Abstract
Background
Real-world data (RWD) have quickly emerged as an important source of information to address uncertainties about new treatments, including novel anticancer therapies. Many stakeholders are using such data and the evidence derived therefrom to answer the questions that remain about the safety and effectiveness of antitumor medicines after their approval by regulators. Our objective was to investigate the academic RWD study landscape and explore to what extent RWD are being integrated into investigator-initiated clinical research.
Materials and methods
We designed an online survey that was distributed between May and August 2022 to representatives of cancer cooperative groups active in Europe, North America, South America, Asia, and/or Oceania.
Results
In total, 125 cooperative groups operating in 58 different countries and conducting research across 13 distinct cancer domains participated in the survey. While most of the responders (67.2%) did not have a formal policy in place to gather and utilize RWD, a majority (68.0%) had carried out studies involving the analysis of such data before, both for exploratory and confirmatory purposes. The groups that were experienced in capturing and interpreting RWD had mainly worked with observational RWD that were not predominantly prospective or retrospective in nature and which originated from disease registries, electronic health records, and patient questionnaires. They perceived the low costs and the large scale of RWD research to be its most significant benefits, and viewed the accompanying methodological and operational challenges as its biggest constraints. However, they did not have a common understanding of what RWD were. Despite their experience with analyzing RWD, their research portfolio still primarily comprised traditional clinical trials; 62.5% of the groups that had never undertaken any RWD studies were nonetheless planning to initiate them in the future.
Conclusions
Cancer cooperative groups are already incorporating RWD studies into their research agendas, but still lack knowledge and expertise in this regard, and do not agree on what RWD are. The conduct of conventional clinical trials continues to be their priority.
Key words: real-world data, real-world evidence, oncology, cancer, cooperative groups, survey
Highlights
-
•
RWD are increasingly being used to support the development and market access of anticancer therapies.
-
•
Cancer cooperative groups around the world are already incorporating RWD studies into their research agendas.
-
•
They have mainly used observational RWD drawn from cancer registries, electronic health records, and patient questionnaires.
-
•
Nevertheless, they continue to prioritize the conduct of traditional clinical trials.
-
•
There is still room for expanding the role of academia in interventional RWD research.
Introduction
Although no consensus exists on its most appropriate definition, the term ‘real-world data’ (RWD) is commonly understood to refer to health data that are collected outside of conventional randomized controlled trials (RCTs), usually as part of routine clinical practice.1 Such data can be derived from various sources, including patient registries, electronic health records, administrative claims, pharmacy records, wearable devices (e.g. smartwatches), and social media.2,3 If subjected to well-chosen statistical analyses, RWD can give rise to so-called real-world evidence (RWE), which may be used to inform and support healthcare-related decision making.4,5 Both observational (e.g. cohort studies) and interventional (e.g. pragmatic trials) study designs are capable of generating RWE, and RWD can be prospective or retrospective in nature.3,6
Traditionally, RWD and RWE have played an important role in the postmarketing surveillance of medicines,7, 8, 9 allowing for the evaluation of long-term safety risks and the identification of severe, infrequently occurring side-effects.10 Aside from applications in the domain of pharmacovigilance, RWD and RWE can also be used to investigate access to and quality of care (e.g. utilization of health services, concordance with medical guidelines), patient and disease characteristics (e.g. disease burden, biology, and progression), and therapeutic outcomes (e.g. treatment performance in specific subpopulations).11 However, their place in the assessment of the comparative effectiveness of health technologies remains a point of contention, owing to the biases that can be present in the results of studies that do not standardly make use of randomization to negate the impact of confounding factors.11 The methodological setup of RCTs renders them more suitable for comparing different therapies in a head-to-head fashion, but such trials typically have major limitations (e.g. their reliance on stringent eligibility criteria for recruiting participants, reducing the external validity of their conclusions), and their conduct may not be feasible in some settings.12
In the field of oncology, the emergence of the precision medicine model has been accompanied by changes in the way pivotal studies are undertaken by pharmaceutical companies. The industry has gradually stepped away from the gold standard of large and rigorously designed RCTs, in favor of a new paradigm centered around smaller and nonrandomized trials that may not feature any comparator arms at all.13 Regulatory mechanisms expediting the approval of promising drugs such as the European Medicines Agency’s conditional marketing authorization scheme have likely contributed to this development by easing the initial evidentiary burden imposed on applicants.14 Irrespective of the legal pathway they follow to get there, novel antineoplastic agents now often enter the market without having demonstrated to improve patients’ quality of life or overall survival.15 Moreover, uncertainties frequently persist with regard to their optimal dosing schedule and their combination with existing interventions.16
While it has been suggested that RWD can be harnessed to fill the gaps in the available evidence,17 some authors18 have rejected this notion, arguing that RCTs are needed to formulate definitive answers to the questions with which regulators and downstream decision makers such as payers, doctors, and patients are faced. The inconsistent relationship that is observed between the findings of RCTs and those of confirmatory studies relying on the analysis of RWD has highlighted the dangers of depending exclusively on RWE to characterize the effects of antitumor treatments.19 Nevertheless, when best practices are taken into consideration (e.g. the target trial principle)20, 21, 22, 23 and population-based datasets are used,24 research involving the collection and interpretation of RWD can offer valuable insights into how well anticancer therapies work in a real-life environment, producing learnings that complement the ones drawn from RCTs.25,26 This implies that RWD should be applied to corroborate or contextualize data originating from RCTs, not serve as a substitute for them.27
RWD and RWE have rapidly become integral components of the business strategies of most companies developing medicinal products. The commercial sector continues to invest a significant amount of resources into projects and initiatives that aim to operationalize RWD and deliver robust RWE, with oncology standing out as a key area of focus.28 Other stakeholders are also increasingly looking to leverage the knowledge contained within the health data of patients with cancer across Europe. For example, the European Commission has made the expanded utilization of RWD a core objective of Europe’s Beating Cancer Plan,29 building on its efforts to establish the European Health Data Space.30 However, the extent to which such data are being incorporated into investigator-initiated cancer clinical research is currently still largely unclear. In this study, we set out to survey the cooperative groups actively carrying out this type of research and explore their policies and experiences with respect to working with RWD. These academic-led groups, which are composed of cancer clinicians collaborating at a regional, national, or international level, are responsible for conducting many practice-changing trials that lacked industry support (e.g. studies of surgical or radiotherapeutic procedures).31,32
Materials and methods
We used the SurveyMonkey platform to design an online questionnaire consisting of a combination of multiple-choice, Likert-type, and open-ended questions, some of which were only shown if specific response options were chosen (Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2023.100878). The survey was subsequently disseminated to representatives (presidents, vice presidents, chairs, vice chairs, secretaries, treasurers, chief executive officers, etc.) of approximately 160 cancer cooperative groups operating in Europe, North America, South America, Asia, and/or Oceania, which were selected based on prior collaborations of the European Organisation for Research and Treatment of Cancer (EORTC) as well as on lists published by the European Society for Medical Oncology (ESMO).33,34 The individuals contacted received an invitation e-mail containing a link to the questionnaire. They were requested to forward this e-mail to the person within their group who they felt would be best suited to contribute to the study. Responses were collected between May and August 2022, with reminder messages being sent out to nonresponders every 2 weeks. Information that could directly identify respondents was not captured. The Ethics Committee Research UZ/KU Leuven reviewed and approved this study (identification number S65201).
The survey data were analyzed using Microsoft (Richmond, VA) Excel (for descriptive analyses) and IBM (New York, NY) SPSS Statistics 28.0 (for inferential analyses). All statistical tests were conducted post hoc. The significance threshold was set at 0.05. For Likert-type questions, the measurement levels of the scales used were converted to numbers (e.g. ‘very low significance’ is equivalent to 1, ‘low significance’ to 2, ‘moderate significance’ to 3, ‘high significance’ to 4, and ‘very high significance’ to 5), which made it possible to determine medians and their corresponding interquartile ranges (IQRs; represented by their lower and upper boundaries). To assess whether the participants held different perceptions of the various items that they were asked to evaluate on a single scale (e.g. ‘operational challenges’ as the first item, ‘technical challenges’ as the second, and ‘methodological challenges’ as the third), Friedman and paired-samples Wilcoxon signed-rank tests were undertaken on the numerically transformed data. One-sample Wilcoxon signed-rank tests were also carried out to verify whether the medians deviated significantly from the midpoint of the scale. Answers to open-ended questions were subjected to thematic analysis.35,36
Results
In total, 125 cancer cooperative groups participated in our survey, of which 91 (72.8%) were active in Europe (e.g. European Lung Cancer Working Party [ELCWP], Fédération Francophone de Cancérologie Digestive [FFCD], Gruppo Oncologico Italiano di Ricerca Clinica [GOIRC], German Breast Group [GBG], British Thoracic Oncology Group [BTOG]), 11 (8.8%) in Oceania (e.g. Australasian Gastro-Intestinal Trials Group [AGITG], Australia New Zealand Gynaecological Oncology Group [ANZGOG], Trans-Tasman Radiation Oncology Group [TROG]), 8 (6.4%) in North America (e.g. Eastern Cooperative Oncology Group - American College of Radiology Imaging Network [ECOG-ACRIN], NSABP-RTOG-GOG Oncology [NRG Oncology], Canadian Cancer Trials Group [CCTG]), 7 (5.6%) in Asia (e.g. Korean Cancer Study Group [KCSG], Japanese Gynecologic Oncology Group [JGOG], Taiwan Cooperative Oncology Group [TCOG]), and 6 (4.8%) in South America (e.g. Latin American Cooperative Oncology Group [LACOG], Grupo Argentino de Investigación Clínica en Oncología [GAICO], Grupo Oncológico Cooperativo Uruguayo [GOCUR]), with the remaining 2 (1.6%) operating on an intercontinental level (International Extranodal Lymphoma Study Group [IELSG] and European Thoracic Oncology Platform - International Breast Cancer Study Group [ETOP-IBCSG]; the full list of responders is provided in Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2023.100878). Nearly all of the responding groups (123/125, 98.4%) completed the questionnaire fully. The response rate was 78.6% (125/159).
Demographic characteristics of the survey sample
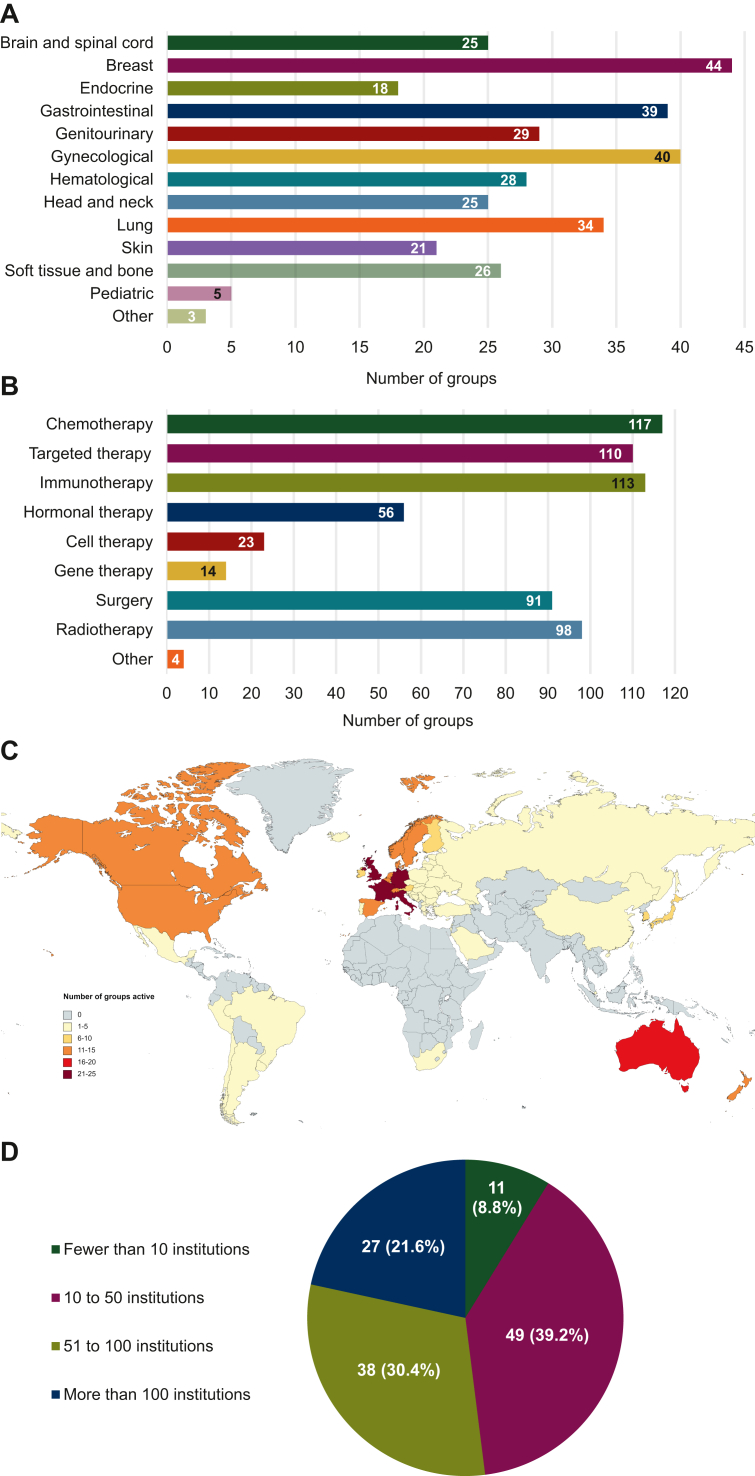
The participating groups carried out research across 13 distinct oncological domains, with breast cancers (44/125, 35.2%), gynecological cancers (40/125, 32.0%), and gastrointestinal cancers (39/125, 31.2%) being the three most common (Figure 1A). The studies they carried out mainly examined pharmacological anticancer treatments, such as chemotherapeutic drugs (117/125, 93.6%), immunotherapeutic agents (113/125, 90.4%), and targeted therapies (110/125, 88.0%). Nevertheless, the groups typically also investigated surgical (91/125, 72.8%) and radiotherapeutic (98/125, 78.4%) interventions (Figure 1B). Their geographical scope spanned 6 continents and 58 countries (Figure 1C), the five biggest hubs of activity being the United Kingdom (25/125, 20.0%), Italy (25/125, 20.0%), Germany (23/125, 18.4%), France (22/125, 17.6%), and Australia (19/125, 15.2%). The survey sample consisted of smaller (i.e. comprising fewer than 50 institutions or hospitals; 60/125, 48.0%) and larger (i.e. comprising more than 50 institutions or hospitals; 65/125, 52.0%) groups to a similar extent (Figure 1D).
Figure 1.
Demographic characteristics. Breakdown of the participating groups by (A) the oncological domains in which they conducted research, (B) the types of anticancer therapies they investigated, (C) the countries in which they were active, and (D) the number of institutions they comprised. The numbers reported for (A), (B), and (C) exceed the total number of responders upon aggregation because multiple response options could be selected for the matching questions.
Policies and experiences with regard to RWD
Approximately two-thirds of the groups (84/125, 67.2%) responding to the questionnaire indicated that they did not have a formal policy in place to collect and use RWD. Of the groups that claimed to standardly gather and utilize such data (41/125, 32.8%), some asserted in an open-ended follow-up question that they were running their own platforms and infrastructures, while others explained that they were dependent on external sources of RWD, such as the national cancer registries of the countries in which they were undertaking research projects.
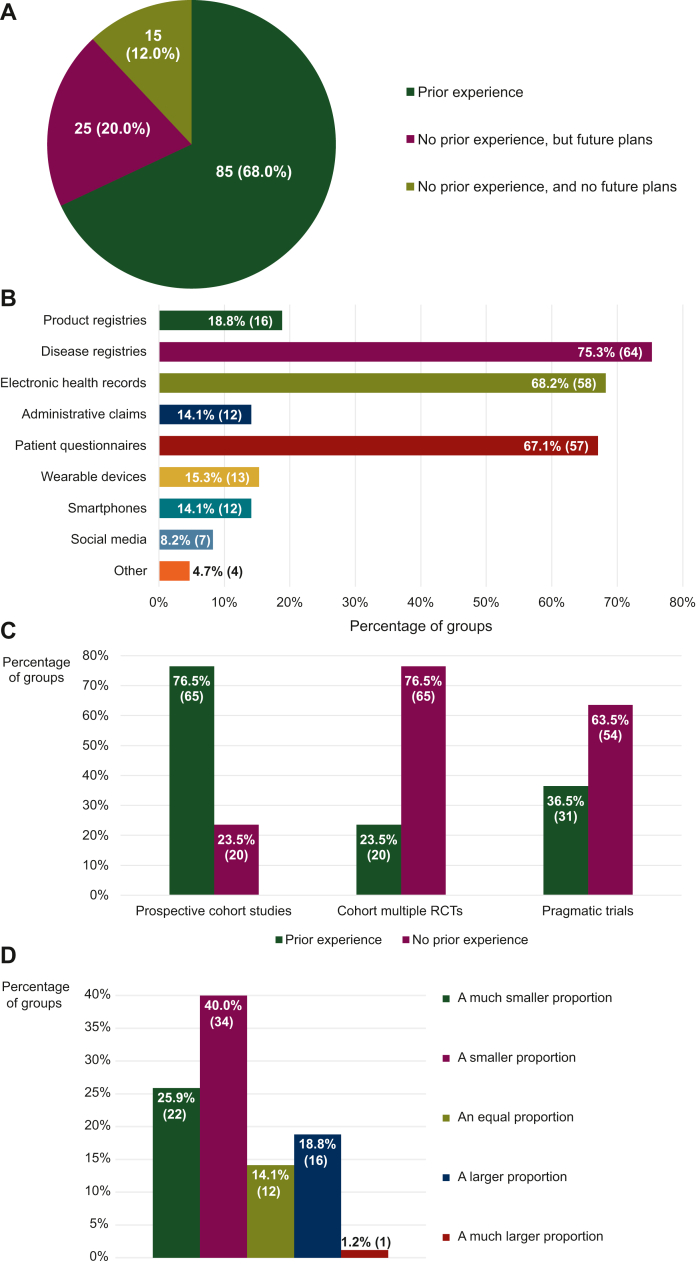
Although the systematic collection and utilization of RWD were rare, a significant majority of the groups (85/125, 68.0%) had experience working with this kind of data (Figure 2A). These experienced groups provided multiple examples of RWD studies that they had launched, some of which are listed in Table 1. For their research activities, they primarily relied on data derived from disease registries (64/85, 75.3%), electronic health records (58/85, 68.2%), and patient questionnaires (57/85, 67.1%). Product registries (16/85, 18.8%), administrative claims (12/85, 14.1%), and wearable devices (13/85, 15.3%) were RWD sources they used less frequently (Figure 2B). The RWD they analyzed were not predominantly retrospective or prospective in nature, with more than half of the groups (49/85, 57.6%) using both types of RWD similarly often.
Figure 2.
Experience with real-world data (RWD) studies and sources. Experience of the participating groups with (A) RWD studies in general, (B) different sources of RWD, (C) specific types of RWD studies, and (D) RWD studies as compared with traditional randomized controlled trials (RCTs), expressed as a proportion of the total number of studies they had carried out. The data shown for (B), (C), and (D) were obtained from a subset of the responders that had worked with RWD before. For (B), (C), and (D), the numbers in brackets indicate the absolute number of groups corresponding with each percentage. The numbers reported for (B) exceed the total number of responders upon aggregation because multiple response options could be selected for the matching question.
Table 1.
Examples of real-world data (RWD) studies. Selected examples cited by the participating cooperative groups of RWD studies that they had initiated
| Cooperative group | Name of study | Primary objective | Country | Design | Reference |
|---|---|---|---|---|---|
| AGITG | RESOLUTE | To assess the clinical benefit of local ablative therapy following initial standard first-line systemic treatment compared with continued standard first-line systemic treatment in patients with unresectable oligometastatic colorectal cancer | Australia | Registry-based trial | 47 |
| ARCAGY-GINECO | ENCOURAGE | To assess the safety profile of first-line bevacizumab therapy for ovarian cancer | France | Prospective cohort study | 48 |
| ISG | TrObs | To evaluate the treatment outcomes of patients with advanced soft tissue sarcomas receiving trabectedin | Italy | Retrospective cohort study | 49 |
| KGOG | KGOG 3052 | To compare survival outcomes between bevacizumab and olaparib maintenance therapy for BRCA-mutated, platinum-sensitive relapsed high-grade serous ovarian carcinoma | South Korea | Retrospective cohort study | 50 |
| LACOG | LACOG 0319 | To evaluate the quality of life of patients with recurrent or metastatic squamous cell carcinoma of the head and neck receiving nivolumab | Brazil | Prospective cohort study | 51 |
AGITG, Australasian Gastro-Intestinal Trials Group; ARCAGY-GINECO, Association de Recherche sur les Cancers dont Gynécologiques - Groupe d’Investigateurs Nationaux pour l’Étude des Cancers Ovariens et du sein; ISG, Italian Sarcoma Group; KGOG, Korean Gynecologic Oncology Group; LACOG, Latin American Cooperative Oncology Group.
However, for 52.9% of the responders experienced in RWD research (45/85), the analysis of interventional RWD was more uncommon than that of observational RWD. In addition, although more than three-quarters of the groups in this subset of the sample (65/85, 76.5%) asserted that they had conducted prospective cohort studies before, only about quarter (20/85, 23.5%) could say the same for cohort multiple RCTs (cmRCTs; i.e. studies designed according to the trials-within-cohorts approach) and a little over a third (31/85, 36.5%) for pragmatic clinical trials (PCTs), illustrating their inexperience with such interventional methodologies that are capable of generating RWE (Figure 2C). Overall, conventional RCTs still remained these groups’ bread and butter: 65.9% (56/85) signaled that RWD studies made up either a smaller (34/85, 40.0%) or a much smaller (22/85, 25.9%) proportion of the research they carried out than traditional clinical trials (Figure 2D).
Most groups that had never worked with RWD before (25/40, 62.5%) were nonetheless planning to set up studies involving the capture and interpretation of such data in the future, though they generally did not appear to have any specific protocols in development at the time of answering the survey questions. The few groups whose plans to integrate RWD into their research agendas seemed to be at a more advanced stage were mainly preparing to build their own comprehensive databases and registries.
Understanding, opportunities, challenges, and applications of RWD
The responders that had undertaken RWD studies prior to their completion of the questionnaire (n = 85) were presented with an additional set of questions to further explore how they perceived and applied RWD. Moreover, they were requested to expand on the benefits and drawbacks of incorporating such data into their research. However, one group did not start this section of the survey and another stopped responding halfway through, so the total number of responders for each question was either 84 or 83.
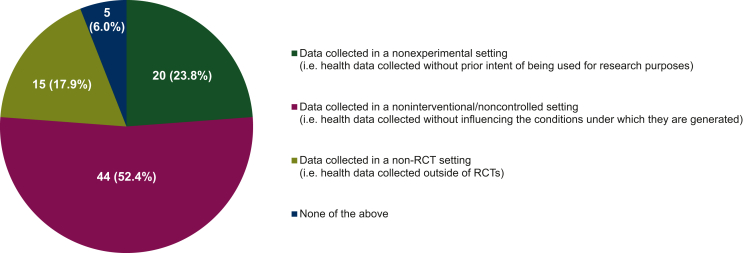
When the groups were asked to identify which of the categories of definitions for RWD described by Makady et al.1 matched the closest with how this term was understood and used within their group, they mostly (44/84, 52.4%) thought that ‘data collected in a noninterventional/noncontrolled setting’ was the best description (Figure 3). Nevertheless, a sizable minority (35/84, 41.7%) selected ‘data collected in a nonexperimental setting’ (20/84, 23.8%) or ‘data collected in a non-RCT setting’ (15/84, 17.9%) instead. The responders that felt none of these categories described RWD adequately (5/84, 6.0%) defined these data more narrowly, specifying that they had to be prospective or population based, for example.
Figure 3.
Understanding of real-world data (RWD). Participating groups’ understanding of RWD, categorized based on the classification system of Makady et al.1 The data shown were obtained from a subset of the responders that had worked with RWD before. The percentages displayed do not add up to exactly 100% due to rounding. RCT, randomized controlled trial.
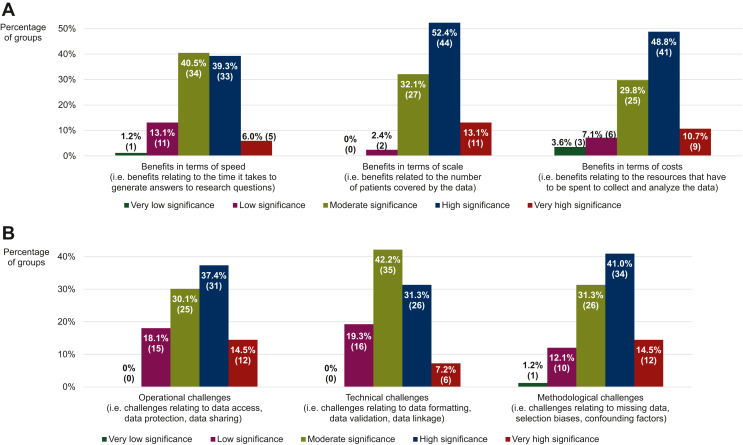
With respect to the advantages associated with the conduct of RWD research, the groups assessed the significance of three of such supposed advantages extracted from the literature37 on a 5-point scale ranging from ‘very low’ (numerically equivalent to 1) to ‘very high’ (numerically equivalent to 5) based on their own experience of working with RWD. It was observed that they regarded some benefits as more significant than others (P = 0.002; Figure 4A), ranking ‘benefits in terms of scale’ (modal and median score of 4 and IQR of 3-4) higher (P < 0.001) than ‘benefits in terms of speed’ (modal and median score of 3 and IQR of 3-4), but at the same level (P = 0.089) as ‘benefits in terms of costs’ (modal and median score of 4 and IQR of 3-4). Nevertheless, each of these advantages received a median score that deviated from the midpoint of the scale (P < 0.001 for all three), indicating that their significance was considered to be relatively high. Additional benefits of RWD that were mentioned by some of the responders centered around the use of such data to (i) characterize the tumor type and the patient population of interest, (ii) formulate hypotheses for future RCTs, (iii) confirm and/or enhance the results of clinical trials (e.g. extension of findings to excluded subpopulations, contextualization of outcomes through inclusion of external control arms), and (iv) gain insight into potential treatment strategies for rare cancers.
Figure 4.
Perceptions of benefits and challenges of real-world data (RWD) research. Participating groups’ perceptions of how significant the (A) benefits and (B) challenges of RWD research are, based on their experience with undertaking RWD studies. The data shown were obtained from a subset of the responders that had worked with RWD before. The percentages displayed may not add up to exactly 100% due to rounding. The numbers in brackets indicate the absolute number of groups corresponding with each percentage.
Concerning the hurdles that can complicate the collection and analysis of RWD, the groups again evaluated the significance of three of these purported hurdles reported in the literature7 on the aforementioned 5-point scale, guided by their previous experiences with carrying out RWD studies. Here as well, it was shown that they viewed some hurdles as more difficult to overcome than others (P = 0.028; Figure 4B), scoring ‘operational challenges’ (i.e. challenges relating to data access, data protection, data sharing, etc.; modal and median score of 4 and IQR of 3-4) and ‘methodological challenges’ (i.e. challenges relating to missing data, selection biases, confounding factors, statistical analyses, etc.; modal and median score of 4 and IQR of 3-4) higher (P = 0.034 and 0.006, respectively) than ‘technical challenges’ (i.e. challenges relating to data formatting, data validation, data linkage, etc.; modal and median score of 3 and IQR of 3-4). The median scores awarded nonetheless differed from the scale’s midpoint (P < 0.001 for ‘operational challenges’ and ‘methodological challenges’, P = 0.006 for ‘technical challenges’), signifying that all three of the hurdles were judged to be of considerable importance. Other barriers to RWD research brought up by the responders related to (i) the insufficient knowledge and expertise they had built up so far in this area, (ii) the lack of funding available to set up and maintain the necessary platforms and infrastructures, (iii) the limited interest on the part of investigators to participate in such research, and (iv) the quality of the data, which was deemed to be low due to the inaccurate or incomplete recording of information.
The groups had mainly initiated RWD studies with the intent of addressing questions pertaining to the (comparative) effectiveness of anticancer therapies (55/83, 66.3%), their safety (47/83, 56.6%), and their optimal target population of patients (47/83, 56.6%). Questions focusing on their optimal dose and dosing regimen (30/83, 36.1%), their costs and economic impact (33/83, 39.8%), and their combination with other interventions (33/83, 39.8%) were less commonly tackled using RWD. A majority of the responders (42/83, 50.6%) claimed to have carried out exploratory (i.e. hypothesis-generating) and confirmatory (i.e. hypothesis-testing) RWD research to a similar extent. Of the groups that asserted to have undertaken either type of research more often than the other (41/83, 49.4%), most (25/41, 61.0%) had carried out a greater number of exploratory RWD studies.
Discussion
To our knowledge, this study is the first to explore the policies and experiences of cancer cooperative groups with respect to the capture and interpretation of RWD, thereby shedding light on the academic RWD research landscape in oncology. As the results indicate, the groups responding to our large, international survey had generally worked with RWD before, but did not standardly collect and use such data. The RWD studies they had carried out had mostly relied on the analysis of observational data sourced from disease registries, electronic health records, or patient questionnaires. The responders saw the large scale and the low costs of RWD research as its main benefits, and perceived the associated methodological and operational hurdles to be its most significant challenges. Nevertheless, despite being experienced in setting up RWD studies, the conduct of traditional clinical trials remained their core business.
We have previously investigated how European and Israeli cancer clinicians perceive RWD and RWE by surveying members of the EORTC network.38 Although this past survey and the present one were not designed with the same objectives in mind, some of their findings align closely with each other. For example, the participants that filled in our earlier questionnaire viewed the methodological complexities of RWD research as more challenging to address than its technical difficulties, a sentiment that the cooperative groups seemed to confirm based on their prior involvement in RWD studies. Moreover, the responders to the two surveys had similar levels of experience working with RWD and broadly agreed on the questions that can be answered using such data, which they most frequently believed could best be defined as data collected in a noninterventional/noncontrolled setting. However, while the individual clinicians thought that the methodological challenges of RWD research were bigger than the operational ones, the representatives of the cooperative groups were of the opinion that both were equally difficult to surmount. Furthermore, contrary to what the clinicians expressed about the circumstances under which they considered the use of RWD to be highly appropriate, the RWD studies that had been undertaken by the groups did not typically tackle questions relating to the economic impact of antitumor treatments, instead focusing on resolving uncertainties with regard to these treatments’ (comparative) effectiveness (it should be mentioned here that comparative effectiveness research also informs health economic analyses).
After conducting interviews with experts in the field and reviewing the available scientific and gray literature on the topic of RWD, Makady et al.1 concluded that data of this kind are most commonly described or interpreted as having originated outside of RCTs. In the current study, responders preferred to characterize RWD as data gathered without influencing the conditions under which they are generated, just like in our previous survey.38 This discrepancy between different stakeholders’ understanding of RWD again demonstrates the need for formulating a standardized and internationally accepted definition for this concept. Establishing such a uniform definition would likely stimulate and facilitate discussion and collaboration in the area of RWD research. The International Coalition of Medicines Regulatory Authorities has recently identified the harmonization of existing RWD and RWE terminologies as a key opportunity for cooperation between regulators around the world.39
A majority of the groups taking part in this survey had never carried out any PCTs or cmRCTs, and those that had experience working with RWD had mainly analyzed observational RWD. These results illustrate that the role of academia in interventional RWD research can still be expanded. As sources of robust and actionable RWE, PCTs and cmRCTs can help bridge some of the evidence gaps that remain after anticancer therapies have entered the market.40,41 In addition, by employing randomization and capturing data in a prospective manner, studies of this nature allow for the methodological and operational constraints of RWD research to be largely overcome, thereby alleviating the most important difficulties that the responders encountered with respect to such research (please note that PCTs can also be nonrandomized or single-arm studies; if the allocation of the PCT’s intervention is not random or if there is no comparator group present in the trial, the methodological challenges of RWD research persist because the risk of drawing biased conclusions from the data increases). Nevertheless, the commercial sector has shown little interest in undertaking PCTs or cmRCTs.42,43 Academic researchers in general are uniquely positioned to set up studies for which industry support is lacking, and cooperative groups in particular already have the network and the infrastructure in place to run large-scale clinical trials. However, it will take time for these groups to grow more accustomed to carrying out PCTs and cmRCTs. The EORTC is currently exploring the possibility of launching studies of this type in the near future.
Our findings suggest that cancer cooperative groups are less inclined to use RWD for the purpose of answering questions relating to an antineoplastic medicine’s (i) optimal dose and dosing regimen, (ii) costs and economic impact, and (iii) combination with other interventions. This could reflect these groups’ overarching research priorities, which do not ordinarily include carrying out health economic analyses, for instance. It could also signify that the survey responders preferred to address such questions through the execution of traditional clinical trials. In any case, to definitively resolve some uncertainties, RCT-derived data are likely required,16,44, 45, 46 which again underscores the importance of increasing the number of academia-sponsored PCTs and cmRCTs in oncology.
This study suffers from a number of limitations. First, we used convenience sampling, so we did not try to recruit every single cancer cooperative group active in Europe, North America, South America, Asia, and/or Oceania. Consequently, our sample may not have been fully representative of all cooperative groups carrying out clinical studies with the aim of investigating oncological treatments, and our observations are not necessarily broadly generalizable in this respect. Nevertheless, by using the network of the EORTC and relying on lists published by ESMO, we likely covered the vast majority of the groups that have undertaken practice-changing research over the years. Second, the individuals who completed the questionnaire may not have been sufficiently knowledgeable of their group’s policies and experiences regarding the collection and analysis of RWD. While the invitation e-mail encouraged the recipients to forward the survey link to someone who was closely familiar with their group’s historical study portfolio, there was no way of verifying whether the responders were able to answer some of the questions accurately. Third, although we provided definitions or descriptions for most of the specialized terms that were mentioned in the questionnaire, it is possible that these were not consulted, because the participants had to actively click on an embedded link to view them. This could imply that they misunderstood or misinterpreted what was meant by these terms, responding incorrectly to certain questions as a result. Lastly, our study was potentially affected by nonresponse bias, given the fact that >20% of the groups targeted did not participate. It is likely that nonresponders decided not to take part in the survey because they had little experience with RWD research, so the conduct of such research may not be as pervasive among cancer cooperative groups as observed in this study.
Conclusions
While the cancer cooperative groups participating in this survey did not have any formal policies in place to collect and analyze RWD, they nonetheless had worked with such data before. More specifically, they had conducted RWD studies for hypothesis-generating and hypothesis-testing purposes to a similar extent, mostly using observational data that had been derived from disease registries, electronic health records, or patient questionnaires, and which were not predominantly prospective or retrospective in nature. Only a minority of the responders had ever undertaken PCTs or cmRCTs. The groups that were experienced in RWD research viewed the large scale and the low costs of such research as its main benefits, and perceived the associated methodological and operational challenges to be its most important constraints. However, they were not in agreement on what the most appropriate definition for RWD was, and their understanding of this term may therefore differ. Despite their often extensive experience with RWD studies, they continued to prioritize the conduct of traditional clinical trials. Of the responders that had never carried out any research involving the collection and interpretation of RWD, a majority were planning to set up studies relying on the analysis of such data in the future. These findings demonstrate that the academic sector is already contributing to the generation of RWE in the field of oncology, but they also show that major barriers remain, including the cooperative groups’ limited knowledge and expertise in this regard, and that there is a need for harmonizing existing RWD and RWE terminologies to avoid confusion. Moreover, the results highlight that there is still room for expanding the role of academia in interventional RWD research.
Acknowledgements
The authors thank the participating cooperative groups for their contribution to the study.
Funding
RS’s work as a Fellow at EORTC Headquarters was supported by a grant from the EORTC Cancer Research Fund (ECRF; no grant number). This publication was supported by a donation from Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society from Belgium.
Disclosure
The authors have declared no conflicts of interest.
Ethics statement
This study was reviewed and approved by the Ethics Committee Research UZ/KU Leuven (identification number S65201). Responders provided their written informed consent to participate in the study.
Supplementary data
References
- 1.Makady A., de Boer A., Hillege H., Klungel O., Goettsch W. on behalf of GetReal Work Package 1. What is real-world data? A review of definitions based on literature and stakeholder interviews. Value Health. 2017;20:858–865. doi: 10.1016/j.jval.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 2.GetReal Consortium RWE Navigator – sources of real-world data; 2020. https://rwe-navigator.eu/use-real-world-evidence/sources-of-real-world-data/ Available at.
- 3.Food and Drug Administration (FDA) Framework for FDA’s Real-World Evidence Program; 2018. https://www.fda.gov/media/120060/download Available at.
- 4.Sherman R.E., Anderson S.A., Dal Pan G.J., et al. Real-world evidence – what is it and what can it tell us? N Engl J Med. 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 5.GetReal Consortium RWE Navigator – real-world evidence; 2020. https://rwe-navigator.eu/use-real-world-evidence/rwe-importance-in-medicine-development/ Available at.
- 6.GetReal Consortium RWE Navigator – generating real-world evidence; 2020. https://rwe-navigator.eu/use-real-world-evidence/generate-real-world-evidence/ Available at.
- 7.Cave A., Kurz X., Arlett P. Real-world data for regulatory decision making: challenges and possible solutions for Europe. Clin Pharmacol Ther. 2019;106:36–39. doi: 10.1002/cpt.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaulieu-Jones B.K., Finlayson S.G., Yuan W., et al. Examining the use of real-world evidence in the regulatory process. Clin Pharmacol Ther. 2020;107:843–852. doi: 10.1002/cpt.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mofid S., Bolislis W.R., Kühler T.C. Real-world data in the postapproval setting as applied by the EMA and the US FDA. Clin Ther. 2022;44:306–322. doi: 10.1016/j.clinthera.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Berlin J.A., Glasser S.C., Ellenberg S.S. Adverse event detection in drug development: recommendations and obligations beyond phase 3. Am J Public Health. 2008;98:1366–1371. doi: 10.2105/AJPH.2007.124537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth C.M., Karim S., Mackillop W.J. Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol. 2019;16:312–325. doi: 10.1038/s41571-019-0167-7. [DOI] [PubMed] [Google Scholar]
- 12.Hemkens L.G., Contopoulos-Ioannidis D.G., Ioannidis J.P.A. Routinely collected data and comparative effectiveness evidence: promises and limitations. Can Med Assoc J. 2016;188:E158–E164. doi: 10.1503/cmaj.150653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pregelj L., Hwang T.J., Hine D.C., et al. Precision medicines have faster approvals based on fewer and smaller trials than other medicines. Health Aff (Millwood) 2018;37:724–731. doi: 10.1377/hlthaff.2017.1580. [DOI] [PubMed] [Google Scholar]
- 14.Wallach J.D., Ross J.S., Naci H. The US Food and Drug Administration’s expedited approval programs: evidentiary standards, regulatory trade-offs, and potential improvements. Clin Trials. 2018;15:219–229. doi: 10.1177/1740774518770648. [DOI] [PubMed] [Google Scholar]
- 15.Davis C., Naci H., Gurpinar E., Poplavska E., Pinto A., Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ. 2017;359:j4530. doi: 10.1136/bmj.j4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saesen R., Lejeune S., Quaglio G., Lacombe D., Huys I. Views of European drug development stakeholders on treatment optimization and its potential for use in decision-making. Front Pharmacol. 2020;11:43. doi: 10.3389/fphar.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichler H., Pignatti F., Schwarzer-Daum B., et al. Randomized controlled trials versus real world evidence: neither magic nor myth. Clin Pharmacol Ther. 2020;109:1212–1218. doi: 10.1002/cpt.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins R., Bowman L., Landray M., Peto R. The magic of randomization versus the myth of real-world evidence. N Engl J Med. 2020;382:674–678. doi: 10.1056/NEJMsb1901642. [DOI] [PubMed] [Google Scholar]
- 19.Soni P.D., Hartman H.E., Dess R.T., et al. Comparison of population-based observational studies with randomized trials in oncology. J Clin Oncol. 2019;37:1209–1216. doi: 10.1200/JCO.18.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franklin J.M., Platt R., Dreyer N.A., et al. When can nonrandomized studies support valid inference regarding effectiveness or safety of new medical treatments? Clin Pharmacol Ther. 2022;111:108–115. doi: 10.1002/cpt.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger M.L., Sox H., Willke R.J., et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE special task force on real-world evidence in health care decision making. Value Health. 2017;20:1003–1008. doi: 10.1016/j.jval.2017.08.3019. [DOI] [PubMed] [Google Scholar]
- 22.Hernán M.A. Methods of public health research – strengthening causal inference from observational data. N Engl J Med. 2021;385:1345–1348. doi: 10.1056/NEJMp2113319. [DOI] [PubMed] [Google Scholar]
- 23.Jaksa A., Wu J., Jónsson P., Eichler H.G., Vititoe S., Gatto N.M. Organized structure of real-world evidence best practices: moving from fragmented recommendations to comprehensive guidance. J Comp Eff Res. 2021;10:711–731. doi: 10.2217/cer-2020-0228. [DOI] [PubMed] [Google Scholar]
- 24.Booth C.M., Tannock I.F. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110:551–555. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Maio M., Perrone F., Conte P. Real-world evidence in oncology: opportunities and limitations. Oncologist. 2020;25:e746–e752. doi: 10.1634/theoncologist.2019-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skovlund E., Leufkens H.G.M., Smyth J.F. The use of real-world data in cancer drug development. Eur J Cancer. 2018;101:69–76. doi: 10.1016/j.ejca.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 27.Karim S., Booth C.M. Effectiveness in the absence of efficacy: cautionary tales from real-world evidence. J Clin Oncol. 2019;37:1047–1050. doi: 10.1200/JCO.18.02105. [DOI] [PubMed] [Google Scholar]
- 28.Deloitte Center for Health Solutions RWE focus is shifting to R&D, early investments begin to pay off; 2020. https://www2.deloitte.com/content/dam/insights/us/articles/6578_CHS-RWE-benchmarking-survey/DI_RWE%20benchmarking%20survey%20(SECURED).pdf Available at.
- 29.European Commission Europe’s Beating Cancer Plan; 2021. https://ec.europa.eu/health/sites/health/files/non_communicable_diseases/docs/eu_cancer-plan_en.pdf Available at.
- 30.European Commission European Health Data Space factsheet; 2022. https://ec.europa.eu/commission/presscorner/api/files/attachment/872447/Factsheet - EHDS.pdf Available at.
- 31.Dignam J.J. The role of cancer cooperative groups within the spectrum of cancer care. Cancer Control. 2004;11:55–63. doi: 10.1177/107327480401100210. [DOI] [PubMed] [Google Scholar]
- 32.Mauer A.M., Rich E.S., Schilsky R.L. In: Cancer Clinical Trials: Proactive Strategies. Leong S.P.L., editor. Springer; Boston, Massachusetts: 2007. The role of cooperative groups in cancer clinical trials; pp. 111–129. [DOI] [PubMed] [Google Scholar]
- 33.European Society for Medical Oncology (ESMO) General research groups; 2020. https://www.esmo.org/research/research-groups-tools-and-database/General-research-groups Available at.
- 34.European Society for Medical Oncology (ESMO) Cancer research groups by type and topic; 2022. https://www.esmo.org/research/research-groups-tools-and-database/Cancer-research-groups-by-type Available at.
- 35.Braun V., Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. [Google Scholar]
- 36.Maguire M., Delahunt B. Doing a thematic analysis: a practical, step-by-step guide for learning and teaching scholars. All Irel J High Educ. 2017;9:335. [Google Scholar]
- 37.De Lusignan S., Crawford L., Munro N. Creating and using real-world evidence to answer questions about clinical effectiveness. J Innov Health Inform. 2015;22:368–373. doi: 10.14236/jhi.v22i3.177. [DOI] [PubMed] [Google Scholar]
- 38.Saesen R., Kantidakis G., Marinus A., Lacombe D., Huys I. How do cancer clinicians perceive real-world data and the evidence derived therefrom? Findings from an international survey of the European Organisation for Research and Treatment of Cancer. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.969778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.International Coalition of Medicines Regulatory Authorities (ICMRA) ICMRA statement on international collaboration to enable real-world evidence (RWE) for regulatory decision-making; 2022. https://icmra.info/drupal/sites/default/files/2022-07/icmra_statement_on_rwe.pdf Available at.
- 40.Kempf E., Bogaerts J., Lacombe D., Liu L. ‘Mind the gap’ between the development of therapeutic innovations and the clinical practice in oncology: a proposal of the European Organisation for Research and Treatment of Cancer (EORTC) to optimise cancer clinical research. Eur J Cancer. 2017;86:143–149. doi: 10.1016/j.ejca.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 41.Simon G.E., Platt R., Hernandez A.F. Evidence from pragmatic trials during routine care – slouching toward a learning health system. N Engl J Med. 2020;382:1488–1491. doi: 10.1056/NEJMp1915448. [DOI] [PubMed] [Google Scholar]
- 42.Buesching D.P., Luce B.R., Berger M.L. The role of private industry in pragmatic comparative effectiveness trials. J Comp Eff Res. 2012;1:147–156. doi: 10.2217/cer.12.9. [DOI] [PubMed] [Google Scholar]
- 43.Relton C. TwiCs – Trials within cohorts (and other groups/data structures); 2018. https://woundsrn.org/wp-content/uploads/2018/05/Clare-Relton.pdf Available at.
- 44.Lacombe D., Bogaerts J., Tombal B., et al. Late translational research: putting forward a new model for developing new anti-cancer treatments that addresses the needs of patients and society. Mol Oncol. 2019;13:558–566. doi: 10.1002/1878-0261.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacombe D., Quaglio G., Lejeune S., Saesen R., Rübig P. Establishing treatment optimisation as part of personalised medicine development. Eur J Cancer. 2019;113:96–97. doi: 10.1016/j.ejca.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Saesen R., Lacombe D., Huys I. Design, organisation and impact of treatment optimisation studies in breast, lung and colorectal cancer: the experience of the European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2021;151:221–232. doi: 10.1016/j.ejca.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Austalasian Gastro-Intestinal Trials Group (AGITG) RESOLUTE. https://gicancer.org.au/clinical-trial/resolute/ Available at.
- 48.Berton D., Floquet A., Lescaut W., et al. Real-world experience of bevacizumab as first-line treatment for ovarian cancer: the GINECO ENCOURAGE cohort of 468 French patients. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.711813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmerini E., Sanfilippo R., Grignani G., et al. Trabectedin for patients with advanced soft tissue sarcoma: a non-interventional, retrospective, multicenter study of the Italian sarcoma group. Cancers (Basel) 2021;13:1053. doi: 10.3390/cancers13051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S.I., Lee J.W., Kim K., et al. Comparisons of survival outcomes between bevacizumab and olaparib in BRCA-mutated, platinum-sensitive relapsed ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG 3052) J Gynecol Oncol. 2021;32:e90. doi: 10.3802/jgo.2021.32.e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latin American Cooperative Oncology Group (LACOG) LACOG 0319; 2022. https://lacogcancerresearch.org/studies/epidemiological-studies/ongoing/lacog-0319/ Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.