Abstract
Meat, poultry, and seafood such as fish are a valuable source of protein, vitamins and minerals. Considering their high consumption in the human diet, it is necessary to study pollutants (such as PAHs) in them. This present study has focused on the PAHs level and probabilistic risk of health in meat, poultry, fish and related product samples by MSPE-GC/MS technique (magnetic solid-phase extraction with gas chromatography-mass spectrometry). The maximum mean of 16 PAH was detected in smoked fish samples (222.7 ± 13.2 μg/kg) and the minimum mean of 16 PAH was detected in chicken (juje) kebab (112.9 ± 7.2 µg/kg μg/kg). The maximum mean of 4PAHs was detected in tuna fish (23.7 ± 2.4 µg/kg) and the minimum mean of 4PAHs was seen in grilled chicken and sausage samples (non-detected). Our results showed the 4PAHs and B[a]P were lower than the EU (European Union) standard levels (these standard levels were 30 and 5 μg/kg, respectively). Furthermore, the correlation among the type and concentrations of PAHs congeners was investigated through cluster analysis by heat map and principal component analysis. The 90th percentile ILCR (incremental lifetime cancer risk) of PAH compounds in fish, poultry, meat and related products samples was 3.39E-06, which was lower than the maximum acceptable level of risk (10–4). Finally, the highest ILCR was related to hamburger (4.45E-06). Therefore, there is no risk in consuming these foods in Iran, but it is necessary to monitor PAHs concentration in different types of foods.
Keywords: Polycyclic aromatic hydrocarbons (PAHs), Meat, Fish, Poultry, Meat products, Health risk assessment, MSPE/GC–MS
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are analytes containing two or further fused aromatic rings, which exist as different isomers. These compounds can be found in the environment as compounds. They are normally colorless to light solids of yellow or white in their purest form [30]. These compounds, such as trace elements, phthalic acid esters, polychlorinated biphenyls (PCBs) and many other pollutants, are obtained from natural disasters like volcanic activities, forest fires, as well as in incomplete combustion processes of fossil fuels and metallurgical furnaces [18], Yaminifar et al.). PAH compounds have been assessed by the EFSA (European Food Safety Authority), JECFA (Committee for Food Additives), and SCF (Scientific Committee for Food) which have identified 16 PAHs in food that includes Benzo[a]pyrene (B[a]P), Naphthalene (Nap), Chrysene (Chr), Fluoranthene (Flu), Benzo[b]fluoranthene (BbF), Acenaphthylene (Acy), Fluorene (Fle), Acenaphthene (Ace), Benzo[ghi]perylene (BghiP), Anthracene (Ant), Pyrene (Pyr), Benzo[a]anthracene (BaA), Phenanthrene (Phe), Benzo[k]fluoranthene (BkF), Indeno[1,2,3-cd]pyrene (IcdP) and Dibenzo[a,h]anthracene (DahA) (Authority 2008, Singh &Agarwal 2018). The IARC (International Agency for Research on Cancer) categorized three PAHs (BaA, DahA and B[a]P) as group 2A (probably carcinogenic to humans) and 3 PAH (BbF, B(k)F and IcdP) as group 2B (possibly carcinogenic to humans).
Raw foods can be contaminated with PAH compounds through environmental pollution (air, soil and water) and contamination of the cooked food is either through the raw food or during the cooking process. For this purpose, during food preparation, numerous factors can form PAHs such as the method of cooking (frying, roasting, and baking), type of fuel (wood, gas, charcoal, and electric power supplies), the temperature of power supply, oil drips on the flame, food fat, time of cooking, and proximity to or direct contact between the flame and food, all affecting the amount of PAH compounds on foods such as kebabs and fish (Singh &Agarwal 2018).
PAHs can be seen in most tissues. These compounds tend to be deposited in kidney and liver tissues, but small amounts are also kept in adrenal glands and spleen. These compounds can be metabolized in the body into more or less toxic compounds. Animal studies show that PAH does not persist in various tissues, and most of these compounds are excreted in the feces and urine after a few days [2], Bansal &Kim 2015).
PAHs can be most commonly found in oils, fats, meat and smoked and non-smoked meat products (meat, fish and oysters), condiments (spices), fruits and vegetables, dairy products, and cereals. The permissible limits of BaP and ∑PAHs (BaA, Chr, B[a]P, BbF) in meat and products of meat are 5 and 30 μg/kg, respectively [17, 22].
Health risk assessment evaluated the health effects of exposure to dangerous materials [13, 28]. Researchers have tried to specify the health risks of PAH in food products by measuring intake rates in many countries. In this connection, research has been conducted on the assessment of the probabilistic risk to human health by the simulation of Monte Carlo for polycyclic aromatic hydrocarbons in cereal products [14], in baby food samples [23], in edible mushrooms [34], in yogurt and butter [17], and in samples of milk and milk powder [33] in commercial samples of coffee and tea [29].
Several studies have reported the probabilistic risk assessment of PAH, in baby food and infant formulae[23], in mushrooms [34], in commercial samples of dairy products [17], in milk powder [33], and in coffee and tea [29]. Accordingly, the mean dietary exposures for grilled and fried meats samples from urban centers in Shandong of China were reported to be 120 and 74.8 ng/kg bw/day, respectively[11]. Jane et al. (2018) reported daily dietary PAH exposures of 634.8 ng/day for meats in the US population.
In Iran, the carcinogenic risk of sausage and burger were reported to be 1E-6 to 1E-4 (at the tolerable risk levels) and > 1E-4 (considerable risk levels), respectively [31].
The most commonly applied techniques for the measurement of these contaminants are GC/MS and HPLC/fluorescence. A technique of GC/MS provides greater selectivity than an HPLC/fluorescence technique and a more precise quantification as a result of the application possibility of categorized compounds of internal standard [10, 22, 26].
During current years, many techniques of sample pretreatment (like SPE (solid-phase extraction), SPME (solid-phase micro-extraction) and SBSE (stir-bar sportive extraction)) have been applied for PAH compounds separation and pre-concentration in types of food [15, 16]. The adsorbents must be located in a cartridge of SPE whose task that is hard, that is one drawback of SPE method. Moreover, the disadvantages of SBSE method are manual operation and memory effects. Concerning SPME, adsorbents from the aqueous phase are gathered using clarification or centrifugation, which might be consuming time (especially in great sample volumes). Additionally, the SPME fibers are comparatively high price and the coatings of the polymer are extremely fragile and delicate [7, 12, 19]. To solve the mentioned problem, CNTs (carbon nano tubs) have been improved with MP (magnetized particles) using chemical procedures applied as MSPE adsorbents [28, 29, 33].
Past studies have focused on one food item (like meat or fish) and in most cases, the carcinogenic risk assessment has not been performed. Therefore, due to the lack of a comprehensive study on the presence of PAH compounds in meat, poultry, fish and related products in Iran and the world, and also considering the high proportion of meat, poultry, fish and related products in the Iranian food basket, it is essential to measure PAH levels in these foods. Therefore, the purposes of this research are as follows: (1) using a reliable, fast and simple procedure (MSPE-GC/MS method) for the determination of PAH compounds in meat, poultry, fish and related product samples (2) using the cancer potency of BaP analyte as a marker to evaluate the potential risk of human health produced by the intake of PAHs (3). Chemometric analysis was also applied to assess the correlation between PAH compounds in meat, poultry, fish and related products.
Materials and methods
Sampling and survey
In our research, 108 samples of 9 types (12 samples of each type) including grilled kebab (containing red meat), Juje kebab, grilled chicken, fried chicken, hamburger, smoked rainbow trout, sausage, boiled chicken and tuna from Tehran-level supermarket was purchased.
Reagents and standards
The mixed standard including sixteen mentioned PAH compounds of were bought from Supel Co (Bellefonte, PA, USA). After that, the solutions of standard, working solutions and internal standards were consulted based on earlier studies [10, 16, 17]. Multi-walled-carbon-nanotubes (MWCNTs) were obtained from Nanoshel (Panchkula, India, diameter 30–60 nm and length 5.0–30 µm) and made operational (magnetic) according to our earlier research [10, 22, 33]. All other chemicals and solvents such as methanol, dichloromethane, biphenyl (as internal standard), acetonitrile, HCl and NaCl were obtained from Merc Co (USA) with the grade of analytical-reagent.
Preparation of sample and analysis
The sample was prepared according to a main three-part method designed by Samiei et al. with some modifications that include the clean-up of the sample, adsorption of PAHs compounds, and desorption of PAHs compounds from the adsorbent [31].
Sample clean-up: Five grams of each sample (frozen) was weighed and 1 mL internal standard (biphenyl 0.05 µg/mL in methanol) was added, and then the mixture was homogenized (for 15 min) with a pestle and mortar. Afterward, the extraction solution containing 7.5 mL methanol-acetonitrile (30%: v/v) and 7.5 mL potassium hydroxide (1 M) was added. In the next step, the mentioned mixture was sonicated in an ultrasonic bath at 40 °C for seven minutes. The mixture (for ten minutes at 8944 × g) was centrifuged and using the freeze lipid filtration method, the fat was removed from the samples. Finally, by adding hydrochloric acid (1 M), the pH of the mixture was adjusted to 6.5.
Adsorption of the analytes: The phase of aqueous was moved to another vessel, after the primary clean-up. In the next step, 500 mg NaCl and ten milligrams of the prepared adsorbent (MWCNT-Fe3O4) were added to the solution which was mixed (vigorously) for five minutes. Finally, by an external magnet, the magnetic adsorbent was gathered to the side of the vial.
Desorption of the analytes from adsorbent: The supernatant was discarded and five milliliters of dichloromethane was added to elute the analytes from the adsorbent with vigorous vortex-mixing for three minutes. Afterward, by an external magnet, the magnetic adsorbent was gathered to the side of the vial. This step was repeated and then the solvent was moved to another vial. It was then evaporated to dryness at 30 °C by a gentle stream of N2 gas. The residue was re-dissolved in 50 mL solution of acetonitrile- methanol (50:50 v/v) and was then vigorously shaken by vortex-mixer for one minute. Lastly, one microliter of the solution was injected into the GC-MS equipment.
Conditions of analytical and instrumental
The brand of GC device was Agilent model: 6890 with a mass detector model: 5973 (PaloAlto, CA, USA). The column of the capillary was DB-5 ms (0.25 µm film thickness, 30 m and 0.25 mm i.d.,). Other analytical and instrumental conditions were set according to earlier studies [10, 16, 17].
Evaluation of the analytical method
The optimum conditions for the examination were the calibration curves (LOQ–150.000 µg/kg) considering the correlation coefficient of 0.988–0.996. The LOQ and LOD of PAHs were 0.14 − 0.240 and 0.035 − 0.080 µg/kg, respectively. In addition, the accuracy, feasibility and reliability of the technique were assessed according to previous research [10, 16, 17]. Additionally, interday precision values for all PAH analytes were lower than 8.9, and the recorded values were 5 − 21% and 4.6 − 11.9% for reproducibility and repeatability, respectively. The estimated recovery of each PAH analyte ranged from 94.2 to 103.8% (Table 1). Finally, there was no peak of interference in the area of PAH compounds and internal standards.
Table 1.
Linear range (µg/Kg), Limit of detection (LOD; µg/Kg), limit of quantification (LOQ; µg/Kg), Coefficient of estimation (r2), repeatability relative standard deviation (RSDr; n = 6), and reproducibility relative standard deviation (RSDR; n = 6)
| Target compound | Linear range (µg/Kg) | Limit of detection (LOD) (µg/Kg) | Limit of quantification (LOQ) (µg/Kg) | Coefficient of estimation (r2) | Recoveries (%) | Repeatability (RSDr) (%) | Reproducibility (RSDR) (%) |
|---|---|---|---|---|---|---|---|
| NA | LOQ–150.000 | 0.035 | 0.14 | 0.988 | 94.9 | 8.9 | 5, 9, 15 |
| Ace | LOQ–150.000 | 0.035 | 0.14 | 0.990 | 94.2 | 11.9 | 9, 14, 21 |
| Ac | LOQ–150.000 | 0.050 | 0.151 | 0.992 | 98.1 | 4.6 | 6, 9,12 |
| F | LOQ–150.000 | 0.035 | 0.14 | 0.996 | 96.5 | 6.7 | 10, 11, 18 |
| Pa | LOQ–150.000 | 0.050 | 0.151 | 0.989 | 98.1 | 8.1 | 12, 15, 17 |
| A | LOQ–150.000 | 0.080 | 0.240 | 0.993 | 103.8 | 10.3 | 6, 9, 13 |
| Fl | LOQ–150.000 | 0.034 | 0.14 | 0.991 | 101.4 | 6.8 | 10, 17, 10 |
| P | LOQ–150.000 | 0.055 | 0.165 | 0.988 | 95.5 | 9.9 | 13, 9, 12 |
| BaA | LOQ–150.000 | 0.035 | 0.14 | 0.989 | 97.7 | 7.5 | 16, 10, 18 |
| Ch | LOQ–150.000 | 0.035 | 0.14 | 0.995 | 102.1 | 8.5 | 9, 10, 12 |
| BbF | LOQ–150.000 | 0.060 | 0.181 | 0.991 | 99.7 | 7.2 | 8, 7, 13 |
| BkF | LOQ–150.000 | 0.050 | 0.151 | 0.988 | 99.6 | 11 | 14, 15, 20 |
| BaP | LOQ–150.000 | 0.07 | 0.220 | 0.989 | 95.5 | 8.3 | 17, 13, 9 |
| IP | LOQ–150.000 | 0.035 | 0.14 | 0.993 | 96.3 | 8.1 | 15, 9, 18 |
| DhA | LOQ–150.000 | 0.055 | 0.165 | 0.996 | 101.2 | 9.3 | 14, 16, 10 |
| BgP | LOQ–150.000 | 0.050 | 0.151 | 0.992 | 99.1 | 8.8 | 12, 15, 18 |
| RSDR of 1 µg/Kg, 5 µg/Kg, and 10 µg/Kg standard value (n = 6) | |||||||
Estimate of dietary exposure
Carcinogenic risk for PAHs mixture compounds was represented using the toxicity equivalency factor (TEF) and BaP equivalent dose (BaPeq) (Table 2) [22]. Therefore, the toxicity equivalent quotient was determined by the sum of TEF multiplied by each PAH concentration based on Eq. (1) [29].
| 1 |
where, Ci is the individual PAH concentration in dissimilar kinds of meat and meat products. The daily dietary PAH exposure level (ED) for each group was determined based on Eq. (2).
| 2 |
Table 2.
PAHs and their toxic equivalent factors (TEFs)
| PAHs | TEF | PAHs | TEF |
|---|---|---|---|
| Benzo(a)pyrene (BaP) | 1 | Anthracene (A) | 0.01 |
| Dibenz(a,h)anthracene (DahA) | 1 | Naphthalene (NA) | 0.001 |
| Benzo(k)fluoranthene (BkF) | 0.1 | Acenaphthylene (AC) | 0.001 |
| Indeno(l,2,3-cd)pyrene (IcdP) | 0.1 | Acenaphthene (ACE) | 0.001 |
| Benz(a)anthracene (BaA) | 0.1 | Phenanthrene (PHE) | 0.001 |
| Benzo(b)fluoranthene (BbF) | 0.1 | Fluorine (FLO) | 0.001 |
| Chrysene (CHR) | 0.01 | Pyrene (PYR) | 0.001 |
| Benzo(g,h,i)perylene (BghiP) | 0.01 | Fluoranthene (FL) | 0.001 |
Based on the mentioned equation, IRi represents the ingestion rate of juje kebab, grilled chicken, fried chicken, kobide kebab, smoked fish, tuna fish, hamburger, sausage, and boiled chicken set as 18.7, 10.8, 10.6, 9.7, 13.8, 7.9, 4, 7.9, 8.7 g/day. per day (g/d), respectively. The meat consumption per person per day was obtained from food frequency questionnaires (FFQs) which were distributed among the citizens of Tehran.
| 3 |
where, ILCR represents contact with PAHs in food and CSF is the oral carcinogenic slope factor for inorganic BaP compound (7.3 per mg/kg/d) [29]. Furthermore, EDi shows exposure duration (70 years), BW the body weight (70 kg), EF the exposure frequency (350 days/years), and AT the average life expectancy (365 × 70) [37]. To improve the accuracy of health risk assessment, the probabilistic statistical analysis was used to compute the uncertainty of the contained parameters by the Monte Carlo simulation (MCS) method.
Statistical analysis
The development of risk assessment methods is connected with an uncertainty that may take place owing to uncertainty in the parameter’s measurement. Thus, the probabilistic examination is needed to obtain a deeper understanding of the result. In the exposure assessment to obtain the randomness of a model’s uncertainty, the statistical uncertainty of Monte Carlo simulations was used according to the USEPA (United States Environmental Protection Agency) methodology in Oracle Crystal Ball (Version. 11.1.2.4.600) [33] and all the statistical analyses were performed by SPSS (Ver 24.0). When PAH compounds were undetectable, 1/2 LOD (half of LOD) was applied to determine the mean concentration. PCA (principal component analysis) was determined the correlation between the features [24]. Heat map analysis was plotted and visualized was performed with the software Clustvis, (https://biit.cs.ut.ee/clustvis/) online program package with Euclidean distance.
Results and discussion
The PAHs concentration in types of meat and related products
The 16 PAH concentration is displayed in Table 3. The EU first considered the B[a]P maximum concentration in different foods (5 µg/kg for smoked meat and meat products) and 4PAHs maximum level in various foods (30 µg/kg for smoked meat and related products).
Table 3.
PAHs in meat, poultry, fish and related product samples collected from Iran (μg/kg)
| PAHs(µg/kg) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAP | ACY | ACE | FLO | PHE | ANT | FLA | PYR | BaA | CHR | BbF | BkF | BaP | DahA | BghiP | IcdP | ∑4 PAHs | ∑16 PAHs | ||
| Mean ± SD | |||||||||||||||||||
| Meat, Poultry, Fish and related products | Juje kebab | 38.4 ± 1.3 | 20.8 ± 1.6 | 19.2 ± 1.4 | 14.8 ± 1.1 | 0.9 ± 0.2 | 6.5 ± 1.5 | 7.5 ± 1.2 | 5.8 ± 1.4 | 2.1 ± 0.6 | 1.3 ± 0.8 | n.d | n.d | n.d | n.d | n.d | n.d | 3.4 ± 0.8 | 112.9 ± 7.2 |
| Koobide kebab | 63.4 ± 5.4 | 8.5 ± 1.3 | 23.7 ± 3.2 | 6.9 ± 0.6 | 4.6 ± 1.6 | 4.7 ± 1.7 | 3.9 ± 0.9 | 3.8 ± 0.6 | n.d | 5.9 ± 1.2 | n.d | n.d | n.d | n.d | n.d | n.d | 5.9 ± 0.9 | 124.4 ± 9.1 | |
| Tuna | 64.9 ± 4.9 | 17.4 ± 1.3 | 21.1 ± 2.2 | 7.6 ± 1.6 | n.d | 8.9 ± 1.9 | 8.6 ± 1.3 | 4.6 ± 1.5 | 19.1 ± 1.4 | 4.6 ± 1.8 | n.d | n.d | n.d | n.d | n.d | n.d | 23.7 ± 2.4 | 156.7 ± 10.0 | |
| Boiled chicken | 42.6 ± 2.2 | 21.4 ± 1.4 | 34.6 ± 3.6 | 10.9 ± 1 | 17.1 ± 1.8 | 14.9 ± 1.5 | 4.9 ± 1.0 | 3.8 ± 0.7 | 1.4 ± 0.4 | 4.9 ± 1.9 | n.d | n.d | n.d | n.d | n.d | n.d | 6.3 ± 1.2 | 144 ± 8.2 | |
| Grilled chicken | 85.5 ± 3.5 | 19.9 ± 2.8 | 35.4 ± 2.3 | 10.9 ± 1.5 | 7.9 ± 1.2 | 11.5 ± 1.2 | 5.9 ± 1.3 | 8.7 ± 1.1 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | nd | 183 ± 13.4 | |
| Hamburger | 53.3 ± 4.3 | 6.7 ± 1.1 | 38.6 ± 2.1 | 2.9 ± 0.9 | 2.9 ± 0.6 | 1.7 ± 0.2 | 4.6 ± 0.6 | 5.4 ± 1.5 | 0.4 ± 0.1 | 1.5 ± 0.05 | n.d | n.d | n.d | n.d | n.d | n.d | 1.9 ± 0.1 | 115.7 ± 9.1 | |
| Fried chicken | 75.4 ± 5.4 | 17.7 ± 1.4 | 29.8 ± 2.7 | 12.4 ± 2.2 | 1.6 ± 0.5 | n.d | 7.3 ± 1.1 | 5.1 ± 1.2 | 0.6 ± 0.1 | 0.7 ± 0.21 | n.d | n.d | n.d | n.d | n.d | n.d | 0.81 ± 0.1 | 148.9 ± 6.4 | |
| Sausage | 73.5 ± 3.5 | 23.9 ± 3.1 | 41.5 ± 32.5 | 12.6 ± 2.3 | 19.8 ± 2.9 | 10.6 ± 1.5 | 15.8 ± 1.8 | 28.9 ± 2.5 | n.d | n.d | n.d | n.d | n.d | n.d | n.d | n.d | nd | 205.4 ± 10.4 | |
| Smoked fish | 105.8 ± 7.3 | 10.6 ± 1.7 | 30.7 ± 2.4 | 35.5 ± 2.5 | 3.5 ± 0.4 | 5.8 ± 1.1 | 8.5 ± 1.5 | 9.4 ± 1.1 | 0.5 ± 0.1 | 15.6 ± 5.2 | n.d | n.d | n.d | n.d | n.d | n.d | 15.6 ± 2.4 | 222.7 ± 13.2 | |
In all samples, NAP was higher than other compounds (from 38.4 ± 1.3 in juje kebab to 105.8 ± 7.3 in smoked fish µg/kg). In all samples, BghiP, BbF, IcdP, B[a]P, BkF and DahA were not detected (nd). The maximum mean of 4PAHs was detected in tuna fish (23.7 ± 2.4 µg/kg) and the minimum mean of 4PAHs was seen in grilled chicken and sausage samples (nd), which could be due to fish contamination (with PAH compounds) used in this process or storage containers. Our results showed that the BaP and 4PAHs were lower than the EU standard levels, which can verify the hygienic conditions of these products in terms of these pollutants. The mean level of 16 PAH compounds in the samples of smoked fish (222.7 ± 13.2 µg/kg) was greater than the other samples and the minimum mean level of 16 PAH compounds was measured in juje kebab (112.9 ± 7.2 µg/kg). The total PAHs rank in the samples was juje kebab < hamburger < koobide kebab < boiled chicken < fried chicken < tuna < grilled chicken < sausage < smoked fish. Higher levels of this contaminant in smoked fish can be due to smoking, which commonly transfers PAHs compounds to food (Oz &Yuzer 2016). The lower PAH levels in grilled chicken (juje kebab) can be due to lower fat content and its earlier cooking [31].
Samiei et al. measured sixteen PAHs in burgers and sausages in Iran (2020) and stated the mean level of 16 PAH compounds in sausage and hamburger was 10.18 − 29.85 and 8.08 − 29.55 μg/kg, respectively, which were lower than the value found in this research [31]. Gorji et al. assessed 16 PAHs in 4 types of Kebab (grilled meat) in Tehran, Iran, and reported that the mean level of ƩPAHs was 7.37 − 17.94 μg/kg and concentration of B[a]P in the samples (0.28–5.81 μg/kg) was higher than that in this research [10]. Husseini et al. measured 4 PAHs in the traditional grilled chicken of Lebanese, and the levels of 4 PAHs was in the range 1.52 to 49.9 μg/kg, which was higher than that found in this research [9]. Terzi et al. measured B[a]P in Turkish döner kebab samples and reported that mean levels of B[a]P was 5.7 μg/kg for gas-fire-cooked meat samples and 24.2 μg/kg for charcoal-fire-cooked meat samples, which was higher than our values [36]. Chen et al. measured B[a]P in various food (treated with heat) from China and reported that B[a]P contents in twelve animal-based foods were larger than the maximum allowable level of Chinese (5 µg/kg) and the highest concentration was 19.75 µg/kg, that was higher than our research [5]. Cho et al. assessed B[a]P in smoked food products in Korea and reported that the average B[a]P level was 0.45 μg/kg and the maximum level of B[a]P was 2.87 μg/kg in the smoked salmon product, that was higher than our results (Cho &Shin 2012). Muyela et al. assessed B[a]P level in smoked and oil-fried fish and reported that variable B[a]P levels were 4.17 − 11.26 µg/kg in oil-fried fish and 7.46 to 18.79 µg/kg in smoked fish, higher than the values of our research [25]. Lee et al. assessed B[a]P in some samples of ready-to-eat food products (Korea) and reported that mean level of B[a]P in these foods was 0.64 μg/kg, higher than out values (Lee &Shin 2019). Oz et al. measured 16 PAHs in beef steak and reported that the total PAH values of samples were nd to 2.63 μg/kg (lower than our outcomes), and B[a]P level was up to 0.29 μg/kg, higher than our findings (Oz &Yuzer 2016). Mastanjević et al. measured 16 PAHs in the traditional dry fermented sausage and stated at the end of the production, the total concentration of the 16 PAHs was 124–679 μg/kg, which was higher than our results [21]. Zachara et al. measured 16 PAHs in smoked fish and meat products and reported that the highest level of B[a]P was 36,510 μg/kg and total level of 4 PAHs 73,010 μg/kg, higher than our findings [40]. Duedahl-Olesen et al. measured 16 PAHs of Danish smoked meat and fish products and found that the total PAHs was 24 μg/kg in smoked meat products (salami) and the highest amount was 64 μg/kg in bacon, while in smoked mackerel prepared with an electric oven it was 22 μg/kg and in herring smoked fish prepared with direct smoking the value was 1387 μg/kg; except for the last case, the other values were lower than our findings. The B[a]P concentration in all samples was below the maximum of EU standard levels that was similar to our research [8]. Santos et al. measured B[a]P levels in traditional smoked meat products (in Portuguese) and reported that B[a]P content was 0.21 − 1.00 μg/kg, standing higher than our research [32].
Among the reasons for the increase in the pollutants in fish, poultry, meat and related products are environmental pollution of food of agricultural origin by soil, water and air contaminated and contamination of food of animal origin by animal feed and contaminated water. Contamination can also occur during the food preparation process such as cooking (especially on charcoal and direct flame). Also, during food preparation, numerous factors are involved in the PAHs formation including the method of cooking (frying, roasting, and baking), type of fuel (wood, gas, charcoal, and electric power supplies), the temperature of power supply, oil drips on the flame, food fat, time of cooking, and proximity to or direct contact between the flame and food, all of which affect the number of pH compounds on foods such as kebabs and fish [10, 22, 26].
Daily exposure estimation of PAHs
To assess the carcinogenic risks from PAHs presence in meat, poultry, fish and related products, the EDi and ILCR for children and adults via ingestion exposure were calculated according to the approach presented by the USEPA by a Simulation of Monte Carlo. According to Table 4, the rank order of the EDi (P90) was hamburger (38.06 mg/kg/day) > smoked fish (8.12 mg/kg/day) > juje kebab (6.22 mg/kg/day) > koobide kebab (5.29 mg/kg/day) > sausage (2.37 mg/kg/day) > tuna (1.65 mg/kg/day) > boiled chicken (1.07 mg/kg/day) > grilled chicken (0.81 mg/kg/day) > fried chicken (0.05 mg/kg/day). The estimated the EDi (P90) of all samples was 63.64 mg/kg/day. According to the recommendations of the Joint Expert Committee on Food Additives (2005), the mean exposures of BaP were considered below 4 ng/kg bw/day and daily intake of 280 ng per person. Additionally, for average dietary consumers, the European Food Safety Authority has advised a mean BaP intake of 235 ng per person and for high dietary consumers 389 ng per day [1]. The rank order of simulated ILCR (P90) for PAHs was hamburger (4.45E-06) > smoked fish (9.43 E -07) > juje kebab (7.10 E -07) > koobide kebab (6.06 E -07) > sausage (2.75 E -07) > tuna (1.89 E -07) > boiled chicken (1.24 E -07) > grilled chicken (9.43 E -08) > fried chicken (5.98 E -09). Regarding the overall uncertainty in the lifetime cancer risk model, a one-in-a-million chance of additional human cancer over a 70-year lifetime from exposure to PAHs (ILCR = 10 -6) is as an acceptable level, while a one-in-a-ten-thousand chance from exposure to PAHs (ILCR = 10 − 4) or greater is a serious level. Our results showed that the hamburger had the highest contribution rate of ILCR, followed by the smoked fish. Likewise, the lowest contribution rate of ILCR was found in fried chicken. The ILCR (P90) to total meat, fish, poultry and related products was 3.39E-06, which was lower than the level of acceptable risk (Table4). Xia et al. assessed 16 PAH in 25 types of 7 foods categories in China and reported that the average values of the ILRC for all groups of people were in the range of 10–6 − 10–5, indicating high potential carcinogenic risk (10–6), lower than the level of priority risk (10–4) [38].
Table 4.
Uncertainly analysis for EDi (mg/kg/d) and ILCRi of PAHs in meat, poultry, fish and related product samples
| EDi (mg/kg/d) | ILCRi | ||||
|---|---|---|---|---|---|
| P 50% | P 90% | P 50% | P 90% | ||
| Meat, Poultry,Fish and related products | Juje kebab | 1.58 | 6.22 | 1.78E-07 | 7.1E-07 |
| Koobide kebab | 0.51 | 5.29 | 5.81E-08 | 6.06E-07 | |
| Tuna | 0.1 | 1.65 | 1.16E-08 | 1.89E-07 | |
| Boiled chicken | 0.32 | 1.07 | 3.64E-08 | 1.24E-07 | |
| Grilled chicken | 0.037 | 0.81 | 4.25E-09 | 9.43E-08 | |
| Hamburger | 2.97 | 38.06 | 3.38E-07 | 4.45E-06 | |
| Fried chicken | 0.001 | 0.05 | 2.04E-10 | 5.98E-09 | |
| Sausage | 0.714 | 2.37 | 8E-08 | 2.75E-07 | |
| Smoked fish | 2.07 | 8.12 | 2.33E-07 | 9.43E-07 | |
| total | 8.302 | 63.64 | 9.36E-07 | 3.39E-06 | |
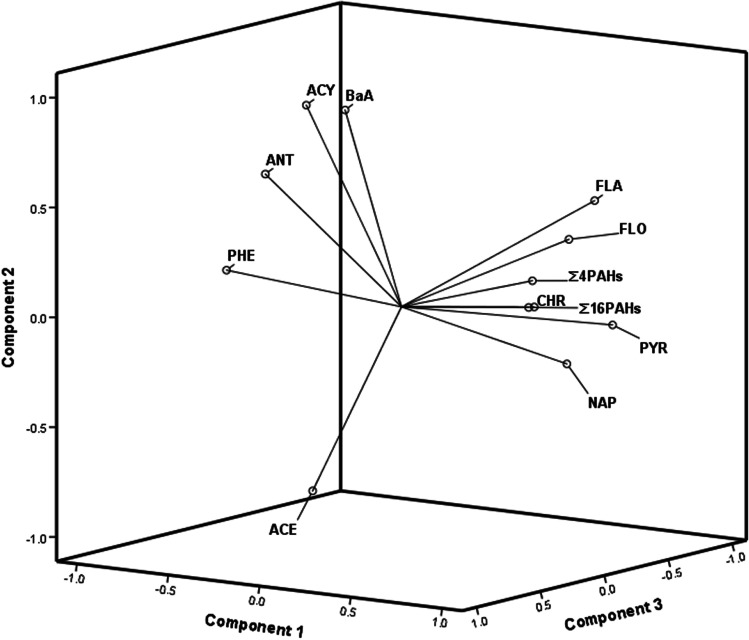
Multivariate data analysis
Investigation of the correlation between the PAHs in meat, poultry, fish and related product samples was performed using PCA. The prioritization of the principal components is based on the coverage of most outcomes of the parameters with the most internal correlations in the data. The result of the first three factors showed 100% of the variance for the data set of meat, fish, poultry and related products samples. Sequentially, the classification analyses by PCA presented PAHs discrimination according to the level and type of contaminant in samples (Fig. 1). The PCA results illustrated the predicted target position and scale using the correlation of the ACY, PNAP, BaA, FLO, ANT, PHE, ACE, PYR, FLA, CHR, ∑4 PAHs and ∑16 PAHs in the samples. In fish, meat and related products samples, the NAP, FLO, ANT, FLA, PYR, ∑4 PAHs and ∑16 PAHs at nearby positions and scale had a strong correlation. While, ACE detected at separately position. The ACY, ANT, PHE and BaA concentrations converged on a particular set in the PCA with a strong correlation (Fig. 1).
Fig. 1.
Principal component analysis loading plot of PAHs in meat, poultry, fish and related products
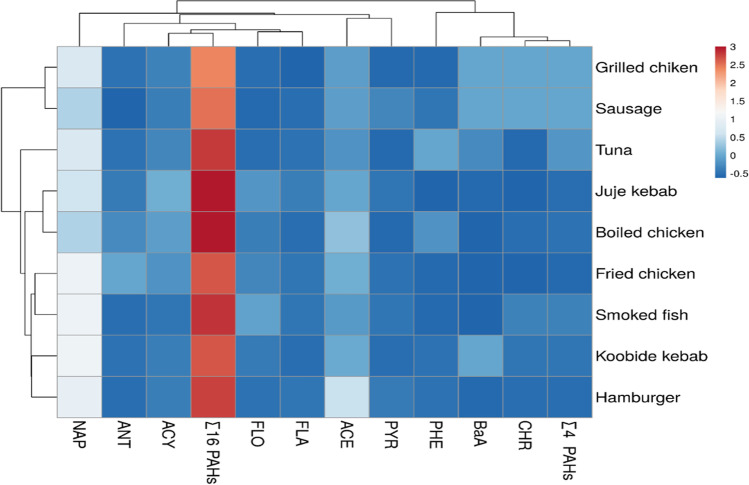
A heat map was used to provide an accurate classification of the data. Further details are shown in Fig. 2 on the analysis process in PAHs in samples by cluster dendrogram. In general terms, for all sites, the closer distance displayed a higher correlation coefficient. In all samples, two distinct clusters were observed. The first cluster includes PHE, BaA, CHR and ∑4 PAHs, and other parameters were fallen in the second cluster. Because of the diverse PAH compounds in the samples, these analytes may be divided into groups based on their amounts, and linked sections can be used to construct easy diagnoses across samples. The structured association and cluster heat map have shown that the smallest and largest data have a similar impression.
Fig. 2.
Heat map of PAHs in meat, poultry, fish and related product samples
Conclusion
This study assessed PAH levels in various samples of fish, poultry, meat and related products collected from the market of Tehran, Iran. MSPE-GC / MS method with recovery higher than 94.4% was used to identify 16 PAH compounds. In all samples, BaP and 4PAHs were below the EU standard for smoked fish and smoked meat products. The total rank of PAHs in the samples was juje kebab < hamburger < koobide kebab < boiled chicken < fried chicken < tuna < grilled chicken < sausage < smoked fish. Our results demonstrated the differences and similarities among the PAHs congeners in fish, poultry, meat and related products by Heat-map and PCA analysis. The estimated daily exposure (P90) of PAHs in dissimilar kinds of fish, meat and related products was 63.64 mg/kg/day. Further, uncertainty analysis for cancer risk of PAHs revealed the ILCR (p90) values to total fish, poultry, meat and related products at 3.39E-06, which was lower than the maximum level of acceptable risk (10–4). However, it is essential that fish, poultry, meat and related products be monitored regularly so that the incidence of these contaminants does not exceed the standard levels.
Acknowledgements
This research was jointly supported by Tehran University of Medical Sciences, Tehran, Iran.
Authors’ contributions
Nabi Shariatifar: Conceptualization, Supervision, Design of study, Writing- Reviewing and Editing. Fariba Khalili: Data curation, Writing- Original draft preparation. Mohammad Hadi Dehghani: Visualization, Investigation. Kamyar Yaghmaeian: Writing- Reviewing and Editing, Software, Ramin Nabizadeh Nodehi: Writing- Reviewing and Editing, Methodology, and Validation, Software, Methodology, Mojtaba Moazzen: Software, Methodology, Mehdi Yaseri: Validation, Methodology, Validation.
Data availability
All data generated or analyzed during current study are included in this article. The datasets analyzed during this study are available from the corresponding author on reasonable request.
Declarations
Competing interests
There is no competitive interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nabi Shariatifar, Email: nshariatifar@ut.ac.ir.
Mohammad Hadi Dehghani, Email: hdehghani@tums.ac.ir.
References
- 1.Abramsson-Zetterberg L, Darnerud PO, Wretling S. Low intake of polycyclic aromatic hydrocarbons in Sweden: Results based on market basket data and a barbecue study. Food Chem Toxicol. 2014;74:107–111. doi: 10.1016/j.fct.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Alomirah H, Al-Zenki S, Al-Hooti S, Zaghloul S, Sawaya W, Ahmed N, Kannan K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control. 2011;22:2028–2035. doi: 10.1016/j.foodcont.2011.05.024. [DOI] [Google Scholar]
- 3.Authority EFS Polycyclic aromatic hydrocarbons in food-scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008;6:724. doi: 10.2903/j.efsa.2008.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal V, Kim K-H. Review of PAH contamination in food products and their health hazards. Environ Int. 2015;84:26–38. doi: 10.1016/j.envint.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y-H, Xia E-Q, Xu X-R, Li S, Ling W-H, Wu S, Deng G-F, Zou Z-F, Zhou J, Li H-B. Evaluation of benzo [a] pyrene in food from China by high-performance liquid chromatography-fluorescence detection. Int J Environ Res Public Health. 2012;9:4159–4169. doi: 10.3390/ijerph9114159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho H-K, Shin H-S. Analysis of benzo [a] pyrene content from smoked food products in Korea. Food Sci Biotechnol. 2012;21:1095–1100. doi: 10.1007/s10068-012-0142-x. [DOI] [Google Scholar]
- 7.Dobaradaran S, Akhbarizadeh R, Mohammadi MJ, Izadi A, Keshtkar M, Tangestani M, Moazzen M, Shariatifar N, Mahmoodi M. Determination of phthalates in bottled milk by a modified nano adsorbent: presence, effects of fat and storage time, and implications for human health. Microchem J. 2020;159:105516. doi: 10.1016/j.microc.2020.105516. [DOI] [Google Scholar]
- 8.Duedahl-Olesen L, White S, Binderup M-L. Polycyclic aromatic hydrocarbons (PAH) in Danish smoked fish and meat products. Polycycl Aromat Compd. 2006;26:163–184. doi: 10.1080/10406630600760527. [DOI] [Google Scholar]
- 9.El Husseini M, Makkouk R, Rabaa A, Al Omar F, Jaber F. Determination of polycyclic aromatic hydrocarbons (PAH4) in the traditional Lebanese grilled chicken: implementation of new, rapid and economic analysis method. Food Anal Methods. 2018;11:201–214. doi: 10.1007/s12161-017-0990-3. [DOI] [Google Scholar]
- 10.Gorji MEh, Ahmadkhaniha R, Moazzen M, Yunesian M, Azari A, Rastkari N. Polycyclic aromatic hydrocarbons in Iranian Kebabs. Food Control. 2016;60:57–63. doi: 10.1016/j.foodcont.2015.07.022. [DOI] [Google Scholar]
- 11.Jiang D, Wang G, Li L, Wang X, Li W, Li X, Shao L, Li F. Occurrence, dietary exposure, and health risk estimation of polycyclic aromatic hydrocarbons in grilled and fried meats in Shandong of China. Food Sci Nutr. 2018;6:2431–2439. doi: 10.1002/fsn3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jira W. A GC/MS method for the determination of carcinogenic polycyclic aromatic hydrocarbons (PAH) in smoked meat products and liquid smokes. Eur Food Res Technol. 2004;218:208–212. doi: 10.1007/s00217-003-0827-8. [DOI] [Google Scholar]
- 13.Karimi F, Shariatifar N, Rezaei M, Alikord M, Arabameri M. Quantitative measurement of toxic metals and assessment of health risk in agricultural products food from Markazi Province of Iran. Int J Food Contam. 2021;8:1–7. doi: 10.1186/s40550-021-00083-0. [DOI] [Google Scholar]
- 14.Khalili F, Shariatifar N, Dehghani MH, Yaghmaeian K, Nodehi RN, Yaseri M, Arabameri M. The analysis and probabilistic health risk assessment of polycyclic aromatic hydrocarbons in cereal products. Environ Sci Pollut Res. 2022;29:31099. doi: 10.1007/s11356-021-17337-1. [DOI] [PubMed] [Google Scholar]
- 15.Kiani A, Ahmadloo M, Shariatifar N, Moazzen M, Baghani AN, Khaniki GJ, Taghinezhad A, Kouhpayeh A, Khaneghah AM, Ghajarbeygi P. Method development for determination of migrated phthalate acid esters from polyethylene terephthalate (PET) packaging into traditional Iranian drinking beverage (Doogh) samples: a novel approach of MSPE-GC/MS technique. Environ Sci Pollut Res. 2018;25:12728–12738. doi: 10.1007/s11356-018-1471-y. [DOI] [PubMed] [Google Scholar]
- 16.Kiani A, Shariatifar N, Shahsavari S, Ahmadloo M, Moazzen M. Investigating the presence of polycyclic aromatic hydrocarbons in Doogh. J Mazandaran Univ Med Sci. 2019;29:10–23. [Google Scholar]
- 17.Kiani A, Ahmadloo M, Moazzen M, Shariatifar N, Shahsavari S, Arabameri M, Hasani MM, Azari A, Abdel-Wahhab MA. Monitoring of polycyclic aromatic hydrocarbons and probabilistic health risk assessment in yogurt and butter in Iran. Food Sci Nutr. 2021;9:2114–2128. doi: 10.1002/fsn3.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiani A, Arabameri M, Moazzen M, Shariatifar N, Aeenehvand S, Khaniki GJ, Abdel-Wahhab M, Shahsavari S. Probabilistic health risk assessment of trace elements in baby food and milk powder using ICP-OES method. Biol Trace Elem Res. 2021b;200:2486–2497. [DOI] [PubMed]
- 19.Kouhpayeh A, Moazzen M, Jahed Khaniki GR, Dobaradaran S, Shariatifar N, Ahmadloo M, Azari A, Nazmara S, Kiani A, Salari M. Extraction and determination of phthalate esters (PAEs) in Doogh. J Mazandaran Univ Med Sci. 2017;26:257–267. [Google Scholar]
- 20.Lee Y-N, Shin H-S Analytical method for the determination of polycyclic aromatic hydrocarbons from various ready-to-eat food products in korea. Polycycl Arom Compd. 2019.
- 21.Mastanjević K, Kartalović B, Petrović J, Novakov N, Puljić L, Kovačević D, Jukić M, Lukinac J, Mastanjević K. Polycyclic aromatic hydrocarbons in the traditional smoked sausage Slavonska kobasica. J Food Compos Anal. 2019;83:103282. doi: 10.1016/j.jfca.2019.103282. [DOI] [Google Scholar]
- 22.Moazzen M, Ahmadkhaniha R, Gorji MEh, Yunesian M, Rastkari N. Magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes for the determination of polycyclic aromatic hydrocarbons in grilled meat samples. Talanta. 2013;115:957–965. doi: 10.1016/j.talanta.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Moazzen M, Shariatifar N, Arabameri M, Hosseini H, Ahmadloo M. Measurement of polycyclic aromatic hydrocarbons in baby food samples in Tehran, Iran with magnetic-solid-phase-extraction and gas-chromatography/mass-spectrometry method: A health risk assessment. Front in Nutr 2022;9. [DOI] [PMC free article] [PubMed]
- 24.Moradi M, Bolandi M, Arabameri M, Karimi M, Baghaei H, Nahidi F, Eslami Kanafi M. Semi-volume gluten-free bread: effect of guar gum, sodium caseinate and transglutaminase enzyme on the quality parameters. J Food Meas Charact. 2021;15:2344–2351. doi: 10.1007/s11694-021-00823-y. [DOI] [Google Scholar]
- 25.Muyela B, Shitandi A, Ngure R. Determination of benzo [a] pyrene levels in smoked and oil fried Lates niloticus. Int Food Res J 2012;19.
- 26.Nachvak SM, Hosseinikia M, Abdollahzad H, Pasdar Y, Oubari F, Hosseinikia R, Shabanpur M. Pattern of kebab intake as a potential carcinogenic risk factor in adults of Kermanshah, Iran: 2015. Int J Hematology-oncol Stem Cell Res. 2018;12:23. [PMC free article] [PubMed] [Google Scholar]
- 27.Oz F, Yuzer MO. The effects of cooking on wire and stone barbecue at different cooking levels on the formation of heterocyclic aromatic amines and polycyclic aromatic hydrocarbons in beef steak. Food Chem. 2016;203:59–66. doi: 10.1016/j.foodchem.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 28.Rezaei H, Moazzen M, Shariatifar N, Khaniki GJ, Dehghani MH, Arabameri M, Alikord M. Measurement of phthalate acid esters in non-alcoholic malt beverages by MSPE-GC/MS method in Tehran city: chemometrics. Environ Sci Pollut Res. 2021;28:51897–51907. doi: 10.1007/s11356-021-14290-x. [DOI] [PubMed] [Google Scholar]
- 29.Roudbari A, Nazari RR, Shariatifar N, Moazzen M, Abdolshahi A, Mirzamohammadi S, Madani-Tonekaboni M, Delvarianzadeh M, Arabameri M. Concentration and health risk assessment of polycyclic aromatic hydrocarbons in commercial tea and coffee samples marketed in Iran. Environ Sci Pollut Res. 2021;28:4827–4839. doi: 10.1007/s11356-020-10794-0. [DOI] [PubMed] [Google Scholar]
- 30.Sahin S, Ulusoy HI, Alemdar S, Erdogan S, Agaoglu S. The presence of polycyclic aromatic hydrocarbons (PAHs) in grilled beef, chicken and fish by considering dietary exposure and risk assessment. Food Science of Animal Resources. 2020;40:675. doi: 10.5851/kosfa.2020.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samiee S, Fakhri Y, Sadighara P, Arabameri M, Rezaei M, Nabizadeh R, Shariatifar N, Khaneghah AM. The concentration of polycyclic aromatic hydrocarbons (PAHs) in the processed meat samples collected from Iran’s market: a probabilistic health risk assessment study. Environ Sci Pollut Res. 2020;27:21126–21139. doi: 10.1007/s11356-020-08413-z. [DOI] [PubMed] [Google Scholar]
- 32.Santos C, Gomes A, Roseiro L. Polycyclic aromatic hydrocarbons incidence in Portuguese traditional smoked meat products. Food Chem Toxicol. 2011;49:2343–2347. doi: 10.1016/j.fct.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 33.Shariatifar N, Dadgar M, Fakhri Y, Shahsavari S, Moazzen M, Ahmadloo M, Kiani A, Aeenehvand S, Nazmara S, Khanegah AM. Levels of polycyclic aromatic hydrocarbons in milk and milk powder samples and their likely risk assessment in Iranian population. J Food Compos Anal. 2020;85:103331. doi: 10.1016/j.jfca.2019.103331. [DOI] [Google Scholar]
- 34.Shariatifar N, Moazzen M, Arabameri M, Moazzen M, Khaniki GJ, Sadighara P. Measurement of polycyclic aromatic hydrocarbons (PAHs) in edible mushrooms (raw, grilled and fried) using MSPE-GC/MS method: a risk assessment study. Appl Biol Chem. 2021;64:1–11. doi: 10.1186/s13765-021-00634-1. [DOI] [Google Scholar]
- 35.Singh L, Agarwal T. Polycyclic aromatic hydrocarbons in diet: Concern for public health. Trends Food Sci Technol. 2018;79:160–170. doi: 10.1016/j.tifs.2018.07.017. [DOI] [Google Scholar]
- 36.Terzi G, Çelik T, Nisbet C. Determination of benzo [alpha] pyrene in Turkish döner kebab samples cooked with charcoal or gas fire. 2008.
- 37.USEPA. Risk assessment guidance for Superfund: volume III part A, process for conducting probabilistic risk assessment. Washington, DC:US Environmental Protection Agency 2001;540-R-02-002:385.
- 38.Xia Z, Duan X, Qiu W, Liu D, Wang B, Tao S, Jiang Q, Lu B, Song Y, Hu X. Health risk assessment on dietary exposure to polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Sci Total Environ. 2010;408:5331–5337. doi: 10.1016/j.scitotenv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Yaminifar S, Aeenehvand S, Ghelichkhani G, Ahmadloo M, Arabameri M, Moazzen M, Shariatifar N. The measurement and health risk assessment of polychlorinated biphenyls in butter samples using the QuEChERS/GC-MS method. Int J Dairy Technol. n/a.
- 40.Zachara A, Gałkowska D, Juszczak L. Contamination of smoked meat and fish products from Polish market with polycyclic aromatic hydrocarbons. Food Control. 2017;80:45–51. doi: 10.1016/j.foodcont.2017.04.024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during current study are included in this article. The datasets analyzed during this study are available from the corresponding author on reasonable request.