Abstract
Background
For patients with stage IV non-small-cell lung cancer with epidermal growth factor receptor (EGFR) exon 19 deletions and exon 21 L858R mutations, osimertinib is the standard of care. Investigating the activity and safety of osimertinib in patients with EGFR exon 18 G719X, exon 20 S768I, or exon 21 L861Q mutations is of clinical interest.
Patients and methods
Patients with stage IV non-small-cell lung cancer with confirmed EGFR exon 18 G719X, exon 20 S768I, or exon 21 L861Q mutations were eligible. Patients were required to have measurable disease, an Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function. Patients were required to be EGFR tyrosine kinase inhibitor-naive. The primary objective was objective response rate, and secondary objectives were progression-free survival, safety, and overall survival. The study used a two-stage design with a plan to enroll 17 patients in the first stage, and the study was terminated after the first stage due to slow accrual.
Results
Between May 2018 and March 2020, 17 patients were enrolled and received study therapy. The median age of patients was 70 years (interquartile range 62-76), the majority were female (n = 11), had a performance status of 1 (n = 10), and five patients had brain metastases at baseline. The objective response rate was 47% [95% confidence interval (CI) 23% to 72%], and the radiographic responses observed were partial response (n = 8), stable disease (n = 8), and progressive disease (n = 1). The median progression-free survival was 10.5 months (95% CI 5.0-15.2 months), and the median OS was 13.8 months (95% CI 7.3-29.2 months). The median duration on treatment was 6.1 months (range 3.6-11.9 months), and the most common adverse events (regardless of attribution) were diarrhea, fatigue, anorexia, weight loss, and dyspnea.
Conclusions
This trial suggests osimertinib has activity in patients with these uncommon EGFR mutations.
Key words: osimertinib, epidermal growth factor receptor mutation, targeted therapy, non-small-cell lung cancer, uncommon EGFR mutations
Highlights
-
•
This trial was closed early due to slow accrual, which illustrates the challenges of trials in rare EGFR mutation subtypes.
-
•
Osimertinib demonstrated activity in patients with EGFR mutations of exon 18 G719X, exon 20 S768I, or exon 21 L861Q.
-
•
No novel adverse events were identified with osimertinib.
Historically, patients with metastatic non-small-cell lung cancer (NSCLC) were treated with platinum-based chemotherapy, which had a modest improvement in overall survival (OS). The field realized that platinum-based chemotherapy had reached a therapeutic plateau, and focused on developing targeted therapies.1 Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors were among the first targeted therapies developed, and they were initially investigated in patients who were not selected based on molecular characteristics.2,3 In the early trials, however, patients with a history of never or light smoking, adenocarcinoma, and Asian ancestry were observed to have a higher response rate.4 These observations led to the identification of sensitizing EGFR mutations, and clinical trials demonstrated the superiority of first- or second-generation EGFR tyrosine kinase inhibitors (TKIs) compared with chemotherapy in patients with a confirmed EGFR exon 19 deletion or exon 21 L858R mutation.5 A subsequent trial demonstrated the superiority of osimertinib compared with gefitinib or erlotinib in this patient population.6 EGFR exon 19 deletions and exon 21 L858R compromise the majority of the EGFR mutations and are especially sensitive to EGFR TKIs.
Additional EGFR mutations were identified as oncogenic drivers but were less sensitive to EGFR TKIs or associated primary resistance to EGFR TKIs (EGFR exon 20 insertion mutations). EGFR mutations, such as exon 18 G719X, exon 20 S768I, and exon 21 L861Q mutations were associated with lower response rates to EGFR TKIs. This subset of EGFR mutations comprise ∼10%-15% of EGFR mutations, and are often referred to as ‘uncommon EGFR mutations’ or ‘atypical EGFR mutations’.7 Preclinical data indicated that the sensitivity to EGFR TKIs varied depending on the EGFR mutation subtype and the specific EGFR TKI.8, 9, 10
When this trial was designed in 2017, the majority of the clinical data available for ‘uncommon’ mutations were from retrospective studies from patients treated in routine clinical care which included a heterogeneous group of EGFR mutations and EGFR TKIs.11, 12, 13 A post hoc analysis of three trials of afatinib in 38 patients with NSCLC with EGFR exon 18 G719X, exon 20 S768I, and exon 21 L861Q mutations reported an objective response rate (ORR) of 71% [95% confidence interval (CI) 54% to 84%], and a median progression-free survival (PFS) of 10.7 months (95% CI 5.6-14.7 months).14 However, approximately 40% of patients treated with afatinib 40 mg daily require a dose reduction, mainly related to EGFR-related adverse events, which raised concerns about the tolerability of this agent.15
Osimertinib, a third-generation EGFR TKI, had demonstrated superiority compared with chemotherapy in patients with disease progression after a first-generation EGFR TKI with an EGFR exon 19 deletion or exon 21 L858R mutation and an exon T790M mutation and subsequently in treatment-naive patients compared with first-generation EGFR TKIs.6,16 In these trials, osimertinib was observed to have a lower rate of EGFR-related adverse events compared with first- and second-generation EGFR TKIs. In the second-line trial, four patients with a G719X and one patient with S768I EGFR mutations received osimertinib, and patients with these mutations were excluded from the first-line trial.
Based on the limited data on the efficacy of osimertinib in patients with ‘uncommon mutations,’ we developed an investigator-initiated phase II trial of osimertinib in patients with specific EGFR mutations (exon 18 G719X, exon 20 S768I, or exon 21 L861Q). These mutations were selected based on the efficacy of afatinib in these specific mutations, and to reduce the heterogeneity in the patient population. The trial was originally designed as a two-stage trial; however, due to slow accrual which was exacerbated by the COVID-19 pandemic the trial closed after completion of the first stage. We report the final results for this trial, and the results are reported descriptively.
Materials and methods
Patients
Patients were required to have stage IV NSCLC, and EGFR mutation testing as carried out on a Clinical Laboratory Improvement Amendment certified laboratory test demonstrating EGFR mutation of exon 18 G719X, exon 20 S768I, or exon 21 L861Q. Patients with compound mutations (also referred to as multiple mutations) were eligible provided one of the specified mutations was present. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, and measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.17 Patients were required to have adequate hepatic, renal, and hematologic function on laboratory parameters. Patients were required to have a left ventricular ejection fraction ≥45% on echocardiogram, and normal QT interval on electrocardiograms (ECG) evaluation QT corrected interval of ≤450 ms in males or ≤470 ms in females obtained from three ECGs. Patients with treated brain metastases were eligible provided >14 days had elapsed from completion of radiotherapy and the treating physician determined the patient’s neurological symptoms were stable, and patients with untreated asymptomatic brain metastases were eligible.
Key exclusion criteria were prior therapy with EGFR TKI therapy, more than two lines of prior systemic therapy for metastatic NSCLC, and untreated and symptomatic brain metastases. Patients with concurrent EGFR mutation with exon 20 T790M, exon 19 deletion, exon 21 L858R mutation, or exon 20 insertion were excluded.
The institutional review boards of all the participating centers approved the study, and this trial was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. Patients were required to provide written informed consent before any study-related procedures.
Treatment administration and assessments
Patients received osimertinib 80 mg daily, and one cycle was defined as 28 days. Adverse events were assessed by the investigator using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 and with each cycle. Repeat ECGs were performed per institutional standard. Dose modifications for adverse events were defined in the protocol. Patients who experienced grade 3, intolerable grade 2 adverse events that did not resolve to grade ≤1 after 21 days, or had recurrent adverse grade 3 or intolerable grade 2 adverse events after dose reduction to 40 mg daily, discontinued study therapy. The patient’s medication diary was collected at each study visit.
Patients underwent computed tomography (CT) scans of the chest and abdomen, and brain magnetic resonance imaging scans were obtained at baseline. Patients had repeat assessment with CT scans of the chest and abdomen every two cycles. Patients without brain metastases underwent repeat brain imaging as clinically indicated, and patients with brain metastases underwent repeat imaging every three cycles.
Patients continued study therapy until disease progression, unacceptable toxicity, or withdrawal of informed consent.
Study outcomes
The primary study outcome was ORR as assessed by the investigator using RECIST 1.1. Secondary endpoints were PFS, which was defined from the start of study therapy until disease progression using RECIST 1.1 or death (whichever occurs first), and OS which was defined as from the start of study therapy until death. Exploratory endpoints were the ORR and PFS in patients with NSCLC with an EGFR mutation of the exon 18 G719X, exon 20 S768I, exon 21 L861Q, and compound mutations, as previously defined. A post hoc analysis of duration of response was carried out, which was defined from the start of study therapy to the end of response.
Study design and statistical analysis
This study was an investigator-initiated, multicenter, open label, single-arm phase II clinical trial. The safety analysis set included all patients who received at least one dose of osimertinib. A modified intent-to-treat was used for the efficacy analysis set, in which patients who withdrew consent or were ineligible before receiving study therapy were not included. The trial was designed to test the null hypothesis that the ORR was ≤20% against the alternative that the ORR was ≥40% with a one-sided type I error rate of 0.10 and ∼90% power. This study used a Simon’s optimal two-stage design, in which 17 patients were to be enrolled in the first stage and if three or fewer responses were observed, the study would be terminated.18 If four or more responses were observed, the study would enroll an additional 20 patients for a total of 37 patients. Due to slow accrual, however, the study was terminated after the first stage. The results are reported descriptively, and formal hypothesis testing was not carried out. Time-to-event endpoints are summarized using the Kaplan–Meier method, and 95% CIs using Greenwood’s formula. The ORR rate was estimated and its 95% CI was computed using the Clopper–Pearson method. Statistical analyses were carried out using SAS version 9.4. This study is registered with ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT03434418).
Results
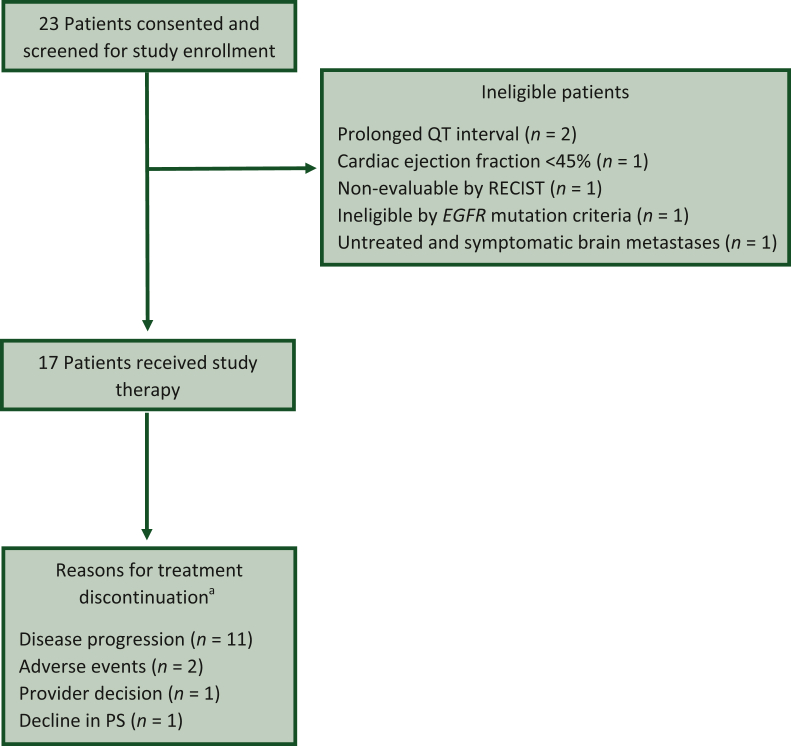
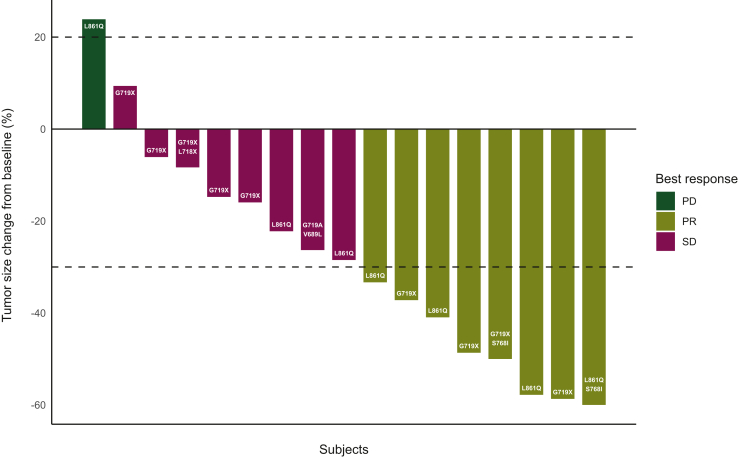
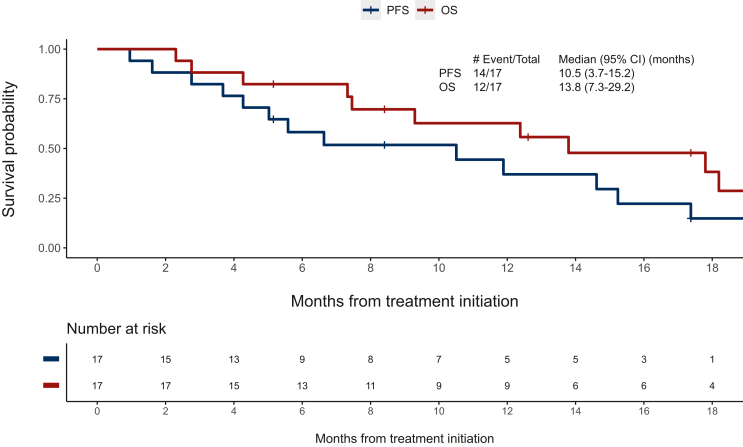
Between May 2018 and March 2020, 23 patients underwent study screening, and 17 eligible patients were enrolled and received study therapy (Figure 1). Data analysis was carried out as of 16 September 2022. The median age of patients was 70 years [interquartile range (IQR) 62-76 years], the majority were female (n = 11), had a PS of 1 (n = 10), and five patients had brain metastases at baseline (Table 1). Of the five patients with baseline brain metastases, three received radiation therapy for the brain metastases and two were asymptomatic and untreated at baseline. The most common EGFR mutation was G719X (n = 7), L861Q (n = 6), and compound mutations (n = 4). The ORR was 47% (95% CI 23% to 72%). Radiographic responses observed were partial response (n = 8), stable disease (n = 8), and progressive disease (n = 1) (Figure 2). With a median follow-up of 12.6 months (range 5.1-31.0 months), 14 of the 17 patients have experienced a PFS event, and the median PFS was 10.5 months (95% CI 5.0-15.2 months), and 12 of the 17 patients have died and the median OS was 13.8 months (95% CI 9.3-29.2 months) (Figure 3). The median duration of response (n = 8) was 8.7 months (range 1.9-13.9 months). The exploratory analysis of the efficacy by mutation subtype is presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101183. In the subset of patients with brain metastases at baseline (n = 5), the ORR was 40% (95% CI 5% to 85%), the median duration of response was 8.8 months (3.8 months in one patient and 13.9 months in one patient), the median PFS was 6.6 months (95% CI 1.6 months to not evaluable), and the median OS was 13.8 (95% CI 4.3 to not evaluable).
Figure 1.
Patient disposition. EGFR, epidermal growth factor receptor; PS, performance status; RECIST, Response Evaluation Criteria in Solid Tumors. aTwo patients remain on treatment at the time of the analysis.
Table 1.
Patient characteristics
| Characteristic | Total patients (n = 17) |
|---|---|
| Median age (years) | 70 (IQR 62-76) |
| Gender | |
| Female | 11 |
| Male | 6 |
| Race | |
| White | 12 |
| Black or African American | 3 |
| Asian | 2 |
| ECOG performance status | |
| 0 | 7 |
| 1 | 10 |
| EGFR mutation subtype | |
| G719X | 7 |
| L861Q | 6 |
| G719X/L718X | 1 |
| G719X/S768I | 1 |
| L861Q/S768I | 1 |
| G719X/V689L | 1 |
| Location of metastasesa | |
| Bone | 8 |
| Brain | 5 |
| Liver | 5 |
| Lung | 4 |
| Lymph node | 8 |
| Pleura | 5 |
IQR, interquartile range.
Patients could have more than one location for metastases.
Figure 2.
Waterfall plot for target lesion tumor size.
PD, progressive disease; PR, partial response; SD, stable disease.
Figure 3.
Progression-free survival (PFS) and overall survival (OS) Kaplan–Meier curves.
CI, confidence interval.
The median duration on treatment was 6.1 months (range 3.6-11.9 months), and the adverse events (regardless of attribution) observed in two or more patients are presented in Table 2. The most common all grade adverse events (regardless of attribution) observed were diarrhea (n = 13), fatigue (n = 9), anorexia (n = 7), weight loss (n = 7), dyspnea (n = 6), vomiting (n = 5), abdominal pain (n = 5), cough (n = 4), acneiform rash (n = 4), and maculopapular rash (n = 4). Two grade 4 events were observed (respiratory failure and thromboembolic event), and no grade 5 events were observed. Of the 17 patients enrolled, 2 patients remain on therapy at the time of analysis and 15 discontinued therapy. The most common reasons for treatment discontinuation were disease progression (n = 11), adverse events (n = 2), decline in PS (n = 1), and provider decision (n = 1) (Figure 1). The specific adverse events that lead to treatment discontinuation were grade 3 QT prolongation and grade 3 infection.
Table 2.
Adverse events
| Adverse event,n(%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Abdominal pain | 1 (6) | 4 (24) | 0 | 0 |
| Anorexia | 2 (12) | 5 (29) | 0 | 0 |
| Bronchial infection | 0 | 3 (18) | 0 | 0 |
| Bruising | 2 (12) | 0 | 0 | 0 |
| Constipation | 2 (12) | 1 (6) | 0 | 0 |
| Cough | 2 (12) | 0 | 1 (6) | 0 |
| Creatinine increase | 1 (6) | 1 (6) | 0 | 0 |
| Depression | 0 | 2 (12) | 0 | 0 |
| Diarrhea | 8 (47) | 4 (24) | 1(6) | 0 |
| Dizziness | 1 (6) | 1 (6) | 0 | 0 |
| Dry mouth | 3 (18) | 1 (6) | 0 | 0 |
| Dry skin | 3 (18) | 1 (6) | 0 | 0 |
| Dysgeusia | 2 (12) | 0 | 0 | 0 |
| Dyspepsia | 1 (6) | 1 (6) | 0 | 0 |
| Dyspnea | 1 (6) | 5 (29) | 0 | 0 |
| Edema (limbs) | 2 (12) | 0 | 0 | 0 |
| Epistaxis | 2 (12) | 0 | 0 | 0 |
| Fall | 1 (6) | 2 (12) | 0 | 0 |
| Fatigue | 5 (29) | 4 (23) | 0 | 0 |
| Flank pain | 2 (12) | 0 | 0 | 0 |
| Post-nasal drip | 2 (12) | 0 | 0 | 0 |
| Productive cough | 2 (12) | 1 (6) | 1 (6) | 0 |
| QT interval prolonged | 0 | 0 | 2 (12) | 0 |
| Rash acneiform | 4 (23) | 0 | 0 | 0 |
| Rash maculopapular | 4 (23) | 0 | 0 | 0 |
| Thromboembolic event | 0 | 0 | 2 (12) | 1 (6) |
| Tumor pain | 1 (6) | 1 (6) | 0 | 0 |
| Urinary tract infection | 0 | 2 (12) | 0 | 0 |
| Vomiting | 2 (12) | 3 (18) | 0 | 0 |
| Weight loss | 5 (29) | 1 (6) | 1 (6) | 0 |
Discussion
Unfortunately, this trial was closed before completing the intended accrual, and the data are observational. The ORR of 47% (95% CI 23% to 72%), and the median PFS of 10.5 months, however, demonstrate promising activity. Of note, the lower limit of the ORR is greater than the ORR of 20% which was set to be not worthy for further investigation specified at the time of study design. We did not accrue enough patients to determine whether there was a trend for greater activity for a specific mutation which limits the value of the exploratory analyses.
The adverse events were collected regardless of attribution, and this decision was based on the premise that attributing causality between treatment- and disease-related adverse events can be difficult. The higher rate of adverse events observed may be related to lower response rate observed in this patient population compared with patients with an EGFR exon 19 deletion and exon 21 L858R mutation resulting in more disease-related adverse events. The most common reason for treatment discontinuation was disease progression, and only two patients discontinued treatment due to adverse events. One of the events, QT prolongation, was likely treatment related and the other event was an infection which was not related to treatment. If we focus on the specific EGFR-related adverse events of rash and diarrhea, the rate of these events is similar to those in other studies of osimertinib.
At the time this trial was designed in 2017, data of the efficacy of osimertinib were lacking, but since that time another single-arm phase II trial revealed the activity of osimertinib in uncommon mutations (n = 36).19 The ORR was 50% (18 of 36 patients; 95% CI 33% to 67%). Median PFS was 8.2 months (95% CI 5.9-10.5 months), similar to our study. The most common adverse events (any grade) observed in that study were rash, pruritus, decreased appetite, diarrhea, and dyspnea. Thus, the two studies reveal similar activity and adverse events.
A retrospective study of 299 patients treated on clinical trials with osimertinib, identified 21 patients with these mutations.20 Ten patients were previously treated with an EGFR TKI, and three had a T790M resistance mutation. The entire cohort ORR was 47.6% (95% CI 25.7% to 70.2%) and the median PFS was 5.5 months (95% CI 4.2-6.7 months). In the cohort of patients with first-line osimertinib (n = 11) the ORR was 63.6% (95% CI 30.8% to 89.1%) and the median PFS was 5.5 months. A retrospective, global, multicenter study reviewed existing health records identified consecutive EGFR TKI-naive patients with uncommon EGFR mutations.21 Two hundred and forty-six patients were identified from nine countries, and only six patients received osimertinib and were assessable for response. These experiences suggest that obtaining data on the activity of osimertinib in retrospective studies will be difficult.
Ultimately, multiple factors likely contributed to the slow accrual. In retrospect, given the prevalence of uncommon EGFR mutations in the United States we should have designed the trial to have 5-10 study sites. An ongoing single-arm phase II trial with a similar design in Japan will enroll 40 patients, and plans on having 30 study sites.22 We also selected the EGFR mutation subtype based on the patients eligible to receive afatinib, so there would be similarity in the molecular characteristics of the patient population. We could have included patients with EGFR exon 18 E709X mutations, which have been reported to be sensitive to first-generation EGFR TKIs.8 One challenge is that there is no agreed upon definition of ‘uncommon’ EGFR mutation, and developing a consensus definition of this term would facilitate future research. Another mechanism of prospectively collecting data in this patient population is international registries which have been used for the patients with RET fusions and NRG1 fusions.23,24 In our trial we limited enrollment to patients with an ECOG PS of 0 or 1, and more recent targeted therapy trials have allowed enrollment of patients with ECOG PS of 2. A broader PS eligibility criterion may have facilitated enrollment and made the study a better reflection of the patients who are seen in routine clinical care.
Although our trial did not complete accrual, we hope the data obtained will help in the development of future trials or be combined with other trials to develop a better understanding of the clinical benefit of osimertinib in this patient population.
Funding
This work was supported by AstraZeneca (no grant number).
Disclosure
LCV reports consulting fees from: Janssen, Daiichi Sankyo, Takeda, Jazz Pharmaceuticals, Bristol Myers Squibb, InterVenn Bio, Sanofi.
EMB reports advisory board and consulting fees from Pfizer.
TFB reports grants to the institution for investigator-initiated trial from Novartis; advisory board from Blueprint Medicines Corporation, EMD Serono Inc., Foundation Medicine, Janssen Scientific Affairs, LLC, Mirati Therapeutics; data and safety monitoring board Advarra, Inc.
JC reports support for clinical trials from Bristol Myers Squibb, Genentech, Spectrum, Adaptimmune, MedPacto, Bayer, AbbVie, Moderna, GlaxoSmithKline, Array, AstraZeneca, Grid Therapeutics, CBMG; advisory board fees from AstraZeneca, Merck, Pfizer, NGM Biopharmaceuticals, Spectrum Genentech Novartis, Turning Point, G1 Therapeutics, Vivacitas, Omega Therapeutics; support for attending meetings and/or travel from Merck, AstraZeneca, Pfizer, NGM Bio; participation on a data safety monitoring board or advisory board from G1 Therapeutics; leadership in other board, society with Lung Cancer Initiative of North Carolina (uncompensated).
JC reports grants or contracts from any entity National Clinical Trials Network (NCTN) Lead Academic Participating Site (LAPS) grant, AstraZeneca, Helsinn, Pfizer (to institution); consulting fees from AstraZeneca, AVEO, Faraday, G1 Therapeutics, Jazz Pharmaceuticals, Merck, Partner Therapeutics, Sandoz; participation on a data safety monitoring board or advisory board from SeaGen, BioAtla, NIA.
GAO reports grants to the institution from Bristol Myers Squibb, Merck, AstraZeneca, Genentech, AbbVie, Revolution Medicine Dizal, Elevation Oncology; participation on a data safety monitoring board Novocure, BeiGene.
SA reports grants from the US Department of Defense and the US National Cancer Institute; royalties or license from Moffitt Cancer Center/Memgen; consulting fees from scientific advisory board for RAPT Therapeutics, Venn Therapeutics, Shoreline Bioscience, Achilles Therapeutics, Memgen, Glympse, Xilis, Immutep; payment for lectures: Society of Immunotherapy for Cancer (SITC); travel support from RAPT Therapeutics; patents: Moffitt Cancer Center licensed to Memgen; data safety monitoring board or advisory board: EMD Serano; stock or stock options: Shoreline Bioscience, RAPT Therapeutics, Achilles Therapeutics, Celsius Therapeutics.
NER reports consulting fees from Bristol Myers, Merck, Regeneron, Roche, G1 Therapeutics, AstraZeneca, Jazz Pharmaceuticals, Janssen.
AJW reports consulting fees from Janssen, Incyte, GlaxoSmithKline, Novocure, Beigene, Bristol Myers Squibb, Daiichi, Spectrum; payment or honoraria for lectures Aptitude Health, OncLive; participation on a data safety monitoring board or advisory board from BeyondSpring, Odonate Therapeutics, Genentech; leadership or fiduciary role in other board, society, committee from MARF Lung Cancer Research Foundation.
TES reports grants from AstraZeneca, Takeda, Regeneron, Seagen, Mirati Therapeutics, Genentech/Roche (to the institution), participation on a data safety monitoring board or advisory board from EMD Serono, Janssen Oncology, Turning Point Therapeutics, Sanofi/Aventis, GlaxoSmithKline, Genentech/Roche, Daiichi Sankyo/AstraZeneca, Takeda, Eisai/H3 Biomedicine, G1 Therapeutics, Spectrum Pharmaceuticals.
All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Schiller J.H., Harrington D., Belani C.P., et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd F.A., Rodrigues Pereira J., Ciuleanu T., et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Thatcher N., Chang A., Parikh P., et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 4.Paez J.G., Janne P.A., Lee J.C., et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Mok T.S., Wu Y.L., Thongprasert S., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 6.Ramalingam S.S., Vansteenkiste J., Planchard D., et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 7.Costa D.B. Kinase inhibitor-responsive genotypes in EGFR mutated lung adenocarcinomas: moving past common point mutations or indels into uncommon kinase domain duplications and rearrangements. Transl Lung Cancer Res. 2016;5:331–337. doi: 10.21037/tlcr.2016.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi Y., Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci. 2016;107:1179–1186. doi: 10.1111/cas.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banno E., Togashi Y., Nakamura Y., et al. Sensitivities to various epidermal growth factor receptor-tyrosine kinase inhibitors of uncommon epidermal growth factor receptor mutations L861Q and S768I: what is the optimal epidermal growth factor receptor-tyrosine kinase inhibitor? Cancer Sci. 2016;107:1134–1140. doi: 10.1111/cas.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun C.H., Boggon T.J., Li Y., et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu C.H., Yang C.T., Shih J.Y., et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol. 2015;10:793–799. doi: 10.1097/JTO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 12.Baek J.H., Sun J.M., Min Y.J., et al. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR-mutated non-small cell lung cancer except both exon 19 deletion and exon 21 L858R: a retrospective analysis in Korea. Lung Cancer. 2015;87:148–154. doi: 10.1016/j.lungcan.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Arrieta O., Cardona A.F., Corrales L., et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer. 2015;87:169–175. doi: 10.1016/j.lungcan.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Yang J.C., Sequist L.V., Geater S.L., et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16:830–838. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 15.Yang J.C., Sequist L.V., Zhou C., et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol. 2016;27:2103–2110. doi: 10.1093/annonc/mdw322. [DOI] [PubMed] [Google Scholar]
- 16.Mok T.S., Wu Y.L., Ahn M.J., et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 19.Cho J.H., Lim S.H., An H.J., et al. Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR mutations: a multicenter, open-label, phase II trial (KCSG-LU15-09) J Clin Oncol. 2020;38:488–495. doi: 10.1200/JCO.19.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eide I.J.Z., Stensgaard S., Helland A., et al. Osimertinib in non-small cell lung cancer with uncommon EGFR-mutations: a post-hoc subgroup analysis with pooled data from two phase II clinical trials. Transl Lung Cancer Res. 2022;11:953–963. doi: 10.21037/tlcr-21-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popat S., Hsia T.C., Hung J.Y., et al. Tyrosine kinase inhibitor activity in patients with NSCLC harboring uncommon EGFR mutations: a retrospective international cohort study (UpSwinG) Oncologist. 2022;27:255–265. doi: 10.1093/oncolo/oyac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuma Y., Shimokawa M., Hashimoto K., et al. Uncommon EGFR mutations conducted with osimertinib in patients with NSCLC: a study protocol of phase 2 study (UNICORN/TCOG1901) Future Oncol. 2022;18:523–531. doi: 10.2217/fon-2021-0892. [DOI] [PubMed] [Google Scholar]
- 23.Drilon A., Duruisseaux M., Han J.Y., et al. Clinicopathologic features and response to therapy of NRG1 fusion-driven lung cancers: the eNRGy1 global multicenter registry. J Clin Oncol. 2021;39:2791–2802. doi: 10.1200/JCO.20.03307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautschi O., Milia J., Filleron T., et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35:1403–1410. doi: 10.1200/JCO.2016.70.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.