Abstract
Background
Pre-operative chemoradiotherapy (CRT) rather than radiotherapy (RT) has resulted in fewer locoregional recurrences (LRRs), but no decrease in distant metastasis (DM) rate for patients with locally advanced rectal cancer (LARC). In many countries, patients receive post-operative chemotherapy (pCT) to improve oncological outcomes. We investigated the value of pCT after pre-operative CRT in the RAPIDO trial.
Patients and methods
Patients were randomised between experimental (short-course RT, chemotherapy and surgery) and standard-of-care treatment (CRT, surgery and pCT depending on hospital policy). In this substudy, we compared curatively resected patients from the standard-of-care group who received pCT (pCT+ group) with those who did not (pCT− group). Subsequently, patients from the pCT+ group who received at least 75% of the prescribed chemotherapy cycles (pCT ≥75% group) were compared with patients who did not receive pCT (pCT−/− group). By propensity score stratification (PSS), we adjusted for the following unbalanced confounders: age, clinical extramural vascular invasion, distance to the anal verge, ypT stage, ypN stage, residual tumour, serious adverse event (SAE) and/or readmission within 6 weeks after surgery and SAE related to pre-operative CRT. Cumulative probability of disease-free survival (DFS), DM, LRR and overall survival (OS) was analysed by Cox regression.
Results
In total, 396/452 patients had a curative resection. The number of patients in the pCT+, pCT >75%, pCT− and pCT−/− groups was 184, 112, 154 and 149, respectively. The PSS-adjusted analyses for all endpoints demonstrated hazard ratios between approximately 0.7 and 0.8 (pCT+ versus pCT−), and 0.5 and 0.8 (pCT ≥75% versus pCT−/−). However, all 95% confidence intervals included 1.
Conclusions
These data suggest a benefit of pCT after pre-operative CRT for patients with high-risk LARC, with approximately 20%-25% improvement in DFS and OS and 20%-25% risk reductions in DM and LRR. Compliance with pCT additionally reduces or improves all endpoints by 10%-20%. However, differences are not statistically significant.
Key words: locally advanced rectal cancer, post-operative chemotherapy, oncological outcomes, propensity score stratification, adjuvant chemotherapy
Highlights
-
•
There might be a benefit of pCT after CRT for patients with LARC.
-
•
pCT improves DFS and OS and reduces DM and LRR by 20%-25%.
-
•
Compliance with pCT results in an additional 10%-20% gain.
Introduction
The introduction of pre-operative (chemo)radiotherapy and total mesorectal excision (TME) has contributed to improved local control in patients with rectal cancer in the curative setting. However, this treatment has not led to a decrease in distant metastasis (DM). For this, post-operative chemotherapy (pCT) has been tested in several randomised trials, but the trials have not unequivocally proven that pCT decreases the risk of recurrence, or improves survival.1,2 Despite the lack of strong evidence, pCT is frequently administered according to several guidelines.3,4
The administration of pCT aims to eradicate micrometastases to reduce the risk of recurrent disease and thereby improve survival.5 Clinical trials have demonstrated improved overall survival (OS) in stage III colon cancer after pCT, which probably also applies to high-risk stage II colon cancer.6 The lack of firm evidence in rectal cancer has generated much debate and, as a result, different treatment algorithms have been developed.7,8
Compared to colon cancer, a disadvantage for rectal cancer patients is the prolonged interval between diagnosis and the start of pCT. This interval is generally ∼2 months in colon cancer and at least 4 months in rectal cancer, depending on the pre-operative treatment strategy.1,8 In addition, post-operative complications, being more frequent following rectal cancer surgery, may result in further delay or even omission of pCT.9,10
Therefore, total neoadjuvant treatment (TNT), with pre-operative chemotherapy in addition to pre-operative (chemo)radiotherapy, has been introduced in rectal cancer as an alternative strategy. The RAPIDO trial randomised patients with locally advanced rectal cancer (LARC) at high risk of recurrence between standard-of-care treatment [chemoradiotherapy (CRT) followed by TME and pCT depending on hospital policy (HP)] and an experimental treatment [short-course radiotherapy (RT) followed by pre-operative chemotherapy, i.e. TNT, and TME]. Significantly decreased disease-related treatment failure (DrTF) and DM rates in favour of TNT have been reported.11 The decision to administer pCT was optional in the standard-of-care group, following local guidelines, but was made at each hospital before trial initiation. To advance knowledge about the value of pCT following CRT and radical surgery in high-risk LARC, patients in the standard-of-care treatment group of the RAPIDO trial were analysed.
Materials and methods
Patient selection and randomisation
The RAPIDO trial is a multicentre, phase III trial at 57 community and academic centres in 7 countries. It was approved by the institutional review boards of participating institutions (2010-023957-12). Inclusion and exclusion criteria have been described.11,12 Briefly, patients aged 18 years or older were randomised (1 : 1) in case of biopsy-proven, newly diagnosed rectal cancer, <16 cm from the anal verge at endoscopy and at least one high-risk criterion on magnetic resonance imaging: cT4a/b, cN2, extramural vascular invasion (EMVI+), involved mesorectal fascia or enlarged lateral lymph nodes considered to be pathological. Patients were randomised to receive the experimental or the standard-of-care treatment. In this substudy, only patients from the standard-of-care group were included. The standard-of-care treatment entailed long-course RT (28-25 × 1.8-2.0 Gy) with concurrent capecitabine (825 mg/m2 twice daily on day 1 to 33-38, depending on the number of fractions) followed by surgery after 8 ± 2 weeks. Before participation in the RAPIDO trial, all hospitals had to specify whether they would administer pCT. According to prespecified HP, patients in the standard-of-care group should or should not receive 8 cycles of CAPOX or 12 cycles of FOLFOX4 post-operatively.
Analyses
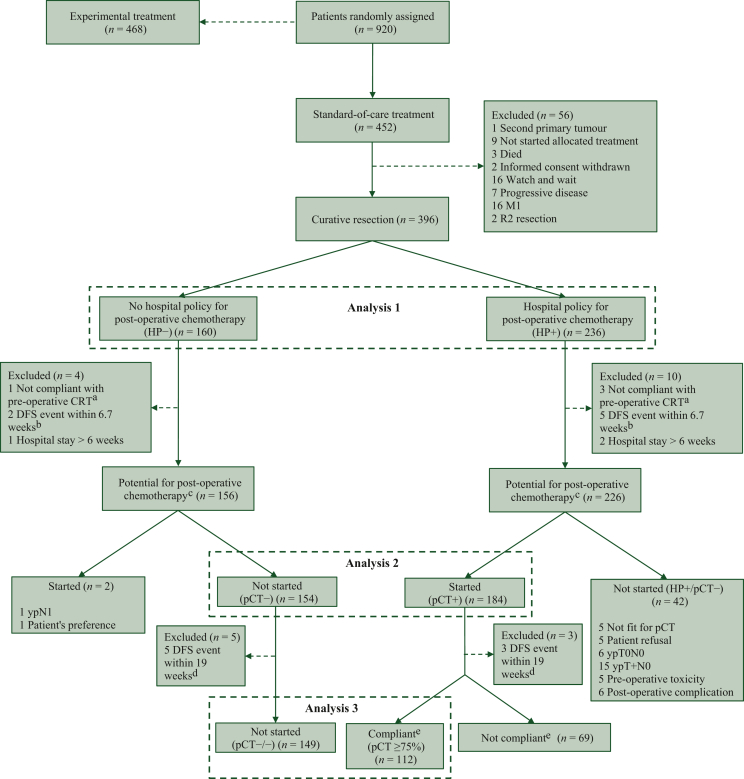
Patients included in the three analyses carried out for this report are presented in Figure 1. Analysis 1 was an intention-to-treat (ITT) analysis including all patients who underwent a curative resection (R0 or R1) within 6 months after the end of CRT and compared all patients treated in a hospital with a policy to provide pCT (HP+ group) with those treated in a hospital with a policy not to provide pCT (HP− group).
Figure 1.
CONSORT diagram. DFS, disease-free survival; HP, hospital policy; M1, distant metastasis; pCT, post-operative chemotherapy; R2 resection, macroscopic residual tumour. aCompliance was defined as receiving at least 45 Gy of the prescribed pre-operative radiotherapy with concurrent capecitabine for at least 25 days. bDefined as a DFS event during the median time from surgery to the start of pCT. cDefined as being able to start treatment with curative intention within 12 weeks after surgery. dDefined as a DFS event during the median time from surgery to receiving at least 75% of the prescribed number of cycles of pCT. eCompliance was defined as receiving at least 75% of the prescribed number of cycles of pCT.
Analysis 2 was a per-protocol analysis and aimed to determine the value of pCT in patients who actually received the intended treatment, so patients who did not receive pCT in the HP− group (pCT−) were compared with patients who actually started pCT in the HP+ group (pCT+). To include only patients who were fit to undergo pCT, we excluded patients not compliant with pre-operative CRT (compliance being defined as having received at least 45 Gy with concurrent capecitabine for at least 25 days), patients with a recurrence or who died before the start of pCT [start of pCT being defined as the median time from surgery to start of pCT (6.7 weeks) to enable similar exclusion in the HP− group] and patients with a post-operative hospital stay exceeding 6 weeks.
Analysis 3 aimed to determine the benefit of compliance to pCT when a dose close to the scheduled could be given and compared patients who received at least 75% of the prescribed cycles pCT (pCT ≥75% group) with those in the HP− group who did not receive any pCT (pCT−/− group). Compliance (pCT ≥75%) was defined as at least 5 courses of CAPOX, 7 courses of FOLFOX4 or at least 4 courses of CAPOX and ≥1 course of capecitabine, or at least 7 courses of chemotherapy in total in case of a switch from CAPOX to FOLFOX4. In case of toxicity, dose reductions were allowed as described in the protocol, without violating the definition of compliance. Before the third analysis, patients were excluded in case recurrence or death within the median time needed to deliver 75% of pCT (∼19 weeks) occurred.
Statistics
Categorical variables were compared using chi-square tests and continuous variables, depending on the distribution of the data, by a t-test or a Mann–Whitney U test. All calculated means were accompanied by a standard deviation and median values by an interquartile range (IQR). All tests were two-tailed, and P values ≤0.050 were considered statistically significant. The median follow-up was calculated by using the reverse Kaplan–Meier method.
In this report, disease-free survival (DFS), DrTF, DM, locoregional recurrence (LRR) and OS were calculated between the groups provided in Figure 1. The primary endpoint of the RAPIDO trial was amended from DFS to DrTF when it became apparent that some patients never became disease free during treatment. However, as this substudy only analysed patients who had a curative resection, it was considered more appropriate to use DFS instead of DrTF and define all endpoints since surgery instead of since randomisation. DFS was defined as the time from surgery till the first occurrence of DM, LRR, a new primary tumour or death by any cause. DrTF was defined as the time from surgery till the first occurrence of DM, LRR, a new primary colorectal tumour or treatment-related death. DM was defined as a recurrence outside the pelvic region and LRR as any pelvic recurrence. Since DrTF was the primary endpoint of the trial and was reported before, and because of the great similarity with DFS, DrTF is provided in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101158.
Propensity score stratification (PSS) was used to adjust for an anticipated imbalance of confounders between the groups in analysis 2 (pCT+ versus pCT−) and 3 (pCT ≥75% versus pCT−/− groups). Propensity scores were generated for each patient using a binary logistic regression in which pCT (yes or no) was the dependent variable and the following were covariates: age, EMVI, tumour distance to the anal verge at baseline, ypT stage, ypN stage, residual tumour classification [resection margin >1 mm (R0) or ≤1 mm (R1)], any serious adverse event (SAE) listed in the study protocol related to pre-operative CRT and any SAE listed in the study protocol and/or readmission within 6 weeks after surgery. These confounders were selected through discussions between principal investigators of the RAPIDO trial. The methods of identifying and selecting confounders and their definitions are explained in detail in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101158.
In the next step, 10 strata were created using visual binning and the range of each stratum was determined based on equal percentages of propensity scores. After stratification, each stratum contained patients of the pCT+ and the pCT− group (or pCT ≥75% and pCT−/− groups in analysis 3) in which the confounders should be equally distributed. This equal distribution of confounders was checked and expressed by calculating a standardised difference (StD), with an StD between −10% and 10% suggesting a good balance between the groups in analysis 2 or 3.13
Using the PSS-adjusted data, the cumulative probabilities of DFS, DrTF, DM, LRR and OS were calculated by stratified Cox regression expressed as hazard ratio (HR) with 95% confidence intervals (CIs). SPSS for Windows (version 28.0, SPSS, Chicago, IL) and R-studio (version 4.1.2, R-Foundation, Vienna, Austria) were used for the statistical analyses.
Results
Study population and compliance
Of the 452 patients randomised to the standard-of-care treatment, 396 (87.6%) underwent a curative resection within 6 months of randomisation (Figure 1). The ITT analysis included 160 patients in the HP− and 236 in the HP+ group (analysis 1). After the exclusion of patients who were not fit for pCT, who started pCT despite HP− or did not start pCT despite HP+, 338/396 (85.4%) patients were included in analysis 2 (n = 154 pCT− versus n = 184 pCT+). For analysis 3, 112/184 (61%) patients received a compliant dose of pCT (i.e. pCT ≥75%) and were compared with 149 patients in the pCT−/− group. At the time of the data lock (11 March 2022), the median follow-up was 5.6 years (IQR 5.4-7.5 years).
Intention-to-treat analysis (analysis 1)
Baseline characteristics of patients in the HP+ and HP− groups are presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101158. Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.101158, shows an overview of which centres followed HP+ or HP−, how many patients per centre were treated in the HP+ and HP− groups, how many patients violated the protocol and reasons for protocol violations. The ITT analysis demonstrated no statistically significant differences between HP+ and HP− patients concerning DFS [HR 1.08 (95% CI 0.78-1.50); P = 0.65], DM [HR 1.17 (95% CI 0.79-1.74); P = 0.43], LRR [HR 1.37 (95% CI 0.51-3.64); P = 0.53] and OS [HR 1.03 (95% CI 0.67-1.61); P = 0.88] (Table 1).
Table 1.
Overview of the HRs with 95% CIs of the three analyses
| Oncological outcome | HR | 95% CI | P value |
|---|---|---|---|
| Analysis 1: HP+ (n = 236) versus HP− (n = 160) | |||
| DFS | 1.08 | 0.78-1.50 | 0.65 |
| DM | 1.17 | 0.79-1.74 | 0.43 |
| LRR | 1.37 | 0.51-3.64 | 0.53 |
| OS | 1.03 | 0.67-1.61 | 0.88 |
| Analysis 2: pCT+ (n = 184) versus pCT− (n = 154) | |||
| DFS | 0.78 | 0.53-1.14 | 0.20 |
| DM | 0.80 | 0.51-1.26 | 0.33 |
| LRR | 0.74 | 0.26-2.15 | 0.58 |
| OS | 0.82 | 0.49-1.37 | 0.44 |
| Analysis 3: pCT ≥75% (n = 112) versus pCT−/− (n = 149) | |||
| DFS | 0.63 | 0.38-1.03 | 0.07 |
| DM | 0.61 | 0.34-1.08 | 0.09 |
| LRR | 0.49 | 0.10-2.38 | 0.38 |
| OS | 0.74 | 0.38-1.44 | 0.38 |
CI, confidence interval; DFS, disease-free survival; DM, distant metastasis; HR, hazard ratio; LRR, locoregional recurrence; OS, overall survival.
The value of pCT on oncological outcomes (analysis 2)
Baseline characteristics of patients in the pCT+ and pCT− groups are presented in Table 2. It shows that 48/154 (31%) patients in the pCT− group and 66/184 (36%) in the pCT+ group had ypN+. Moreover, it shows significant differences in several characteristics, for which PSS adjustment was carried out. In Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.101158, the distribution of the selected confounders and the accompanying StD values before and after PSS are presented. Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.101158, graphically illustrates that all confounders have an StD between −10% and 10% after PSS, representing an equal distribution of confounders between the pCT+ and pCT− groups, whereby potential bias is strongly diminished.
Table 2.
Baseline, surgical and pathological characteristics of eligible patients
| pCT− (n = 154) |
pCT+ (n = 184) |
P value pCT+ versus pCT− | |
|---|---|---|---|
| Gender | 0.68 | ||
| Male | 107 (70) | 124 (67) | |
| Female | 47 (31) | 60 (33) | |
| Age (years) | 0.66a | ||
| Mean (SD) | 60 (10) | 61 (10) | |
| ECOG at baseline | 0.30 | ||
| 0 | 126 (82) | 142 (77) | |
| 1 | 28 (18) | 42 (23) | |
| High-risk criteriab | |||
| cT4 | 34 (22) | 65 (35) | 0.008 |
| cN2 | 103 (67) | 131 (71) | 0.39 |
| Enlarged lateral nodes | 20 (13) | 30 (16) | 0.39 |
| EMVI+ | 34 (22) | 80 (44) | <0.0001 |
| MRF+ | 98 (64) | 134 (73) | 0.07 |
| Number of high-risk criteria | <0.0001 | ||
| 1 | 67 (44) | 39 (21) | |
| 2 | 54 (35) | 63 (34) | |
| 3 | 21 (14) | 58 (32) | |
| 4 | 9 (6) | 19 (10) | |
| 5 | 3 (2) | 5 (3) | |
| Distance from anal verge (cm) | 0.15c | ||
| <5 | 52 (34) | 46 (25) | |
| 5-10 | 50 (33) | 69 (38) | |
| ≥10 | 52 (34) | 69 (38) | |
| Type of approach | <0.0001 | ||
| Laparoscopic | 97 (63) | 59 (32) | |
| Open | 44 (29) | 114 (62) | |
| Laparoscopic → open | 13 (8) | 11 (6) | |
| Type of resection | 0.050 | ||
| Anterior resection, PME | 6 (4) | 23 (13) | |
| LAR, TME | 83 (54) | 90 (49) | |
| APR, TME | 58 (38) | 65 (35) | |
| Hartmann’s procedure | 5 (3) | 5 (3) | |
| Other | 2 (1) | 1 (1) | |
| Radicality of resection | 0.001 | ||
| R0 >1 mm | 148 (96) | 157 (85) | |
| R1 ≤1 mm | 6 (4) | 27 (15) | |
| pCR | 0.51 | ||
| No | 130 (84) | 160 (87) | |
| Yes | 24 (16) | 24 (13) | |
| Differentiation grade | 0.031c | ||
| Well + moderate | 104 (68) | 132 (72) | |
| Poor | 11 (8) | 15 (8) | |
| No tumour | 32 (21) | 26 (14) | |
| Unknown | 7 (5) | 11 (6) | |
| Pathological T stage | 0.018 | ||
| ypT0 | 32 (21) | 26 (14) | |
| ypTis | 1 (1) | — | |
| ypT1 | 7 (5) | 9 (5) | |
| ypT2 | 43 (28) | 36 (20) | |
| ypT3 | 62 (40) | 99 (54) | |
| ypT4 | 9 (6) | 14 (8) | |
| Pathological N stage | 0.31 | ||
| ypN0 | 106 (69) | 118 (64) | |
| ypN1 | 32 (21) | 41 (22) | |
| ypN2 | 16 (10) | 25 (14) |
Data are presented as n (%). Percentages may not equal 100 due to rounding. Bold values represent statistically significant differences.
APR, abdominoperineal resection; ECOG, Eastern Cooperative Oncology Group; EMVI, extramural vascular invasion; LAR, low anterior resection; MRF, mesorectal fascia; MRI, magnetic resonance imaging; pCR, pathological complete response; pCT−, no hospital policy for post-operative chemotherapy (pCT) and did not receive pCT; pCT+, hospital policy for pCT and received pCT; PME, partial mesorectal excision; SD, standard deviation; TME, total mesorectal excision.
Calculated with independent sample t-test.
MRI defined.
P value calculated over the known values.
The Cox regression of analysis 2 demonstrated no statistically significant differences between the pCT+ and pCT− groups in the cumulative probability of any endpoint: DFS [HR 0.78 (95% CI 0.53-1.14); P = 0.20], DM [HR 0.80 (95% CI 0.51-1.26); P = 0.33], LRR [HR 0.74 (95% CI 0.26-2.15); P = 0.58] and OS [HR 0.82 (95% CI 0.49-1.37); P = 0.44] (Table 1).
The value of a compliant dose of pCT on oncological outcomes (analysis 3)
The distribution of confounders in the pCT ≥75% and pCT−/− groups is presented in Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.101158. After PSS all confounders had an StD between −10% and 10% (Supplementary Table S4 and Figure S2, available at https://doi.org/10.1016/j.esmoop.2023.101158).
The Cox regression of analysis 3 demonstrated no statistically significant differences in the cumulative probability of any of the endpoints: DFS [HR 0.63 (95% CI 0.38-1.03); P = 0.07], DM [HR 0.61 (95% CI 0.34-1.08); P = 0.09], LRR [HR 0.49 (95% CI 0.10-2.38); P = 0.38] and OS [HR 0.74 (95% CI 0.38-1.44); P = 0.38] (Table 1).
An overview of all HRs, 95% CIs and P values of analyses 1, 2 and 3 is provided in Table 1 and in Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2023.101158, including DrTF.
Discussion
In this substudy, we explored the value of pCT after pre-operative CRT for patients with high-risk LARC in the RAPIDO trial. The PSS-adjusted analyses suggest a potentially beneficial effect of pCT regarding all endpoints. The risk of a DFS event, DM, LRR and death appears to be reduced by ∼20%-25% by pCT during a median follow-up of 5 years. Compliance with pCT may reduce the risk by another 10%-20%. However, results must be interpreted with caution since they were not based on a randomised comparison and the differences observed were not statistically significant.
The rationale to administer fluoropyrimidine (FU) and oxaliplatin (Ox) as pCT in rectal cancer is mainly based on evidence from trials in colon cancer.14,15 FU-based pCT improved DFS in stage II/III colon cancer.14,15 FU/Ox− versus FU-based pCT additionally improved DFS, with an HR of 0.80 (95% CI 0.69-0.93)16 and similar HRs were observed in two other landmark studies.17,18 The risk reduction regarding DFS events in our analysis [HR 0.78 (95% CI 0.53-1.14)] is somewhat smaller, but similar to those in stage II/III colon cancer trials, which suggests that the addition of pCT after pre-operative CRT in stage II and III rectal cancer might reduce recurrence risks and, thus, be of value.
Trials on pCT in rectal cancer can be characterised into two groups: (i) surgery followed by pCT or not and (ii) pre-operative CRT (or RT alone) and surgery followed by pCT or not. In the first category, a Cochrane analysis demonstrated an added value of pCT.19 However, its current relevance could be questioned because of heterogeneity between the studies, the chemotherapy mostly used is presently not considered adequate and TME was not standard of care in any study. In the second category, two systematic reviews from 2015, one from 2016 and one from 2022 compared different regimens of pCT which did not yield statistically significant differences in DFS and OS when FU or FU/Ox was compared to observation,1,2,20,21 except for Zhao et al. [HR of DFS 0.85 (95% CI 0.73-0.98)] when FU/Ox was compared to observation.20 Despite the absence of firm evidence, several clinical guidelines make a (robust) proposal in favour of the adoption of pCT for rectal cancer patients after CRT and surgery3,4 and remarkably, pCT is extensively used worldwide.
A disadvantage of administering chemotherapy post-operatively is that post-operative complications and decreased physical condition may delay or even lead to the omission of pCT. In our study, pCT was omitted in 34% of the patients who had a curative resection in the HP+ group. In randomised trials, pCT was omitted in ∼25%.21, 22, 23 Full compliance with pCT varies between 43% and 74%.1,22,24, 25, 26, 27 Although our study did not show statistically significantly improved oncological outcomes in compliant patients, the HRs for all endpoints were reduced/improved by 25%-40% compared to 20%-25% of the patients who received any chemotherapy cycle (analysis 3 versus analysis 2). Thus, as compliance to chemotherapy appears to improve oncological outcome and the RAPIDO trial convincingly showed improved compliance with pre-operative chemotherapy versus pCT (84% versus 61%), this may explain the superior results of the experimental treatment of the RAPIDO trial.11
If pCT has favourable effects after CRT and surgery in rectal cancer, the option not to provide it to all patients in the standard-of-care group would have disfavoured the results of this group in the RAPIDO trial. Thus, the differences previously reported between the standard-of-care and experimental treatment in RAPIDO (in favour of the experimental treatment) could be interpreted as exaggerated. This hypothesis was demonstrated by a recent sensitivity analysis of Jimenez-Fonseca et al.28 and validated by a sensitivity analysis of the RAPIDO collaborative.29 However, even if pCT had been mandatory in the standard-of-care treatment, less than two-thirds of the patients would have been treated (due to omission of 34% in our trial) with poor compliance, opposed to no omission and excellent compliance with pre-operative chemotherapy in the RAPIDO trial. Therefore, it is our opinion that chemotherapy can be effective for some patients post-operatively but is more effective for more patients pre-operatively. This is further substantiated by a sensitivity analysis that used the outcomes of this study to analyse the effect of the experimental compared to the standard-of-care treatment, had more patients been treated with pCT (i.e. more hospitals chosen to provide pCT) in the standard-of-care treatment.29
The first article of the RAPIDO collaborative reported that HP on pCT did not statistically significantly affect the primary and secondary outcomes, which may seem contradictory to the results of this study.11 However, the previous results—a sensitivity analysis and a forest plot11—analysed the effect of HP, while analysis 2 and 3 of this article analysed the effect when pCT was initiated or provided to a compliant level (chosen as at least 75% of the number of cycles). The analysis in this article used 5-year follow-up data, with correction for confounders and exclusion of ineligible patients for pCT and those having a recurrence before/during pCT.
Our study is accompanied by some limitations. This report is based on a subgroup analysis of a non-randomised set of patients, which inevitably leads to cohorts with unequal characteristics. However, the analyses were adjusted for unbalanced confounders by using PSS. Moreover, the analyses were based on small cohorts resulting in a great degree of statistical uncertainty. However, the HRs are clearly below 1 and larger sample sizes may have obtained narrower CIs, potentially not including 1. If these risk reductions are true, they are also considered clinically relevant. Further, there might be a bias between countries, e.g. early in the trial there was a difference in attention to EMVI between nations and, as a result, EMVI was probably underreported in the Netherlands (which is the main country in which pCT was not given). If EMVI had been more consistently reported, the PSS groups might have been different. Besides, selecting confounders for the PSS analysis is an arbitrary process, often led by expert opinions. Other experts might select other confounders, possibly altering the outcomes. Nonetheless, the selected covariates are commonly considered important confounders in literature. Furthermore, PSS analyses cannot correct for unmeasured variables and, therefore, ‘unmeasured bias’ may remain. Lastly, the decision to administer pCT was optional in the standard-of-care group, following national or regional guidelines, but was made before trial initiation.
In conclusion, the PSS-adjusted data of the RAPIDO trial suggest a potential, although not statistically significant, benefit of pCT after pre-operative CRT and TME for patients with high-risk LARC. This benefit seems to exist for the group of patients who could be treated within 6-12 weeks after curative surgery, which applies to ∼80% of the patients. Our results add to the still limited evidence from randomised trials of a small gain in preventing recurrences, not sufficient to result in an OS gain as in colon cancer.
Acknowledgements
We thank all patients, treating physicians and research support staff at all participating centres.
Funding
This work was supported by the Dutch Cancer Foundation [grant number CKS-2011-4997]; the Swedish Cancer Society; the Swedish Research Council (project number K2014-99X-22481-01-3); the Spanish Ministry of Economy and Competitiveness through the Carlos III Health Institute [grant number EC11-423]; and by the Spanish Clinical Research Network [grant numbers SCReN-PT13/0002/0031, PT17/0017/0003; co-financed by European Regional Development Fund ‘A way to make Europe’].
Disclosure
PJN reports honoraria from Ethicon, Johnson & Johnson and Amgen. GAPH reports consulting fees from Roche, MSD, Amgen and Novartis; consulting fees and research support to their institution from Bristol Myers Squibb; and research support to their institution from Seerave Foundation. AGHR and CJHvdV were partially funded by the EU’s Horizon 2020 research and innovation program under a Marie Skłodowska Curie grant award (H2020MSCAITN2019, grant agreement number 857894; project acronym: CAST). MPH reports consulting fees from MSD. JC reports consulting fees, travel expenses and research support to their institution from Pfizer, Ipsen and Eisai; consulting fees and research support to their institution from Bayer, Novartis and Advanced Accelerator Applications; consulting fees from Sanofi, Exelixis and Merck Serono; and research support to their institution from AstraZeneca. BG reports research support from the Swedish Cancer Society. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Breugom A.J., Swets M., Bosset J.F., et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16(2):200–207. doi: 10.1016/S1470-2045(14)71199-4. [DOI] [PubMed] [Google Scholar]
- 2.Bujko K., Glimelius B., Valentini V., Michalski W., Spalek M. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta-analysis of randomized trials comparing surgery ± a fluoropyrimidine and surgery + a fluoropyrimidine ± oxaliplatin. Eur J Surg Oncol. 2015;41:713–723. doi: 10.1016/j.ejso.2015.03.233. [DOI] [PubMed] [Google Scholar]
- 3.Glynne-Jones R., Wyrwicz L., Tiret E., et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:22–40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 4.Benson A.B., Venook A.P., Al-Hawary M.M., et al. Rectal cancer, version 6.2020: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(7):807–815. doi: 10.6004/jnccn.2020.0032. [DOI] [PubMed] [Google Scholar]
- 5.Osterman E., Hammarström K., Imam I., Osterlund E., Sjöblom T., Glimelius B. Recurrence risk after radical colorectal cancer surgery—less than before, but how high is it? Cancers (Basel) 2020;12:1–31. doi: 10.3390/cancers12113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyers B.M., Cosby R., Quereshy F., Jonker D. Adjuvant chemotherapy for stage II and III colon cancer following complete resection: a cancer care Ontario systematic review. Clin Oncol. 2017;29(7):459–465. doi: 10.1016/j.clon.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Valentini V., van Stiphout R.G.P.M., Lammering G., et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29(23):3163–3172. doi: 10.1200/JCO.2010.33.1595. [DOI] [PubMed] [Google Scholar]
- 8.Glimelius B. Adjuvant chemotherapy in rectal cancer: state of the art and future perspectives. Curr Opin Oncol. 2020;32:377–383. doi: 10.1097/CCO.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 9.van der Valk M.J.M., Marijnen C.A.M., van Etten B., et al. Compliance, acute toxicity and postoperative complications of short-course radiotherapy followed by chemotherapy and surgery for high-risk rectal cancer. Results of the randomized RAPIDO-trial. Eur J Surg Oncol. 2020;46(2) doi: 10.1016/j.radonc.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho C., Glynne-Jones R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol. 2017;18:e354–e363. doi: 10.1016/S1470-2045(17)30346-7. [DOI] [PubMed] [Google Scholar]
- 11.Bahadoer R.R., Dijkstra E.A., van Etten B., et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson P.J., van Etten B., Hospers G.A.P., et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer - the RAPIDO trial. BMC Cancer. 2013;13:279. doi: 10.1186/1471-2407-13-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson E.J., Forbes A. Introduction to propensity scores. Respirology. 2014;19:625–635. doi: 10.1111/resp.12312. [DOI] [PubMed] [Google Scholar]
- 14.O’Connell M.J., Mailliard J.A., Kahn M.J., et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15(1):246–250. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 15.Labianca R., Marsoni S., Pancera G., et al. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet. 1995;345(8955):939–944. [PubMed] [Google Scholar]
- 16.Schmoll H.J., Tabernero J., Maroun J., et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol. 2015;33(32):3733–3740. doi: 10.1200/JCO.2015.60.9107. [DOI] [PubMed] [Google Scholar]
- 17.Yothers G., O’Connell M.J., Allegra C.J., et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29(28):3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.André T., de Gramont A., Vernerey D., et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33(35):4176–4187. doi: 10.1200/JCO.2015.63.4238. [DOI] [PubMed] [Google Scholar]
- 19.Petersen S.H., Harling H., Kirkeby L.T., Wille-Jørgensen P., Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev. 2012;2012:CD004078. doi: 10.1002/14651858.CD004078.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L., Liu R., Zhang Z., et al. Oxaliplatin/fluorouracil-based adjuvant chemotherapy for locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery: a systematic review and meta-analysis of randomized controlled trials. Colorectal Dis. 2016;18:763–772. doi: 10.1111/codi.13381. [DOI] [PubMed] [Google Scholar]
- 21.Rödel C., Graeven U., Fietkau R., et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–989. doi: 10.1016/S1470-2045(15)00159-X. [DOI] [PubMed] [Google Scholar]
- 22.Bosset J.F., Collette L., Calais G., et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 23.Sainato A., Cernusco Luna Nunzia V., Valentini V., et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT) Radiother Oncol. 2014;113(2):223–229. doi: 10.1016/j.radonc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 24.QUASAR Collaborative Group. Gray R., Barnwell J., et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z., Mohile S.G., Tejani M.A., et al. Poor compliance with adjuvant chemotherapy use associated with poorer survival in patients with rectal cancer: an NCDB analysis. Cancer. 2017;123:52–61. doi: 10.1002/cncr.30261. [DOI] [PubMed] [Google Scholar]
- 26.Hong Y.S., Kim S.Y., Lee J.S., et al. Oxaliplatin-based adjuvant chemotherapy for rectal cancer after preoperative chemoradiotherapy (ADORE): long-term results of a randomized controlled trial. J Clin Oncol. 2019;37:3111–3123. doi: 10.1200/JCO.19.00016. [DOI] [PubMed] [Google Scholar]
- 27.Mari G.M., Maggioni D., Crippa J., et al. Compliance to adjuvant chemotherapy of patients who underwent surgery for rectal cancer: report from a multi-institutional research network. World J Surg. 2019;43(10):2544–2551. doi: 10.1007/s00268-019-05060-5. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Fonseca P., Salazar R., Valenti V., Msaouel P., Carmona-Bayonas A. Is short-course radiotherapy and total neoadjuvant therapy the new standard of care in locally advanced rectal cancer? A sensitivity analysis of the RAPIDO clinical trial. Ann Oncol. 2022;33:786–793. doi: 10.1016/j.annonc.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Dijkstra E.A., Zwart W.H., Putter H., et al. Authors’ reply—A sensitivity analysis of the RAPIDO clinical trial. Ann Oncol. 2023;34(4):446–447. doi: 10.1016/j.annonc.2022.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.