Abstract
Background
Apart for infantile fibrosarcoma (IFS), very little is known about NTRK-rearranged mesenchymal tumors (NMTs). The objective of this study is to describe the distribution, characteristics, natural history, and prognosis of NMT.
Patients and methods
This study was carried out as a translational research program, retrospectively from a cohort of 500 soft tissue sarcoma (STS; excluding IFS) and prospectively both in routine practice and from the RNASARC molecular screening program (N = 188; NCT03375437).
Results
Using RNA-sequencing, NTRK fusion was detected in 16 patient tumors diagnosed as STS: 8 samples of sarcoma with simple genomics (4 NTRK-rearranged spindle cell neoplasm, 3 ALK/ROS wild-type inflammatory myofibroblastic tumor, and 1 quadruple Wild-type gastrointestinal stromal tumor) and 8 samples of sarcomas with complex genomics (dedifferentiated liposarcoma, intimal sarcoma, leiomyosarcoma, undifferentiated pleomorphic sarcoma, high-grade uterine sarcoma, malignant peripheral nerve sheath tumor). Among the eight patients with simple genomics, four were treated with tyrosine receptor kinase inhibitor (TRKi) at different stages of the disease and all benefited from the treatment, including one complete response. Among the eight other patients, six evolved with metastatic spreading and the median metastatic survival was 21.9 months, as classically reported in these tumor types. Two of them received a first-generation TRKi without objective response.
Conclusions
Our study confirms low frequency and histotype diversity of NTRK fusion in STS. While the activity of TRKi in simple genomics NMT is confirmed, our clinical data encourage subsequent studies focusing on the biological relevance of NTRK fusions in sarcomas with complex genomics together with the efficacy of TRKi in this population.
Key words: sarcoma, mesenchymal tumor, NTRK rearrangement, TRK inhibitor
Highlights
-
•
NTRK fusions are identified in adult and pediatric sarcomas at a frequency of ≈1% and in a wide range of histotypes.
-
•
A significant activity of TRKi in NMT with simple genomics is confirmed.
-
•
The functional significance of NTRK fusion and the relevance of TRKi in NMT with complex genomics remain elusive.
-
•
Studies to address the biological relevance of NTRK fusions in SCG, together with the efficacy of TRKi, are required.
Introduction
Soft tissue sarcomas (STSs) are rare and heterogeneous malignant tumors accounting for <1% of all adult malignant tumors.1, 2, 3 From a genetic perspective, they have been classified into two main categories. One group is characterized by near-diploid karyotypes and simple genetic alterations including translocations or oncogene activating mutations that are essential to sarcomagenesis [simple genomic sarcomas (SGSs)]. The second includes sarcomas with complex karyotypes and chromosomal instability [complex genomic sarcomas (CGSs)].4 This classification does not reflect the genetic diversity across tumors of a given group and each category includes a wide diversity of sarcoma subtypes.
Major advances in molecular characterization have driven further refinements in the fifth WHO (World Health Organization) Classification of Soft Tissue and Bone Tumors and several new entities have been included5; >150 distinct mesenchymal tumors are now described. Among SGSs, infantile fibrosarcoma (IFS) is an ultra-rare subtype of STS mainly characterized by ETV6::NTRK3 oncogenic fusion.6 Other subtypes of SGS with NTRK fusion have been reported, including inflammatory myofibroblastic tumor (IMT)7 and ‘NTRK-rearranged spindle cell neoplasm’.6,8,9 The latter category describes a group morphologically, molecularly, and phenotypically defined by a monotonous proliferation of spindle cells, NTRK fusion, and frequent expression of CD34 and S100, respectively. This emerging category includes tumors previously diagnosed as ‘lipofibromatosis-like neural tumor’ (LNT)7 and tumors that closely resemble peripheral nerve sheath tumors (PNSTs).5 By contrast, recurrent chromosomal fusion events involving the carboxy-terminal kinase domain of NTRK and various upstream amino-terminal partners have been identified across various types of CGS.9,10
NTRK fusions lead to overexpression of the chimeric tyrosine receptor kinase (TRK) protein, resulting in constitutively active downstream signaling.11 Phase I trials and phase II ‘basket’ studies reported an interesting benefit–risk profile of TRK inhibitors (TRKis) in patients with NTRK fusion-positive cancer, regardless of tumor type, including patients with sarcoma.12, 13, 14
Management of STS is challenging due to its rarity and the clinical and biological heterogeneity of the disease. Although there have been progresses in local therapy and management through standardized approaches in expert centers, the standard treatment of advanced/metastatic disease remains single-agent anthracycline chemotherapy, providing objective responses in 12%-26% of patients and a median progression-free survival (PFS) of 4-6 months.15,16 Beyond the front-line setting, several systemic agents are approved including pazopanib and trabectedin, with modest improvements in PFS, but the prognosis of advanced disease remains poor with a median overall survival (OS) of 18 months.17,18
In this context, the use of TRKis for patients with ‘NTRK-rearranged mesenchymal tumors’ (NMTs) including STS represents a major therapeutic opportunity. Apart for IFS, very little is known about their characteristics, natural history, and prognosis.19, 20, 21 Therefore we investigated the distribution and prognostic significance of NMT including STS in an ambispective series of mesenchymal tumors, using RNA sequencing.
Patients and methods
Selection of cases
This study was carried out between March 2019 and March 2021 at three French sarcoma reference centers (Centre Léon Bérard, Oscar Lambret, and Gustave Roussy) as an ambispective translational research program. Tumor samples were tested for NTRK fusions using whole-exome RNA-sequencing: (i) from a retrospective cohort of 500 patients with STS (excluding IFS) of the NETSARC database (https://netsarc.sarcomabcb.org/; cohort 1); (ii) from a prospective cohort of 188 patients with STS included in the RNASARC molecular screening program (NCT03375437; cohort 2); and (iii) prospectively in routine practice (cohort 3). The inclusion criteria were histopathological diagnosis of STS and sufficiently available tumor material. All tumor samples were obtained from the pathology department and consisted of formalin-fixed paraffin-embedded tissues samples at initial diagnosis or at recurrence, obtained by biopsy or surgical excision to establish the diagnosis of the disease. Each case was reviewed by a pathological expert of sarcoma from the RRePS network. All patients of the prospective cohort signed an informed consent to participate in the research according to the French laws. An institutional review board (The Centre Léon Bérard Clinical Trial Review Committee) reviewed and agreed with the study protocol and the informed consent form.
Clinical review
Patients and tumors characteristics, management, and follow-up data were collected from the medical record. The following data were collected: center, birthdate, gender, date of primary tumor diagnosis, tumor status at diagnosis (localized/locally advanced/metastatic), date of relapse, date of last follow-up, status at last follow-up (alive without disease/alive with disease/dead), characteristics of the primary tumor [pathological diagnosis, size, grade, site, (neo)adjuvant therapy, surgery, quality of resection], NTRK fusion results [date, type of sample, NTRK stain by immunohistochemistry (IHC), other relevant molecular alterations, fusion partner gene], local recurrence (date, location, treatment), metastatic spreading (date, metastases location, systemic treatment, best response, date of last cycle, reason for discontinuation, progression date), and TRKi administration (TRKi name, clinical trial/special access program, start date, stop date, best response, reason for discontinuation, progression date). Follow-up was calculated starting from the date of the diagnosis to the date of last follow-up. Survival was calculated from the date of diagnosis to the date of death or last follow-up.
RNA sequencing and gene expression analysis
All cases of the series were reviewed by expert sarcoma pathologists from the NETSARC network. Integrative whole-exome RNA sequencing and transcriptomic analysis were carried out as previously described.22
Results
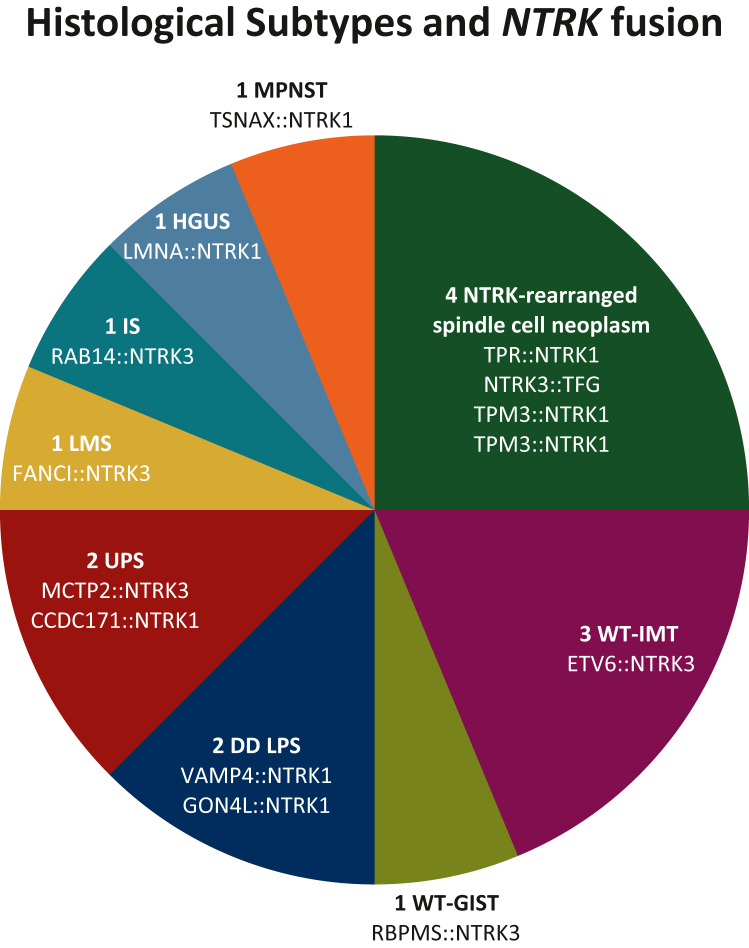
This study identified 16 cases of patients with a fusion of NTRK: five cases from cohort 1 (5/500, 1%), three cases from cohort 2 (3/188, 1.6%), and eight cases detected prospectively in routine practice (cohort 3), which included 2642 solid tumors at the time of the analysis. Table 1 describes the characteristics of the patients and tumor. Seven were women and nine were men. The median age at the time of diagnosis was 47.6 years (range 10 months to 76 years). Histological subtypes according to the fifth WHO Classification of Soft Tissue and Bone Tumors4 were the following: four NTRK-rearranged spindle cell neoplasm (including two cases diagnosed as LNT, one as tumor resembling PNST, and one as cutaneous spindle cell mesenchymal tumor), three ALK/ROS wild-type (WT) IMT (Figure 1), one quadruple WT-gastrointestinal stromal tumor (GIST), two dedifferentiated liposarcomas (DD LPSs), two undifferentiated pleomorphic sarcoma (UPS), one leiomyosarcoma (LMS), one intimal sarcoma (IS), one high-grade uterine sarcoma (HGUS), and one neurofibromatosis type 1-associated malignant PNST (NF1-MPNST). NTRK IHC was carried out in six cases, which was negative in three (UPS, LMS, and IS) and positive in three (PNST, LNT, and cutaneous spindle cell).
Table 1.
Patients and tumor characteristics of the whole cohort (N = 16)
| Characteristics | Simple genomic sarcoma (n = 8) | Complex genomic sarcoma (n = 8) |
|---|---|---|
| Sex | ||
| Male | 4 | 5 |
| Female | 4 | 3 |
| Age at initial diagnosis | ||
| Median (range), years | 37.7 (0.8-74) | 60.5 (21.8-76) |
| Histological subtypes | 4 NTRK-rearranged spindle cell neoplasm | 2 DD LPS |
| 2 LNT | 2 UPS | |
| 1 tumor resembling PNSTs | 1 LMS | |
| 1 cutaneous spindle cell mesenchymal tumor | 1 IS | |
| 3 ALK/ROS WT-IMT | 1 HGUS | |
| 1 quadruple WT-GIST | 1 NF1-associated MPNST | |
| Tumor size at diagnosis | ||
| Median (range), mm | 80 (20-120) | 100 (8-155) |
| Primary tumor location | 2 scapula | 2 lower limb |
| 2 thoracic | 1 upper limb | |
| 1 hand | 1 uterine | |
| 1 peritoneal | 1 pulmonary artery | |
| 1 duodenal | 1 mediastinal | |
| 1 spermatic cord | ||
| FNCLCC primary tumor grade | ||
| NA | 5 | 1 |
| Grade I | 2 | 1 |
| Grade II | 1 | 1 |
| Grade III | - | 5 |
| Disease status | ||
| Localized/metastatic at diagnosis | 8/0 | 7/1 |
| Local recurrence | 1 | 1 |
| Metastatic recurrence | 2 | 6 |
| No recurrence | 5 | 1 |
| Relapse-free survival | ||
| Median (range), months | 21.1 (10.7-51.1) | 10 (6.3-48) |
| Overall survival | ||
| Median (range), months | 35.6 (3.3-144.4) | 34.9 (9.3-127.2) |
DD LPS, dedifferentiated liposarcoma; HGUS, high-grade uterine sarcoma; IS, intimal sarcoma; LMS, leiomyosarcoma; LNT, lipofibromatosis-like neural tumor; MPNST, malignant peripheral nerve sheath tumor; NF1, neurofibromatosis 1; PNST, peripheral nerve sheath tumor; UPS, undifferentiated pleiomorphic sarcoma; WT-GIST, wild-type gastrointestinal stromal tumor; WT-IMT, wild-type inflammatory myofibroblastic tumor.
Figure 1.
Distribution of histological subtypes and NTRK fusion.
DD LPS, dedifferentiated liposarcoma; HGUS, high-grade uterine sarcoma; IS, intimal sarcoma; LMS, leiomyosarcoma; MPNST, malignant peripheral nerve sheath tumor; UPS, undifferentiated pleomorphic sarcoma; WT-GIST, quadruple KIT/PDGFR/NF1-Ras/SDH wild-type gastrointestinal stromal tumor; WT-IMT, ALK/ROS wild-type inflammatory myofibroblastic tumor.
The distribution of histological subtypes and NTRK fusion is described in Figure 1. All gene rearrangements resulted in the 3′ region of NTRK (NTRK1, n = 7; NTRK3, n = 9) joining with the 5′ end of various fusion partner genes (LMNA, ETV6, RAB14, FANCI, MCTP2, CCDC171, TPR, TPM3, TSNAX, VAMP4, GON4L, RBPMS, and TFG). Based on the histopathological diagnosis referring to the fifth WHO Classification of Soft Tissue and Bone Tumors, two groups of NMT emerged: eight cases of SGS (ALK/ROS WT-IMT, quadruple WT-GIST, and NTRK-rearranged spindle cell neoplasm) and eight cases of CGS (DD LPS, HGUS, LMS, IS, and MPNST).
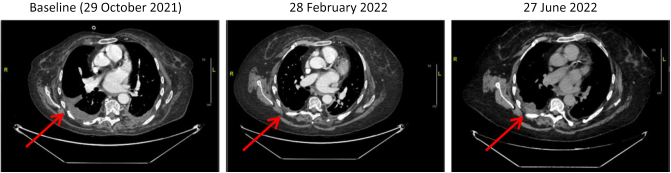
The description of the eight patients with SGS is detailed in the Table 2. Four patients presented with localized disease that did not recur following locoregional treatment after 4, 5, 13, and 57 months, respectively. One patient with a locally advanced nonoperable IMT was included in the Navigate phase II trial (NCT02576431) and received larotrectinib for 6 months. This treatment led to a 66% tumor shrinkage allowing the tumor to be surgically resected later. The pathological report described a 35-mm tumor in complete histological response (no viable tumor cells). No recurrence occurred after a 3.7-year follow-up. A patient with a LNT had his primary tumor resected in 2010. Local recurrences occurred in 2012 and 2014, both treated with surgical resection. In 2018, the patient presented with a third local recurrence without possibility of conservative surgery. Identification of NTRK fusion allowed his inclusion in the “STARTRK-2” clinical trial (NCT02568267) in May 2019 and entrectinib led to a complete response that is still ongoing. The patient with WT-GIST had complete surgical resection of high-risk duodenal GIST in 2015 (8 cm, 10 mitoses/mm2). She received imatinib as adjuvant treatment and presented liver and peritoneal recurrence while receiving the adjuvant treatment. Her drug regimen was changed to sunitinib during 5 months without benefit and she was then treated with cabozantinib (which targets TRK) in the CABOGIST clinical trial (NCT02216578). She had clinical and radiological response to treatment with a prolonged 15-month PFS. NTRK testing was carried out retrospectively, after this patient died of disease. The patient with the tumor resembling PNST relapsed in the lung and pleura after adjuvant radiotherapy and received entrectinib in the STARTRK-2 trial with a 78% tumor shrinkage, with the response lasting 8 months (as illustrated in Figure 2). He is now waiting to be treated with repotrectinib, a next-generation TRKi.
Table 2.
Description of patients, tumor, and management of simple genomic sarcoma
| Age at diagnosis | Stage and histological subtype | Tumor location | Treatment | TRKi | Follow-up |
|---|---|---|---|---|---|
| 44 years | Localized LNT | Shoulder | Surgery | — | 4 months CR |
| 13.4 years | Localized LNT | Hand | Surgery three times for local relapse | Entrectinib at the third local relapse | 3.4 years CR |
| 31 years | Localized cutaneous NTRK-rearranged spindle neoplasm | Shoulder | R1 surgery | — | 5 months CR |
| 65.4 years | Metastatic PNST | Lung and pleura metastases | R1 surgery + RT | Entrectinib at metastatic relapse | 8 months PFS |
| 10 months | Locally advanced IMT | Peritoneum | R1 surgery | Neoadjuvant larotrectinib | 3.7 years CR |
| 5.4 years | Localized IMT | Lung | R0 surgery | — | 1.1 years CR |
| 45.8 years | Localized IMT | Lung | R0 surgery | — | 4.8 years CR |
| 74 years | Metastatic GIST | Liver and peritoneal metastases | Adjuvant imatinib + first-line sunitinib | Cabozantinib as the second line | 15 months PFS |
CR, complete response; GIST, Gastro intestinal stromal tumor; IMT, inflammatory myofibroblastic tumor; LNT, lipofibromatosis neural tumor; PFS, progression-free survival; PNST, peripheral nerve sheath tumor; RT, radiotherapy; UK, unknown.
Figure 2.
Thorax computed tomography scan at pre- and post-treatment of patients with peripheral nerve sheath tumor treated with tyrosine receptor kinase inhibitor.
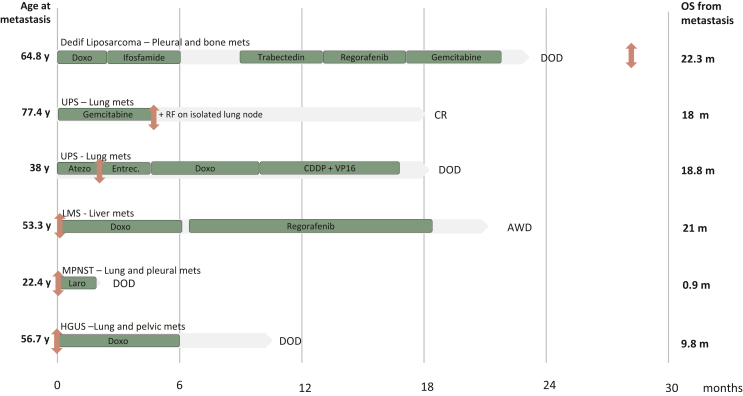
In the group of eight CGSs, two patients had localized disease with no recurrence after locoregional management. One patient with a 100-mm right DD LPS was treated with R0 resection and adjuvant radiotherapy; he did not recur after 10.5-year follow-up. One patient with IS experienced a local relapse at 1 year treated with radiotherapy and metronomic cyclophosphamide. This treatment led to complete response after 3.4-year follow-up. In both cases tumor harbored MDM2 amplification. For six patients, the disease evolved in a metastatic mode and Figure 3 describes these patients, their tumor, and their management in the advanced setting. The patient with HGUS was metastatic at diagnosis while for the others, the median time to relapse was 10 months (range 6.3-48). The median OS after the diagnosis of metastasis was 21.9 months (range 0.9-33.4). The metastatic sites were the lung in four patients, the liver in one patient, the peritoneum in one patient, the pleura in two patients, and the pelvis in one patient. For one patient, NTRK fusion was identified after death, as part of the retrospective program. For one patient, it was identified when the patient was in complete remission after a combination of chemotherapy and local treatment of a unique metastasis. In two cases, NTRK fusion was identified at the beginning of metastatic disease, but the patients had no access to TRKi. Finally, both patients with UPS and NF1-MPNST, respectively, did not respond to TRKi.
Figure 3.
Description of patients, tumor, and management of metastatic complex genomic sarcoma.
AWD, alive with disease; Atezo, Atezolizumab; CR, complete remission; DD, dedifferentiated; DOD, dead of disease; Doxo, doxorubicin; Entrec, Entrectinib; GIST, gastrointestinal stromal tumor; HGUS, high-grade uterine sarcoma; Laro, Larotrectinib; LMS, leiomyosarcoma; m, months; Mets, metastases; MPNST, malignant peripheral nerve sheath tumor; OS, overall survival; RF, radiofrequency; UPS, undifferentiated pleiomorphic sarcoma; y, years.  , Diagnosis of NTRK fusion.
, Diagnosis of NTRK fusion.
Discussion
With 16 patients included, this multi-institutional ambispective study provides the largest cohort so far, to describe the characteristics, pattern of care, and outcomes of mesenchymal tumors harboring NTRK fusion. In both studied cohorts (cohorts 1 and 2) for which the incidence of NTRK fusion could be determined, the incidence of 1% and 1.6%, respectively, is consistent with data from the literature. A description of the distribution of histotypes included in these three cohorts would be of great interest to get a more precise assessment of NMT incidence according to sarcoma subtype. Such an analysis is planned to be carried out but not available for the current work. Regarding such an unusual event, screening of all mesenchymal tumors cannot be considered and the need for clue to select patients to screen is therefore essential. These clues may be clinical, histopathological, or biological. Collecting more data to increase knowledge of these entities is essential to refine the testing strategy. In both phase II clinical trials reporting the efficacy of TRKi, no description of the histological subtypes of mesenchymal tumors included is available beyond IFS12,13 and the scientific community aims at identifying those mesenchymal tumors likely to harbor NTRK fusion.14,23,24
From a molecular genetics perspective, NMTs of our series may be split into two categories. A first category of eight NMTs belongs to the group of SGS, including ALK/ROS WT-IMT, quadruple WT-GIST, and NTRK-rearranged spindle cell neoplasm. NTRK IHC was positive for the three cases tested in this group. Half of them were treated with TRKi at different stages of the disease: all had a clear benefit from the treatment. TRKi made an unresectable tumor to become resectable; another one presents a long-lasting complete response after a third locoregional recurrence. TRKi led to 8-month PFS and partial response as first-line therapy in a metastatic tumor resembling PNST, to put into perspective with the historical 4.5-month median PFS obtained in metastatic STS treated with doxorubicin. In second line of a metastatic GIST, after imatinib as adjuvant therapy and sunitinib as first line, a 15-months PFS was achieved with cabozantinib, to compare with the 5.5-month median PFS reported in the clinical trial.23 The other four tumors of this group did not present any recurrence. A second category of eight NMT belongs to the group of CGS, including DD LPS, LMS, UPS, IS, HGUS, and NF1-MPNST. All these cases were high-grade sarcomas (NTRK IHC was negative for the three cases tested in this group); six of them evolved with metastatic spreading and a 21.9-month median metastatic survival as classically reported in advanced STS. Four patients of this group did not receive TRKi due to delayed molecular diagnosis or rapid fatal evolution. Surprisingly, the patients with UPS and NF1-associated MPNST did not respond to TRKi.
The experts from the World Sarcoma Network (WSN) proposed an algorithm to guide diagnosis testing, based on fusion frequency.21 In their recommendations, the WSN proposes ALK-ROS1 WT-IMT to be tested for NTRK fusion in high-priority, which is consistent with our data. Indeed, this recommendation is justified not only by the high-fusion frequency but also by its clinical impact. Otherwise, quadruple WT-GISTs are considered with intermediate priority, as well as SCG. Given the rarity of quadruple WT-GIST and the therapeutic consequences in the advanced setting, one can wonder if they should not also be included in this ‘high-priority’ testing group. On the contrary, our data may question the pathogenicity of NTRK rearrangement in CGS, owing to frequent chromosomal breaks in their genome. We definitely need to carry out integrative genomic analysis of these tumors to better define the role of identified NTRK fusion in a context of genomic instability. In consequence, the predictive impact of the presence of the NTRK fusion should not be the same in all tumor types and the algorithm to guide diagnostic testing should be based both on the fusion frequency and its predictive value.
The WSN also suggests that MDM2/CDK4-amplified liposarcoma (together with sarcoma with recurrent gene fusions and KIT, PDGFR, SDH, NF1, or BRAF-altered GIST) may be excluded from routine NTRK-fusion testing. In the present study, we report two patients with DD LPS and one patient with an IS of the pulmonary artery, all three harboring both MDM2/CDK4 amplification and NTRK fusion. These results suggest that sarcomas with molecular alteration identified as oncogenic driver (MDM2 and/or CDK4 amplification for example) may in addition harbor NTRK fusion. Nevertheless, there is no formal proof that these fusions are relevant and that patients might benefit from TRKi. That is why as of today, those patients should not be tested for NTRK fusion in routine practice. Further and larger cohorts are needed to determine the frequency of NTRK fusion in the different sarcoma subtypes and better guide the optimal approach to NTRK-fusion screening.
Since 2019-2020, larotrectinib and entrectinib are approved by the US Food and Drug Administration (FDA) and European Medicines Agency for use in adult and pediatric patients with advanced/metastatic solid tumors harboring an NTRK gene fusion. Nevertheless, the French transparency committee [Haute Autorité de santé (HAS)] transmitted a favorable opinion for reimbursement of the TRKi larotrectinib only for pediatric patients with advanced IFS or other NMTs. TRKi demonstrated efficacy in several phase I/II trials irrespective of age and this cut-off (< versus >18 years) does not seem to be based on any scientific rationale, except that pediatrics sarcomas are usually SSG while SCG mostly affect adults.
Furthermore, the HAS justified their decision by the lack of comparative data with standard treatment and the lack of data on a potential prognostic impact of the presence of the fusion. Given the rarity of these entities, conducting randomized studies will be challenging in NMT to compare the efficacy of TRKi with standard treatment. A randomized study including NTRK-fusion tumor of any histotype will hardly be considered given the lack of standard treatment across different histotypes. With the increasing fragmentation of histologic and molecular subtypes driven by biology in sarcoma and tumor in general, the regulators should take into account the difficulty to conduct randomized trials in these entities with smaller and smaller populations of patients.
Conclusions
Our study confirms that NTRK fusions are identified in adult and pediatric sarcomas at a frequency of ≈1% and in a wide range of sarcoma histotypes. While the significant activity of TRKi in NMT with simple genomics (such as IMT or GIST) is once more confirmed, the functional significance of NTRK fusion and the relevance of TRKi in NMT with complex genomics remain elusive. Therefore the recommendation to screen all sarcomas for NTRK fusions is not supported. Finally, our study encourages subsequent studies to address the biological relevance and sarcomagenic potential of NTRK fusions in sarcomas with complex genomics, together with the efficacy of TRKi in this population.
Acknowledgments
Funding
Bayer, LYRIC-INCA DGOS4664, Lyrican INCa_INSERM_DGOS_12563, InterSARC grants, Infosarcome [grant number: I-1636-01], Roche company.
Disclosure
AD declares research support from Bayer and GSK. JYB declares research honoraria from Novartis, GSK, Bayer, Roche, Deciphera, Ignyta, BMS, MSD, PharmaMar, and Karyopharm; and research support from Novartis, GSK, Bayer, Roche, Deciphera, Ignyta, BMS, MSD, PharmaMar, and Karyopharm. ALC declares honoraria from Bayer, PharmaMar, and Deciphera. MB declares honoraria from Bayer and Amgen and accommodations, expenses from PharmaMar. Other authors have declared no conflicts of interest.
References
- 1.Cormier J.N., Pollock R.E. Soft tissue sarcomas. CA Cancer J Clin. 2004;54(2):94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.de Pinieux G., Karanian M., Le Loarer F., et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours. International Agency for Research on Cancer; Lyon, France: 2020. p. 607. [Google Scholar]
- 5.Davis J.L., Lockwood C.M., Stohr B., et al. Expanding the spectrum of pediatric NTRK-rearranged mesenchymal tumors. Am J Surg Pathol. 2019;43(4):435–445. doi: 10.1097/PAS.0000000000001203. [DOI] [PubMed] [Google Scholar]
- 6.Alassiri A.H., Ali R.H., Shen Y., et al. ETV6-NTRK3 is expressed in a subset of ALK-negative inflammatory myofibroblastic tumors. Am J Surg Pathol. 2016;40(8):1051–1061. doi: 10.1097/PAS.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 7.Agaram N.P., Zhang L., Sung Y.S., et al. Recurrent NTRK1 gene fusions define a novel subset of locally aggressive lipofibromatosis-like neural tumors. Am J Surg Pathol. 2016;40(10):1407–1416. doi: 10.1097/PAS.0000000000000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haller F., Knopf J., Ackermann A., et al. Paediatric and adult soft tissue sarcomas with NTRK1 gene fusions: a subset of spindle cell sarcomas unified by a prominent myopericytic/haemangiopericytic pattern. J Pathol. 2016;238(5):700–710. doi: 10.1002/path.4701. [DOI] [PubMed] [Google Scholar]
- 9.Brahmi M., Dufresne A., Verret B., Tirode F., Blay J.Y. NTRK fusion in soft tissue sarcomas harboring MDM2/CDK4 amplification: three case reports. Ann Oncol. 2021;32(6):813–814. doi: 10.1016/j.annonc.2021.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Chiang S., Cotzia P., Hyman D.M., et al. NTRK fusions define a novel uterine sarcoma subtype with features of fibrosarcoma. Am J Surg Pathol. 2018;42(6):791–798. doi: 10.1097/PAS.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocco E., Scaltriti M., Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demetri G.D., De Braud F., Drilon A., et al. Updated integrated analysis of the efficacy and safety of entrectinib in patients with NTRK fusion-positive solid tumors. Clin Cancer Res. 2022;28(7):1302–1312. doi: 10.1158/1078-0432.CCR-21-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong D.S., DuBois S.G., Kummar S., et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drilon A., Laetsch T.W., Kummar S., et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tap W.D., Wagner A.J., Papai Z., et al. ANNOUNCE: a randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox) + olaratumab versus dox + PBO in patients (pts) with advanced soft tissue sarcomas (STS) J Clin Oncol. 2019;37(suppl 18):LBA3. [Google Scholar]
- 16.Judson I., Verweij J., Gelderblom H., et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 17.van der Graaf W.T.A., Blay J.Y., Chawla S.P., et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 18.Le Cesne A., Blay J.Y., Cupissol D., et al. Results of a prospective randomized phase III T-SAR trial comparing trabectedin (T) vs best supportive care (BSC) in patients with pretreated advanced soft tissue sarcoma (ASTS): a French Sarcoma Group (FSG) trial. J Clin Oncol. 2018;36(suppl 15) [Google Scholar]
- 19.Solomon J.P., Linkov I., Rosado A., et al. NTRK fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Mod Pathol. 2020;33(1):38–46. doi: 10.1038/s41379-019-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silvertown J.D., Lisle C., Semenuk L., et al. Prevalence of NTRK fusions in Canadian solid tumour cancer patients. Mol Diagn Ther. 2023;27(1):87–103. doi: 10.1007/s40291-022-00617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klink A.J., Kavati A., Gassama A., Kozlek T., Gajra A., Antoine R. Treatment patterns of real-world patients with TRK fusion cancer treated by US community oncologists. Target Oncol. 2022;17(5):549–561. doi: 10.1007/s11523-022-00909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macagno N., Pissaloux D., de la Fouchardière A., et al. Wholistic approach: transcriptomic analysis and beyond using archival material for molecular diagnosis. Genes Chromosomes Cancer. 2022;61(6):382–393. doi: 10.1002/gcc.23026. [DOI] [PubMed] [Google Scholar]
- 23.Demetri G.D., Antonescu C.R., Bjerkehagen B., et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: expert recommendations from the World Sarcoma Network. Ann Oncol. 2020;31(11):1506–1517. doi: 10.1016/j.annonc.2020.08.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonescu C.R. Emerging soft tissue tumors with kinase fusions: an overview of the recent literature with an emphasis on diagnostic criteria. Genes Chromosomes Cancer. 2020;59(8):437–444. doi: 10.1002/gcc.22846. [DOI] [PMC free article] [PubMed] [Google Scholar]