et al. (2010) and Pearson et al. (2000) who used mosses as biomonitors along main routes (RN6 and A43) in the Maurienne Valley (France) and the urban environment of London (UK), respectively. On the contrary, Pinho et al. (2017) reported more negative δ15N values (-15.59‰ to -11.24‰) in Parmotrema hypoleucinum, sampled in the urban area of Sines (Portugal), linked to varying sources of NOx and NHx. Indeed, differences in recorded δ15N-values could be related to the species used in these biomonitoring studies, it is generally accepted that δ15N of epiphytic lichens reflect existing nitrogen compounds from the atmosphere, allowing analysis of predominant nitrogen sources (Boltersdorf and Werner 2014; Fogel et al. 2008; Tozer et al. 2005).
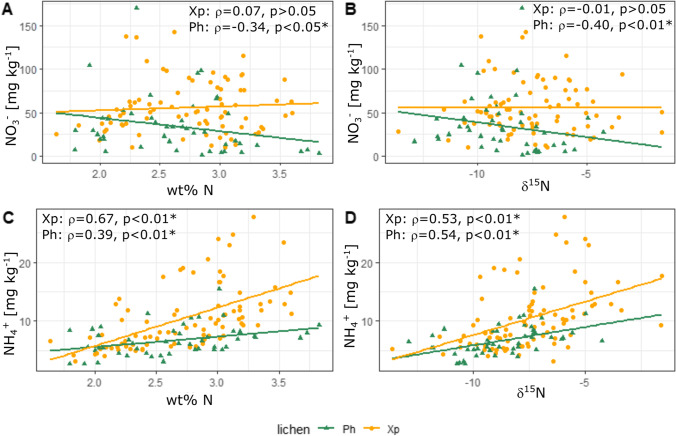
To further investigate potential impacts by nitrogen compounds on lichens, as reported by Pinho et al. (2017), N wt% and δ15N was linked to lichen NO3− and NH4+ (Fig. 7), using the nitrogen speciation method described by Niepsch et al. (2021b). Species-specific responses to nitrogen compounds, particularly for NO3− were recorded, with Physcia spp. wt% N and δ15N being statistically significant correlated with NO3− concentrations, whereas no such relationship was recorded for X. parietina (Fig. 7). In lichens, the mycobiont is responsible for nitrate assimilation, which has been found to be less effective compared to ammonium assimilation (Dahlman et al. 2004; Gaio-Oliveira et al. 2005b; Hauck 2010; Palmqvist and Dahlman 2006; Pavlova and Maslov 2008). Such an inter-species difference should be considered, when applying a lichen biomonitoring approach and comparing (urban) environments.
Fig. 7.
Scatterplots of nitrate (A and B) and ammonium (C and D) concentrations for comparison with (A and C) nitrogen contents [wt%] and (B and D) δ15N [‰] in X. parietina (Xp) and Physcia spp. (Ph; analytical precision, lichen CRM BCR-482-derived for N wt% ± 0.04; δ15N ± 0.23; NO3− ± 0.45 and NH4.+ ± 0.25: not shown in figure); displayed with correlation statistics (Spearman ρ, statistically significant correlation marked with *)
On the contrary, it is evident that NH4+, compared to NO3−, has a significantly positive impact on lichen wt% N and δ15N values. Elevated levels of ammonia/ammonium have been reported in urban areas, due to three-way catalyst cars being connected to increases in airborne ammonia and subsequently NH4+ (Bishop and Stedman 2015; Cape et al. 2004; Sun et al. 2017). Comparably, NH3 from vehicular emissions and industrial sources (Fig. 7) were found in more positive ranges, compared to highly negative ranges of agricultural nitrogen compound isotopic signatures (i.e. pig farm and livestock; Fig. 6, that may have impacted on recorded lichen δ15N. Additionally, NH4+ from precipitation (Fig. 6) could have impacted on recorded lichen δ15N values. For instance, Xu et al. (2021) reported that lichen δ15N lower than -7.8‰ indicate a dominance of 15N-depleted NH4+ relative to 15N-enriched NO3− in wet deposition. Moreover, they reported a higher contribution from volatilization NH3 (e.g. from waste and fertilizers) than combustion NH3 (e.g. from vehicular emissions, coal combustion and biomass burning) to X. parietina δ15N in the Beijing-Tianjin-Hebei region, China. However, δ15N values from lichen samples across Manchester suggest local sources (e.g. vehicular emissions) of nitrogen pollution, although not allowing for a clear distinction of particular pollution sources. Hence, a more complex influence of NOx and NHx compounds across Manchester is suggested.
Nonetheless, lichen wt% N and δ15N stable-isotope-ratio signatures can provide beneficial information on spatial variability of nitrogen compounds (ii), particularly at locations that are not regularly covered by automated air quality monitoring stations. Moreover, lichen NO3− and NH4+ concentrations, when combined with wt% N and δ15N allow for finer detail of air quality and aid identification of areas with poor air quality and potential increased human health risks (iii). To further pinpoint vehicular-related influences, traffic marker metal (e.g., Pb and Zn) concentrations in lichens could be combined with δ15N signatures (Bermejo-Orduna et al. 2014; Gerdol et al. 2002; Pearson et al. 2000).
Lichen sulfur stable-isotope ratios (δ34S)
Across Manchester δ34S values in X. parietina and Physcia spp. ranged between 1.3 and 10.3‰ and 1.5 and 9.6‰, respectively. Studies reported that δ34S-values < 10‰ are related to anthropogenic sulfur sources (Wadleigh 2003; Wadleigh and Blake 1999; Wiseman and Wadleigh 2002). Moreover, low δ34S values (< 10‰), together with increases in wt% S, have been associated with anthropogenic sulfur sources (Wadleigh and Blake 1999), as is evident for both lichens utilised in this study (X. parietina and Physcia spp.; Fig. 6 f), i.e. a statistically significant negative correlation (X. parietina: p < 0.01, Physcia spp: p < 0.05) was recorded for δ34S and wt% S. In this study, X. parietina samples were more enriched in 34S in more residential surroundings in the northeast and southwest of the research area, whereas lower δ34S values were recorded in the city centre area (Fig. 8), i.e. where higher traffic counts and surrounding building heights (Table 2) suggest localised influences. For instance, sulfur-containing compounds and particulates, e.g. from residential combustion (using petroleum coke; NAEI 2018b) and vehicular emissions (AQEG 2012; Clean Air Greater Manchester 2021) may have impacted on lichen stable-isotope-ratio signatures.
Sulfur enters the atmosphere from different sources and SO2 can be widely distributed (Pinho et al. 2008). However, lichen sulfur-isotope-ratio signatures are related to their proximal atmosphere, and little stable-isotope fractionation during assimilatory processes has been demonstrated, allowing the use of δ34S values to fingerprint natural versus anthropogenic sulfur sources (Batts et al. 2004; Wadleigh 2003; Wadleigh and Blake 1999; Wiseman and Wadleigh 2002). Manchester lichen δ34S ranges are in ranges of anthropogenic sources, e.g. fuel combustion and vehicular emissions (Fig. 9), whereas natural sources (e.g. bacterial H2S and seawater SO42−) δ34S are in highly negative and positive ranges, respectively. Additionally, low recorded atmospheric SO2 concentrations (at Manchester Piccadilly) and an annual reduction of approximately 5% (each year) between 2000 and 2017 (Sicard et al. 2021), suggest that lichen sulfur-isotope-ratio signatures are likely to represent the anthropogenic sources and “local” sulfur concentrations for the urban environment of Manchester (Spiro et al. 2002; Wadleigh 2003; Wadleigh and Blake 1999; Wiseman and Wadleigh 2002). Thus, this study’s findings support the use of lichen wt% S and δ34S signatures to evaluate spatial variability in airborne sulfur pollution, and potentially fingerprint potential sources in an urban environment (ii).
Fig. 9.
δ34S values of potential atmospheric pollutants and pollution sources reported in the literature (Case and Krouse 1980; Cortecci and Longinelli 1970; Norman 2004; Wadleigh 2003; Wadleigh and Blake 1999; Wiseman and Wadleigh 2002) to undertake lichen δ34S value source apportionment; displayed with δ-values of lichens (X. parietina and Physcia spp.) sampled across Manchester (rural X. parietina are displayed as red solid line on bar for comparison)
Meteorological conditions (e.g., wind dispersion and wash-off effects by precipitation; Spiro et al. 2002) as well other sulfur compounds (e.g., organosulfur from biological process; Perraud et al. 2015) might have impacted on lichen δ34S values, but were beyond the scope of this work. Detailed characterisation of lichen δ34S values directly proximal to potential sulfur sources, as well as analysis of different fuel types used in energy production and residential heating across Manchester, would improve further source apportionment using δ34S.
Comparison of CNS contents and stable-isotope ratios in urban and rural X. parietina samples and the implications for lichen biomonitoring studies
X. parietina samples (N = 12; Fig. S3) were used to compare the contrasting urban and rural environments and to evaluate specific differences in CNS contents and stable-isotope-ratio signatures (individual rural lichen wt% CNS and δ-values in Table S7). Overall, wt% C and wt% N were higher and significantly (p < 0.01) different in rural X. parietina lichens, compared to their urban counterparts (Fig. S8 a and b), whereas wt% S contents were lower in rural X. parietina samples (Fig. S8 c). Comparison of stable-isotope-ratio signatures (δ13C, δ15N and δ34S), showed significant (p < 0.01) differences between rural and urban X. parietina, with enrichment in 13C, 15N and 34S in rural lichens (Fig. S8 d, e and f).
For wt% C and δ13C (Fig. S8 a and d), recorded differences are most likely linked to environmental conditions in the analysed environment (i.e. urban and rural) and intra- and inter-variability of carbon contents in lichens (Beck and Mayr 2012; Johansson et al. 2010; Máguas et al. 2013). Further, ranges of (organic) carbon contents (% dry weight) in poultry manure (from different poultry in the UK) were reported between 24.2–34.8% (Nicholson et al. 1996), suggesting potential deposition related influences on lichens proximal to the poultry farm.
In contrast, elevated wt% N in rural X. parietina (Fig. S8 b) is in accordance with lichen studies undertaken in agricultural surroundings and livestock areas (Boltersdorf and Werner 2013; Franzen-Reuter 2004; Frati et al. 2007), being linked to nitrogen emissions from poultry manure and nitrogen-containing fertilizers (AQEG 2018; Nahm 2005). Moreover, wt% N decreased with increased distance from the poultry farm (r = -0.61; p < 0.05), suggesting increased availability of nutrients (e.g. NOx and NHx), increased lichen biomass, increased chlorophyll content and subsequent increased photosynthetic capacity, as well as net gain from CO2 assimilation, and thus potentially explaining elevated wt% C in rural X. parietina (Dahlman et al. 2002; Gaio-Oliveira et al. 2005b; Hauck 2010; Johansson et al. 2010; Nybakken et al. 2009). Interestingly, rural lichen δ15N values (Fig. S8 e) were more positive than urban samples, with δ15N particularly enriched in close proximity to the poultry farm. More negative δ15N was expected in the agricultural area, compared to Manchester, due to greater contribution of reduced nitrogen, i.e. NHx (Boltersdorf et al. 2014; Pearson et al. 2000; Sutton et al. 2004). However, poultry farm emissions might also be isotopically different compared to other livestock; e.g., poultry farm emitted NO2 has been reported to have a δ15N value of -8.5‰ (Felix and Elliott 2014; Liu et al. 2016) that could explain enrichment in 15N in the rural X. parietina analysed in this study. Consequently, comparison of lichen δ15N in the analysed urban (Manchester) and rural (poultry farm) environments has not allowed for a clear distinction of NOx (i.e. urban) and/or NHx-related (i.e. rural) influences and additional research on the δ15N values of NOx and NHx compounds (and their impact on lichen stable-isotope ratios) in these environments is required.
Differences in wt% S between rural and urban lichen sampled (Fig. S8 c) are most likely related to lower SO2 concentrations in the rural environment. For instance, SO2 emissions in the UK from agriculture accounted for small amounts (11.58 kilotons in 2016) of total emissions, compared to anthropogenic sources (168 kilotons in 2016) (NAEI 2018b). Additionally, δ34S in urban samples were less positive, compared to rural samples (Fig. S8 d) and δ34S did not decrease with increasing wt% S in rural samples, suggesting minor anthropogenic influences. Hence, δ34S (as well as wt% S) in rural lichen samples may well simply record the ‘background/natural’ sulfur concentrations in the rural environment, which is less affected by anthropogenic sources. Hence, the applied lichen biomonitoring approach was considered a useful tool to assess and compare these contrasting environments for (anthropogenic) sources of sulfur compounds.
In general, lichen wt% N and wt% S allow for comparison of atmospheric pollution in contrasting urban and rural environments; e.g., poultry farm and agricultural emissions for rural lichen wt% N and combustion sources for urban lichen wt% S. However, lichen δ13C and δ15N values suggest more complex airborne pollutant influences on lichens, with δ34S solely allowing for differentiation of anthropogenic impacts in these contrasting environments. However, a lichen biomonitoring approach facilitates a finer spatial detail of air quality assessment and can help to identify urban areas where improvements to air quality are required, thereby further supporting existing automated air quality measurements. Nonetheless, X. parietina samples, obtained around a poultry farm might not represent optimal sites for comparison with urban environments, when using CNS contents and stable-isotope-ratio signatures, because agricultural influences, particularly for nitrogen compounds, may have impacted on recorded wt% N and δ15N values that warrant further investigation. To further improve the comparison between environments and fingerprinting of potential sources, more pristine (if possible) remote lichen samples (e.g. sampled from areas with lower concentrations of atmospheric pollutants) are suggested. Additional environmental compartments, e.g. soil and atmospheric compound wt% CNS their associated stable-isotope-ratio compositions would provide additional information and aid contribution of these compounds to lichen pollutant loadings and isotopic compositions.
Conclusion
In this study two lichens (X. parietina and Physcia spp.) were used as biomonitors to identify high-resolution spatial variability of air quality, and also possible pollution source apportionment, across the Manchester (UK) urban environment. Spatial variability of airborne nitrogen and sulfur compounds across Manchester was identified using these lichens, whereas identification of deteriorated air quality by carbon compounds was not completely possible (i.e., due to temporal change superimposed on spatial variability of lichen signals).
Central Manchester’s complex urban layout has impacted on recorded lichen carbon, nitrogen and sulfur loadings and associated stable-isotope-ratio signatures, allowing for identification of areas of deteriorated air quality, and thus human health concern, where ameliorative actions should be targeted. Species-specific differences in lichen chemistry were recorded and such variability ought to be considered when using lichens as biomonitors of spatial variability of airborne pollution; use of a ‘single’ lichen species is suggested, particularly when studying urban environments.
Source apportionment of airborne pollution in Manchester was not possible from lichen δ13C and δ15N values, but a more complex impact of nitrogen compounds (NOx and NHx) was found that warrants further investigation, i.e. using lichen NO3− and NH4+ concentrations. In contrast, combined Manchester lichen wt% S and δ34S values can primarily be attributed to anthropogenic sources of sulfur, such as from domestic heating emissions. Additional research on stable-isotope-ratio signatures of possible anthropogenic sources, e.g. particulates and gaseous compounds (Berner and Felix 2020), would significantly improve source apportionment studies.
This study contributes to further development of an easy-to-use, relatively quick, and low-cost lichen biomonitoring approach, applicable to the assessment of urban airborne pollutants at a finer spatial detail which is transferable to comparable urban areas. Such an approach can be undertaken in areas without automated air quality measurements, or lichen biomonitoring of this type can support, and be ground-truthed, by automated monitoring programmes. A lichen biomonitoring approach using wt% N and S wt% with their associated stable-isotope ratios, can provide a promising tool for local authorities, stakeholders and NGOs to identify ‘hotspots’ of poor air quality and areas of human health concern. Thereby, supporting development of potential airborne pollution amelioration and mitigation strategies, e.g. emission reductions, pollution taxes and ‘clean air zones’.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dave McKendry for assistance during CN analysis and the SUERC laboratories to allow analysis of lichen material using their equipment.
The authors would like to thank Dr Michael Flynn (Whitworth Observatory, University of Manchester) for permission to use meteorological data. Further, the authors would like to thank the independent reviewers for their valuable feedback that helped to improve the manuscript.
Author’s contribution
DN: Writing – original draft, Conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualisation; LJC: Conceptualization, funding acquisition, project administration, resources, methodology, supervision, writing – reviewing & editing; JN: resources, data curation and analysis, writing reviewing & editing; KT: writing reviewing & editing; GC: writing reviewing & editing.
Funding
DN was supported by a Manchester Metropolitan University Environmental Science Research Centre (MMU-ESRC 2015–02) PhD studentship.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
All data generated or analysed during this study are included in the article and its supplementary information.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interest to disclose.
Footnotes
Highlights
• Low-cost approach for high spatial resolution air quality monitoring.
• Lichen wt% C and d13C values not considered a useful tool for source apportionment.
• Lichen wt% N and d15N values suggest complex influences by NOx and NHx compounds.
• Lichen wt% S and d34S value suggest anthropogenic sulfur pollution sources.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdull N, Yoneda M, Shimada Y. Traffic characteristics and pollutant emission from road transport in urban area. Air Qual Atmos Heal. 2020 doi: 10.1007/s11869-020-00830-w. [DOI] [Google Scholar]
- Academy of Science of South. Africa Brazilian Academy Of Science. German National Academy of Sciences Leopoldina. U. S. National Academy of Medicine. U. S. National Academy of Sciences Air Pollution and Health – A Science-Policy Initiative. Ann Glob Heal. 2019;85:1–9. doi: 10.5334/aogh.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AQEG (2012) Fine particulate matter (PM2.5) in the United Kingdom. Air quality expert group, report (PB13837; pages 1–191) prepared for: department for environment, food and rural affairs; Scottish Executive; Welsh Government; and Department of the Environment in Northern Ireland
- AQEG (2018) Air pollution from agriculture. Air quality expert group, report (PB14509; pages 1–46) prepared for department for environmental, food and rural affairs; Scottish Government; Welsh Government; and Department of the Environment in Northern Ireland
- Batts JE, Calder LJ, Batts BD. Utilizing stable isotope abundances of lichens to monitor environmental change. Chem Geol. 2004;204:345–368. doi: 10.1016/j.chemgeo.2003.11.007. [DOI] [Google Scholar]
- Beck A, Mayr C. Nitrogen and carbon isotope variability in the green-algal lichen Xanthoria parietina and their implications on mycobiont-photobiont interactions. Ecol Evol. 2012;2:3132–3144. doi: 10.1002/ece3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Orduna R, McBride JR, Shiraishi K, Elustondo D, Lasheras E, Santamaría JM. Biomonitoring of traffic-related nitrogen pollution using Letharia vulpina (L.) Hue in the Sierra Nevada, California. Sci Total Environ. 2014;490:205–212. doi: 10.1016/j.scitotenv.2014.04.119. [DOI] [PubMed] [Google Scholar]
- Berner AH, Felix JD. Investigating ammonia emissions in a coastal urban airshed using stable isotope techniques. Sci Total Environ. 2020;707:134952. doi: 10.1016/j.scitotenv.2019.134952. [DOI] [PubMed] [Google Scholar]
- Biazrov LG. Values of stable carbon isotopes (δ13C) in the thalli of the arid vagrant lichen Xanthoparmelia camtschadalis along an altitudinal gradient in the Khangai Plateau as a reflection of the spatial and ecological heterogeneity of the semiari. Arid Ecosyst. 2012;2:54–60. doi: 10.1134/S2079096112010039. [DOI] [Google Scholar]
- Bishop GA, Stedman DH. Reactive Nitrogen Species Emission Trends in Three Light-/Medium-Duty United States Fleets. Environ Sci Technol. 2015;49:11234–11240. doi: 10.1021/acs.est.5b02392. [DOI] [PubMed] [Google Scholar]
- Boltersdorf SH, Werner W. Source attribution of agriculture-related deposition by using total nitrogen and δ15N in epiphytic lichen tissue, bark and deposition water samples in Germany. Isotopes Environ Health Stud. 2013;49:197–218. doi: 10.1080/10256016.2013.748051. [DOI] [PubMed] [Google Scholar]
- Boltersdorf SH, Werner W. Lichens as a useful mapping tool?—an approach to assess atmospheric N loads in Germany by total N content and stable isotope signature. Environ Monit Assess. 2014;186:4767–4778. doi: 10.1007/s10661-014-3736-3. [DOI] [PubMed] [Google Scholar]
- Boltersdorf SH, Pesch R, Werner W. Comparative use of lichens, mosses and tree bark to evaluate nitrogen deposition in Germany. Environ Pollut. 2014;189:43–53. doi: 10.1016/j.envpol.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Britter RE, Hanna SR. Flow and Dispersion in Urban Areas. Annu Rev Fluid Mech. 2003;35:469–496. doi: 10.1146/annurev.fluid.35.101101.161147. [DOI] [Google Scholar]
- Broad K (1989) Lichens in southern woodlands, forestry commission, Handbook 4. HMSO Books, London. Accessible via: https://www.forestresearch.gov.uk/publications/archive-lichens-in-southern-woodlands/. Accessed 23 Nov 2022
- Cape JN, Tang YS, Van Dijk N, Love L, Sutton MA, Palmer SCF. Concentrations of ammonia and nitrogen dioxide at roadside verges, and their contribution to nitrogen deposition. Environ Pollut. 2004;132:469–478. doi: 10.1016/j.envpol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Cariolet JM, Colombert M, Vuillet M, Diab Y. Assessing the resilience of urban areas to traffic-related air pollution: Application in Greater Paris. Sci Total Environ. 2018;615:588–596. doi: 10.1016/j.scitotenv.2017.09.334. [DOI] [PubMed] [Google Scholar]
- Carreras HA, Pignata ML. Biomonitoring of heavy metals and air quality in Cordoba City, Argentina, using transplanted lichens. Environ Pollut. 2002;117:77–87. doi: 10.1016/S0269-7491(01)00164-6. [DOI] [PubMed] [Google Scholar]
- Case JW, Krouse HR. Variations in sulphur content and stable sulphur isotope composition of vegetation near a SO2 source at Fox Creek, Alberta, Canada. Oecologia. 1980;44:247–257. doi: 10.1007/BF00572687. [DOI] [PubMed] [Google Scholar]
- Ciężka MM, Górka M, Trzyna A, Modelska M, Łubek A, Widory D. The multi-isotope biogeochemistry (S, C, N and Pb) of Hypogymnia physodes lichens: air quality approach in the Świętokrzyski National Park, Poland. Isotopes Environ Health Stud. 2022;58:340–362. doi: 10.1080/10256016.2022.2110591. [DOI] [PubMed] [Google Scholar]
- City of Trees (2018) All our trees – greater Manchester’s tree and woodland strategy [WWW Document]. https://www.cityoftrees.org.uk/sites/default/files/8082_All_our_trees_report_Dr8_MW.pdf. Accessed 13-08-2022
- Clean Air Greater Manchester (2021) Types and sources of air pollution [WWW Document]. URL https://cleanairgm.com/air-pollution/ (accessed 12.5.21)
- Coplen TB. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectrom. 2011;25:2538–2560. doi: 10.1002/rcm.5129. [DOI] [PubMed] [Google Scholar]
- Cortecci G, Longinelli A. Isotopic composition of sulfate in rain water, Pisa, Italy. Earth Planet Sci Lett. 1970 doi: 10.1016/0012-821X(70)90096-8. [DOI] [Google Scholar]
- Cyrys J, Eeftens M, Heinrich J, Ampe C, Armengaud A, Beelen R, Bellander T, Beregszaszi T, Birk M, Cesaroni G, Cirach M, Dimakopoulou K, Eriksen K, Galassi C, Gra R, Grivas G, Gruzieva O, Gustafsson AH, Hoffmann B, Iakovides M, Ineichen A, Krämer U, Lanki T, Lozano P, Madsen C, Meliefste K, Modig L, Mölter A, Mosler G, Nieuwenhuijsen M, Nonnemacher M, Oldenwening M, Peters A, Pontet S, Probst-hensch N, Quass U, Raaschou-nielsen O, Ranzi A, Sugiri D, Stephanou EG, Taimisto P, Tsai M, Vaskövi É, Villani S, Wang M, Brunekreef B, Hoek G (2012) Variation of NO2 and NOx concentrations between and within 36 European study areas : Results from the ESCAPE study 62:374–390. 10.1016/j.atmosenv.2012.07.080
- Dahlman L, Palmqvist K, Näsholm T. Growth, nitrogen uptake, and resource allocation in the two tripartite lichens Nephroma arcticum and Peltigera aphthosa during nitrogen stress. New Phytol. 2002;153:307–315. doi: 10.1046/j.0028-646X.2001.00321.x. [DOI] [Google Scholar]
- Dahlman L, Persson J, Näsholm T, Palmqvist K. Carbon and nitrogen distribution in the green algal lichens Hypogymnia physodes and Platismatia glauca in relation to nutrient supply. Planta. 2003 doi: 10.1007/s00425-003-0977-8. [DOI] [PubMed] [Google Scholar]
- Dahlman L, Persson J, Palmqvist K, Näsholm T. Organic and inorganic nitrogen uptake in lichens. Planta. 2004;219:459–467. doi: 10.1007/s00425-004-1247-0. [DOI] [PubMed] [Google Scholar]
- DEFRA (2018a) Air Quality England - webpage: Oxford Road [WWW Document]. URL http://www.airqualityengland.co.uk/site/data?site_id=MAN1 (accessed 9.25.18)
- DEFRA (2018b) Air Quality England - webpage: Manchester Piccadilly [WWW Document]. URL http://www.airqualityengland.co.uk/site/latest?site_id=MAN3 (accessed 9.25.18)
- DEFRA (2020) Auromatic Urban and Rural network (AURN) [WWW Document]. URL https://uk-air.defra.gov.uk/networks/network-info?view=aurn (accessed 7.5.20)
- DEFRA (2022) National statistics Emissions of air pollutants in the UK – Sulphur dioxide (SO2) [WWW Document]. URL https://www.gov.uk/government/statistics/emissions-of-air-pollutants/emissions-of-air-pollutants-in-the-uk-sulphur-dioxide-so2 (accessed 12.13.22)
- DEFRA, DfT (2017) UK plan for tackling roadside nitrogen dioxide concentrations: Detailed plan 2017
- DfT (2017) Road traffic statistics [WWW Document]. URL https://www.gov.uk/government/publications/road-traffic-estimates-great-britain-jan-to-mar-q1-2014/5Cnhttps://www.gov.uk/government/collections/road-traffic-statistics. Accessed 02-06-2018
- Dobson FS. Lichens - An Illustrated Guide to the British and Irish Species. 6. Slough: The Richmond Publishing Co., Ltd.; 2011. [Google Scholar]
- Elliott EM, Kendall C, Wankel SD, Burns DA, Boyer EW, Harlin K, Bain DJ, Butler TJ. Nitrogen isotopes as indicators of NOx source contributions to atmospheric nitrate deposition across the midwestern and northeastern United States. Environ Sci Technol. 2007;41:7661–7667. doi: 10.1021/es070898t. [DOI] [PubMed] [Google Scholar]
- EPA (2008) Emission facts: idling vehicle emissions for passenger cars, light-duty trucks, and heavy-duty trucks. U. S. environmental protection agency, office of transportation and air quality - EPA420-F-08-025, October 2008
- Erisman JW, Vermetten AWM, Asman WAH, Waijers-Ijpelaan A, Slanina J. Vertical distribution of gases and aerosols: The behaviour of ammonia and related components in the lower atmosphere. Atmos Environ. 1988;22:1153–1160. doi: 10.1016/0004-6981(88)90345-9. [DOI] [Google Scholar]
- EU Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Off J Eur Union. 2008;152:1–44. [Google Scholar]
- Felix JD, Elliott EM. Isotopic composition of passively collected nitrogen dioxide emissions: Vehicle, soil and livestock source signatures. Atmos Environ. 2014;92:359–366. doi: 10.1016/j.atmosenv.2014.04.005. [DOI] [Google Scholar]
- Felix JD, Elliott EM, Shaw SL. Nitrogen isotopic composition of coal-fired power plant NOx: Influence of emission controls and implications for global emission inventories. Environ Sci Technol. 2012;46:3528–3535. doi: 10.1021/es203355v. [DOI] [PubMed] [Google Scholar]
- Felix JD, Elliott EM, Gish TJ, McConnell LL, Shaw SL. Characterizing the isotopic composition of atmospheric ammonia emission sources using passive samplers and a combined oxidation-bacterial denitrifier approach. Rapid Commun Mass Spectrom. 2013;27:2239–2246. doi: 10.1002/rcm.6679. [DOI] [PubMed] [Google Scholar]
- Fogel ML, Wooller MJ, Cheeseman J, Smallwood BJ, Roberts Q, Romero I, Meyers MJ. Unusually negative nitrogen isotopic compositions (δ15N) of mangroves and lichens in an oligotrophic, microbially-influenced ecosystem. Biogeosciences. 2008;5:1693–1704. doi: 10.5194/bg-5-1693-2008. [DOI] [Google Scholar]
- Forbes PBC, van der Wat L, Kroukamp EM (2015) Biomonitors - chapter 3. In: Forbes P (ed) Monitoring of air pollutants: sampling, sample preparation and analytical techniques, comprehensive analytical chemistry. Elsevier B.V., pp 53–108. 10.1016/bs.coac.2015.09.003
- Forbes PBC (2015) Monitoring of air pollutants: sampling, sample preparation and analytical techniques. In: Forbes PBC (ed) Comprehensive analytical chemistry. Elsevier B.V., pp 3–372. 10.1016/S0166-526X(15)00016-1
- Franzen-Reuter I (2004) Untersuchungen zu den Auswirkungen atmosphärischer Stickstoffeinträge auf epiphytische Flechten und Moose im Hinblick auf die Bioindikation. PhD thesis. Rheinischen Friedrich-Wilhelms-Universität Bonn
- Frati L, Caprasecca E, Santoni S, Gaggi C, Guttova A, Gaudino S, Pati A, Rosamilia S, Pirintsos SA, Loppi S. Effects of NO2 and NH3 from road traffic on epiphytic lichens. Environ Pollut. 2006;142:58–64. doi: 10.1016/j.envpol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Frati L, Santoni S, Nicolardi V, Gaggi C, Brunialti G, Guttova A, Gaudino S, Pati A, Pirintsos SA, Loppi S. Lichen biomonitoring of ammonia emission and nitrogen deposition around a pig stockfarm. Environ Pollut. 2007;146:311–316. doi: 10.1016/j.envpol.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Freer-Smith PH, Holloway S, Goodman A. The uptake of particulates by an urban woodland: Site description and particulate composition. Environ Pollut. 1997 doi: 10.1016/S0269-7491(96)00119-4. [DOI] [PubMed] [Google Scholar]
- Fry B. Stable Isotope Ecology. 3. LLC, New York: Springer Science + Business Media; 2006. [Google Scholar]
- Gadsdon SR, Dagley JR, Wolseley PA, Power SA. Relationships between lichen community composition and concentrations of NO2 and NH3. Environ Pollut. 2010;158:2553–2560. doi: 10.1016/j.envpol.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Gaio-Oliveira G, Dahlman L, Palmqvist K, Máguas C. Ammonium uptake in the nitrophytic lichen Xanthoria parietina and its effects on vitality and balance between symbionts. Lichenol. 2004;36:75–86. doi: 10.1017/S0024282904014124. [DOI] [Google Scholar]
- Gaio-Oliveira G, Dahlman L, Palmqvist K, Máguas C. Responses of the lichen Xanthoria parietina (L.) Th. Fr. to varying thallus nitrogen concentrations. Lichenologist. 2005;37:171–179. doi: 10.1017/S0024282904014598. [DOI] [Google Scholar]
- Gaio-Oliveira G, Dahlman L, Palmqvist K, Martins-Loução MA, Máguas C. Nitrogen uptake in relation to excess supply and its effects on the lichens Evernia prunastri (L.) Ach and Xanthoria parietina (L.) Th. Fr. Planta. 2005;220:794–803. doi: 10.1007/s00425-004-1396-1. [DOI] [PubMed] [Google Scholar]
- Galimov EM. Carbon isotope composition of Antarctic plants. Geochim Cosmochim Acta. 2000;64:1737–1739. doi: 10.1016/S0016-7037(99)00328-2. [DOI] [Google Scholar]
- Gaston KJ, Davies ZG, Edmondson JL. Urban environments and ecosystem functions. In: Gaston KJ, editor. Urban Ecology. Cambridge University Press; 2010. pp. 35–52. [Google Scholar]
- Gerdol R, Bragazza L, Marchesini R, Medici A, Pedrini P, Benedetti S, Bovolenta A, Coppi S. Use of moss (Tortula muralis Hedw.) for monitoring organic and inorganic air pollution in urban and rural sites in Northern Italy. Atmos Environ. 2002 doi: 10.1016/S1352-2310(02)00298-4. [DOI] [Google Scholar]
- Gerdol R, Marchesini R, Iacumin P, Brancaleoni L. Monitoring temporal trends of air pollution in an urban area using mosses and lichens as biomonitors. Chemosphere. 2014;108:388–395. doi: 10.1016/j.chemosphere.2014.02.035. [DOI] [PubMed] [Google Scholar]
- GMCA (2018) 2017 air quality annual status report (ASR) for greater Manchester. LAQM Annual Status Report 2017 (GMASR2017), Manchester, 26th September 2018
- Gombert S, Asta J, Seaward MR. Correlation between the nitrogen concentration of two epiphytic lichens and the traffic density in an urban area. Environ Pollut. 2003;123:281–290. doi: 10.1016/S0269-7491(02)00367-6. [DOI] [PubMed] [Google Scholar]
- González CM, Casanovas SS, Pignata ML. Biomonitoring of air pollutants from traffic and industries employing Ramalina ecklonii (Spreng.) Mey. and Flot. in Córdoba, Argentina. Environ Pollut. 1996;91:269–277. doi: 10.1016/0269-7491(95)00076-3. [DOI] [PubMed] [Google Scholar]
- Górka M, Lewicka-Szczebak D. One-year spatial and temporal monitoring of concentration and carbon isotopic composition of atmospheric CO2 in a Wrocław (SW Poland) city area. Appl Geochemistry. 2013;35:7–13. doi: 10.1016/j.apgeochem.2013.05.010. [DOI] [Google Scholar]
- Górka M, Kosztowniak E, Lewandowska AU, Widory D. Carbon isotope compositions and TC/OC/EC levels in atmospheric PM10 from Lower Silesia (SW Poland): Spatial variations, seasonality, sources and implications. Atmos Pollut Res. 2020;11:1099–1114. doi: 10.1016/j.apr.2020.04.003. [DOI] [Google Scholar]
- GraphPad Software Inc. (2018) GraphPad prism 7 for windows [Computer Software], San Diego, California. www.graphpad.com
- Grimmond CSB, Oke TR. Aerodynamic Properties of Urban Areas Derived from Analysis of Surface Form. J Appl Meteorol. 1999;38:1262–1292. doi: 10.1175/1520-0450(1999)038<1262:APOUAD>2.0.CO;2. [DOI] [Google Scholar]
- Gu B, Zhang L, Van Dingenen R, Vieno M, Van Grinsven HJ, Zhang X, Zhang S, Chen Y, Wang S, Ren C, Rao S, Holland M, Winiwarter W, Chen D, Xu J, Sutton MA (2021) Abating ammonia is more cost-effective than nitrogen oxides for mitigating PM2.5 air pollution. Science (80). 374:758–762. 10.1126/science.abf8623 [DOI] [PubMed]
- Harrison RM, Jones AM, Lawrence RG. Major component composition of PM10 and PM2.5 from roadside and urban background sites. Atmos Environ. 2004;38:4531–4538. doi: 10.1016/j.atmosenv.2004.05.022. [DOI] [Google Scholar]
- Hauck M. Ammonium and nitrate tolerance in lichens. Environ Pollut. 2010;158:1127–1133. doi: 10.1016/j.envpol.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Heaton THE. Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: A review. Chem Geol Isot Geosci Sect. 1986;59:87–102. doi: 10.1016/0168-9622(86)90059-X. [DOI] [Google Scholar]
- Heaton THE, Spiro B, Robertson SMC. Potential canopy influences on the isotopic composition of nitrogen and sulphur in atmospheric deposition. Oecologia. 1997;109:600–607. doi: 10.1007/s004420050122. [DOI] [PubMed] [Google Scholar]
- Hernández-Paniagua IY, Lowry D, Clemitshaw KC, Fisher RE, France JL, Lanoisellé M, Ramonet M, Nisbet EG. Diurnal, seasonal, and annual trends in atmospheric CO2 at southwest London during 2000–2012: Wind sector analysis and comparison with Mace Head, Ireland. Atmos Environ. 2015;105:138–147. doi: 10.1016/j.atmosenv.2015.01.021. [DOI] [Google Scholar]
- Highway Forecasting and Analytical Services (2015) HFAS report 1843 - transport statistics Manchester 2014, appendix 3: traffic flow, road accidents and congestion plots. Transport for Greater Manchester, Manchester. Accessible via: https://www.gmtu.gov.uk/reports/transport2014/DSD%20Report%201843%20Transport%20Statistics%20Manchester%202014%20Appendix%203.pdf. Accessed 04-05-2018
- Hoefs J. Stable Isotope Geochemistry. 6. Berlin, Heidelberg: Springer Springer Verlag; 2009. [Google Scholar]
- Janhäll S. Review on urban vegetation and particle air pollution – Deposition and dispersion. Atmos Environ. 2015;105:130–137. doi: 10.1016/j.atmosenv.2015.01.052. [DOI] [Google Scholar]
- Johansson O, Nordin A, Olofsson J, Palmqvist K. Responses of epiphytic lichens to an experimental whole-tree nitrogen-deposition gradient. New Phytol. 2010;188:781–795. doi: 10.1111/J.1469-8137.2010.03426.x. [DOI] [PubMed] [Google Scholar]
- Jones AM, Harrison RM. Interpretation of particulate elemental and organic carbon concentrations at rural, urban and kerbside sites. Atmos Environ. 2005;39:7114–7126. doi: 10.1016/j.atmosenv.2005.08.017. [DOI] [Google Scholar]
- Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151:362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Karamizadeh S, Abdullah SM, Halimi M, Shayan J, Rajabi MJ (2014) Advantage and drawback of support vector machine functionality, in: 2014 International Conference on Computer, Communications, and Control Technology (I4CT). IEEE, pp. 63–65. 10.1109/I4CT.2014.6914146
- Kim SY, Peel JL, Hannigan MP, Dutton SJ, Sheppard L, Clark ML, Vedal S. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ Health Perspect. 2012;120:1094–1099. doi: 10.1289/ehp.1104721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum U, Wirth V. Flechten erkennen - Umwelt bewerten. 3. Wiesbaden: Hessisches Landesamt für Umwelt und Geologie; 2010. [Google Scholar]
- Kubota T, Miura M, Tominaga Y, Mochida A. Wind tunnel tests on the relationship between building density and pedestrian-level wind velocity: Development of guidelines for realizing acceptable wind environment in residential neighborhoods. Build Environ. 2008;43:1699–1708. doi: 10.1016/j.buildenv.2007.10.015. [DOI] [Google Scholar]
- Kurppa M, Hellsten A, Auvinen M, Raasch S, Vesala T, Järvi L. Ventilation and air quality in city blocks using large-eddy simulation-urban planning perspective. Atmosphere (basel) 2018;9:1–27. doi: 10.3390/atmos9020065. [DOI] [Google Scholar]
- Laffray X, Rose C, Garrec JP. Biomonitoring of traffic-related nitrogen oxides in the Maurienne valley (Savoie, France), using purple moor grass growth parameters and leaf 15N/14N ratio. Environ Pollut. 2010;158:1652–1660. doi: 10.1016/j.envpol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Larsen Vilsholm R, Wolseley PA, Søchting U, Chimonides PJ. Biomonitoring with lichens on twigs. Lichenologist. 2009;41:189–202. doi: 10.1017/S0024282909007208. [DOI] [Google Scholar]
- Lee YI, Lim HS, Yoon HI. Carbon and nitrogen isotope composition of vegetation on King George Island, maritime Antarctic. Polar Biol. 2009;32:1607–1615. doi: 10.1007/s00300-009-0659-5. [DOI] [Google Scholar]
- Liu J, Zhang Y, Liu X, Tang A, Qiu H, Zhang F. Concentrations and isotopic characteristics of atmospheric reactive nitrogen around typical sources in Beijing, China. J Arid Land. 2016;8:910–920. doi: 10.1007/s40333-016-0020-0. [DOI] [Google Scholar]
- López-Veneroni D. The stable carbon isotope composition of PM2.5 and PM10 in Mexico City Metropolitan Area air. Atmos Environ. 2009;43:4491–4502. doi: 10.1016/j.atmosenv.2009.06.036. [DOI] [Google Scholar]
- Máguas C, Pinho P, Branquinho C, Hartard B, Lakatos M. Carbon-Water-Nitrogen relationships between lichens and the atmosphere: Tools to understand metabolism and ecosystem change. MycoKeys. 2013;6:95–106. doi: 10.3897/mycokeys.6.4814. [DOI] [Google Scholar]
- Manchester City Council (2016) Manchester’s state of the city report 2016 - transport. Manchester City Council, Manchester, pp 42–49
- Manchester City Council (2018) Air quality information and campaigns - nature and sources of air pollutants [WWW Document]. URL https://secure.manchester.gov.uk/info/100006/environmental_problems/2942/air_quality_information_and_campaigns/5. Accessed 12 Dec 2018
- Manchester City Council (2019) State of the city Rreport 2019. Manchester City Council [WWW Document], pp 1–197. URL https://www.manchester.gov.uk/downloads/download/7121/state_of_the_city_report_2019_whole_document. Accessed 24 Apr 2021
- Martin CL, Allan JD, Crosier J, Choularton TW, Coe H, Gallagher MW. Seasonal variation of fine particulate composition in the centre of a UK city. Atmos Environ. 2011;45:4379–4389. doi: 10.1016/j.atmosenv.2011.05.050. [DOI] [Google Scholar]
- Masiol M, Squizzato S, Formenton G, Harrison RM, Agostinelli C. Air quality across a European hotspot: Spatial gradients, seasonality, diurnal cycles and trends in the Veneto region, NE Italy. Sci Total Environ. 2017;576:210–224. doi: 10.1016/j.scitotenv.2016.10.042. [DOI] [PubMed] [Google Scholar]
- Met Office (2015) UK-climate: regional climates [WWW Document]. URL http://www.metoffice.gov.uk/climate/uk/regional-climates/nw (accessed 3.3.16)
- Met Office (2022) UK climate averages - Rochdale Climate Station (Greater Manchester) [WWW Document]. URL https://www.metoffice.gov.uk/research/climate/maps-and-data/uk-climate-averages/gcw2ymd6s (accessed 1.8.23)
- Minitab LLC (2019) Minitab 19 [Computer Software]. Available from: https://www.minitab.com
- Mitchell LE, Lin JC, Bowling DR, Pataki DE, Strong C, Schauer AJ, Bares R, Bush SE, Stephens BB, Mendoza D, Mallia D, Holland L, Gurney KR, Ehleringer JR. Long-term urban carbon dioxide observations reveal spatial and temporal dynamics related to urban characteristics and growth. Proc Natl Acad Sci. 2018;115:2912–2917. doi: 10.1073/pnas.1702393115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldanová J, Grennfelt P, Jonsson A, Simpson D, Spranger T, Aas W, Munthe J, Rabl A (2011) Nitrogen as a threat to European air quality. In: Stutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt P. van Grinsven H, Grizzetti B (Eds.), The European Nitrogen Assessment - Sources, Effects and Policy Perspectives. Cambridge, pp. 405–433. 10.1029/2003JD004173.Aires
- NAEI (2016) UK emissions data selector - Ammonia [WWW Document]. URL http://naei.beis.gov.uk/data/data-selector-results?q=114381 (accessed 10.1.19)
- NAEI (2018a) Pollutant Information: Carbon Monoxide [WWW Document]. URL http://naei.beis.gov.uk/overview/pollutants?pollutant_id=4 (accessed 7.13.18)
- NAEI (2018b) Pollutant Information: Sulphur Dioxide [WWW Document]. URL http://naei.beis.gov.uk/overview/pollutants?pollutant_id=8 (accessed 7.13.18)
- Nahm KH. Evaluation of the nitrogen content in poultry manure. Worlds Poult Sci J. 2005;59:77–88. doi: 10.1079/wps20030004. [DOI] [Google Scholar]
- Nash TH., III . Lichen Biology. 2. Cambridge, New York, Melbourne, Madrid, Cape Town, Sangapore, Sao Paulo, Delhi: Cambridge University Press; 2008. [Google Scholar]
- Nicholson FA, Chambers BJ, Smith KA. Nutrient composition of poultry manures in England and Wales. Bioresour Technol. 1996;58:279–284. doi: 10.1016/S0960-8524(97)86087-7. [DOI] [Google Scholar]
- Niepsch D, Clarke LJ, Tzoulas K, Cavan G. Spatiotemporal variability of nitrogen dioxide (NO2) pollution in Manchester (UK) city centre (2017–2018) using a fine spatial scale single-NOx diffusion tube network. Environ Geochem Health. 2021 doi: 10.1007/s10653-021-01149-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepsch D, Clarke LJ, Tzoulas K, Cavan G. Distinguishing atmospheric nitrogen compounds (nitrate and ammonium) in lichen biomonitoring studies. Environ Sci Process Impacts. 2021;23:2021–2036. doi: 10.1039/d1em00274k. [DOI] [PubMed] [Google Scholar]
- Norman A-L. Insights into the biogenic contribution to total sulphate in aerosol and precipitation in the Fraser Valley afforded by isotopes of sulphur and oxygen. J Geophys Res. 2004;109:D05311. doi: 10.1029/2002JD003072. [DOI] [Google Scholar]
- Nybakken L, Johansson O, Palmqvist K. Defensive compound concentration in boreal lichens in response to simulated nitrogen deposition. Glob Chang Biol. 2009;15:2247–2260. doi: 10.1111/j.1365-2486.2009.01853.x. [DOI] [Google Scholar]
- Olsen HB, Berthelsen K, Andersen HV, Søchting U. Xanthoria parietina as a monitor of ground-level ambient ammonia concentrations. Environ Pollut. 2010;158:455–461. doi: 10.1016/j.envpol.2009.08.025. [DOI] [PubMed] [Google Scholar]
- ONS (2021) Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland (Mid-2020) [Dataset], Office for National Statistics, accessible via: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland. Accessed 01-11-2022
- OriginLab (2018) Origin 2019, Version 9.6.0 [Computer Software]. OriginLab Corporation, Northampton, MA
- Palmqvist K, Dahlman L. Responses of the green algal foliose lichen Platismatia glauca to increased nitrogen supply. New Phytol. 2006;171:343–356. doi: 10.1111/j.1469-8137.2006.01754.x. [DOI] [PubMed] [Google Scholar]
- Paoli L, Vannini A, Fačkovcová Z, Guarnieri M, Bačkor M, Loppi S. One year of transplant: Is it enough for lichens to reflect the new atmospheric conditions? Ecol Indic. 2018;88:495–502. doi: 10.1016/j.ecolind.2018.01.043. [DOI] [Google Scholar]
- Pavlova EA, Maslov AI. Nitrate uptake by isolated bionts of the lichen Parmelia sulcata. Russ J Plant Physiol. 2008;55:475–479. doi: 10.1134/S1021443708040079. [DOI] [Google Scholar]
- Pazdur A, Kuc T, Pawełczyk S, Piotrowska N, Sensuła B, Rozanski K. Carbon isotope composition of atmospheric carbon dioxide in southern Poland: Imprint of anthropogenic CO2 emissions in regional biosphere. Radiocarbon. 2013;55:848–864. doi: 10.2458/azu_js_rc.55.16286. [DOI] [Google Scholar]
- Pearson J, Wells DM, Seller KJ, Bennett A, Soares A, Woodall J, Ingrouille MJ. Traffic exposure increases natural 15N and heavy metal concentrations in mosses. New Phytol. 2000;147:317–326. doi: 10.1046/j.1469-8137.2000.00702.x. [DOI] [Google Scholar]
- Pereira GW, Valente DSM, de Queiroz DM, Coelho de ALF, Costa MM, Grift T (2022) Smart-Map: An Open-Source QGIS Plugin for Digital Mapping Using Machine Learning Techniques and Ordinary Kriging. Agronomy 12. 10.3390/agronomy12061350
- Perraud V, Horne JR, Martinez AS, Kalinowski J, Meinardi S, Dawson ML, Wingen LM, Dabdub D, Blake DR, Gerber RB, Finlayson-Pitts BJ. The future of airborne sulfur-containing particles in the absence of fossil fuel sulfur dioxide emissions. Proc Natl Acad Sci U S A. 2015;112:13514–13519. doi: 10.1073/pnas.1510743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho P, Augusto S, Martins-Loução MA, Pereira MJ, Soares A, Máguas C, Branquinho C. Causes of change in nitrophytic and oligotrophic lichen species in a Mediterranean climate: Impact of land cover and atmospheric pollutants. Environ Pollut. 2008;154:380–389. doi: 10.1016/j.envpol.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Pinho P, Branquinho C, Cruz C, Tang YS, Dias T, Rosa AP, Máguas C, Martins-Loução MAS, Parto M-A, Sutton MA. Assessment of Critical Levels of Atmospheric Ammonia for Lichen Diversity in Cork-Oak Woodland, Portugal. In: Sutton MA, Reis S, Baker S, editors. Atmospheric Ammonia: Detecting Emission Changes and Environmental Impacts. Netherlands, Dordrecht: Springer; 2009. pp. 109–123. [Google Scholar]
- Pinho P, Barros C, Augusto S, Pereira MJ, Máguas C, Branquinho C. Using nitrogen concentration and isotopic composition in lichens to spatially assess the relative contribution of atmospheric nitrogen sources in complex landscapes. Environ Pollut. 2017;230:632–638. doi: 10.1016/j.envpol.2017.06.102. [DOI] [PubMed] [Google Scholar]
- QGIS Development Team (2022) QGIS geographic information system, Version 3.22.3 [Computer Software]. QGIS Association. http://www.qgis.org
- Quevauviller P, Herzig R, Muntau H (1996) The certification of the contents (mass fractions) of Al, As, Cd, Cr, Cu, Hg, Ni, Pb and Zn in lichen CRM 482. European Commission, bcr information - reference materials (EUR 1684 EN), Brussels, Luxembourg
- Ranković B, Kosanić M (2019) Lichens as a potential source of bioactive secondary metabolites. In: Ranković, Branislav (ed) Lichen secondary metabolites: bioactive properties and pharmaceutical potential. Springer, Cham. 10.1007/978-3-319-13374-4_1
- Razali NM, Wah YB. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J Stat Model Anal. 2011;2:21–33. doi: 10.1515/bile-2015-0008. [DOI] [Google Scholar]
- Red Rose Forest (2008) Manchester tree audit phase 2 (MTA2): the location and function of trees in the city - executive summary. Red Rose Forest and Manchester City Council, Salford, Manchester
- Regan D (2018) Manchester public health annual report 2018. Manchester City Council, Manchester Health & Care Commissioning (MHCC), Manchester, pp 1–34
- Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Sources of fine organic aerosol. 3. Road dust, tire debris, and organometallic brake lining dust: roads as sources and sinks. Environ Sci Technol. 1993;27:1892–1904. doi: 10.1021/es00046a019. [DOI] [Google Scholar]
- RStudio Team (2021) RStudio: integrated development for R [Computer Software]. RStudio, PBC, Boston, MA. http://www.rstudio.com/
- Salmond JA, Williams DE, Laing G, Kingham S, Dirks K, Longley I, Henshaw GS. The influence of vegetation on the horizontal and vertical distribution of pollutants in a street canyon. Sci Total Environ. 2013;443:287–298. doi: 10.1016/j.scitotenv.2012.10.101. [DOI] [PubMed] [Google Scholar]
- Salmond JA, McKendy IG (2009) Influences of Meteorology on Air Pollution Concentrations and Processes in Urban Areas. In: Harrison RM, Hester RE (Eds.), Air Quality in Urban Environments, Issues in Environmental Science and Technology. Royal Society of Chemistry, Cambridge, pp. 23–42. 10.1039/9781847559654
- Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung S-H, Mortimer K, Perez-Padilla R, Rice MB, Riojas-Rodriguez H, Sood A, Thurston GD, To T, Vanker A, Wuebbles DJ. Air Pollution and Noncommunicable Diseases; A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. Chest. 2019;155:409–416. doi: 10.1016/j.chest.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp Z (2017) Principles of Stable Isotope Geochemistry, 2nd ed. Pearson. 10.5072/FK2GB24S9F
- Shukla V, Upreti DK, Bajpai R. Lichens to Biomonitor the Environment, Lichens to Biomonitor the Environment. New Delhi: Springer India; 2014. [Google Scholar]
- Sicard P, Agathokleous E, De Marco A, Paoletti E, Calatayud V (2021) Urban population exposure to air pollution in Europe over the last decades. Environ Sci Eur 33. 10.1186/s12302-020-00450-2 [DOI] [PMC free article] [PubMed]
- Son JY, Lee JT, Kim KH, Jung K, Bell ML. Characterization of fine particulate matter and associations between particulate chemical constituents and mortality in Seoul, Korea. Environ Health Perspect. 2012;120:872–878. doi: 10.1289/ehp.1104316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrius LB. Response of epiphytic lichen communities to decreasing ammonia air concentrations in a moderately polluted area of The Netherlands. Environ Pollut. 2007;146:375–379. doi: 10.1016/j.envpol.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Spiro B, Morrison J, Purvis OW. Sulphur Isotopes in Lichens as Indicators of Sources. In: Nimis PL, Scheidegger C, Wolseley PA, editors. Monitoring with Lichens - Monitoring Lichens. Dordrecht: Springer Science + Business Media B.V; 2002. pp. 311–315. [Google Scholar]
- Stritzke F, Diemel O, Wagner S. TDLAS-based NH3 mole fraction measurement for exhaust diagnostics during selective catalytic reduction using a fiber-coupled 2.2-µm DFB diode laser. Appl Phys B. 2015;119:143–152. doi: 10.1007/s00340-015-6073-5. [DOI] [Google Scholar]
- Sturm P, Leuenberger M, Valentino FL, Lehmann B, Ihly B. Measurements of CO2, its stable isotopes, O2/N2, and Rn-222 at Bern, Switzerland. Atmos Chem Phys. 2006;6:1991–2004. doi: 10.5194/acp-6-1991-2006. [DOI] [Google Scholar]
- Sun K, Tao L, Miller DJ, Pan D, Golston LM, Zondlo MA, Griffin RJ, Wallace HW, Leong YJ, Yang MM, Zhang Y, Mauzerall DL, Zhu T. Vehicle Emissions as an Important Urban Ammonia Source in the United States and China. Environ Sci Technol. 2017;51:2472–2481. doi: 10.1021/acs.est.6b02805. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Pitcairn CER, Whitfield CP (2004) Bioindicator and biomonitoring methods for assessing the effects of atmospheric nitrogen on statutory nature conservation sites, JNCC Report No. 356. Peterborough. Accessible via: https://data.jncc.gov.uk/data/ff165313-018d-46b9-9c4d-3f3dc25d9a27/JNCC-Report-356-FINAL-WEB.pdf. Accessed 21 June 2016
- TfGM, GMCA (2016) Greater manchester low-emission strategy. Transport for greater manchester, greater manchester combined authority, greater Manchester. Accessible via: https://www.greatermanchester-ca.gov.uk/media/1276/low-emission-strategy-dec-2016.pdf. Accessed 15 Dec 2019
- The Royal College of Physicians (2016) Every breathe we take: the lifelong impact of air pollution - Report of a working party. London accessible via: https://www.rcplondon.ac.uk/projects/outputs/every-breath-we-take-lifelong-impact-air-pollution. Accessed 21 Jan 2017
- Tozer WC, Hackell D, Miers DB, Silvester WB. Extreme isotopic depletion of nitrogen in New Zealand lithophytes and epiphytes; the result of diffusive uptake of atmospheric ammonia? Oecologia. 2005;144:628–635. doi: 10.1007/s00442-005-0098-0. [DOI] [PubMed] [Google Scholar]
- Van der Wat L, Forbes PBC. Lichens as biomonitors for organic air pollutants. Trends Anal Chem. 2015;64:165–172. doi: 10.1016/j.trac.2014.09.006. [DOI] [Google Scholar]
- Van Herk CM. A changing lichen flora The effects of short and long distance nitrogen deposition on epiphytic lichens. In: Lambley P, Wolseley PA, editors. Lichens in a Changing Pollution Environment: English Nature Research Reports. Peterborough: English Nature; 2003. pp. 13–20. [Google Scholar]
- Van Herk CM, Mathijssen-Spiekman EAM, De Zwart D. Long distance nitrogen air pollution effects on lichens in Europe. Lichenologist. 2003;35:347–359. doi: 10.1016/S0024-2829(03)00036-7. [DOI] [Google Scholar]
- Vardoulakis S, Solazzo E, Lumbreras J. Intra-urban and street scale variability of BTEX, NO2 and O3 in Birmingham, UK: Implications for exposure assessment. Atmos Environ. 2011;45:5069–5078. doi: 10.1016/j.atmosenv.2011.06.038. [DOI] [Google Scholar]
- Vingiani S, Adamo P, Giordano S. Sulphur, nitrogen and carbon content of Sphagnum capillifolium and Pseudevernia furfuracea exposed in bags in the Naples urban area. Environ Pollut. 2004;129:145–158. doi: 10.1016/j.envpol.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Wadleigh MA. Lichens and atmospheric sulphur: What stable isotopes reveal. Environ Pollut. 2003;126:345–351. doi: 10.1016/S0269-7491(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Wadleigh MA, Blake DM. Tracing sources of atmospheric sulphur using epiphytic lichens. Environ Pollut. 1999;106:265–271. doi: 10.1016/S0269-7491(99)00114-1. [DOI] [PubMed] [Google Scholar]
- WHO . Regional Office for Europe: Air Quality Guidelines for Europe. 2. Switzerland: Geneva; 2000. [Google Scholar]
- WHO (2005) Air quality guidelines: global update 2005. Report on a working group meeting, Bonn, Germany, 8-20 October 2005. (EUR/05/5046029)
- WHO (2013) Health risks of air pollution in Europe – HRAPIE project. Recommendations for concentration–response functions for cost–benefit analysis of particulate matter, ozone and nitrogen dioxide. World Health Organization, Regional Office for Europe, Copenhagen
- WHO (2018) Air Pollution [WWW Document]. URL http://www.who.int/airpollution/en/ (accessed 6.11.18)
- Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag New York. https://ggplot2.tidyverse.org
- Widory D. Combustibles, fuels and their combustion products: A view through carbon isotopes. Combust Theory Model. 2006;10:831–841. doi: 10.1080/13647830600720264. [DOI] [Google Scholar]
- Widory D. Nitrogen isotopes: Tracers of origin and processes affecting PM10 in the atmosphere of Paris. Atmos Environ. 2007;41:2382–2390. doi: 10.1016/j.atmosenv.2006.11.009. [DOI] [Google Scholar]
- Wiseman RD, Wadleigh MA. Lichen response to changes in atmospheric sulphur: Isotopic evidence. Environ Pollut. 2002;116:235–241. doi: 10.1016/S0269-7491(01)00133-6. [DOI] [PubMed] [Google Scholar]
- Xu SY, Huang H, Song W, Liu XY (2021) Lichen nitrogen concentrations and isotopes for indicating nitrogen deposition levels and source changes. Sci Total Environ 787. 10.1016/j.scitotenv.2021.147616
- Zereini F, Wiseman CLS. Urban Airborne Particulate Matter, Urban Airborne Particulate Matter: Origin Chemistry, Fate and Health Impacts, Environmental Science and Engineering. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. [Google Scholar]
- Zhang Y, Tang A, Wang D, Wang Q, Benedict K, Zhang L, Liu D, Li Y, Collett JL, Jr, Sun Y, Liu X. The vertical variability of ammonia in urban Beijing, China. Atmos Chem Phys. 2018;18:16385–16398. doi: 10.5194/acp-18-16385-2018. [DOI] [Google Scholar]
- Zhou X, Zhang X, Wang B (2016) Online Support Vector Machine: A Survey. pp. 269–278. 10.1007/978-3-662-47926-1_26
- Zimnoch M, Florkowski T, Necki JM, Neubert REM. Diurnal variability of δ13C and δ18O of atmospheric CO2 in the urban atmosphere of Kraków, Poland. Isotopes Environ Health Stud. 2004;40:129–143. doi: 10.1080/10256010410001670989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in the article and its supplementary information.