Abstract
Background
Acquired resistance limits long-term epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) efficacy in patients with EGFR mutation-positive non-small-cell lung cancer (NSCLC) in whom anti-programmed death-ligand 1 (PD-L1) efficacy is also limited. We hypothesized that combining atezolizumab with erlotinib could enhance antitumor immunity and extend efficacy in these patients.
Patients and methods
This open-label phase Ib trial was conducted in adults aged ≥18 years who had advanced, unresectable NSCLC. Stage 1 (safety evaluation) enrolled EGFR TKI-naive patients regardless of EGFR status. Stage 2 (expansion) enrolled patients with EGFR-mutant NSCLC treated with ≤1 prior non-EGFR TKI therapy. Patients received 150 mg erlotinib orally once daily. After a 7-day erlotinib run-in, atezolizumab 1200 mg was administered intravenously every 3 weeks. The primary endpoint was the safety and tolerability of the combination in all patients; secondary endpoints included antitumor activity per RECIST 1.1 in stage 2 patients.
Results
At the data cut-off on 7 May 2020, 28 patients (8 in stage 1, 20 in stage 2) were assessable for safety. No dose-limiting toxicities or grade 4 or 5 treatment-related adverse events occurred. Grade 3 treatment-related adverse events occurred in 46% of patients; the most common were increased alanine aminotransferase, diarrhea, pyrexia, and rash (each in 7% of patients). Serious adverse events occurred in 50% of patients. Pneumonitis (grade 1) was reported in a single patient (4%). The objective response rate was 75% [95% confidence interval (CI) 50.9% to 91.3%]), median response duration was 18.9 months (95% CI 9.5-40.5 months), median progression-free survival was 15.4 months (95% CI 8.4-39.0 months), and median overall survival was not estimable (NE) (95% CI 34.6-NE).
Conclusions
Atezolizumab combined with erlotinib demonstrated a tolerable safety profile and encouraging, durable clinical activity in patients with advanced EGFR mutation-positive NSCLC.
Key words: EGFR-mutant NSCLC, PD-L1 inhibitor, immune checkpoint inhibitor, tyrosine kinase inhibitor
Highlights
-
•
We hypothesized that combining atezolizumab and erlotinib may enhance antitumor immunity and efficacy in EGFR+ NSCLC.
-
•
This open-label phase Ib trial of atezolizumab plus erlotinib involved 28 patients with advanced pretreated NSCLC.
-
•
No dose-limiting or grade 4 or 5 treatment-related adverse events (AEs) occurred; serious AEs occurred in 50% of patients.
-
•
Response rate was 75%, progression-free survival 15.4 months, and median overall survival not reached in EGFR+ patients.
-
•
Atezolizumab plus erlotinib was tolerable and showed durable clinical activity in some patients with advanced EGFR+ NSCLC.
Introduction
Lung cancer remains the leading cause of cancer mortality worldwide, with non-small-cell lung cancer (NSCLC) accounting for 80% to 85% of cases.1 Epidermal growth factor receptor (EGFR) mutations are present in ∼15% of patients with NSCLC in the USA and in up to 50% of patients of Asian descent; 90% of all patients with EGFR-mutant NSCLC have mutations associated with sensitivity to EGFR tyrosine kinase inhibitors (TKIs).2
TKI therapy is recommended as first-line treatment of patients with NSCLC whose tumors harbor an oncogenic EGFR mutation.3,4 Approved EGFR TKIs include the first-generation agents erlotinib and gefitinib, second-generation agents afatinib and dacomitinib, and the third-generation agent osimertinib.5 Substantial efficacy has been seen with these agents, with objective response rates (ORRs) of 56% to 83%, median progression-free survival (PFS) of 9.2-18.9 months, and median overall survival (OS) of 19.3-38.6 months.5,6 Nevertheless, despite the high-level activity observed with these TKIs in patients with EGFR-mutant NSCLC, the resulting clinical benefits are almost always transient; acquired resistance is typically inevitable regardless of the agent used in the first-line setting,7, 8, 9, 10 highlighting the need for more effective treatment options for these patients.
Recent advances in the development of immune checkpoint inhibitors (ICIs), including monoclonal antibodies targeting programmed death-ligand 1 (PD-L1) and programmed cell death protein 1 (PD-1), have significantly improved treatment outcomes in NSCLC. Patients whose tumors harbor EGFR mutations, however, experience limited efficacy with PD-L1/PD-1 inhibition relative to those with wild-type tumors. Single-agent PD-L1 and PD-1 inhibitors have not shown a significant survival benefit compared with cytotoxic agents such as docetaxel in patients with previously treated EGFR-mutant disease.11, 12, 13, 14
The transient nature of TKI benefit has prompted attempts to improve the durability of response to EGFR TKIs. One such strategy, combining EGFR TKIs with PD-L1/PD-1 inhibition, is predicated on preclinical and correlative analyses demonstrating that PD-L1 is frequently up-regulated in NSCLC tumors with activating EGFR mutations, and that activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung cancer models.15, 16, 17, 18 Initial studies evaluating combinations of EGFR TKIs and PD-L1/PD-1 inhibitors in NSCLC suggested rare durable responses,19 but have been limited by unanticipated combinatorial toxicities, including grade 3/4 liver toxicity in >40% of patients treated with gefitinib and either durvalumab or pembrolizumab, and interstitial lung disease (ILD) in patients treated with osimertinib and durvalumab.20, 21, 22 The high incidence of ILD in particular resulted in early termination of the CAURAL trial, which had been designed to test osimertinib and durvalumab versus osimertinib monotherapy.23
Atezolizumab is an anti-PD-L1 monoclonal antibody that inhibits binding of PD-L1 to its receptors, PD-1 and B7-1.24 Atezolizumab is currently approved as first-, second-, and later-line treatment of advanced or metastatic NSCLC, and as adjuvant treatment in patients with PD-L1-positive early-stage NSCLC, after demonstrating efficacy and safety alone and in combination with chemotherapy, with or without bevacizumab.14,25, 26, 27, 28, 29, 30 Atezolizumab monotherapy is approved in both the USA and Europe for treatment of patients with EGFR-mutant NSCLC who have had disease progression on an approved TKI therapy.25,31, 32, 33
In the global, randomized phase III IMpower150 trial, first-line treatment with atezolizumab, bevacizumab, carboplatin, and paclitaxel prolonged PFS and OS versus bevacizumab, carboplatin, and paclitaxel in non-squamous advanced or metastatic NSCLC, including for patients with EGFR-mutant tumors.26,34 Subgroup analyses of IMpower150 patients suggested an OS benefit for atezolizumab, bevacizumab, carboplatin, and paclitaxel versus bevacizumab, carboplatin, and paclitaxel in patients with sensitizing EGFR mutations, including those with prior TKI failures.34,35 These results support the hypothesis that combining atezolizumab with other targeting agents such as EGFR TKIs could offer durable treatment benefits for patients with EGFR-mutant NSCLC.
This open-label, multicenter phase Ib study was conducted to evaluate the safety and clinical activity of atezolizumab in combination with erlotinib in EGFR TKI treatment-naive patients with advanced NSCLC. Erlotinib inhibits epidermal growth factor-dependent proliferation of cells at submicromolar concentrations, blocks cell cycle progression in the G1 phase, and can promote cell death in EGFR-dependent tumors.36 Additionally, like other EGFR TKIs, erlotinib can influence immune/inflammatory responses by directly modulating major histocompatibility complex expression, which may enhance priming and activation of effector T-cell responses.37 Therefore, we hypothesized that combining erlotinib with atezolizumab might provide durable antitumor effects in patients with EGFR-mutant NSCLC.
Methods
Study design, patients, and treatment
This open-label, multicenter, global phase Ib trial (NCT02013219) was conducted to investigate the safety, tolerability, and clinical activity of atezolizumab combined with erlotinib in patients with advanced, unresectable NSCLC. During the safety evaluation stage (stage 1), patients with advanced NSCLC who were EGFR TKI treatment-naive were enrolled regardless of EGFR mutation status. This stage was designed to establish the safety and tolerability of the combination and to identify a recommended phase II dose (RP2D) and schedule for atezolizumab in combination with erlotinib. The expansion stage (stage 2) was conducted to evaluate the dose combination established as RP2D in stage 1 in an expansion cohort of patients with EGFR mutation-positive advanced NSCLC who had not been previously treated or who had received one prior treatment that was not an EGFR TKI.
Eligible patients were ≥18 years of age and had histologically or cytologically documented advanced or metastatic NSCLC, measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 and an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Patients in stage 1 were not required to have tumors with EGFR mutations and could have received prior systemic treatment (except for EGFR TKIs) for advanced or metastatic disease. Patients in stage 2 were required to have EGFR-mutant tumors and could have received at most one prior systemic treatment (except for EGFR TKIs) for advanced or metastatic disease. Key exclusion criteria included prior treatment with any EGFR TKI, known primary central nervous system metastases, and prior treatment with approved anticancer agents within 3 weeks of study initiation or with any other investigational agent(s) within 4 weeks of randomization.
The study conformed to Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Independent ethics committees or institutional review boards approved the study protocol at each site, and all patients provided written informed consent to participate in the study. The trial was registered with ClinicalTrials.gov (NCT02013219).
The study design called for the first patient in stage 1 to be given a starting dose of 150 mg erlotinib (i.e. the recommended single-agent dose) orally once daily. Following a 1-week run-in period of erlotinib, a starting dose of 1200 mg atezolizumab was to be administered intravenously every 3 weeks. If the first patient did not experience unacceptable toxicity during the first 3 weeks of receiving the combination therapy, the remaining patients would be enrolled into stage 1 and receive the same combination regimen. If the starting combination regimen was not tolerated, alternative dosing and/or schedules of atezolizumab and erlotinib would be tested to define the RP2D.
The dose-limiting toxicity (DLT) window in stage 1 was 21 days, starting from the first administration of atezolizumab plus erlotinib. A DLT was defined as treatment-related grade ≥4 neutropenia lasting ≥7 days; grade ≥4 thrombocytopenia lasting ≥7 days or associated with bleeding; grade ≥3 non-hematologic, non-hepatic organ toxicity; grade ≥3 symptomatic hepatic toxicities lasting ≥48 h; or grade ≥3 asymptomatic hepatic toxicities lasting >7 days, with exceptions. The highest dose level(s) at which less than one-third of the first dose cohort planned to be enrolled in stage 1 experienced a DLT was declared the combination maximally tolerated dose and defined the RP2D. All patients enrolled in stage 2 began treatment at the RP2D for this combination.
Treatment could continue in both stages for as long as a patient experienced clinical benefit, as determined by the investigator after assessment of radiographic data, biopsy results, and the patient’s clinical status. Patients who discontinued atezolizumab for reasons other than disease progression could continue erlotinib treatment on study until disease progression per RECIST 1.1.
Assessments and endpoints
The primary safety endpoint was the incidence and nature of DLTs, with secondary endpoints being the incidence, nature, and severity of adverse events (AEs) according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, as well as changes in vital signs and laboratory abnormalities. AEs of any cause were recorded until 30 days after the last dose of the study treatment or until study discontinuation/termination, initiation of subsequent anticancer therapy, consent withdrawal, or death, whichever occurred first. Patients continuing to experience treatment-related adverse events (TRAEs) beyond 30 days following the last dose of study treatment were followed until the event had resolved to baseline grade, the event was stable per investigator assessment, or the patient had begun a subsequent anticancer treatment, whichever occurred first.
Clinical activity endpoints evaluated were investigator-assessed ORR and PFS per RECIST 1.1, as well as duration of confirmed objective response [duration of response (DOR)] and OS.
Tumors were assessed by computed tomography scan at baseline; 7 weeks after treatment start (cycle 1, day 1); every 6 weeks thereafter through week 37; and every 12 weeks thereafter until radiologic disease progression per RECIST 1.1, patient death, consent withdrawal, or termination of study by sponsor, whichever occurred first. Responses were documented as complete response (CR), partial response (PR), stable disease, or progressive disease per RECIST 1.1.
Statistical analysis
It was planned that approximately 26 to 32 patients would be enrolled into the study, with 6-12 patients in the safety evaluation stage and ∼20 in the expansion stage. Determination of sample sizes was based on goals of obtaining preliminary safety, efficacy, pharmacokinetic, and pharmacodynamic data; explicit power and type I error considerations were not made for this study.
Analyses were carried out in either the safety-evaluable population, defined as all patients who received any amount of any study treatment, or the efficacy-evaluable population, defined as all patients who received at least one dose of atezolizumab plus erlotinib, and had measurable disease per RECIST 1.1. Means, standard deviations, medians, and ranges were used to summarize continuous variables, and categorical variables were summarized using counts and percentages. All summaries were tabulated or listed by dose cohort. Safety was assessed through summaries of AEs by mapped term and National Cancer Institute Common Terminology Criteria for Adverse Events toxicity grade, changes in laboratory test results, and changes in vital signs.
Data on ORR, DOR, PFS, and OS were listed for all patients by study stage and summarized overall. ORR was defined as the proportion of patients with a PR or CR, as determined by investigator assessment per RECIST 1.1 and confirmed by repeat assessments ≥4 weeks after initial documentation. Patients who did not meet these criteria, including those without any post-baseline tumor assessment, were considered to be non-responders. DOR for patients who achieved an objective response was defined as the time from the initial CR or PR to the time of disease progression or death, whichever occurred first. DOR for patients who did not experience disease progression or death before the end of the study was censored at the day of the last tumor assessment. PFS was defined as the time from the first day of study treatment to disease progression or death, whichever occurred first; PFS was censored at the day of the last tumor assessment for patients who did not experience disease progression or death at the end of the study. OS was defined as the time from the first dose of study treatment to the time of death from any cause during the study. Time to event analyses for DOR, PFS, and OS were done using the Kaplan–Meier method, with accompanying 95% confidence intervals (CIs) constructed using the Brookmeyer and Crowley method.
Results
Patient characteristics
Between 3 April 2014 and 12 August 2015, 28 patients were enrolled into this study at 10 sites in Spain, France, Great Britain, Hong Kong, and the USA; 8 patients were enrolled into stage 1 and 20 patients into stage 2. The median age of the study population was 61 years (range 47-84 years); 20 patients (71%) were women, 21 (75%) had ECOG PS of 1, and 12 (43%) had never smoked (Table 1). Ten patients (36%) had prior cancer therapy and 12 (48%) had prior radiotherapy. Two patients (28%) in stage 1 had EGFR mutations. In stage 2, which enrolled only patients with EGFR mutations, the most common were exon 19 deletions in 11 of 20 tested patients (55%) and L858R mutations in 5 patients (25%) (Table 1).
Table 1.
Baseline characteristics
| Characteristic | Stage 1 (n = 8) | Stage 2 (n = 20) | All patients (n = 28) |
|---|---|---|---|
| Median age (range), years | 72 (47-84) | 57.5 (47-74) | 61 (47-84) |
| Sex, n (%) | |||
| Male | 4 (50) | 4 (20) | 8 (29) |
| Female | 4 (50) | 16 (80) | 20 (71) |
| Race, n (%) | |||
| Asian | 0 | 6 (30) | 6 (21) |
| Black/African American | 1 (13) | 2 (10) | 3 (11) |
| White | 6 (75) | 8 (40) | 14 (50) |
| Other | 0 | 1 (5) | 1 (4) |
| Unknown | 1 (13) | 3 (15) | 4 (14) |
| ECOG performance status, n (%) | |||
| 0 | 0 | 7 (35) | 7 (25) |
| 1 | 8 (100) | 13 (65) | 21 (75) |
| Smoking status, n (%) | |||
| Current | 0 | 1 (5) | 1 (4) |
| Prior | 8 (100) | 7 (35) | 15 (54) |
| Never | 0 | 12 (60) | 12 (43) |
| Histology type | |||
| Squamous | 1 (13) | 0 | 1 (4) |
| Non-squamous | 7 (88) | 20 (100) | 27 (96) |
| Prior cancer therapy | 7 (88) | 3 (15) | 10 (36) |
| Prior radiotherapy | 5 (63) | 7 (35) | 12 (43) |
| EGFR mutation, n | 5 | 20 | 25 |
| Exon 19 deletion | 1 (14) | 11 (55) | 12 (48) |
| Exon 20 insertion | 0 | 1 (5) | 1 (4) |
| L858R | 0 | 5 (25) | 5 (20) |
| L861Q | 0 | 1 (5) | 1 (4) |
| Other | 1 (14) | 2 (10) | 3 (12) |
| Not done | 3 (60) | 0 | 3 (12) |
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
Safety
The median treatment duration in the safety population of 28 patients was 314 days (range 1-1592 days). The median number of atezolizumab doses was 13 (range 1-75).
The safety profile of the combination of atezolizumab and erlotinib was acceptable, with no DLTs, no grade 4 or 5 AEs, and no grade 4 or 5 TRAEs reported. Although TRAEs were reported by all patients (Table 2), they were mostly grade 1 and 2. Thirteen patients experienced grade 3 TRAEs; each occurred only in one patient, except for increased alanine aminotransferase (ALT), pyrexia, and rash, which each occurred in two patients (Table 2).
Table 2.
Safety summary and highest grade of TRAEs
| n (%) | Stage 1 (n = 8) | Stage 2 (n = 20) | All patients (n = 28) |
|---|---|---|---|
| Any AE | 8 (100) | 20 (100) | 28 (100) |
| Grade 3 AE | 5 (63) | 15 (75) | 20 (71) |
| Grade 4 or 5 AE | 0 | 0 | 0 |
| Serious AE | 5 (63) | 9 (45) | 14 (50) |
| Any TRAE | 8 (100) | 20 (100) | 28 (100) |
| Grade 3 TRAEa | 3 (38) | 10 (50) | 13 (46) |
| Grade 4 or 5 TRAE | 0 | 0 | 0 |
| Treatment-related SAEb | 2 (25) | 6 (30) | 8 (27) |
| AE leading to atezolizumab withdrawal | 1 (13) | 5 (25) | 6 (21) |
| AE leading to erlotinib withdrawal | 2 (25) | 1 (5) | 3 (11) |
| AE leading to dose modification/interruption | 6 (75) | 15 (75) | 21 (75) |
| Grade 3 AEs related to atezolizumabc | |||
| Rash | 0 | 2 (10) | 2 (7) |
| ALT increase | 0 | 2 (10) | 2 (7) |
| Pyrexia | 0 | 2 (10) | 2 (7) |
| Any AESI | 7 (88) | 20 (100) | 27 (96) |
| Grade 3 AESI | 2 (25) | 7 (35) | 9 (32) |
| Liver-related AESI | |||
| Immune-mediated hepatitis (diagnosis and laboratory abnormalities) | 1 (13) | 9 (45) | 10 (36) |
| Grade 3 immune-mediated hepatitis (diagnosis and laboratory abnormalities)d | 0 | 4 (20) | 4 (14)d |
| Lung-related AESI | |||
| Pneumonitise | 0 | 1 (5) | 1 (4) |
AE, adverse event; AESI, adverse events of special interest; ALT, alanine aminotransferase; SAE, serious adverse events; TRAE, treatment-related adverse event.
No grade 4 or 5 TRAEs occurred.
Atezolizumab-related serious AEs were colitis, diarrhea, hemorrhoidal hemorrhage, pyrexia, increased liver function test, myopathy, occipital neuralgia, and deep vein thrombosis (each in 1 patient).
Occurring in ≥2 patients.
ALT increased (n = 2), blood bilirubin increased (n = 1), and liver function test increased (n = 1).
Grade 1 or 2.
AEs that led to atezolizumab withdrawal in six patients (21%) were myopathy, pyrexia, low-grade chest pain, arthralgia, hemorrhoidal hemorrhage, and acneiform dermatitis. AEs that led to erlotinib withdrawal in three patients (11%) were dyspnea, deep vein thrombosis, and increased ALT. AEs resulted in dose modification or interruption in 12 patients (75%).
Adverse events of special interest (AESIs) for atezolizumab occurred in 27 patients (46%) and in 9 patients (32%) at grade 3 (Table 2). The only grade 3 AESIs associated with liver function were ALT increased in two patients (7%), blood bilirubin increased in one patient (4%), and liver function tests increased in one patient (4%); all other AESIs associated with liver function occurred at grade ≤2, and none led to treatment withdrawal.
No grade ≥3 lung-associated AESIs were reported in this study, although grade 1 pneumonitis, discovered in routine computed tomography imaging, occurred in one patient (4%; Table 2). This was an asymptomatic radiology finding described as ‘increased right basilar clustered opacities, probably infectious/inflammatory,’ with an indeterminate right middle lobe opacity ‘possibly also infectious/inflammatory with/without component of mucoid impaction.’ The patient was treated with a 15-day course of prednisone 20 mg daily and the radiological findings improved on subsequent computed tomography scans. Treatment with erlotinib continued throughout; one cycle of atezolizumab was skipped, after which atezolizumab treatment resumed, and the patient remained on erlotinib with atezolizumab for another 9 months.
Efficacy
Efficacy analysis focused on the 20 patients with EGFR-mutated NSCLC enrolled in stage 2, and data are presented here based on a data cut-off of 7 May 2020. After a median 44.8 months of survival follow-up, 15 patients had a confirmed PR; hence, the ORR of atezolizumab plus erlotinib was 75% (Table 3). The disease control rate was 90%. In addition to the 15 patients (75%) who had a PR, 3 patients (15%) had durable stable disease (≥24 weeks). Four patients had an ongoing response at the time of study discontinuation.
Table 3.
Summary of efficacy in patients with EGFR-mutated NSCLC
| Stage 2 (n = 20) | |
|---|---|
| Confirmed ORR, n (%) | 15 (75) |
| [95% CI] | [50.9-91.34] |
| CR, n (%) | 0 |
| [95% CI] | [0.00-16.84] |
| PR, n (%) | 15 (75) |
| [95% CI] | [50.9-91.34] |
| SD, n (%) | 3 (15.0) |
| [95% CI] | [3.21-37.89] |
| PD, n (%) | 2 (10) |
| [95% CI] | [1.23-31.7] |
| DCRa, n (%) | 18 (90) |
| [95% CI] | [68.3-98.77] |
| Median DOR, months | 18.9 |
| [95% CI for median] | [9.7-40.5] |
| Range (censored) | 4.2-50.4 |
| Median PFS, months | 15.4 |
| [95% CI for median] | [8.4-39.0] |
| Range (censored) | 1.6-53.3 |
| Median OS, months | NE |
| [95% CI for median] | [34.6-NE] |
| Range (censored) | 11.4-53.3 |
CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; EGFR, epidermal growth factor receptor; NE, not evaluable; NSCLC, non-small-cell lung cancer; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
CR + PR + SD ≥24 weeks.
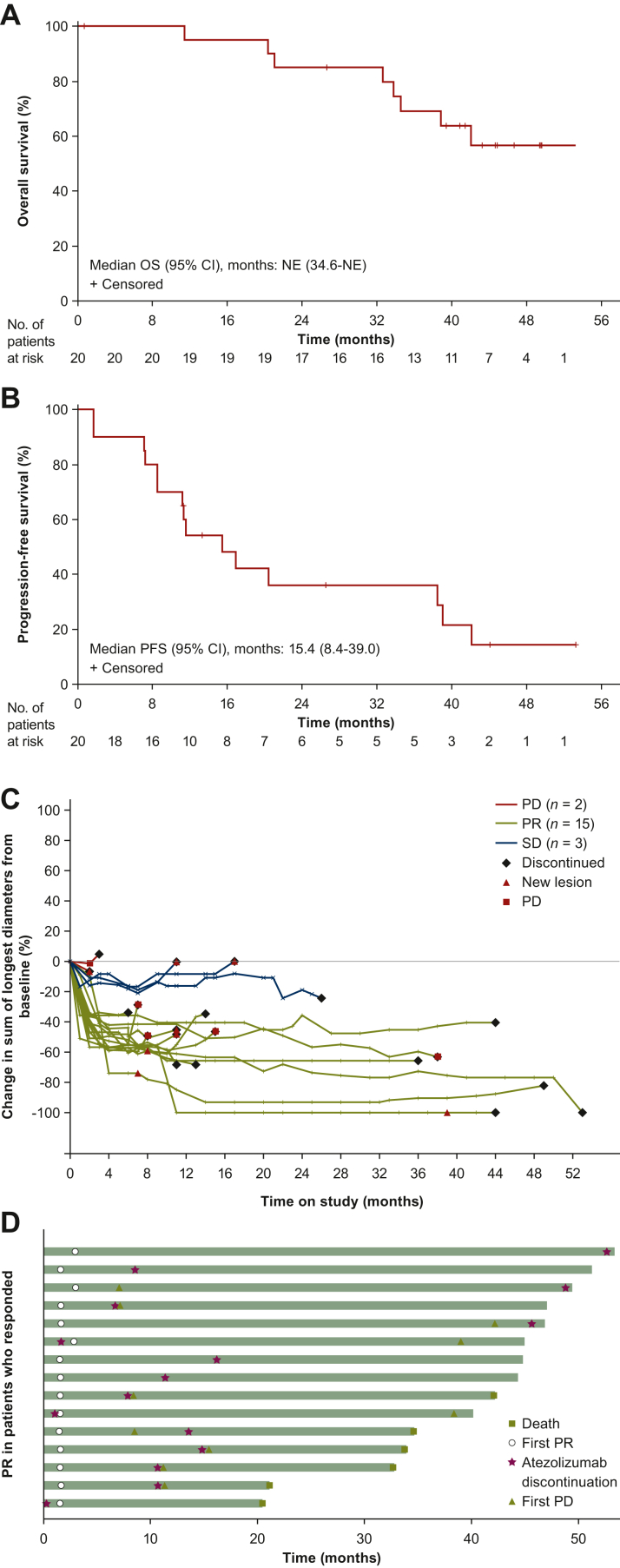
Median OS was not evaluable (95% CI 34.6-not evaluable), and duration of survival ranged from 11.4 to 53.3 (censored) months (Figure 1A). Median PFS was 15.4 months (95% CI 8.4-39.0 months), ranging from 1.6 to ≥53.3 (censored) months (Figure 1B). Clinical responses to this treatment combination were durable: the median DOR was 18.9 months (95% CI 9.7-40.5 months), ranging from 4.2 to ≥50.4 (censored) months (Figure 1C and D). In three patients, clinical responses continued after discontinuation of atezolizumab treatment throughout the duration of follow-up (Figure 1D).
Figure 1.
Clinical activity in stage 2 (n = 20). Kaplan–Meier curves of (A) overall survival and (B) progression-free survival. (C) Spaghetti plot of tumor burden over time by investigator-confirmed response per RECIST 1.1. (D) Swimlane plot showing duration of investigator-assessed confirmed partial responses per Response Evaluation Criteria in Solid Tumors 1.1.
CI, confidence interval; CR, complete response; NE, not evaluable; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Discussion
This is the first study to investigate the safety and clinical activity of combining atezolizumab immunotherapy with the TKI erlotinib in patients with advanced NSCLC. The safety and efficacy findings from this final analysis after an additional median 18 months of follow-up were consistent with those reported after 26 months of follow-up.38 The median DOR in patients whose NSCLC had EGFR mutations was 18.9 months and the median OS remains not evaluable, with 40% of events having occurred. Whereas the median OS was not reached, the lower bound of the 95% CI was 34.6 months, however, with many of the censored events occurring at 40 months or later. Recent studies of erlotinib monotherapy in patients with EGFR-mutant NSCLC have reported median OS >45 months.39,40 The median PFS and ORR with this combination compared favorably with those observed with erlotinib monotherapy,41,42 and also with ORRs reported in other phase I/II trials of EGFR TKIs combined with ICIs in patients with EGFR-mutant NSCLC, which ranged from 42% to 70% in the first-line setting and from 15% to 67% in the second-line setting.19, 20, 21, 22
Atezolizumab plus erlotinib demonstrated a tolerable safety profile, with the majority of TRAEs being non-severe and manageable. Although treatment-limiting liver22 and lung21 toxicities have been reported with other combinations of immunotherapy and EGFR TKIs, these were notably not observed in this study. In the phase I/II KEYNOTE-021 study that evaluated the feasibility of combining erlotinib or gefitinib with pembrolizumab, pembrolizumab combined with gefitinib was found not to be feasible because grade 3/4 liver toxicity occurred in five of seven patients (71.4%), leading to permanent treatment discontinuation in four of them.22 Liver toxicity was not observed in the patients treated with pembrolizumab plus erlotinib, consistent with our data using atezolizumab.
ILD was another potential concern, given that more ILD events were reported with osimertinib plus durvalumab compared with either drug alone in the phase Ib TATTON study in patients with EGFR-mutant NSCLC.21 In that study, ILD occurred in 13 of 34 patients (38%) receiving combination treatment and in 2.9% and 2%, respectively, of patients receiving osimertinib and durvalumab alone. Due to the increased incidence of ILD in TATTON, recruitment into CAURAL (NCT02454933), a phase III study of osimertinib plus durvalumab versus osimertinib monotherapy, was terminated early.23 A total of 1 of 14 patients (7.1%) randomized to the combination arm reported grade 2 ILD while receiving osimertinib monotherapy after discontinuing durvalumab therapy, having received only one dose. Pneumonitis was not a concern when erlotinib was combined with nivolumab or pembrolizumab in the phase I CheckMate 012 (NCT01454102)19 or phase I/II KEYNOTE-021 (NCT02039674)22 studies. Together these data suggest that erlotinib may represent a more attractive TKI backbone for immunotherapy combinations for treatment of EGFR-mutant NSCLC. Further research will be needed to determine whether the low rate of pneumonitis reported here and in other small studies of erlotinib with ICIs can be confirmed in larger trials. Overall, the data from this study suggest that atezolizumab can be safely combined with erlotinib and may be associated with durable benefit in some patients.
In preclinical studies, activation of the EGFR pathway induced PD-L1 expression and other immunosuppressive factors that limit host antitumor immune response, suggesting an active role for EGFR in remodeling the tumor microenvironment.16 EGFR mutation has been correlated with increased PD-L1 expression, lack of CD8+ T-cell infiltration, and lower tumor mutation burden in pooled clinical trial and sequence database analyses.43 Elevated PD-L1 expression has also been correlated with poor response and de novo resistance to EGFR TKIs among patients with EGFR-mutated NSCLC.44 These data suggest that the immune microenvironment is an important factor in responses to targeted therapy and imply that patients with poor responses to EGFR TKIs, especially those with de novo resistance, might in particular benefit from the addition of ICI.
In conclusion, atezolizumab combined with erlotinib demonstrated a tolerable safety profile and encouraging, durable clinical activity in patients with NSCLC harboring EGFR mutations.
Acknowledgements
Medical writing assistance for this manuscript was provided by Derrick Afful, PhD, and Samantha Santangelo, PhD, of Health Interactions, Inc. and was funded by F. Hoffmann-La Roche, Ltd.
Funding
This work was supported by F. Hoffmann-La Roche (no grant number), Ltd/Genentech, a member of the Roche Group.
Disclosure
CMR has received personal fees from AbbVie, Amgen, AstraZeneca, D2G, Daiichi Sankyo, Epizyme, Genentech/Roche, Ipsen, Jazz, Kowa, Lilly, Merck, Syros, Bridge Medicines, Earli, and Harpoon Therapeutics outside the submitted work.
AC has received grants from Genentech for clinical trials development during the conduct of the study, and grants for clinical trials development from Merck Serono, Roche, BeiGene, Bayer, Servier, Lilly, Novartis, Takeda, Astellas, Natera, Bristol Myers Squibb (BMS), and Merck Sharp & Dohme (MSD) outside the submitted work.
AD has served on advisory boards for AstraZeneca and Ipsen.
BB has received grants from D Pharma, AbbVie, Amgen, Aptitude Health, AstraZeneca, BeiGene, Blueprint Medicines, Boehringer Ingelheim, Celgene, Cergentis, Chugai Pharmaceutical, Cristal Therapeutics, Daiichi Sankyo, Eli Lilly, Eisai, Genzyme Corporation, GlaxoSmithKline, Inivata, Ipsen, Janssen, Onxeo, OSE Immunotherapeutics, Pfizer, Genentech/Roche, Sanofi, Takeda, Tolero Pharmaceuticals, and Turning Point Therapeutics.
DBC has received consulting fees and honoraria from Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer, Blueprint Medicines, and Janssen, institutional research support from Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer, MSD, Merrimack Pharmaceuticals, BMS, Clovis Oncology, Spectrum Pharmaceuticals, Tesaro, and Daiichi Sankyo, and consulting fees from Teladoc and Grand Rounds by Included Health.
PS has received honoraria or consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Pfizer, Puma, Roche, Eisai, and Celgene; and grants or institutional funding from Astellas, AstraZeneca, Genentech, Novartis, Oncogenex, Roche, and Medivation.
RH has received institutional research support from AbbVie, Agios, Corvus, Daiichi Sankyo, Erasca, Exelixis, Genentech, Incyte, Lilly, Mirati, Novartis, and Turning Point; and consulting fees from AbbVie, Daiichi Sankyo, Novartis, EMD Serono, and Regeneron.
VMV reports receiving personal fees from Sanofi, Tgen, AstraZeneca, Novocure, and BMS; and stock ownership in Johnson & Johnson.
JS is an employee of Genentech/Roche and holds stocks in the company.
SL is an employee of Genentech/Roche and holds stocks in the company.
EC is an employee of Genentech/Roche and holds stocks in the company.
GJR reports receivin m Roche/Genentech during the conduct of the study; and outside the submitted work, he has received grants from Novartis, GlaxoSmithKline, Infinity Pharmaceuticals, and Merck; grants and non-financial support from Roche/Genentech, Pfizer, Mirati Therapeutics, and Takeda; and the Memorial Sloan Kettering Cancer Center has submitted a patent application covering pulsatile use of erlotinib to treat or prevent brain metastases.
SG reports receiving grants from BMS, Roche/Genentech, NextCure, Takeda/Ariad and Iovance outside the submitted work.
All authors received medical writing assistance with preparation of the manuscript provided by Samantha Santangelo, PhD, and Derrick Afful, PhD, of Health Interactions, Inc, funded by F. Hoffmann-La Roche.
BM has declared no conflicts of interest.
Data sharing
For eligible studies qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Midha A., Dearden S., McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII) Am J Cancer Res. 2015;5:2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 3.Hanna N., Johnson D., Temin S., Masters G. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update summary. J Oncol Pract. 2017;13:832–837. doi: 10.1200/JOP.2017.026716. [DOI] [PubMed] [Google Scholar]
- 4.Planchard D., Popat S., Kerr K., et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 5.Calles A., Riess J.W., Brahmer J.R. Checkpoint blockade in lung cancer with driver mutation: choose the road wisely. Am Soc Clin Oncol Educ Book. 2020;40:372–384. doi: 10.1200/EDBK_280795. [DOI] [PubMed] [Google Scholar]
- 6.Duma N., Santana-Davila R., Molina J.R. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi S., Boggon T.J., Dayaram T., et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 8.Leonetti A., Sharma S., Minari R., Perego P., Giovannetti E., Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–737. doi: 10.1038/s41416-019-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehlman C., Cadranel J., Rousseau-Bussac G., et al. Resistance mechanisms to osimertinib in EGFR-mutated advanced non-small-cell lung cancer: a multicentric retrospective French study. Lung Cancer. 2019;137:149–156. doi: 10.1016/j.lungcan.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Yu H.A., Arcila M.E., Rekhtman N., et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisberg A., Cummings A., Goldman J.W., et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor Naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13:1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 14.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng S., Wang R., Zhang X., et al. EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer. 2019;18:165. doi: 10.1186/s12943-019-1073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbay E.A., Koyama S., Carretero J., et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Fang W., Zhan J., et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10:910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 18.Azuma K., Ota K., Kawahara A., et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 19.Gettinger S., Hellmann M.D., Chow L.Q.M., et al. Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J Thorac Oncol. 2018;13:1363–1372. doi: 10.1016/j.jtho.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Creelan B.C., Yeh T.C., Kim S.W., et al. A phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. Br J Cancer. 2021;124:383–390. doi: 10.1038/s41416-020-01099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oxnard G.R., Yang J.C., Yu H., et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31:507–516. doi: 10.1016/j.annonc.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Yang J.C.H., Gadgeel S.M., Sequist L.V., et al. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thorac Oncol. 2019;14:553–559. doi: 10.1016/j.jtho.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Yang J.C.H., Shepherd F.A., Kim D.W., et al. Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M-positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J Thorac Oncol. 2019;14:933–939. doi: 10.1016/j.jtho.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Herbst R.S., Soria J.C., Kowanetz M., et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 26.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 27.Ardizzoni A., Azevedo S., Rubio-Viqueira B., et al. Primary results from TAIL: a global single-arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2020-001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehrenbacher L., Spira A., Ballinger M., et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 29.West H., McCleod M., Hussein M., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 30.Felip E., Altorki N., Zhou C., et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344–1357. doi: 10.1016/S0140-6736(21)02098-5. [DOI] [PubMed] [Google Scholar]
- 31.NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. V6; 2020. [Google Scholar]
- 32.TECENTRIQ (atezolizumab). Package insert. Genentech, Inc; South San Francisco, CA: 2020. [Google Scholar]
- 33.TECENTRIQ (atezolizumab) Roche GmbH; Germany: 2020. Summary of product characteristics. Grenzach-Wyhlen. [Google Scholar]
- 34.Reck M., Mok T.S.K., Nishio M., et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 35.Nogami N., Barlesi F., Socinski M.A., et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol. 2022;17:309–323. doi: 10.1016/j.jtho.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Gridelli C., Bareschino M.A., Schettino C., Rossi A., Maione P., Ciardiello F. Erlotinib in non-small cell lung cancer treatment: current status and future development. Oncologist. 2007;12:840–849. doi: 10.1634/theoncologist.12-7-840. [DOI] [PubMed] [Google Scholar]
- 37.Pollack B.P., Sapkota B., Cartee T.V. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res. 2011;17:4400–4413. doi: 10.1158/1078-0432.CCR-10-3283. [DOI] [PubMed] [Google Scholar]
- 38.Rudin C., Cervantes A., Dowlati A., et al. MA15.02 Long-term safety and clinical activity results from a phase Ib study of erlotinib plus atezolizumab in advanced NSCLC. J Thorac Oncol. 2018;13:S407. [Google Scholar]
- 39.Saito H., Fukuhara T., Furuya N., et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto N., Seto T., Nishio M., et al. Erlotinib plus bevacizumab vs erlotinib monotherapy as first-line treatment for advanced EGFR mutation-positive non-squamous non-small-cell lung cancer: survival follow-up results of the randomized JO25567 study. Lung Cancer. 2021;151:20–24. doi: 10.1016/j.lungcan.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 41.Rosell R., Carcereny E., Gervais R., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y.L., Zhou C., Liam C.K., et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26:1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 43.Dong Z.Y., Zhang J.T., Liu S.Y., et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su S., Dong Z.Y., Xie Z., et al. Strong programmed death ligand 1 expression predicts poor response and de novo resistance to EGFR tyrosine kinase inhibitors among NSCLC patients with EGFR mutation. J Thorac Oncol. 2018;13:1668–1675. doi: 10.1016/j.jtho.2018.07.016. [DOI] [PubMed] [Google Scholar]