Abstract
Historically women were frequently excluded from clinical trials and drug usage to protect unborn babies from potential harm. As a consequence, the impact of sex and gender on both tumour biology and clinical outcomes has been largely underestimated. Although interrelated and often used interchangeably, sex and gender are not equivalent concepts. Sex is a biological attribute that defines species according to their chromosomal makeup and reproductive organ, while gender refers to a chosen sexual identity. Sex dimorphisms are rarely taken into account, in either preclinical or clinical research, with inadequate analysis of differences in outcomes according to sex or gender still widespread, reflecting a gap in our knowledge for a large proportion of the target population. Underestimation of sex-based differences in study design and analyses has invariably led to ‘one-drug’ treatment regimens for both males and females. For patients with colorectal cancer (CRC), sex also has an impact on the disease incidence, clinicopathological features, therapeutic outcomes, and tolerability to anticancer treatments. Although the global incidence of CRC is higher in male subjects, the proportion of patients presenting right-sided tumours and BRAF mutations is higher among females. Concerning sex-related differences in treatment efficacy and toxicity, drug dosage does not take into account sex-specific differences in pharmacokinetics. Toxicity associated with fluoropyrimidines, targeted therapies, and immunotherapies has been reported to be more extensive for females with CRC than for males, although evidence about differences in efficacy is more controversial. This article aims to provide an overview of the research achieved so far into sex and gender differences in cancer and summarize the growing body of literature illustrating the sex and gender perspective in CRC and their impact in relation to tumour biology and treatment efficacy and toxicity. We propose endorsing research on how biological sex and gender influence CRC as an added value for precision oncology.
Key words: colorectal cancer, gender, sex, tumour biology, treatment, toxicity
Highlights
-
•
Sexual dimorphisms in cancer influence incidence, clinicopathological features, therapeutic outcomes, and tolerability.
-
•
Historically, female patients have been underrepresented in research, requiring extrapolation from male-based studies.
-
•
Concerning CRC, incidence is higher in males, but right-sided tumours and BRAF mutations are more frequent among females.
-
•
Toxicity with approved drugs for CRC is higher in females, but differences in efficacy are controversial.
-
•
The balance between efficacy and toxicity for CRC may be improved by the development of sex-specific dosing strategies.
Introduction
With the exception of thyroid cancer, incidence of non-reproductive tumours is higher in males than in females, while mortality rates in males doubling those in females.1 Sex does not only influence cancer incidence, but also clinicopathological features of disease, differences in treatments, therapeutic outcomes, and tolerability. These sex-associated differences are known as sexual dimorphisms. However, it was not until recently that sex disparities in oncology have been acknowledged.
Although interrelated and often used interchangeably, sex and gender are not equivalent concepts. Sex is a biological attribute that defines species, including humans, as male, female, and/or intersex according to their chromosomal makeup and reproductive organs.2 Gender, on the other hand, is a chosen sexual identity and represents a social construct that refers to the norms, identities, and relations that structure our societies and organizations, and shape behaviours, products, technologies, environments, and knowledge.3 Gender is a dynamic concept that varies from society to society and can change throughout an individual’s lifetime. Although appropriate reporting of sex and gender in oncology practices is vital, the incorporation of gender variables in clinical research and patients’ medical histories remains limited,4 restricting us from adequately addressing the impact of gender-influenced behaviours on health outcomes, which are different from those influenced by biological sex.
Colorectal cancer (CRC) is the third most common cancer worldwide and the fourth leading cause of cancer death in the world.1 As in other tumours, there are differences in incidence according to sex. Worldwide estimation of incident cases in 2020 was 547 619 and 288 852 cases of colon and rectum cancer in females, respectively, and 600 896 and 443 358 cases of colon and rectum cancer in males, respectively.
This review provides context for understanding sex and gender differences in cancer and summarizes the growing body of literature illustrating the sex and gender perspective in CRC and their impact in relation to tumour biology and treatment efficacy and toxicity.
Historical overview of sex- and gender-based cancer research
Women were historically largely excluded as subjects of investigations in non-reproductive clinical research, resulting in the extrapolation of data from male-based investigations to women.5,6 However, underlying and fundamental differences in biology are likely to affect disease development and pharmacokinetics, and impact treatment efficacy and toxicity, which have been widely described.
Following the scandals resulting from the use of thalidomide in women during pregnancy, warnings about fetal risks led to the labelling of pregnant women by the National Commission for the Protection of Human Subjects and Biomedical and Behavioural Research as vulnerable research subjects. In 1977, the United States Food and Drug Administration (FDA) issued a guidance document entitled “General Considerations for the Clinical Evaluation of Drugs” advising that women of childbearing potential should be excluded from early-phase clinical research, with the exception of trials testing drugs for life-threatening illnesses.7 Women could be included in later phase II and III trials for drugs with a favourable risk–benefit ratio, as long as studies about teratogenicity and fertility had been accomplished. However, the term ‘woman capable of becoming pregnant’ covered a broad range of women, since it could include premenopausal single abstinent women, women using contraceptives, and women with sterile partners, whereas it did not account for the reproductive desires of women and their partners. Thus, advocacy groups criticized the 1977 FDA guideline by arguing that it deprived women of opportunities, and did not focus on women’s independence to make decisions. They also voiced that women were capable of endorsing drug development about sex differences through clinical research participation; furthermore, this policy had an unintended consequence of causing underrepresentation of women in clinical research.
In 1986, the National Institutes of Health (NIH) reinforced the movement by establishing a policy that urged the inclusion of women in clinical trials and finally, in 1993, the FDA reversed the 1977 guidance and lifted the ban that prevented women of childbearing potential from being enrolled in early-phase trials and promulgated “Guidelines for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs”. The document pointed out that clinical trial subjects should be representative of the patient population to which the drug would likely be prescribed after approval, and highlighted the importance of exploring differences in terms of safety, efficacy, pharmacokinetics, and pharmacodynamics among subpopulations.8
In 1994, the FDA furthered its commitment with the creation of an Office of Women’s Health to address the health of women through policy, science, and outreach and to promote the inclusion of women in clinical studies as well as subanalyses of sex, gender, and subpopulations.9 In 1998, the FDA amended its regulations pertaining to new drug applications (NDAs) and announced the publication of a new document “Presentation of Safety and Effectiveness Data for Certain Subgroups of the Population in Investigational New Drug Application Reports and New Drug Applications” that specifically stated that safety and efficacy data for important populations, including sex, age, and racial subgroups, were mandatory for NDAs.10 In 2000, the FDA issued the “Investigational New Drug Applications: Amendment to Clinical Hold Regulations for Products Intended for Life-Threatening Disease and Conditions” that permitted placing on hold any trial for a life-threatening condition that excluded patients only because of their reproductive potential.11
Although today women are systematically included in clinical trials, inadequate analysis of differences in outcomes according to sex or gender remains widespread and reflects an unmet need deserving of attention and deeper knowledge.
Sex disparities in preclinical research
The powerhouse of clinical cancer research springs from in vitro workhorse models using cell lines and validation in in vivo animal models, including patient-derived models. However, historical ignorance of the role that sex plays across this process has widely jeopardized bench-to-bedside cancer research, obscuring fundamental sex differences that may guide clinical studies.
Male cell lines stocked in repositories of human non-reproductive cancer cell lines outnumber female cell lines, leading to single-sex analyses. Furthermore, few in vitro cell-based experiments report the sex of the cell line, posing a potential risk to analysis and interpretation and, even when investigators do acknowledge the sex origin of a cell line, the original sexual identity may transform over the course of routine cell culture passaging.12,13 Added to this, the sex of cultured cells and that of cell culture media are rarely matched. In fact, the effect of culture media components on the hormonal environment of cultured cells is seldom considered.14 Hormone concentrations in calf fetus sera may differ depending on whether the serum is sourced from a single sex or a mixture, whereas hormone levels are not routinely measured and sex identity is usually not reported. Finally, the estrogenic contribution of plastic labware commonly used for cell culture or that of the common pH dye indicator phenol red is also rarely considered.15, 16, 17 Phenol red, present in most commercial media, turns yellow in response to acidification of the medium during cell growth; however, binding and activation of estrogen receptors in multiple cell lines in a dose-dependent manner has been reported.
In the case of in vivo experiments using animal models, bias concerning the sex parameter also exists. Species-specific sex differences, and the fact that these vary between species [e.g. that fewer genes escape X chromosome inactivation (XCI) in female mice than in humans, and X-linked gene regulation] should be taken into consideration.18,19 However, research invariably fails to investigate sex disparity by the differential use of models of male and female animals20 and furthermore, when using xenografts, investigators seldom account for matching the sex of transplanted cells and their animal recipients, even though this is not consistent with reliable modelling. Plus, as males present aggressive behaviour that requires cage separation, cost concerns often result in favouring a single-sex study in co-caged female mice of premenopausal age, even though human cancers are predominantly diagnosed at a late age.21,22
In light of this and striving for the inclusion of both sexes in preclinical research, in 2014 the NIH unveiled a new policy requiring federally funded scientists to include both males and females in cell and animal studies.23
Sex and gender disparities in cancer development
Exposome
The exposome, defined as the repertoire of exposures and associated interactions of a given individual during their lifetime, may differ among individuals according to their gender. The exposome is known to have an impact on cancer development, with obesity, smoking habits, and inflammation having a direct and strong correlation with carcinogenesis in multiple tumour types24, 25, 26 (Table 1).
Table 1.
Summary of the complex interplay that the exposome, sex chromosomes, sex hormones, and immunity can have in intrinsic control of cancer-initiating cell populations and cancer development
| Sex and gender disparities in cancer development | |
|---|---|
| Exposome | Exposure to known risk factors for cancer (dietary habits, exercise, smoking, drinking, socioeconomic status, social support, and industrial pollution) can differ according to gender, while strength and direction of the correlation might be dependent on sex and/or gender (i.e. stronger associations between obesity and increased risk of overall CRC have been found in men, compared with women). |
| Sex chromosomes | X chromosome inactivation process in females is normally random, cells within females have a mosaic expression of either the maternal or paternal chromosome:
|
| Sex hormones | Sex hormones exert pleiotropic functions on multiple tissues. Cancer cells secrete vascular endothelial growth factor A (VEGFA) and platelet-activating factor (PAF) in response to estrogens, enhancing proliferation and migration. ERα is up-regulated in T cells while ERβ is highly expressed in B cells. |
| Immunity | Approximately 50 X-linked genes, including FOXP3 and TLR8, are involved in adaptive and innate immunity. Females are able to mount stronger immune responses than males and immunity decline against cancer occurs at later age in females. |
CRC, colorectal cancer; ERα, estrogen receptor-α; XIST, X-inactive specific transcript.
Concerning CRC, external exposures such as dietary habits, exercise, smoking, drinking, socioeconomic status, social support, and industrial pollution are known risk factors for colorectal carcinogenesis.27, 28, 29, 30, 31, 32, 33, 34, 35 Exposure can differ according to gender, while strength and direction of the correlation might be dependent on sex and/or gender. As such, stronger associations between obesity and increased risk of overall CRC have been found in men, compared with women. Patterns also differ: weight gain later in life seems to be an important risk factor for CRC in men, while in women, early life obesity is a known risk factor.36, 37, 38 However, to date, sex-specific differences have not been sufficiently investigated for CRC. Findings about dietary patterns, which are related to obesity, and their contribution to CRC have been reported mainly from female-specific prospective cohort studies such as the Nurses’ Health.39 However, unfortunately, large cohort studies that included both women and men mostly have not reported sex-specific estimates. To date, investigations have focused on tumour location and analysed sex differences in terms of risk by colorectal subsite according to high carbohydrate intake, concluding that it might increase right-sided colon cancer in women, while increasing rectal cancer in men.40 A high inflammatory profile (proinflammatory diet plus sedentarism plus obesity) has been associated with higher risk for colon cancer in men and no significant association in women in the European Prospective Investigation into Cancer and nutrition study (EPIC), while in another study, soy consumption (a known phytoestrogen) was not associated with risk of CRC in males, but risk reduction in females was reported.41,42
However, even after adjusting for external exposures, incidence of non-reproductive cancers is lower in females than in males, suggesting sexual dimorphisms.43,44 In the following sections, we focus on the complex role that sex chromosomes and sex hormones appear to have in this context.
Sex chromosomes and cancer
Male and female development diverges under the influence of both X and Y allosomes (sexual chromosomes) and autosomes (non-sexual chromosomes) and the contribution of sex steroid hormones. However, these sexual dimorphisms might also contribute to sex disparities in cancer development.
Since female cells harbour two entire X chromosomes, a critical XCI to avoid simultaneous expression of two entire X chromosomes must occur at early cell division in implanted embryos. This phenomenon is mediated by the long non-coding RNA X-inactive specific transcript (XIST), which silences one X pair at a random.45 As a consequence, XX cells present a silenced and inactive X chromosome (Xi) and an expressed and active one (Xa). As XX cells express either the maternal or the paternal X arbitrarily, females and males with Klinefelter syndrome present distinct X gene repertoires, resulting in mosaicism and phenotypic diversity. This contrasts with the exclusive expression of the only X chromosome in XY cells from males.
However, some genes escape and are expressed from both the Xa and the Xi, conferring protective benefits from cancer and other diseases, but also potential risks. X-linked oncogene activation or loss of a tumour suppressor would not be expressed in all cells due to mosaicism, while in males it would lead to obligatory expression of the same alteration in the maternal X-linked gene. Tumour suppressor genes that escape from X-inactivation, termed EXITS, are ATRX, CNKSR2, DDX3X, KDM5C, KDM6A, and MAGEC3, and might be responsible for the lower propensity of cancer in females, in comparison with males.46,47 On the other hand, the loss of XIST expression may promote tumour development, as the deletion of XIST expression causes the reactivation of the Xi chromosome, triggering unfavourable genome-wide changes, including involvement in DNA replication, chromosome segregation, cell cycle checkpoints, and haematopoietic genetic disorders.48, 49, 50 In males, loss of Y chromosome expression might also constitute a sex-specific biomarker, as it is related to six Y-linked genes known to serve as tumour suppression genes (KDM6C, KDM5D, DDX3Y, EIF1AY, RPS4Y1, and ZFY). Reduced transcription levels of these genes have been found in 12 non-reproductive tumour types to date.51,52
Immunity and cancer
Influenced by sex hormones, sex chromosomes also have an impact on cancer immune defences.53 In general, females are able to mount stronger innate and adaptive immune responses than males, which results in faster clearance of pathogens and greater vaccine efficacy in females than in males, but also explains the greater incidence of inflammatory and autoimmune diseases in females.54,55
Approximately 50 X-linked genes are involved in adaptive and innate immunity.56 These X-linked genes code for proteins including pattern recognition receptors [such as Toll-like receptor 7 (TLR7) and TLR8], cytokine receptors [e.g. Interleukin 2 receptor subunit gamma (IL2RG) and Interleukin 13 receptor subunit alpha 2 (IL13RA2)], and transcriptional factors (e.g. FOXP3). The X-linked Forkhead box P3 (FOXP3), expressed in regulatory T (Treg) cells, is critical for immune homeostasis, as it limits the adaptive immune responses. In comparison with female visceral adipose tissue (VAT), male VAT is enriched in FOXP3+ Treg cells and presents a distinct molecular profile that is enforced by a sex hormone-dependent niche.57 The X-linked TLR8 also performs a central role in Treg cell biology. Activation of TLR8 in Treg cells triggers selective inhibition of glucose uptake leading to their senescence, relieving Treg cell inhibition of effector T cells. In the case of the TLR7 gene, TLR7 may escape XCI in immune cells, leading to enhanced TLR7 expression owing to biallelism in XX cells, which contributes to the higher risk of developing autoimmune disorders in women and in men with Klinefelter syndrome.58 Concerning lymphoid cell subsets, adult females present a higher frequency of B cells during adulthood, whereas higher CD4+ T-cell counts, higher CD4/CD8 ratios, and lower presence of CD8+ T cells are reported throughout life, compared with age-matched males.59, 60, 61, 62
Immune defence against cancer declines with age and shows sexual dimorphisms. Reduction in T-cell numbers occurs in both sexes with age, but a disproportionate decrease in T-cell and B-cell populations is more evident in older males. Two waves of epigenetic regulation depleting immune cell functions have been identified, the first one in the late 30s, with a similar impact across the sexes. Genomic differences between sexes increase after the age of 65 years, with a second wave in males in their early 60s that results in increased proinflammatory activity and innate immunity and lower adaptive immunity. This is delayed by 5-6 years in females, who exhibit greater adaptive immunity.63
A pan-cancer analysis to evaluate the sex-based variance of different genomic immune-related factors using The Cancer Genome Atlas showed differences in tumour mutation burden, neoantigen burden, tumour purity, cytolytic activity, CD8+ T cell, and expressions of immune checkpoint genes according to sex.64
Sex hormones and cancer
Sex hormones exert pleiotropic functions on multiple tissues. The estrogen effect depends on the activation of the estrogen receptor-α (ERα) and -β (ERβ). Activation of ERα promotes expansion and mobilization of haematopoietic stem cells, favours skin wound healing, promotes angiogenesis and endothelial cell precursor mobilization, reduces hepatocyte proliferation, and restrains the inflammatory role of macrophages. ERβ also blocks macrophage activation and, contrary to ERα, negatively regulates vessel formation. The androgen receptor promotes angiogenesis, liver cell proliferation, and macrophage activation through the stimulation of tumour necrosis factor and CC-chemokine ligand 4, while it also suppresses wound healing. Hormone pathways are interrelated, since androgenic hormones are converted into estrogens through the action of aromatase, resulting in indirect control of pathways affected by ERα.
In the tumour microenvironment, cancer cells secrete vascular endothelial growth factor (VEGF) A and platelet-activating factor in response to estrogens, enhancing proliferation and migration.65,66 In addition, estrogens promote mobilization of bone marrow-derived precursors to cancer stroma in breast tumours.67 Sex hormones are partially responsible for the sex-related differences in immune response as ERα and ERβ are expressed by many types of immune cells, including T cells, B cells, dendritic cells, macrophages, neutrophils, and natural killer (NK) cells68 and these receptors present differential expression among immune cell subsets, as ERα is up-regulated in T cells while ERβ is highly expressed in B cells.69 Finally, age-related changes in sex steroid concentrations and sex steroid receptor signalling might subsequently contribute to age-associated changes of immune function and populations.70
Sexual dimorphisms in CRC tumour biology
The tumour biology of CRC has been proven to be different in males and females as a result of the sex hormones and sex chromosomes that can influence immunity.71 In addition, key proliferative pathways in CRC tumourigenesis are regulated through estrogens. Estrogens have been reported to control the activity of a class of Kv channels (KCNQ1 : KCNE3), which regulate fundamental ion transport functions of the colon and ultimately promote cell proliferation and epithelial–mesenchymal transition through bi-directional interactions with the Wnt/β-catenin signalling pathway. At the same time, estrogen modulates proliferative responses to hypoxia via the novel membrane estrogen receptor G protein-coupled estrogen receptor (GPER), as well as by Hypoxia-inducible factor 1-alpha (HIF1A) and VEGF signalling. Differences in oncogene expression, such as a higher frequency of mutations of STK11 in males, and sexual dimorphisms in proteomes of CRC cells may contribute to the disparities in tumour biology according to sex in these tumours.72, 73, 74
Lastly, sexual dimorphisms in the tumour microenvironment of colorectal tumours have been investigated using tissue microarrays comprising primary tumour, tumour-infiltrated lymph nodes, and uninvolved colon.75 Differential gene expression was observed in pathways related to Treg function, T-cell activity, and T-cell exhaustion, amongst several others, in females compared to males.
Sexual dimorphisms in clinicopathological features of CRC
Whilst globally females diagnosed with CRC have better overall survival compared with males,1,76 in some countries the 5-year survival rate among women has been reported to be lower than among men, especially after the age of 70 years.77 The proportion of patients presenting right-sided tumours is higher among females than males, as it is the proportion of BRAF-mutated tumours. Right-sided colon tumours are often at a more advanced stage at diagnosis and are less differentiated, which might partially explain this lower 5-year survival rate in females.78
Sex hormones may explain the higher frequency of right-sided CRC in females. It has been proposed that exposure to estrogen is protective against the development of tumours with microsatellites instability (MSI), while the lack of estrogen in older females might increase the risk of MSI-high CRC.79 PIK3CA mutations, associated with poorer prognosis, and methylation of CpG island in the 5ʹ region of the p16INKa tumour suppressor also occur at a higher frequency in females.80,81
Sex-associated differences in the microbiome have also been reported in healthy individuals during their life span, some of which are mediated by sex hormones and conditioning the estrobolome (a gene repertoire of intestinal microbiota able to carry out estrogen metabolism).82,83 Plus, the microbiome is highly conditioned by the exposome, which might also vary according to gender. In patients with CRC, sexual dimorphism of microbiome has also been reported.84 The microbiome might be more stable in the male gut than in the female gut. The male gut shows an enrichment of rare species that may contribute to the stability of microbial communities, whereas in the female gut there is a loss of species that could be responsible for the vulnerable microbial communities with the development of CRC.
Sex-related differences in treatment efficacy and toxicity in CRC
Chemotherapy agents
Fluoropyrimidines are the backbone of chemotherapy in CRC management. Dosage is based on body surface area but does not take into account sex differences. However, a substantial body of literature indicates sex-associated differences in pharmacokinetics of 5-fluorouracil (5-FU).85 Plasma clearance, plasma clearance per kilogram, and dose have been found to be lower in females compared with males, whereas plasma life and area under the plasma concentration–time curve might be higher in females. On the contrary, volume of distribution, volume of distribution per kilogram, and dose per kilogram do not differ significantly between sexes. These differences may have an impact on outcomes and toxicity.
Toxicity associated with 5-FU has been reported to be more extensive for females than for males in terms of the number of different types of toxicity, maximum toxicity grade, and incidence of severe toxicities, including haematologic toxicities such as leukopaenia, neutropaenia, and thrombocytopaenia, and self-reported toxicity such as stomatitis, diarrhoea, nausea, vomiting, or hand-foot syndrome.86, 87, 88 This increased toxicity might be due to lower clearance of 5-FU leading to higher plasma levels in females compared to males, as previously reported.89 Concerning patient-reported outcomes, an investigation exploring the occurrence and severity of self-reported physical and psychological co-occurring symptoms in patients with stage IV CRC receiving different 5-FU-based chemotherapy schemes reported more severe worrying, lack of energy, and nausea in women.90
Similar data about toxicity have been reported with the use of capecitabine. In a cohort of 299 patients (163 males, 136 females) receiving capecitabine in the adjuvant setting, females had significantly higher dose-limiting toxicity than men. Incidence of all common toxicities was higher in females and required significantly more dose reductions than males, leading to statistically significant lower relative dose intensities in females.91 Consistent with these results, an analysis of 36 640 patients with colon cancer receiving adjuvant fluoropyrimidine-based chemotherapy in the ACCENT database showed that incidence of grade 3 or grade 4 non-haematological toxicity (nausea, vomiting, stomatitis, diarrhoea, peripheral neuropathy, and transaminitis) and haematological toxicity (neutropaenia and leukopaenia) was statistically significant and clinically relevant higher in females.92
Differential efficacy according to sex has only recently been studied. The SOLSTICE trial, comparing standard capecitabine and bevacizumab versus TAS102 and bevacizumab as frontline therapy for patients with metastatic CRC (mCRC) not candidates for intensive chemotherapy, did not meet its primary point in terms of progression-free survival (PFS). But interestingly, statistical differences for PFS were found in the subgroup analyses for males treated with the experimental treatment in comparison with those treated with capecitabine and bevacizumab.93 A subgroup analysis of the VALENTINO trial, a multicentre, randomized, phase II trial, investigating two panitumumab-based maintenance strategies following first-line panitumumab plus FOLFOX in RAS wild-type mCRC patients, showed no significant differences in PFS, overall survival, overall response rate, or clinical benefit rate according to sex, but a significantly higher rate of grade 3-4 toxicity in females, compared to males.94 Similarly, in the phase III trials TRIBE and TRIBE2 investigating first-line FOLFOXIRI/bevacizumab or a doublet (FOLFIRI or FOLFOX)/bevacizumab, no statistical differences were reported depending on age or sex, although an increased risk of grade 3-4 toxicity was found in elderly females.95 However, it must be noted that these studies aimed to explore overall differences between sexes but were not specifically designed to compare efficacy of each arm between males and females.
These results suggest that patient sex should be taken into account and that conventional methods of using body surface area for dosing may be inaccurate. Therefore, therapeutic drug monitoring has been proposed as an alternative dosage of 5-FU as it might lead to therapeutic plasma levels with a maximized risk/benefit ratio.96
Targeted therapies
The use of targeted therapies for patients with CRC has been limited to antiangiogenic agents or epidermal growth factor receptor inhibitors in combination with chemotherapy for years. The impact of sex and gender on efficacy and toxicity of these therapies might therefore be obscured by the concomitant use of chemotherapy. Regorafenib, a multi-kinase inhibitor, obtained approval for refractory mCRC in monotherapy. More frequent toxicity in terms of fatigue, anorexia, hypertension, and rash, as well as severe toxicity have been reported in females compared with males, which might lead to lesser adherence to treatment.97,98
Only recently, the use of BRAF inhibitors without chemotherapy has proved efficient in patients with mCRC harbouring BRAF V600 mutations. Influence of sex on efficacy and toxicity with the use of BRAF targeted therapy has been reported with the use of encorafenib plus cetuximab, with or without binimetinib. Among patients with mCRC harbouring BRAF V600E mutations treated with these combinations, a trend for superior clinical benefit in females, particularly with the triplet combination but with a higher toxicity cost, was observed.99
Ongoing trials testing the efficacy of targeted therapies, such as KRAS G12C inhibitors or anti-human epidermal growth factor receptor 2 (HER2) therapies, should specifically address these sex-associated differences.
Immunotherapy
Efficacy of immunotherapy according to sex across different tumour types has been investigated with inconsistent conclusions.100,101 Given that the use of immunotherapy in CRC is limited to mCRC presenting MSI, analyses exploring this phenomenon usually include only a small number of patients. Better overall survival has been reported in males compared with females in patients with mCRC treated with immune checkpoint inhibitors.102 However, this analysis included only 99 patients, 45 of whom were females and with an uncertain number of patients with MSI tumours.103
In an analysis exploring symptomatic and objective toxicities across multiple cancer types, including gastrointestinal tumours, and patients treated with immunotherapy, the risk of symptomatic and haematologic adverse events was higher for females receiving immunotherapy compared with males, while the risk of objective non-haematologic toxicity was similar for both groups.104 Concerning the mechanism of action of immunotherapy, females receiving immune checkpoint inhibitors and immune system modulators had a higher risk of symptomatic toxicity, but this association was not observed for asymptomatic toxicity.
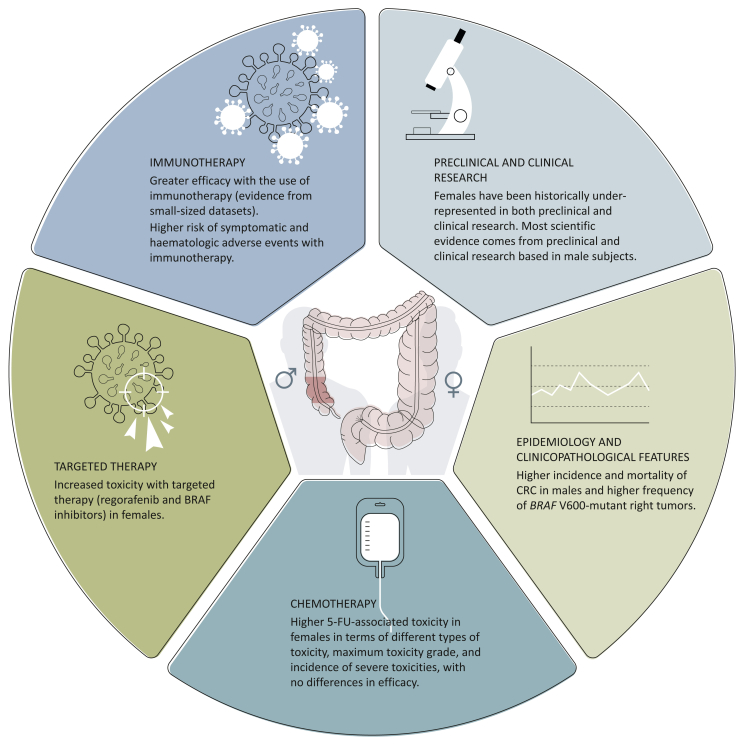
Taken together, these data support designing trials addressing specifically sex and gender, as the balance between efficacy and toxicity may be improved by the development of sex-specific dosing strategies (Table 2, Figure 1).
Table 2.
Summary of the investigations exploring the influence of sex in the management of CRC
| Treatment of investigation | Type of treatment | Author | Study population | N (male/female) | Aim of the study | Conclusions |
|---|---|---|---|---|---|---|
| 5-FU | Chemotherapy | Chansky et al.86 | Adjuvant setting (SWOG-8572, SWOG-8591) and advanced CRC (SWOG-8611, SWOG-8905) | 505 (260/245) | Toxicity | Greater number of different types of toxicity, higher maximum grade toxicity, and higher incidence of severe toxicity (≥ grade 3) in females |
| 5-FU | Chemotherapy | Sloan et al.87,88 | NCCTG trials between 1980 and 1995 in the setting of adjuvant and advanced CRC | 2448 (1355/1093) | Toxicity | Higher rates of toxicity in females (stomatitis, leukopaenia, alopecia, nausea, vomiting, and diarrhoea) |
| 5-FU-based regimens | Chemotherapy | Mueller et al.89 | Patients with CRC, pancreatic or cholangiocellular cancer | 31 (31/10) | Elimination of 5-FU | Lower clearance of 5-FU in females |
| 5-FU | Chemotherapy | Röhrl et al.90 | Curative chemotherapy (neoadjuvant or adjuvant) and palliative chemotherapy for CRC | 120 (73/47) | Patient-reported outcomes | More severe worrying, lack of energy, and nausea in females |
| Capecitabine | Chemotherapy | Ilich et al.91 | Adjuvant setting CRC | 299 (163/136) | Toxicity | Higher incidence of all common toxicities and of dose-limiting toxicity in females. Higher frequency of dose reduction and lower dose intensity in females |
| Fluoropyrimidine-based regimens | Chemotherapy | Wagner et al.92 | ACCENT database | 34 640 (18 664/15 976) | Toxicity | Higher incidence of ≥ grade 3 non-haematological (nausea, vomiting, stomatitis, diarrhoea, peripheral neuropathy and transaminitis) and haematological toxicities (neutropaenia and leukopaenia) in females |
| TAS102 + bevacizumab versus capecitabine + bevacizumab | Chemotherapy + targeted therapy | André et al.93 | Frontline mCRC, not candidates for intensive chemotherapy (SOLSTICE clinical trial) | 856 (466/390) | Subgroup analysis | Greater efficacy of TAS102 + bevacizumab in males (versus males treated with capecitabine + bevacizumab) |
| FOLFOX + panitumumab | Chemotherapy + targeted therapy | Raimondi et al.94 | Frontline RAS wild-type mCRC | 229 (152/77) | Efficacy and toxicity | No differences in ORR, OS, PFS, or clinical benefit. Higher rate severe toxicity, G3-4 thrombocytopaenia, any grade and G3-4 neutropaenia, and any grade conjunctivitis in females. Higher incidence of any grade skin rash and hypomagnesaemia in males |
| FOLFOXIRI + bevacizumab versus FOLFOX/FOLFIRI + bevacizumab | Chemotherapy + targeted therapy | Marmorino et al.95 | Frontline mCRC (TRIBE and TRIBE2 clinical trials) | 1187 (693/494) | Efficacy and toxicity | No differences in ORR or PFS. Any grade and G3-4 chemotherapy-related adverse events more frequent in females. Any grade nausea and vomiting and G3-4 nausea and vomiting more frequent in females. No differences in bevacizumab-related adverse events |
| Regorafenib | Targeted therapy | Kawakami et al.97 | Refractory mCRC | 96 (46/50) | Adherence to treatment | Higher rates of toxicity in females, associated with lower adherence to regorafenib |
| Regorafenib | Targeted therapy | Vandeputte et al.98 | Refractory mCRC | 136 (78/58) | Toxicity | More frequent G3-4 toxicity in females. Higher rates of fatigue, anorexia, hypertension, and rash in females |
| Encorafenib + cetuximab ± binimetinib | Targeted therapy | Ros-Montana et al.99 | Previously treated BRAF-mutant CRC | 59 (23/36) | Efficacy (OS and PFS) and toxicity | No statistical differences in efficacy (although greater clinical benefit in females with the triplet). Higher rates of toxicity in females, requiring dose modifications more frequently |
| Immune checkpoint inhibitors | Immunotherapy | Ye et al.and Samstein et al.102,103 | mCRC (microsatellite status not specified) | 99 (54/45) | Efficacy | Better OS in males (HR = 0.53, P = 0.041) |
5-FU, 5-fluorouracil; G, grade; HR, hazard ratio; mCRC, metastatic colorectal cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
Figure 1.
Sex and gender differences in colorectal cancer (CRC). 5-FU, 5-fluorouracil.
Conclusions
The consequence of failing to include sex-based differences in study design and analyses has repeatedly led to ‘one-drug’ treatment regimens for both males and females.The impact of gender on health outcomes, which are different from those influenced by biological sex, is even more unknown, since gender variables are seldom included in patients’ medical histories. In the literature, these terms are often used interchangeably, but the results of the investigations that have been achieved so far exploring the sexual differences are mainly focused on sex indeed. This is due to lack of information about gender, that, because of its nature, must be specifically obtained by directly questioning the patient. In this sense, professionals should be trained in the importance of data collection about gender identity as a first step to be able to move forward in this field.
Precision oncology is not limited to exploring molecular biomarkers and it requires deeper understanding of biological sex and gender differences. If the long-term goal of personalizing treatments for patients with CRC is effective treatment for all individuals, then the sex and gender must be accounted for. Despite the calls from the NIH, FDA, industry, and advocacy, the path ahead is long. Since basic science and translational research serves as the cornerstone for clinical research and medical decision making, sex disparities in physiology and pathophysiology cannot be neglected.
In our perspective, barriers to enrolment of females in clinical trials in oncology should be addressed through partnership with all stakeholders, including patients, investigators, referring clinicians, health care systems, and social community. Interventional clinical trials with the focus on investigating sex-specific differences in efficacy and toxicity (including both objective toxicity and measurement of patient-reported outcomes) as a primary endpoint and the evaluation of specific dosing regimens according to sex are needed to improve outcomes. In endorsing research on how biological sex and gender influence CRC, we have the opportunity for greater precision, as it might usher in the development of sex-specific therapeutics with greater efficacy and safer toxicity profile for our patients.
Acknowledgements
We sincerely thank Sarah MacKenzie, PhD, for writing assistance.
Funding
None declared.
Disclosure
IB has received accommodation and travel expenses from Amgen, Merck, Sanofi, and Servier. JR has received personal speaker honoraria from Sanofi and Amgen, and accommodation expenses from Pierre-Fabre, Servier, Amgen, and Merck. NS has received accommodation and travel expenses from Amgen and Merck. FS reports personal financial interests, honoraria for advisory role, travel grants, research grants (past 5 years): Hoffman La-Roche, Sanofi Aventis, Amgen, Merck Serono, Servier, Bristol-Myers Squibb. MRC has received personal speaker honoraria from ROVI and accommodation expenses from BMS, Amgen, and Merck. JT reports personal financial interest in the form of scientific consultancy role for Array Biopharma, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, Inspirna Inc, IQVIA, Lilly, Menarini, Merck Serono, Merus, MSD, Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Scandion Oncology, Scorpion Therapeutics, Seattle Genetics, Servier, Sotio Biotech, Taiho, Tessa Therapeutics, TheraMyc, and Tolremo Therapeutics. Stocks: Oniria Therapeutics and also educational collaboration with Imedex/HMP, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education, and Physicians Education Resource (PER). EÉ has received personal speaker honoraria from Organon and Novartis; and personal advisory board honoraria from Amgen, Bayer, Hoffman-La Roche, Merck Serono, Sanofi, Pierre Fabre, MSD, and Servier. Her institution has received research funding from Amgen Inc, Array Biopharma Inc, AstraZeneca Pharmaceuticals LP, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Debiopharm International SA, F. Hoffmann-La Roche Ltd, Genentech Inc, HalioDX SAS, Hutchison MediPharma International, Janssen-Cilag SA, MedImmune, Menarini, Merck Health KGAA, Merck Sharp & Dohme, Merus NV, Mirati, Novartis Farmacéutica SA, Pfizer, Pharma Mar, Sanofi Aventis Recherche & Développement, Servier, Taiho Pharma USA Inc. She held/holds non-remunerated roles as Coordinator of the SEOM +MIR Section of Residents and Young Assistants of the Sociedad Española de Oncología Médica (SEOM), Speaker of the ESMO Academy of the European Society for Medical Oncology (ESMO), and Volunteer member of the ASCO Annual Meeting Scientific Program Committee: Developmental Therapeutics – Immunotherapy of the American Society of Clinical Oncology (ASCO). AG has declared no conflicts of interest.
Contributor Information
I. Baraibar, Email: ibaraibar@vhio.net.
E. Élez, Email: meelez@vhio.net.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Haig D. The inexorable rise of gender and the decline of sex: social change in academic titles, 1945-2001. Arch Sex Behav. 2004;33(2):87–96. doi: 10.1023/b:aseb.0000014323.56281.0d. [DOI] [PubMed] [Google Scholar]
- 3.Money J. Hermaphroditism, gender and precocity in hyperadrenocorticism: psychologic findings. Bull Johns Hopkins Hosp. 1955;96(6):253–264. [PubMed] [Google Scholar]
- 4.Kamen C.S., Pratt-Chapman M.L., Meersman S.C., et al. Sexual orientation and gender identity data collection in Oncology Practice: findings of an ASCO survey. JCO Oncol Pract. 2022;18(8):e1297–e1305. doi: 10.1200/OP.22.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carnes M., Morrissey C., Geller S.E. Women’s health and women’s leadership in academic medicine: hitting the same glass ceiling? J Womens Health. 2008;17(9):1453–1462. doi: 10.1089/jwh.2007.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu K.A., Dipietro Mager N.A. Women’s involvement in clinical trials: historical perspective and future implications. Pharm Pract (Granada) 2016;14(1):708. doi: 10.18549/PharmPract.2016.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.General Considerations for the Clinical Evaluation of Drugs | FDA. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-considerations-clinical-evaluation-drugs Available at.
- 8.Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs | FDA. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/study-and-evaluation-gender-differences-clinical-evaluation-drugs Available at.
- 9.Office of Women’s Health | FDA. https://www.fda.gov/about-fda/office-commissioner/office-womens-health Available at.
- 10.Investigational new drug applications and new drug applications-FDA. Final rule. Fed Regist. 1998;63(28):6854–6862. [PubMed] [Google Scholar]
- 11.Investigational new drug applications; amendment to clinical hold regulations for products intended for life-threatening diseases and conditions; final rule. Fed Regist. 2000;65(106):34963–34971. [PubMed] [Google Scholar]
- 12.Shah K., McCormack C.E., Bradbury N.A. Do you know the sex of your cells? Am J Physiol Cell Physiol. 2014;306(1):C3–C18. doi: 10.1152/ajpcell.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durkin A.S., Cedrone E., Sykes G., Boles D., Reid Y.A. Utility of gender determination in cell line identity. Vitro Cell Dev Biol Anim. 2000;36(6):344–347. doi: 10.1290/1071-2690(2000)036<0344:UOGDIC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Kim J., Kim M., Nahm S.S., Lee D.M., Pokharel S., Choi I. Characterization of gender-specific bovine serum. Anim Cells Syst (Seoul) 2011;15(2):147–154. [Google Scholar]
- 15.Ishikawa T., Takano K., Yasufuku-Takano J., et al. Estrogenic impurities in labware. Nat Biotechnol. 2001;19(9):812. doi: 10.1038/nbt0901-812. [DOI] [PubMed] [Google Scholar]
- 16.Soto A.M., Justicia H., Wray J.W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environ Health Perspect. 1991;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthois Y., Katzenellenbogen J.A., Katzenellenbogen B.S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peeters S.B., Cotton A.M., Brown C.J. Variable escape from X-chromosome inactivation: identifying factors that tip the scales towards expression. Bioessays. 2014;36(8):746–756. doi: 10.1002/bies.201400032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasserman W.W., Palumbo M., Thompson W., Fickett J.W., Lawrence C.E. Human-mouse genome comparisons to locate regulatory sites. Nat Genet. 2000;26(2):225–228. doi: 10.1038/79965. [DOI] [PubMed] [Google Scholar]
- 20.Yoon D.Y., Mansukhani N.A., Stubbs V.C., Helenowski I.B., Woodruff T.K., Kibbe M.R. Sex bias exists in basic science and translational surgical research. Surgery. 2014;156(3):508–516. doi: 10.1016/j.surg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Fane M., Weeraratna A.T. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89–106. doi: 10.1038/s41568-019-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur A., Webster M.R., Marchbank K., et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532(7598):250–254. doi: 10.1038/nature17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clayton J.A., Collins F.S. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lortet-Tieulent J., Goding Sauer A., Siegel R.L., et al. State-level cancer mortality attributable to cigarette smoking in the United States. JAMA Intern Med. 2016;176(12):1792–1798. doi: 10.1001/jamainternmed.2016.6530. [DOI] [PubMed] [Google Scholar]
- 25.Stone T.W., McPherson M., Gail Darlington L. Obesity and cancer: existing and new hypotheses for a causal connection. EBioMedicine. 2018;30:14–28. doi: 10.1016/j.ebiom.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furman D., Campisi J., Verdin E., et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carr P.R., Weigl K., Edelmann D., et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a population-based study. Gastroenterology. 2020;159(1):129–138.e9. doi: 10.1053/j.gastro.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi J., Jia G., Wen W., Shu X.O., Zheng W. Healthy lifestyles, genetic modifiers, and colorectal cancer risk: a prospective cohort study in the UK Biobank. Am J Clin Nutr. 2021;113(4):810–820. doi: 10.1093/ajcn/nqaa404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang P., Liu Y. A reasonable diet promotes balance of intestinal microbiota: prevention of precolorectal cancer. Biomed Res Int. 2019;2019 doi: 10.1155/2019/3405278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkins T., McMechan D., Talukder A. Colorectal cancer screening and prevention. Am Fam Physician. 2018;97(10):658–665. [PubMed] [Google Scholar]
- 31.Egeberg R., Halkjaer J., Rottmann N., Hansen L., Holten I. Social inequality and incidence of and survival from cancers of the colon and rectum in a population-based study in Denmark, 1994-2003. Eur J Cancer. 2008;44(14):1978–1988. doi: 10.1016/j.ejca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Brooke H.L., Talbäck M., Martling A., Feychting M., Ljung R. Socioeconomic position and incidence of colorectal cancer in the Swedish population. Cancer Epidemiol. 2016;40:188–195. doi: 10.1016/j.canep.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Sarma E.A., Kawachi I., Poole E.M., et al. Social integration and survival after diagnosis of colorectal cancer. Cancer. 2018;124(4):833–840. doi: 10.1002/cncr.31117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-Pérez J., Fernández de Larrea-Baz N., Lope V., et al. Residential proximity to industrial pollution sources and colorectal cancer risk: a multicase-control study (MCC-Spain) Environ Int. 2020;144 doi: 10.1016/j.envint.2020.106055. [DOI] [PubMed] [Google Scholar]
- 35.Jenwitheesuk K., Peansukwech U., Jenwitheesuk K. Accumulated ambient air pollution and colon cancer incidence in Thailand. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-74669-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H., Giovannucci E.L. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control. 2017;28(1):1–4. doi: 10.1007/s10552-016-0831-5. [DOI] [PubMed] [Google Scholar]
- 37.Liu P.H., Wu K., Ng K., et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 2019;5(1):37. doi: 10.1001/jamaoncol.2018.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ning Y., Wang L., Giovannucci E.L. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev. 2010;11(1):19–30. doi: 10.1111/j.1467-789X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 39.Hur J., Otegbeye E., Joh H.K., et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut. 2021;70(12):2330–2336. doi: 10.1136/gutjnl-2020-323450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borugian M.J., Sheps S.B., Whittemore A.S., Wu A.H., Potter J.D., Gallagher R.P. Carbohydrates and colorectal cancer risk among Chinese in North America. Cancer Epidemiol Biomarkers Prev. 2002;11(2):187–193. [PubMed] [Google Scholar]
- 41.Jakszyn P., Cayssials V., Buckland G., et al. Inflammatory potential of the diet and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. 2020;147(4):1027–1039. doi: 10.1002/ijc.32870. [DOI] [PubMed] [Google Scholar]
- 42.Yan L., Spitznagel E.L., Bosland M.C. Soy consumption and colorectal cancer risk in humans: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(1):148–158. doi: 10.1158/1055-9965.EPI-09-0856. [DOI] [PubMed] [Google Scholar]
- 43.OuYang P.Y., Zhang L.N., Lan X.W., et al. The significant survival advantage of female sex in nasopharyngeal carcinoma: a propensity-matched analysis. Br J Cancer. 2015;112(9):1554–1561. doi: 10.1038/bjc.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wisnivesky J.P., Halm E.A. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol. 2007;25(13):1705–1712. doi: 10.1200/JCO.2006.08.1455. [DOI] [PubMed] [Google Scholar]
- 45.Brown C.J., Ballabio A., Rupert J.L., et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 46.Dunford A., Weinstock D.M., Savova V., et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat Genet. 2017;49(1):10–16. doi: 10.1038/ng.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haupt S., Caramia F., Herschtal A., et al. Identification of cancer sex-disparity in the functional integrity of p53 and its X chromosome network. Nat Commun. 2019;10(1):5385. doi: 10.1038/s41467-019-13266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirchia S.M., Ramoscelli L., Grati F.R., et al. Loss of the inactive X chromosome and replication of the active X in BRCA1-defective and wild-type breast cancer cells. Cancer Res. 2005;65(6):2139–2146. doi: 10.1158/0008-5472.CAN-04-3465. [DOI] [PubMed] [Google Scholar]
- 49.Kawakami T., Zhang C., Taniguchi T., et al. Characterization of loss-of-inactive X in Klinefelter syndrome and female-derived cancer cells. Oncogene. 2004;23(36):6163–6169. doi: 10.1038/sj.onc.1207808. [DOI] [PubMed] [Google Scholar]
- 50.Richardson A.L., Wang Z.C., de Nicolo A., et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9(2):121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Loftfield E., Zhou W., Yeager M., Chanock S.J., Freedman N.D., Machiela M.J. Mosaic Y loss is moderately associated with solid tumor risk. Cancer Res. 2019;79(3):461–466. doi: 10.1158/0008-5472.CAN-18-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cáceres A., Jene A., Esko T., Pérez-Jurado L.A., González J.R. Extreme downregulation of chromosome Y and cancer risk in men. J Natl Cancer Inst. 2020;112(9):913–920. doi: 10.1093/jnci/djz232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gubbels Bupp M.R., Potluri T., Fink A.L., Klein S.L. The confluence of sex hormones and aging on immunity. Front Immunol. 2018;9:1269. doi: 10.3389/fimmu.2018.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 55.Amadori A., Zamarchi R., de Silvestro G., et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1(12):1279–1283. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 56.Meester I., Manilla-Muñoz E., León-Cachón R.B.R., et al. SeXY chromosomes and the immune system: reflections after a comparative study. Biol Sex Differ. 2020;11(1):3. doi: 10.1186/s13293-019-0278-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasanthakumar A., Chisanga D., Blume J., et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature. 2020;579(7800):581–585. doi: 10.1038/s41586-020-2040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Souyris M., Cenac C., Azar P., et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19) doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 59.Abdullah M., Chai P.S., Chong M.Y., et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. 2012;272(2):214–219. doi: 10.1016/j.cellimm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Lee B.W., Yap H.K., Chew F.T., et al. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry. 1996;26(1):8–15. doi: 10.1002/(SICI)1097-0320(19960315)26:1<8::AID-CYTO2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 61.Lisse I.M., Aaby P., Whittle H., Jensen H., Engelmann M., Christensen L.B. T-lymphocyte subsets in West African children: impact of age, sex, and season. J Pediatr. 1997;130(1):77–85. doi: 10.1016/s0022-3476(97)70313-5. [DOI] [PubMed] [Google Scholar]
- 62.Uppal S.S., Verma S., Dhot P.S. Normal values of CD4 and CD8 lymphocyte subsets in healthy Indian adults and the effects of sex, age, ethnicity, and smoking. Cytometry B Clin Cytom. 2003;52(1):32–36. doi: 10.1002/cyto.b.10011. [DOI] [PubMed] [Google Scholar]
- 63.Márquez E.J., Chung C.H., Marches R., et al. Sexual-dimorphism in human immune system aging. Nat Commun. 2020;11(1):751. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang P.F., Song H.F., Zhang Q., Yan C.X. Pan-cancer immunogenomic analyses reveal sex disparity in the efficacy of cancer immunotherapy. Eur J Cancer. 2020;126:136–138. doi: 10.1016/j.ejca.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Seo K.H., Lee H.S., Jung B., et al. Estrogen enhances angiogenesis through a pathway involving platelet-activating factor-mediated nuclear factor-κB activation. Cancer Res. 2004;64(18):6482–6488. doi: 10.1158/0008-5472.CAN-03-2774. [DOI] [PubMed] [Google Scholar]
- 66.Stoner M., Wormke M., Saville B., et al. Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor alpha and SP proteins. Oncogene. 2004;23(5):1052–1063. doi: 10.1038/sj.onc.1207201. [DOI] [PubMed] [Google Scholar]
- 67.Gupta P.B., Proia D., Cingoz O., et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67(5):2062–2071. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- 68.Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giefing-Kröll C., Berger P., Lepperdinger G., Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abancens M., Bustos V., Harvey H., McBryan J., Harvey B.J. Sexual dimorphism in colon cancer. Front Oncol. 2020;10:2765. doi: 10.3389/fonc.2020.607909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan Y., Liu L., Chen H., et al. Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell. 2016;29(5):711–722. doi: 10.1016/j.ccell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Y., Liu Y., Zhou Y., You H. Molecular mechanism of LKB1 in the invasion and metastasis of colorectal cancer. Oncol Rep. 2018;41(2):1035–1044. doi: 10.3892/or.2018.6877. [DOI] [PubMed] [Google Scholar]
- 74.Hasáková K., Bezakova J., Vician M., Reis R., Zeman M., Herichova I. Gender-dependent expression of leading and passenger strand of miR-21 and miR-16 in human colorectal cancer and adjacent colonic tissues. Physiol Res. 2017;66(suppl 4):S575–S582. doi: 10.33549/physiolres.933808. [DOI] [PubMed] [Google Scholar]
- 75.Geddes A.E., Ray A.L., Nofchissey R.A., et al. An analysis of sexual dimorphism in the tumor microenvironment of colorectal cancer. Front Oncol. 2022;12:5700. doi: 10.3389/fonc.2022.986103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y., Wang G., He J., et al. Gender differences in colorectal cancer survival: a meta-analysis. Int J Cancer. 2017;141(10):1942–1949. doi: 10.1002/ijc.30827. [DOI] [PubMed] [Google Scholar]
- 77.Park H.C., Shin A., Kim B.W., et al. Data on the characteristics and the survival of korean patients with colorectal cancer from the Korea central cancer registry. Ann Coloproctol. 2013;29(4):144–149. doi: 10.3393/ac.2013.29.4.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petrelli F., Tomasello G., Borgonovo K., et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3(2):211–219. doi: 10.1001/jamaoncol.2016.4227. [DOI] [PubMed] [Google Scholar]
- 79.Slattery M.L., Potter J.D., Curtin K., et al. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res. 2001;61(1):126–130. [PubMed] [Google Scholar]
- 80.Phipps A.I., Makar K.W., Newcomb P.A. Descriptive profile of PIK3CA-mutated colorectal cancer in postmenopausal women. Int J Colorectal Dis. 2013;28(12):1637–1642. doi: 10.1007/s00384-013-1715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiencke J.K., Zheng S., Lafuente A., et al. Aberrant methylation of p16INK4a in anatomic and gender-specific subtypes of sporadic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1999;8(6):501–506. [PubMed] [Google Scholar]
- 82.Yoon K., Kim N. Roles of sex hormones and gender in the gut microbiota. J Neurogastroenterol Motil. 2021;27(3):314–325. doi: 10.5056/jnm20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valeri F., Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol. 2021;61 doi: 10.1016/j.yfrne.2021.100912. [DOI] [PubMed] [Google Scholar]
- 84.Liao H., Li C., Ai Y., Kou Y. Gut microbiome is more stable in males than in females during the development of colorectal cancer. J Appl Microbiol. 2021;131(1):435–448. doi: 10.1111/jam.14943. [DOI] [PubMed] [Google Scholar]
- 85.Gusella M., Crepaldi G., Barile C., et al. Pharmacokinetic and demographic markers of 5-fluorouracil toxicity in 181 patients on adjuvant therapy for colorectal cancer. Ann Oncol. 2006;17:1656–1660. doi: 10.1093/annonc/mdl284. [DOI] [PubMed] [Google Scholar]
- 86.Chansky K., Benedetti J., Macdonald J.S. Differences in toxicity between men and women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer. 2005;103(6):1165–1171. doi: 10.1002/cncr.20878. [DOI] [PubMed] [Google Scholar]
- 87.Sloan J.A., Goldberg R.M., Sargent D.J., et al. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol. 2002;20(6):1491–1498. doi: 10.1200/JCO.2002.20.6.1491. [DOI] [PubMed] [Google Scholar]
- 88.Sloan J.A., Loprinzi C.L., Novotny P.J., Okuno S., Nair S., Barton D.L. Sex differences in fluorouracil-induced stomatitis. J Clin Oncol. 2000;18(2):412–420. doi: 10.1200/JCO.2000.18.2.412. [DOI] [PubMed] [Google Scholar]
- 89.Mueller F., Büchel B., Köberle D., et al. Gender-specific elimination of continuous-infusional 5-fluorouracil in patients with gastrointestinal malignancies: results from a prospective population pharmacokinetic study. Cancer Chemother Pharmacol. 2013;71(2):361–370. doi: 10.1007/s00280-012-2018-4. [DOI] [PubMed] [Google Scholar]
- 90.Röhrl K., Guren M.G., Småstuen M.C., Rustøen T. Symptoms during chemotherapy in colorectal cancer patients. Support Care Cancer. 2019;27(8):3007–3017. doi: 10.1007/s00520-018-4598-y. [DOI] [PubMed] [Google Scholar]
- 91.Ilich A.I., Danilak M., Kim C.A., et al. Effects of gender on capecitabine toxicity in colorectal cancer. J Oncol Pharm Pract. 2016;22(3):454–460. doi: 10.1177/1078155215587345. [DOI] [PubMed] [Google Scholar]
- 92.Wagner A.D., Grothey A., Andre T., et al. Sex and adverse events of adjuvant chemotherapy in colon cancer: an analysis of 34 640 patients in the ACCENT database. J Natl Cancer Inst. 2021;113(4):400–407. doi: 10.1093/jnci/djaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.André T., Falcone A., Shparyk Y., et al. VP11-2021: Trifluridine/tipiracil plus bevacizumab vs capecitabine plus bevacizumab as first line treatment for patients with metastatic colorectal cancer (mCRC) ineligible for intensive therapy: the phase III randomized SOLSTICE study. Ann Oncol. 2022;33(2):229–230. [Google Scholar]
- 94.Raimondi A., Fucà G., Leone A.G., et al. Impact of age and gender on the efficacy and safety of upfront therapy with panitumumab plus FOLFOX followed by panitumumab-based maintenance: a pre-specified subgroup analysis of the Valentino study. ESMO Open. 2021;6(5) doi: 10.1016/j.esmoop.2021.100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marmorino F., Rossini D., Lonardi S., et al. Impact of age and gender on the safety and efficacy of chemotherapy plus bevacizumab in metastatic colorectal cancer: a pooled analysis of TRIBE and TRIBE2 studies. Ann Oncol. 2019;30(12):1969–1977. doi: 10.1093/annonc/mdz403. [DOI] [PubMed] [Google Scholar]
- 96.Zhou X., Chang Y., Qian J., et al. Clinical benefit of therapeutic drug monitoring in colorectal cancer patients who received fluorouracil-based chemotherapy. Med Sci Monit. 2021;27 doi: 10.12659/MSM.929474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawakami K., Wakatsuki T., Soejima A., et al. Factors associated with regorafenib adherence with metastatic colorectal cancer. Patient Prefer Adherence. 2019;13:1745–1750. doi: 10.2147/PPA.S217835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vandeputte C., Bregni G., Gkolfakis P., et al. Sex and regorafenib toxicity in refractory colorectal cancer: safety analysis of the RegARd-C trial. Clin Colorectal Cancer. 2021;20(4):326–333. doi: 10.1016/j.clcc.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Ros-Montana F.J., Navarro V., Comas C., et al. Influence of sex on safety and efficacy in BRAF-V600E mutated metastatic colorectal cancer (mCRC) treated with encorafenib-cetuximab +/-binimetinib. Ann Oncol. 2022;33(suppl 7):S136–S196. [Google Scholar]
- 100.Wallis C.J.D., Butaney M., Satkunasivam R., et al. Association of patient sex with efficacy of immune checkpoint inhibitors and overall survival in advanced cancers: a systematic review and meta-analysis. JAMA Oncol. 2019;5(4):529–536. doi: 10.1001/jamaoncol.2018.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Conforti F., Pala L., Bagnardi V., et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):737–746. doi: 10.1016/S1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 102.Ye Y., Jing Y., Li L., et al. Sex-associated molecular differences for cancer immunotherapy. Nat Commun. 2020;11(1):1779. doi: 10.1038/s41467-020-15679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Samstein R.M., Lee C.H., Shoushtari A.N., et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Unger J.M., Vaidya R., Albain K.S., et al. Sex Differences in risk of severe adverse events in patients receiving immunotherapy, targeted therapy, or chemotherapy in cancer clinical trials. J Clin Oncol. 2022;40(13):1474–1486. doi: 10.1200/JCO.21.02377. [DOI] [PMC free article] [PubMed] [Google Scholar]