Abstract
Background
Single-arm trials (SATs) can sometimes be used to support marketing authorization of anticancer medicinal products in the European Union. The level and durability of antitumor activity of the product as well as context are important aspects to determine the relevance of trial results. The aim of this study is to provide details on the contextualization of trial results and to evaluate the magnitude of benefit of medicinal products approved based on SATs.
Materials and methods
We focused on anticancer medicinal products for solid tumors approved on the basis of SAT results (2012-2021). Data were retrieved from European public assessment reports and/or published literature. The benefit of these medicinal products was evaluated via the European Society for Medical Oncology (ESMO)-Magnitude of Clinical Benefit Scale (MCBS).
Results
Eighteen medicinal products were approved based on 21 SATs—few medicinal products were supported by >1 SAT. For the majority of clinical trials, a clinically relevant treatment effect was (pre)specified (71.4%) and most often an accompanying sample size calculation was provided. For 10 studies, each testing a different medicinal product, a justification for the threshold for a clinically relevant treatment effect could be identified. At least 12 out of 18 applications included information to facilitate the contextualization of trial results, including six supportive studies. Of the pivotal SATs analyzed (n = 21), three were assigned an ESMO-MCBS score of 4, which corresponds to ‘substantial’ benefit.
Conclusions
The clinical relevance of the treatment effects shown by medicinal products for solid tumors tested in SATs is dependent on the effect size and context. To better facilitate regulatory decision making, prespecifying and motivating a clinically relevant effect and aligning the sample size to that effect is important. External controls may facilitate in the contextualization process, but the associated limitations must be addressed.
Key words: single-arm trials, oncology, European Medicines Agency, clinical benefit, contextualization
Highlights
-
•
The European Commission approved 18 medicinal products for solid tumors on the basis of results from 21 SATs (2012-2021).
-
•
Thresholds to be ruled out by the statistical test were regularly justified based on historical data.
-
•
For the majority of SATs, results were contextualized by additional information provided by the applicant.
-
•
Of the SATs included in our study, only three were assigned an ESMO-MCBS score of ≥4 (substantial benefit).
-
•
It is important to discuss among stakeholders what can be considered the clinical benefit of medicines tested in SATs.
Introduction
Randomized controlled trials (RCTs) are referred to as the ‘gold standard’ in testing medicinal products.1 These trials have several advantages over clinical trials with other designs due to their design features. For example, randomization facilitates subjects in the experimental and control groups being comparable at baseline. Randomization and blinding are useful techniques to determine whether there is a cause–effect relation between treatment and outcome.2,3 RCTs are the preferred trials to be included in applications for marketing authorization, as laid down in Directive 2001/83/EC. In this directive, it is stated that clinical trials relevant to the indication “shall be done as ‘controlled clinical trials’ if possible, randomised; any other design shall be justified”.4 Yet, it is not always possible to conduct an RCT, and, consequently, clinical trials with other designs need to be considered for registrational purposes.5 The latter includes the use of single-arm trials (SATs).
Tenhunen et al. identified that, between 2010 and 2019, the European Commission (EC) approved 22 medicinal products for the treatment of solid tumors or hematological malignancies on the basis of SAT results.6 Many of the medicinal products included in their study received ‘conditional marketing authorization’ (CMA).6 This type of approval was introduced in the past to address an unmet medical need, and is based on less complete data than are usually required for standard approval.7 It should be mentioned, however, that SATs can also support standard approvals—albeit less common. Examples are the approvals of engineered autologous T-cell immunotherapies.8, 9 However, demonstrating that an investigational medicinal product provides clinical benefit can be challenging when it is tested solely in an SAT. Trials like these are associated with different forms of bias, including selection bias.10,11 Besides, surrogate endpoints such as objective response rate (ORR) are commonly used in SATs, at least when focusing on cancer research.12,13 ORR is not a direct measure of clinical benefit. Yet, it is a measure of (antitumor) activity, as spontaneous regression occurs infrequently in cancer.13
Some guidance exists on the use of SATs for regulatory purposes. It is stated in the “Guideline on the clinical evaluation of anticancer medicinal products” of the European Medicines Agency (EMA) that resorting to a non-randomized design should be justified by, among others, a large treatment effect on ORR and duration of response (DoR), effects that will likely translate into clinical benefit.5 Moreover, in the same guideline, it is stated that contextualization of results is an important topic for SATs, particularly for less evident cases.5 Indirect comparisons with available therapies are often made for these purposes.14,15 While it is not the task of regulatory agencies to ensure comparative efficacy,16 there is a general need to ensure that new medicinal products are not worse—in terms of efficacy and/or safety—than standard of care. Importantly, the aspects described above, such as the size and durability of the treatment effect and context, will help to determine the clinical relevance of trial results.
The aim of this study was to provide details on how clinical benefit of anticancer medicinal products tested in SATs was determined, including the methods used to contextualize the trial results. In addition, we were interested in how many of the authorized medicinal products based on SATs showed ‘substantial’ benefit. We started with investigating whether a threshold for the relevant treatment effect was (pre)specified in the pivotal trials—for example, in a power calculation. Subsequently, we determined if applicants submitted additional evidence to contextualize the SAT results. Finally, by limiting this study to medicinal products for the treatment of solid tumors, we evaluated the magnitude of benefit of the medicinal products included in our study via a validated tool, the European Society for Medical Oncology (ESMO)-Magnitude of Clinical Benefit Scale (MCBS).
Materials and methods
Medicinal products
An overview of all human medicines that were granted approval by the EC was retrieved from the EMA database (https://www.ema.europa.eu/en/medicines). Products were identified on the basis of their Anatomic Therapeutic Chemical (ATC) codes, that is, L01-04 for antineoplastic and immunomodulating agents. We focused on medicinal products for the treatment of solid tumors authorized between 2012 and 2021—a 10-year period. The inclusion criterion for our analysis was initial approvals based on an SAT(s). Approvals based on RCTs were excluded. Approvals of generic and biosimilar products were also excluded.
Data sources
The main data source was the European public assessment reports (EPARs). These reports were obtained from the EMA database (https://www.ema.europa.eu/en/medicines). EPARs contain information on the scientific evaluation conducted by the Committee for Medicinal Products for Human Use (CHMP)—a committee of the EMA. The scientific evaluation forms the basis for the EC decision on approval. Another data source was published literature on pivotal clinical trials. Relevant publications were identified via PubMed and/or ClinicalTrials.gov.
Data collection
Data were retrieved from EPARs and/or scientific publications. We focused on pivotal trials, meaning that clinical pharmacology and dose-finding studies were not included. We collected the following information on the pivotal trials: the study design, dosing regimen, study population, planned sample size, statistical methods, primary/secondary endpoints, clinical outcomes, and type of authorization. It was also determined whether applicants made additional efforts to contextualize the results of the SAT(s), i.e. the use of external evidence to facilitate the interpretation of trial results. This concerned analyses (e.g. within-patient analysis) and/or evidence such as publications and additional studies that were included in the EPAR as supportive evidence. In addition to EPARs, scientific publications, including publicly available protocols that were supplementary to these publications, were used to complement information on the statistical methods.
Determining clinical benefit
The ESMO created the ESMO-MCBS, a validated tool to evaluate the magnitude of clinical benefit.17 The ESMO-MCBS scores already assigned to clinical trials (i.e. ESMO publications or EMSO-MCBS scorecards) were identified. The remaining SATs included in our analysis were assigned an ESMO-MCBS score independently by two researchers (VSB and JM). This was done according to EMSO instructions.18 Scientific publications were used for this purpose. In case a CMA was converted to standard marketing authorization (SMA) at the time of data analysis, an ESMO-MCBS score was assigned to the confirmatory trial. For non-curative therapies, ESMO-MCBS scores ≥4 represent substantial benefit.19
Results
Approval of medicinal products for the treatment of solid tumors
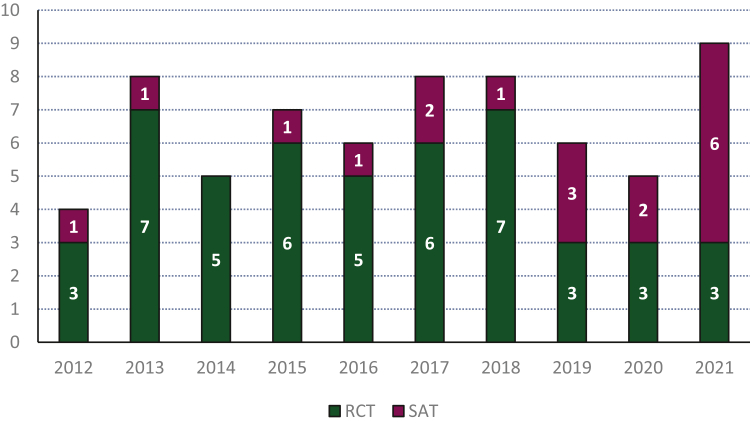
A total of 731 medicinal products received EC approval between 2012 and 2021. Of these, 66 (9.0%) were granted approval for the treatment of solid tumors—excluding generics or biosimilars (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.101209). Over the recent years, the proportion of approvals for solid tumors based on SATs increased compared to prior years (Figure 1). In total, 18 (2.5%) medicinal products were approved based on 21 SATs (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101209). Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.101209, shows the intended patient populations for which the medicinal products were approved. Half of the medicinal products were approved (also) for the treatment of advanced non-small-cell lung cancer (NSCLC). The approvals of alectinib, avapritinib, and crizotinib were on the basis of results from an SAT(s) with top-line results from an RCT—albeit not always in a similar treatment setting (e.g. different line of therapy). However, as the SATs remained the pivotal trial(s) supporting these applications, the three products were retained in our analyses.
Figure 1.
Number of medicinal products for the treatment for solid tumors approved by the European Commission per year. Generic and biosimilar medicinal products were excluded. In purple the number of approvals based solely on single-arm trials (SATs) and in green the number of approvals based on randomized controlled trials (RCTs).
All 18 medicinal products approved based on an SAT(s) were granted CMA. At the time of data analysis, eight CMAs were converted to SMAs (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.101209). For one of the CMAs, i.e. rucaparib, the benefit–risk balance was no longer considered favorable by the CHMP based on the confirmatory trial. The marketing authorization holder (MAH) requested to remove the indication.
Single-arm trials and thresholds for clinically relevant treatment effect
Most approvals were supported by one pivotal trial. The approvals of alectinib, osimertinib, and rucaparib were supported by two SATs. For the approvals of entrectinib and larotrectinib, integrated analyses by pooling data across clinical trials were used for the evaluation of efficacy (three trials each). For all trials or integrated analyses, the primary endpoint was ORR (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101209).
For the majority of clinical trials or integrated analyses [15 out of 21 (71.4%)], a clinically relevant treatment effect was (pre)specified and most often an accompanying sample size calculation was provided (Table 1). The test for a relevant effect was often defined as the lower bound of the 95% confidence interval (CI) for ORR exceeding a (predefined) value, which is equivalent to testing a null hypothesis corresponding to that value. Protocols confirmed the results, which were publicly available (i.e. supplementary to publication) for all SATs except for those studying alectinib (both trials), avelumab, osimertinib (AURA 2), and rucaparib (CO-338-010). For the trials investigating entrectinib, larotrectinib, pemigatinib, and selpercatinib, a clinically relevant lower boundary of the 95% CI for ORR was defined, but the null and alternative hypotheses were not explicitly mentioned in the EPARs/publications. For the trials testing ceritinib, crizotinib, lorlatinib, and rucaparib, no power calculations were carried out based on the information presented in the EPARs and/or publications. Regarding trial CO-338-017, one of the SATs testing rucaparib, some sample size assumptions were made for subgroup allocation (part 1 and 2) and comparison (part 1) of the trial, but no calculations were made based on expected treatment effects.
Table 1.
Statistical aspects of SATs
| Medicinal product | Trial(s) | Therapeutic area | Biomarker-based indication | Available therapies in treatment setting | Sample size calculationsa | Lower bound of the 95% CI for ORR to be ruled out | Justification | Ref. |
|---|---|---|---|---|---|---|---|---|
| Alectinib | NP28761 | Lung cancer | Yes | Chemotherapy | Yes | 35% | Not provided | 20 |
| NP28673 | Lung cancer | Chemotherapy | Yes | 35% | Not provided | 21 | ||
| Amivantamab | EDI1001 | Lung cancer | Yes | Chemotherapy or immunotherapy | Yes | 12% | Single-agent chemotherapy as the benchmark | 22 |
| Avapritinib | BLU-285-1101 | Sarcoma | Yes | Tyrosine kinase inhibitors | Yes | 10% | Benchmarked against available therapies | 23 |
| Avelumab | EMR100070-003 | Skin cancer | No | Chemotherapy | Yes | 20% | Absence of literature documenting treatment outcomes for second-line patients | 24 |
| Cemiplimab | 2810-ONC-1540 | Skin cancer | No | EGFR inhibitors and/or chemotherapy | Yes |
laCSCC 25% |
Based on previous studies | 25 |
| Yes | mCSCC 15% |
Based on previous studies | 26 | |||||
| Ceritinib | CLDK378X2101 | Lung cancer | Yes | Chemotherapy | No | Not specified | Not applicable | 27 |
| Crizotinib | A8081001 | Lung cancer | Yes | Chemotherapy | No | Not specified | Not applicable | 28 |
| Dostarlimab | 4010-01-001 | Endometrial cancer | Yes | Chemotherapy or bevacizumab | Yes | 20% | Expected ORR for conventional therapy | 29 |
| Entrectinibb | ALKA-372-001, RXDX-101-01, and RXDX-101-03 | Lung cancer | Yes | Crizotinib | Yes (on precision and implicitly on power) | 50% | Observed with standard-of-care ROS1 fusion-positive NSCLC treatment | 30 |
| Cancer | No appropriate available therapies | Yes (on precision and implicitly on power) | 30% | Not provided | 31 | |||
| Larotrectinibb | LOXO-TRK-14001, LOXO-TRK-15002, and LOXO-TRK-15003 | Cancer | Yes | No appropriate available therapies | Yes | 30% | Consistent with the response rates seen with approved targeted therapies in genetically defined patient populations who have progressed on prior therapies | 32 |
| Lorlatinib | B7461001 | Lung cancer | Yes | (Platinum-based) chemotherapy/immunotherapy | No | Not specified | Not applicable | 33 |
| Osimertinibc | AURA extension | Lung cancer | Yes | (Platinum-based) chemotherapy or tyrosine kinase inhibitor rechallenge | Yes (based on precision) | Not specified | Not applicable | 34 |
| AURA 2 | (Platinum-based) chemotherapy or tyrosine kinase inhibitor rechallenge | Yes (based on precision) | Not specified | Not applicable | 35 | |||
| Pemigatinib | INCB 54828-202 | Bile duct cancer | Yes | Chemotherapy | Yes | 15% | Proportions of patients with an objective response reported by previous studies | 36 |
| Pralsetinib | BLU-667-1101 | Lung cancer | Yes | (Platinum-based) cytotoxic chemotherapy and/or immunotherapy | Yes | 48% | Not provided | 37 |
| Chemotherapy ± ramucirumab or immunotherapy | Yes | 23% | Not provided | |||||
| Rucaparibb | CO-338-010 | Ovarian cancer | Yes | Chemotherapy | No | Not specified | Not applicable | 38 |
| CO-338-017 | Chemotherapy | No | Not specified | Not applicable | 39 | |||
| Selpercatinib | LOXO-RET-17001 | Lung cancer | Yes | Chemotherapy ± ramucirumab or immunotherapy | Yes | NSCLC 30% |
Consistent with the response rates seen with approved targeted therapies in molecularly defined populations who failed prior therapies | 40 |
| Thyroid cancer | Treatment options in these settings are limited—tyrosine kinase inhibitors rechallenge—or even lacking | Yes | MTC 20% |
The limited treatment options | 41 | |||
| No | TC Not specified |
Not applicable | 41 | |||||
| Trastuzumab deruxtecan | DS8201-A-U201 | Breast cancer | Yes | HER2-targeted therapy in combination with chemotherapy | Yes | 20% | Not provided | 42 |
| Vismodegib | SHH4476g | Skin cancer | No | Radiation therapy or chemotherapy | Yes | mBCC 10% |
No therapeutic options exist for these patients and spontaneous responses have not been reported in this diseased | 43 |
| Yes | aBCC 20% |
No therapeutic options exist for these patients and spontaneous responses have not been reported in this diseased | 43 |
Therapeutic areas are depicted in color: lung cancer in orange, skin cancer in yellow, cancer (general) in blue, and remaining areas in green. Information was retrieved from EPARs and complemented by scientific publications and protocols, if available and necessary.
aBCC, advanced basal cell carcinoma; CI, confidence interval; HER2, human epidermal growth factor receptor 2; laCSCC, locally advanced cutaneous squamous cell carcinoma; mBCC, metastatic basal cell carcinoma; mCSCC, metastatic cutaneous squamous cell carcinoma; MAH, marketing authorization holder; MTC, medullary thyroid cancer; NSCLC, non-small-cell lung cancer; ORR, objective response rate; ROS1, c-ros oncogene 1; TC, thyroid cancer.
Sample size calculations were based on power unless otherwise specified.
Integrated analysis was carried out based on two or three trials.
Separated and integrated analysis was carried out for trials AURA and AURA2.
Based on protocol.
For 10 out of 21 trials (47.6%), each testing a different medicinal product, justification for the threshold to the statistical test could be extracted from EPARs/publications/protocols (Table 1). Mostly, the treatment effect of available therapies was used as a benchmark (n = 5). Other justifications were ‘consistent with the response rates seen with approved targeted therapies in genetically defined patient populations who have progressed on prior therapies’ (n = 2), ‘limited treatment options’ (n = 1), and ‘absence of literature documenting treatment outcomes for second-line patients’ (n = 1).
Pralsetinib and selpercatinib were tested in trials that included patients with RET fusion-positive NSCLC who previously received platinum-based chemotherapy. The specified clinically relevant lower bound of the 95% CI for ORR was different between the two trials, namely 23% and 30%, respectively (Table 1). Larotrectinib and entrectinib were tested in clinical trials that included patients with NTRK gene fusion-positive tumors. For both applications, the lower bound of the 95% CI for ORR was 30% for the integrated analysis across clinical trials (Table 1).
Contextualization
The type and amount of information that was included for contextualization purposes varied between the applications for marketing authorization for the 18 medicinal products. At least 12 out of 18 applications (71.4%) included some additional information for contextualization purposes (Table 2, Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.101209). Six out of 18 applications included supportive studies (Table 2, Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.101209). One of these supportive studies concerned a bibliographic reference, namely the Dermatologic Cooperative Oncology Group (DeCOG) study. Other supportive studies were of a retrospective nature, and included real-world data from various sources. From the supportive studies included in the applications of trastuzumab deruxtecan and entrectinib, i.e. the Unicancer study and WO40977, respectively, matched populations were generated. In the latter study, a comparative analysis with a matched crizotinib arm derived from real-world data was conducted.
Table 2.
Information provided by applicants to contextualize SAT results
| Medicinal product | Information included in the European public assessment report |
|---|---|
| Amivantamab | Supportive study 61186372NSC100 |
| Avapritinib | A comparison of trial versus natural history data |
| Supportive study BLU-285-1002 | |
| Avelumab | Best response on the last prior anticancer drug therapy for metastatic disease |
| Supportive study 100070-Obs001 | |
| Cemiplimab | Supportive study Dermatologic Cooperative Oncology Group |
| Crizotinib | Indirect comparison versus other treatmenta |
| Results to previous treatment | |
| Dostarlimab | Best overall response from last platinum-containing prior anticancer therapy |
| Entrectinib | Supportive study WO40977 |
| Larotrectinib | Comparison of larotrectinib with available systemic treatment for cancer |
| Lorlatinib | A comparison between time to tumor progression on lorlatinib and the time to tumor progression on last treatment before lorlatinib |
| Pemigatinib | An analysis of second-line treatment |
| Rucaparib | Results from prospective studies in platinum-sensitive disease that included third-line treatment |
| Trastuzumab deruxtecan | A literature-based analysis to understand the historical context |
| Supportive study Unicancer |
Data of the indirect comparison were not shown in the EPAR.
Evaluating benefit
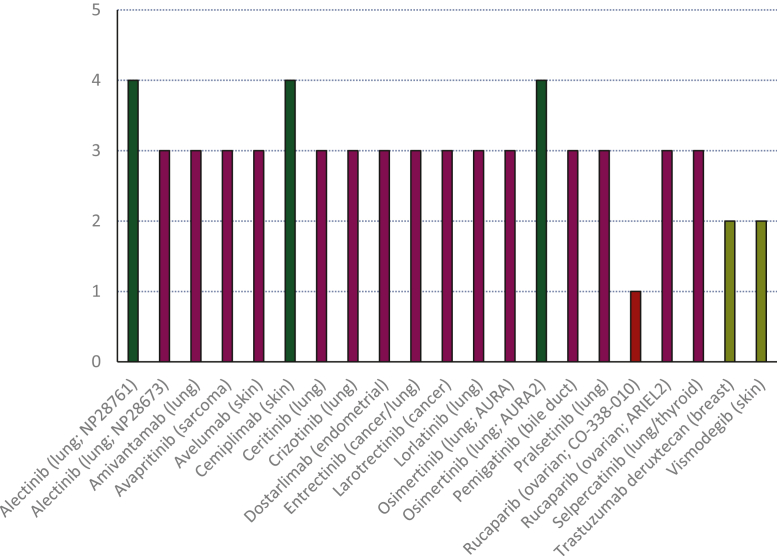
Figure 2 and Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2023.101209, show the ESMO-MCBS scores for the pivotal SATs (n = 21), either assigned by us or already published by ESMO. For all the SATs included in our study, three SATs were assigned an ESMO-MCBS score of ‘4’. Fifteen SATs were assigned an EMSO-MCBS score of ‘3’, two SATs were assigned an ESMO-MCBS score of ‘2’, and one SAT was assigned an ESMO-MCBS score of ‘1’. ESMO-MCBS scores of ‘4’ were assigned as a result of the score upgrades for quality of life (QoL), meaning the investigators reported improvements in QoL.
Figure 2.
European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS) scores assigned to pivotal single-arm trials. In dark green the trials are depicted that were assigned a high ESMO-MCBS score. Scores were either made publicly available by the ESMO or were assigned by us. Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.101209, provides additional scoring details and reference to publications or scorecards for the scores assigned by ESMO.
Five out of eight CMAs were converted to SMA based on an RCT, i.e. reaching a comprehensive level of evidence. Of these RCTs, four were assigned an ESMO-MCBS score of ‘4’ and one was assigned a score of ‘2’ (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2023.101209).
Discussion
In specific situations, medicinal products may receive (expedited) regulatory approval on the basis of results from SATs. In this study, we analyzed pivotal SAT-based applications for anticancer medicinal products in the European Union between 2012 and 2021. In this period, 18 medicinal products for the treatment of solid tumors received an approval based on 21 SATs. At least 12 out of 18 applications included additional information to contextualize the results from the pivotal trials, which included supportive studies, external evidence, information on response to prior therapy, and/or a within-patient comparison. Of all the SATs or integrated analyses supporting the 18 EC approvals, three were assigned an ESMO-MCBS score of ‘4’, that is, a score indicating substantial benefit.
SATs are generally initiated to determine whether an investigational product has sufficient activity to continue development.44,45 Often statistical testing is used to determine whether the treatment effect is above a prespecified threshold, which is reflected in whether the null hypothesis related to the threshold is rejected.46 Our results indicate that a justification for this threshold was not always reported in the EPAR (or scientific publication). Tenhunen et al. reported that the threshold for ‘success’ in pivotal SATs is relatively uniform—20% ORR—and often not scientifically justified.6 Our study does not confirm their results, as thresholds varied—ranging from 10% to 50%. This might, however, be explained by the partial differences in datasets. The threshold for success is often based on historical data or clinical judgment, which reflects ORRs by available treatment or standard of care.47 However, determining this threshold can be challenging. For instance, historical data can be inconsistent with regard to the observed ORRs. Studies with doxorubicin plus ifosfamide in soft tissue sarcoma showed varied ORRs (i.e. 16%-35%).48 Also, historical data might be absent or derived from studies that differ in, but not limited to, design or study population in comparison to the SAT.48 The latter being particularly relevant for biomarker-driven SATs, which concerns the majority of SATs included in our study. Overall, it is important to select an appropriate threshold before conducting an SAT, but even more to provide argumentation why having a lower bound of the 95% CI above this threshold constitutes a clinically relevant outcome.
Our results show that the ORR to be ruled out at a particular significance level is not always ambitious. For example, thresholds based on historical data were sometimes lower than thresholds in absence of treatment options. Also, similar historical data sometimes led to different thresholds. Another point to mention is that the ORR used for sample size/power calculations, i.e. the effect under the alternative hypothesis, is rarely justified to be clinically relevant or corresponding to an effect one would not want to miss (data not shown), the latter, for instance, in the context of a go/no-go decision for proceeding with drug development program.49 Simply rejecting the null hypothesis may not be sufficient for regulatory decision making. As already highlighted a few decades ago, the meaningfulness of ORR depends on whether this translates into ‘true’ benefit (e.g. improvement in survival).50 Oxnard et al. showed that an ORR statistically exceeding 30% (or higher) is associated with regulatory approval, at least for monotherapies tested in SATs.51 However, not only ORR but also DoR will be important for regulatory decision making. For example, the CHMP was of the opinion that the activity of pralsetinib, indicated by a high percentage of durable responses in the pivotal trial, would translate into clinical meaningful benefit.52 The observed ORR of entrectinib shown in the integrated analysis was below the assumed ORR used for the sample size calculation. However, the observed ORR, in combination with DoR, was considered of clinical relevance by the CHMP.53 In contrast, the ORR and DoR shown by retifanlimab in the pivotal SAT were not considered clinically relevant by the CHMP. In fact, the criterion for ‘success’ was not met in this SAT—i.e. ruling out an ORR of 13%—and the applicant withdrew the application for marketing authorization.54 If it is justified to use an SAT for regulatory purposes, it will be key to motivate which effect would constitute an (minimal) important effect from a clinical point of view, not merely ruling out a, sometimes unimpressive, historical ORR.
During the approval process, context may be sought via indirect comparisons with (well-)documented outcomes for clinical trials testing available therapies. This is also relevant considering that new data may have become available after initiation of the SAT. We demonstrate that applications frequently include information for contextualization purposes, including results from supportive studies. There are, however, limitations associated with cross-trial comparisons,55 which necessitate caution when interpreting these results. For example, differences between study populations may lead to inappropriate comparisons.56 One approach to (partly) overcome these limitations is to use patient-level data to generate a matched external control.56 Interestingly, a recent study carried out by Schröder et al. demonstrated that external controls generated from electronic health record-derived databases were successful in replicating a control arm from an RCT in metastatic colorectal cancer.57 However, matched comparisons with external controls are rare—at least in our dataset. Only two comparative matched analyses with standard of care were carried out. External controls, however, cannot be corrected for confounders that are unknown or unmeasured.57 There is some regulatory guidance available to reduce potential bias with external controls.2, 5 However, after addressing all the limitations as much as possible, the issue remains that, if there is a high chance for residual bias, the outcome in an SAT has to be convincing to compensate for the potential bias. Importantly, the quality of data will likely determine the extent to which external controls can be used for regulatory decision making.58
Pignatti et al. highlighted that the definition of clinical value is different between stakeholders, which may lead to different conclusions.59 While the CHMP concluded that the benefit of the medicinal products included in our analysis was clinically relevant, stakeholders other than regulators might appreciate benefit differently. For instance, the ESMO considers benefit as ‘living longer and/or living better’, which resonates in the ESMO-MCBS form for SATs.19,17 This is evident by our results, as the benefit of the majority of products was ‘modest’ on the basis of the ESMO-MCBS scores. Tibau et al. stated that large treatment effects in combination with an improvement in QoL (or data from post-marketing studies) are needed for SATs to be assigned a high ESMO-MCBS score.60 However, QoL is not always a secondary endpoint in clinical trials, and one of the shortcomings of the ESMO-MCBS is that it does not take into account delayed publications or publication bias for QoL.61 Besides, the CHMP repeatedly stated in assessment reports that no firm conclusion can be drawn from QoL data generated by SATs.62, 63, 64, 65 Thus, QoL is of lesser importance in regulatory decision making on SATs.
There are other tools to evaluate the benefit of approved anticancer medicinal products. For instance, a committee of the Dutch Society of Medical Oncology created the PASKWIL criteria for non-randomized trials, for which the ESMO-MCBS was used as a basis.66 In comparison to the ESMO-MCBS, QoL and safety are not incorporated in this instrument, and benefit is based on predefined ORR and DoR thresholds.66 Other criteria are that the medicinal product is authorized by the EC, the disease is rare, the patient population is adequately selected, and there is a biological rationale for therapy.67 As tools are created on a national level that do not completely align with the EMSO-MCBS, there might be a need to fine-tune what can be considered benefit on an European level. Consistency among tools may warrant further discussion among stakeholders so as to prevent potential inequality in care.
All medicinal products included in our study received a CMA. When the MAH intends to fulfill the specific obligation(s) associated with the CMA, the benefit–risk balance will be re-assessed on a more complete dataset, preferably results from an RCT. However, Tenhunen et al. showed that post-authorization measures associated with CMAs are not always to submit results from an RCT.6 Of course, the level of evidence to be generated in the post-marketing setting depends on, amongst others, feasibility to conduct large trials. Recently, Fashoyin-Aje et al. informed that a ‘comprehensive strategy’ for confirmatory trials is needed, which focusses on the so-called on-ramp (e.g. trial design, patient population, etc.) and off-ramp considerations (i.e. verify clinical benefit).67 The authors highlight that, for accelerated approvals, efforts should be made to timely and adequately address remaining uncertainties regarding the benefit–risk balance. Similarly, Bloem et al. highlight that RCTs should be ongoing when a CMA is granted, ensuring rapid access to a more complete dataset.68 Important to mention is that re-assessment of the ESMO-MCBS score is possible when results from confirmatory trials are published. This may lead to an improvement in ESMO-MCBS score—as also seen in our study. Furthermore, extended follow-up for the SATs themselves may also improve the EMSO-MCBS score. For example, we previously assigned an ESMO-MCBS score of ‘2’ to the SAT investigating cemiplimab.69 However, our current research shows a score of ‘4’ (from an ESMO-MCBS scorecard), which is based on a more recent publication.70
While this study provides insights into the contextualization process of SAT results, it is limited to SATs supporting initial approvals. While extensions of therapeutic indication(s) can in principle be based on SATs, this is rare and such applications are not included in our analysis. For an extension of indication, there is already existing knowledge on the benefits and risks of the concerned medicinal product due to the initial marketing authorization, which might impact decision making. In addition, we did not include withdrawals of SAT-based applications, as these numbers (n = 4) were too limited for a meaningful analysis. It can also be considered a limitation that we restricted our research to publicly available documents. However, we assume that all information relevant to the benefit–risk assessment is incorporated in the EPARs, as it is a reflection of the core documents included in an application, as well as in literature and/or protocols, the latter being available for most SATs. Another limitation is that confirmatory trials were ongoing for some of the products included in our study. The ESMO-MCBS score could, therefore, not yet be re-assessed for these products. Finally, we focused only on SAT-based applications submitted to the EMA. It would be interesting to compare regulatory decision making between agencies, such as the Food and Drug Administration and EMA.
In conclusion, we found that 18 medicinal products were approved for the treatment of solid tumors based on one or more SAT(s). For the majority of clinical trials or integrated analyses supporting these approvals, a threshold to be ruled out was (pre)specified, and most often accompanied by a sample size calculation based on an assumed ORR. However, a justification for the threshold and the assumed ORR could not be identified for all cases. The majority of applications included additional information for contextualization purposes. Determining the benefit–risk balance of medicinal products tested in SATs is challenging and benefit can be appreciated differently by various stakeholders. The clinical relevance of the treatment effects shown by medicinal products tested in SATs is dependent on the activity, its durability and context, especially if other therapies are available that provide benefit. As general recommendations, prespecifying and motivating a clinically relevant effect and aligning the sample size to that effect is of importance for regulatory decision making. External controls may facilitate in the contextualization process, but the limitations associated with such comparisons must be (adequately) addressed. Preferably, such comparisons should be preplanned. It is of relevance that information on these aspects is presented in the EPAR, as this provides transparency on regulatory decision making toward stakeholders. Finally, it is considered of value to further discuss among stakeholders what can be considered clinical benefit in the context of SATs and thus when approval on the basis of lower levels of evidence is justified. This is considered of importance, as SATs will likely continue to form the basis of authorization of part of the new medicinal products.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Disclaimer
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of, or reflecting the position of the EMA or one of its committees, working parties, or any of the national agencies.
Supplementary data
References
- 1.Jones D.S., Podolsky S.H. The history and fate of the gold standard. Lancet. 2015;385:1502–1503. doi: 10.1016/S0140-6736(15)60742-5. [DOI] [PubMed] [Google Scholar]
- 2.ICH Harmonised Tripartite Guideline Choice of Control Group and Related Issues in Clinical Trials E10. https://database.ich.org/sites/default/files/E10_Guideline.pdf Available at.
- 3.Sabbald B., Roland M. Understanding controlled trials. Why are randomized controlled trials important? BMJ. 1998;316:201. doi: 10.1136/bmj.316.7126.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Commission. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf Available at.
- 5.European Medicines Agency. Guideline on the Clinical Evaluation of Anticancer Medicinal Products. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-evaluation-anticancer-medicinal-products-man-revision-6_en.pdf Available at.
- 6.Tenhunen O., Lasch F., Schiel A., Turpeinen M. Single-arm clinical trials as pivotal evidence for cancer drug approvals: a retrospective cohort study of centralized European marketing authorization between 2010 and 2020. Clin Pharmacol Ther. 2020;108:653–660. doi: 10.1002/cpt.1965. [DOI] [PubMed] [Google Scholar]
- 7.Commission Regulation (EC) No 507/2006 of 29 March 2006 on the Conditional Marketing Authorisation for Medicinal Products for Human Use Falling within the Scope of Regulation (EC) No 726/2004 of the European Parliament and of the Council. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R0507&from=IT Available at.
- 8.European Medcines Evaluation. Kymriah Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/kymriah-epar-public-assessment-report_en.pdf Available at.
- 9.European Medcines evaluation. Yescarta Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/yescarta-epar-public-assessment-report_en.pdf Available at.
- 10.George S.L. Selection bias, phase II trials, and the FDA accelerated approval process. J Natl Cancer Inst. 2003;95:1351–1352. doi: 10.1093/jnci/djg070. [DOI] [PubMed] [Google Scholar]
- 11.Glassman R.H., Kim G., Kahn M.J. When are results of single-arm studies dramatic? Nat Rev Clin Oncol. 2020;17:651–652. doi: 10.1038/s41571-020-00429-1. [DOI] [PubMed] [Google Scholar]
- 12.Seymour L., Ivy S.P., Sargent D., et al. The design of phase II clinical trials testing cancer therapeutics: consensus recommendations from the clinical trial design task force of the national cancer institute investigational drug steering committee. Clin Cancer Res. 2010;16:1764–1769. doi: 10.1158/1078-0432.CCR-09-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George S.L. Response rate as an endpoint in clinical trial. J Natl Cancer Inst. 2007;99:98–99. doi: 10.1093/jnci/djk024. [DOI] [PubMed] [Google Scholar]
- 14.Carrigan G., Whipple S., Capra W.B., et al. Using electronic health records to derive control arms for early phase single-arm lung cancer trials: proof-of-concept in randomized controlled trials. Clin Pharmacol Ther. 2020;107:369–377. doi: 10.1002/cpt.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yap T.A., Jacobs I., Andre E.B., Lee L.J., Beaupre D., Azoulay L. Application of real-world data to external control groups in oncology clinical trial drug development. Front Oncol. 2022;11:695936. doi: 10.3389/fonc.2021.695936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLoughery E.P., Prasad V. The US Food and Drug Administration’s use of regular approval for cancer drugs based on single-arm studies: implications for subsequent evidence generation. Ann Oncol. 2018;29:527–529. doi: 10.1093/annonc/mdy008. [DOI] [PubMed] [Google Scholar]
- 17.Cherny N.I., Dafni U., Bogaerts J., et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28:2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 18.European Society for Medical Oncology. ESMO-Magnitude of Clinical Benefit Scale V1.1 Instructions. Available at Version-1-1-Instructions. Available at https://www.esmo.org/content/download/117394/2059186/1/ESMO-MCBS-Version-1-1-Instructions.pdf. Accessed April 9, 2022.
- 19.Cherny N.I., Sullivan R., Dafni U., et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) Ann Oncol. 2015;26:1547–1573. doi: 10.1093/annonc/mdv249. [DOI] [PubMed] [Google Scholar]
- 20.Shaw A.T., Gandhi L., Gadgeel S., et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ou S.H., Ahn J.S., De Petris L., et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661–668. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 22.Park K., Haura E.B., Leighl N.B., et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39:3391–3402. doi: 10.1200/JCO.21.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinrich M.C., Jones R.L., von Mehren M., et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol. 2022;21:935–946. doi: 10.1016/S1470-2045(20)30269-2. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman H.L., Russell J., Hamid O., et al. Avelumab in patients with chemotherapy-refractory metastatic merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Migden M.R., Khushalani N.I., Chang A.L.S., et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21:294–305. doi: 10.1016/S1470-2045(19)30728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migden M.R., Rischin D., Schmults C.D., et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 27.Shaw A.T., Kim D.W., Mehra R., et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwak E.L., Bang Y.J., Camidge D.R., et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oaknin A., Tinker A.V., Gilbert L., et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol. 2020;6:1766–1772. doi: 10.1001/jamaoncol.2020.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drilon A., Siena S., Dziadziuszko R., et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doebele R.C., Drilon A., Paz-Ares L., et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drilon A., Laetsch T.W., Kummar S., DuBois S.G., Lassen U.N., Demetri G.D. Efficacy of larotrectinib in TRK Fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon B.J., Besse B., Bauer T.M., et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 34.Jänne P.A., Yang J.C.H., Kim D.W., et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 35.Goss G., Tsai C.M., Shepherd F.A., et al. Osimertinib for pretreated EGFR Thr790MET-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–1652. doi: 10.1016/S1470-2045(16)30508-3. [DOI] [PubMed] [Google Scholar]
- 36.Abou-Alfa G.K., Sahai V., Hollebecque A., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gainor J.F., Curigliano G., Kim D.W., et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22:959–969. doi: 10.1016/S1470-2045(21)00247-3. [DOI] [PubMed] [Google Scholar]
- 38.Kristeleit R., Shapiro G.I., Burris H.A., et al. A phase I-II study of the oral PARP inhibitor rucaparib in patients with germline BRCA1/2-mutated ovarian carcinoma or other solid tumors. Clin Cancer Res. 2017;23:4095–4106. doi: 10.1158/1078-0432.CCR-16-2796. [DOI] [PubMed] [Google Scholar]
- 39.Swisher E.M., Lin K.K., Oza A.M., et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 40.Drilon A., Oxnard G.R., Tan D.S.W., et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med. 2020;383:813–824. doi: 10.1056/NEJMoa2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirth L.J., Sherman E., Robinson B., et al. Efficacy of selpercatinib in RET-altered thyroid cancer. N Engl J Med. 2020;383:825–835. doi: 10.1056/NEJMoa2005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modi S., Saura C., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekulic A., Migden M.R., Oro A.E., et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2021;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 45.Mariani L., Marubini E. Design and analysis of phase II cancer trials: a review of statistical methods and guidelines for medical researchers. Int Stat Rev. 1996;64:61–88. [Google Scholar]
- 46.Khan I., Sarker S.-J., Hackshaw A. Smaller sample sizes for phase II trials based on exact tests with actual error rates by rading-off their nominal levels of significance and power. Br J Cancer. 2012;20:1801–1809. doi: 10.1038/bjc.2012.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wason J.M., Jaki T. A review of statistical designs for improving the efficiency of phase II studies in oncology. Stat Methods Med Res. 2016;25:1010–1021. doi: 10.1177/0962280215588247. [DOI] [PubMed] [Google Scholar]
- 48.Taylor J.M.G., Braun T.M., Li Z. Comparing an experimental agent to a standard agent: relative merits of a one-arm or randomized two-arm Phase II design. Clin Trials. 2006;3:335–348. doi: 10.1177/1740774506070654. [DOI] [PubMed] [Google Scholar]
- 49.Senn S. Minimally Important Differences: Definitions, Ambiguities and Pitfalls. Available at https://www.ideal.rwth-aachen.de/wp-content/uploads/2014/02/Minimally-Important-Differences-v2.pdf.
- 50.Simon R., Wittes R.E., Ellenberg S.S. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69:1375–1381. [PubMed] [Google Scholar]
- 51.Oxnard G.R., Wilcox K.H., Gonen M., Polotsky M., Hirsch B.R., Schwartz L.H. Response rate as a regulatory end point in single-arm studies of advanced solid tumors. JAMA Oncol. 2016;2:772–779. doi: 10.1001/jamaoncol.2015.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.European Medicines Agency Gavreto Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/gavreto-epar-public-assessment-report_en.pdf Available at.
- 53.European Medicines Agency Rozlytrek Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/rozlytrek-epar-public-assessment-report_en.pdf Available at.
- 54.European Medicines Agency Zynyz Withdrawal Assessment Report. https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-zynyz_en.pdf Available at.
- 55.Ray E.M., Carey L.A., Reeder-Hayes K.E. Leveraging existing data to contextualize phase II clinical trial findings in oncology. Ann Oncol. 2020;31:1591–1593. doi: 10.1016/j.annonc.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Rahman R., Ventz S., McDunn J., et al. Leveraging external data in the design and analysis of clinical trials in neuro-oncology. Lancet Oncol. 2021;22:e456–e465. doi: 10.1016/S1470-2045(21)00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schröder C., Lawrance M., Li C., et al. Building external control arms from patient-level electronic health record data to replicate the randomized IMblaze370 control arm in metastatic colorectal cancer. JCO Clin Cancer Inform. 2021;5:450–458. doi: 10.1200/CCI.20.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishra-Kalyani P.S., Amiri Kordestani L., Rivera D.R., et al. External control arms in oncology: current use and future directions. Ann Oncol. 2022;33:376–383. doi: 10.1016/j.annonc.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 59.Pignatti F., Wilking U., Postmus D., Wilking N., Delgado J., Bergh J. The value of anticancer drugs – a regulatory view. Nat Rev Clin Oncol. 2022;19:207–215. doi: 10.1038/s41571-021-00584-z. [DOI] [PubMed] [Google Scholar]
- 60.Tibau A., Molto C., Borrell M., et al. Magnitude of clinical benefit of cancer drugs approved by the US Food and Drug Administration based on single-arm trials. JAMA Oncol. 2018;4:1610–1611. doi: 10.1001/jamaoncol.2018.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gyawali B., de Vries E.G.E., Dafni U., et al. Biases in study design, implementation, and data analysis that distort the appraisal of clinical benefit and ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) scoring. ESMO Open. 2021;6:100117. doi: 10.1016/j.esmoop.2021.100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.European Medicines Agency Libtayo Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/libtayo-epar-public-assessment-report_en.pdf Available at.
- 63.European Medicines Ageny Tagrisso Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/tagrisso-epar-public-assessment-report_en.pdf Available at.
- 64.European Medicines Agency Vitrakvi Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/vitrakvi-epar-public-assessment-report_en.pdf Available at.
- 65.European Medicines Agency Pemazyre Assessment Report. https://www.ema.europa.eu/en/documents/assessment-report/pemazyre-epar-public-assessment-report_en.pdf Available at.
- 66.BOM Voorstel PASKWIL-Criteria Niet-Gerandomiseerde Studies. https://medischeoncologie.nl/artikelen/2021/mei/editie-4/we-hebben-de-lat-hoog-gelegd Available at.
- 67.Fashoyin-Aje L.A., Mehta G.U., Beaver J.A., Pazdur R. The on- and off-ramps of oncology accelerated approval. N Engl J Med. 2022;387:1439–1442. doi: 10.1056/NEJMp2208954. [DOI] [PubMed] [Google Scholar]
- 68.Bloem L.T., Bot R.E., Mantel-Teeuwisse A.K., et al. Pre-approval and post-approval availability of evidence and clinical benefit of conditionally approved cancer drugs in Europe: a comparison with standard approved cancer drugs. Br J Clin Pharmacol. 2022;88:2169–2179. doi: 10.1111/bcp.15141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mulder J., Pasmooij A.M.G., Stoyanova-Beninska V., Schellens J.H.M. Breakthrough therapy-designated oncology drugs: are they rightfully criticized? Drug Discovery Today. 2020;25:1580–1584. doi: 10.1016/j.drudis.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 70.Cemiplimab ESMO-MCBS Scorecards. https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-scorecards/scorecard-360-1 Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.