Abstract
Introduction
Nearly all existing respiratory syncytial virus (RSV) incidence estimates are based on real-time polymerase chain reaction (RT–PCR) testing of nasal or nasopharyngeal (NP) swabs. Adding testing of additional specimen types to NP swab RT–PCR increases RSV detection. However, prior studies only made pairwise comparisons and the synergistic effect of adding multiple specimen types has not been quantified. We compared RSV diagnosis by NP swab RT–PCR alone versus NP swab plus saliva, sputum, and serology.
Methods
This was a prospective cohort study over two study periods (27 December 2021 to 1 April 2022 and 22 August 2022 to 11 November 2022) of patients aged ≥ 40 years hospitalized for acute respiratory illness (ARI) in Louisville, KY. NP swab, saliva, and sputum specimens were collected at enrollment and PCR tested (Luminex ARIES platform). Serology specimens were obtained at acute and convalescent timepoints (enrollment and 30–60-day visit). RSV detection rate was calculated for NP swab alone and for NP swab plus all other specimen type/test.
Results
Among 1766 patients enrolled, 100% had NP swab, 99% saliva, 34% sputum, and 21% paired serology specimens. RSV was diagnosed in 56 (3.2%) patients by NP swab alone, and in 109 (6.2%) patients by NP swab plus additional specimens, corresponding to a 1.95 times higher rate [95% confidence interval (CI) 1.62, 2.34]. Limiting the comparison to the 150 subjects with all four specimen types available (i.e., NP swab, saliva, sputum, and serology), there was a 2.60-fold increase (95% CI 1.31, 5.17) compared to NP swab alone (3.3% versus 8.7%). Sensitivities by specimen type were: NP swab 51%, saliva 70%, sputum 72%, and serology 79%.
Conclusions
Diagnosis of RSV in adults was several-fold greater when additional specimen types were added to NP swab, even with a relatively low percentage of subjects with sputum and serology results available. Hospitalized RSV ARI burden estimates in adults based solely on NP swab RT–PCR should be adjusted for underestimation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00805-1.
Keywords: Respiratory syncytial virus, Acute respiratory illness, Polymerase chain reaction, Disease diagnosis
Key Summary Points
| Why carry out this study? |
| Adding the collection and testing of additional specimen types to NP swab RT–PCR increases RSV detection, but prior studies only made pairwise comparisons and the synergistic effect of adding multiple specimen types has not yet been quantified. |
| We sought to compare RSV diagnosis by PCR testing of NP swab alone versus NP swab plus saliva, sputum, and serology. |
| What was learned from the study? |
| RSV was diagnosed in 56 (3.2%) patients by NP swab alone, and in 109 (6.2%) patients by NP swab plus additional specimens, corresponding to a 1.95 times higher diagnosis rate (95% CI 1.62, 2.34). |
| Approximately half of identified positives were missed by NP swab testing, even with a relatively low percentage of subjects with sputum and serology results available. |
| Hospitalized RSV ARI burden estimates in adults based solely on NP swab RT–PCR should be adjusted for underestimation. |
Introduction
Respiratory Syncytial Virus (RSV) is a leading cause of respiratory illness in adults, with older adults and those with compromised cardiac, pulmonary, or immune systems most at risk of severe disease [1–5]. Although underrecognized, estimated RSV disease burden is comparable to the burden of influenza in older adults, with both viruses contributing to a similar number of symptomatic illnesses, hospitalizations, and death overall, despite substantial variability in the relative burden of the two viruses from year to year [6]. Due to the nonspecific clinical manifestations of RSV, which often overlap with those of other viral and bacterial causes of acute respiratory illness (ARI), and can contribute to exacerbations of common illnesses such as COPD or CHF, laboratory testing is required for confirmation of RSV infection [7].
Published incidence estimates of RSV disease in adult patients hospitalized with ARI have primarily relied on reverse transcription polymerase chain reaction (RT–PCR) testing of NP swabs [8–10]. However, the results of upper respiratory tract testing using NP swabs in adults may be discordant with positive lower respiratory tract (LRT) testing [11]. Possible explanations for this finding include: (1) a decreased viral concentration in the nasopharynx due to sampling late in the infection at a time when virus may still be present at higher concentrations in the lower respiratory tract [12], (2) lower viral concentrations in adult nasal secretions when compared with children [12], and (3) inadequate NP swab samples due to dry nasal mucosa and operational reasons.
Adding the collection and testing of an additional specimen type to NP/nasal swab RT–PCR has been documented to increase RSV detection in pairwise comparisons. A recent metaanalysis quantified the percent increase in RSV diagnosis by specimen type added: 52% increase for sputum RT–PCR, 44% for paired serology testing, and 28% for oropharyngeal swab RT–PCR [11]. However, the synergistic effect of adding multiple specimen types to NP swab testing has not yet been quantified. Furthermore, saliva has recently been shown to be a high yield specimen for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT–PCR testing [13], but it has not been evaluated directly head-to-head against NP or nasal swab RT–PCR for RSV testing.
The quantification of RSV underestimation associated with sole use of NP swab for diagnosis will allow for adjustment of published RSV incidence rates to estimate the true burden of RSV disease. These more accurate burden of disease estimates will facilitate appropriate decision making regarding the use of RSV disease preventive interventions, such as vaccination. The objective of this study was to define the underestimation in RSV diagnosis by comparing RSV diagnosis rates with NP swab RT–PCR alone to RSV diagnosis rates with the addition of saliva, sputum, and/or serology testing.
Methods
Study Design, Setting, and Patients
This was a prospective cohort study of patients hospitalized with ARI in four adult acute care hospitals in Louisville, KY during two study periods from 27 December 2021 to 1 April 2022, and 22 August 2022 to 11 November 2022. Study periods were chosen to align with real-time RSV activity in Louisville, KY. Patients were eligible for inclusion if they (1) were aged 40 years or older, (2) were hospitalized with an ARI, defined as the presence of at least one of the following: (a) new onset or increase from baseline in any of following nine signs and symptoms—nasal congestion, rhinorrhea, sore throat, hoarseness, cough, sputum production, dyspnea, wheezing, hypoxemia, or (b) admitting diagnosis suggestive of ARI or (c) exacerbation of underlying cardiopulmonary disease involving acute respiratory symptoms, and (3) consented to have NP swab plus at least one other specimen obtained. These criteria for ARI are consistent with previous prospective incidence studies [2, 5].
The age cutoff was selected to include older adults, as well as some middle-aged adults, with a higher likelihood of having underlying conditions such as cardiopulmonary disease, whose prevalence increases with advancing age. Patients were excluded from the study if they developed signs and symptoms of ARI after being hospitalized for 48 h or more, had onset of symptoms more than 21 days before hospital admission, or were previously enrolled in this study within the 45 days prior of their current admission.
This study was approved (#21-N0325) by WCG IRB. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. After informed consent was obtained, we sought to collect NP swab, saliva, sputum, and an acute blood specimen on all subjects. Respiratory samples on any given subject were collected on the day of enrollment. Patients were scheduled for a follow up between 30 and 60 days to collect convalescent blood specimens. In subjects that were unable to produce saliva, a saline mouth wash was obtained.
Study Definitions
RSV Diagnosis
RSV detection by RT–PCR from NP swab, saliva, and sputum specimens was defined as a positive RSV case. Evidence of concurrent RSV infection was defined as a four-fold increase between acute and convalescent paired blood specimens in antibodies to any of four RSV antigens tested, consistent with previously published RSV serology studies [9].
RSV Diagnosis Rates
RSV diagnosis rate from NP swab alone was calculated as the number of subjects with RSV detected from NP swab specimens divided by the number of subjects in the study. RSV diagnosis rate from NP swab plus other specimens was calculated as the number of subjects with RSV diagnosed by any specimen divided by the number of patients in the study.
RSV diagnosis rate increased using additional specimens
The ratio of RSV diagnosis was calculated by dividing the proportion of RSV diagnosis from NP swab plus other specimens by the proportion of RSV diagnosis from NP swab alone. The inverse of this ratio was used to determine the underestimation of RSV diagnosis. Additionally, the ratio of RSV diagnosis was calculated for each combination of sample types in addition to NP swab. This was calculated for the study population overall, regardless of specific results available, and for each subset of the study population with specific results available to illustrate what the increase in diagnosis might be in ideal conditions with high levels of sample collection.
Study Variables
Data on demographics, social, and medical history, vaccination status, standard of care SARS-CoV-2 testing, clinical diagnosis, as well as hospital course, including length of stay and patient mortality, were collected from patient questionnaires and from electronic medical records.
Specimen Processing and Testing
NP swab
NP swab specimens were tested using the ARIES Luminex FluA/B/RSV panel and processed in accordance with standard operating procedures [14].
Sputum
Sputum specimens were processed and tested using the ARIES Luminex FluA/B/RSV platform. Briefly, sputum specimens were diluted to 50% water solution, and mixed by vortex. A swab of this solution was mixed into 700 uL of sterile water and mixed by vortex a second time. From this mixed solution, 200 uL was pipetted into the Luminex cartridge vial.
Saliva
Saliva specimens were processed and tested using the ARIES Luminex FluA/B/RSV platform. Briefly, saliva was handled separately depending on viscosity. For normal saliva, the specimen was mixed by vortex and 200 uL was pipetted into the Luminex cartridge vial. Saliva that was too thick or viscous to pipette was processed the same way as sputum (above).
Serology
Serology was performed by Pfizer central laboratory using Luminex-based total antibody RSV assays. Briefly, the 4-plex assay included spectrally distinct magnetic microspheres coated with recombinant matrix protein, nucleoprotein, and peptide sequences unique to the G protein for RSVA and RSVB (Cambridge Research Biochemicals) [15]. Antigen specific antibodies were detected with a goat-anti-human total Ig Phycoerythrin labeled antibody (Southern Biotech) [16]. Fluorescence was expressed as median fluorescence intensity and results are calculated using a serum reference standard. Persons who received intravenous immunoglobulin (IVIG) treatment with positive serology results were considered false positives and removed from analysis.
Statistical Analysis
Only patients with an NP swab and at least one other specimen result were included in this analysis. Patient characteristics were reported as medians and interquartile ranges (for continuous data) and frequencies and percentages (for categorical data). Venn and Euler diagrams were produced to show RSV diagnosis by specimen type. RSV diagnosis rates were calculated and reported as percentage positive. The RSV detection rate by NP swab alone was used as the baseline RSV rate for comparison, and RSV diagnosis ratios were calculated as percent increase from baseline, with 95% confidence intervals (CIs) calculated. The sensitivity of each assay for diagnosing RSV was calculated for each sample type and combination, with 95% confidence intervals calculated as well. P-values less than 0.05 were considered statistically significant. Sensitivity calculations for each specimen type were limited to subjects that had results for that specimen type. The proportion of all positives detected by a given specimen type was also calculated to capture the real-world benefit of adding a given specimen type, given its operational feasibility and sensitivity. All analysis was performed using R version 4.1.2 [17] and SAS version 9.4 (SAS 9.4, Cary, NC. SAS Institute Inc.).
Results
Patient Population
A total of 1766 participants provided informed consent and enrolled in the study. Figure 1 depicts the study flowchart. Among enrolled participants hospitalized for ARI, most were female (55%), white (70%), and were community-dwelling (95%). Diabetes mellitus and chronic obstructive pulmonary disease were the most frequent comorbidities (39% and 38% of subjects, respectively), and 28% (n = 494) of participants were immunocompromised. Table 1 depicts patient characteristics for study participants.
Fig. 1.
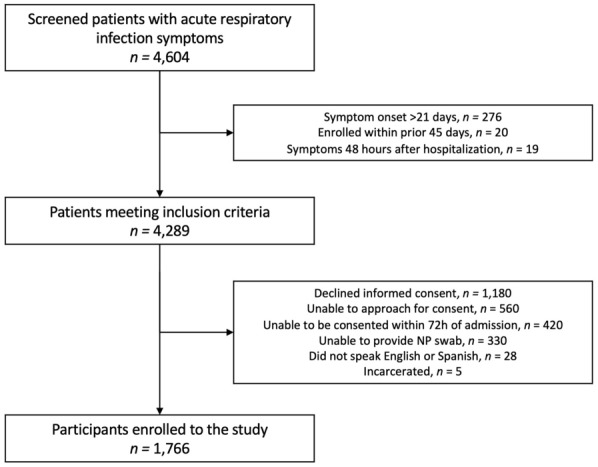
Study flowchart
Table 1.
Study specimen collection, RSV prevalence results, and sensitivity by specimen type
| Study specimens | N | Percentage with this specimen type |
n positive by specimen |
% positive overall |
Percentage of all positives identified by this specimen type |
95% CI | RSV positives from any source among patients with results by that specimen type |
Sensitivity* | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 1766 | 100.0% | 109 | 6.2% | N/A | N/A | 109 | N/A | N/A |
| NP swab | 1766 | 100.0% | 56 | 3.2% | 51.4% | 42.0–60.8% | 109 | 51.4% | 42.0–60.8% |
| Saliva** | 1740 | 98.5% | 75 | 4.3% | 68.8% | 60.1–77.5% | 107 | 70.1% | 61.4–78.8% |
| Sputum | 606 | 34.3% | 41 | 6.8% | 37.6% | 28.5–46.7% | 57 | 71.9% | 60.3–83.6% |
| Serology | 367 | 20.8% | 23 | 6.3% | 21.1% | 13.4%-28.8% | 29 | 79.3% | 64.6–94.1% |
*Sensitivity is limited to those with results with that specimen type, i.e., count of positives by that specimen type divided by count of all positives from any source among those with results by that specimen type
**In subjects that were unable to produce saliva, a saline mouth wash was obtained
RSV Diagnosis
Among 1766 participants enrolled, 100% had NP swab (n = 1766), 99% saliva (n = 1740), 34% sputum (n = 606), and 21% (n = 367) paired serology specimens tested. Overall, RSV was diagnosed in 109 participants using any/all specimens. Figure 2 depicts RSV detection by sample type for the entire study population for respiratory specimens alone (panel A) and all four specimen types (panel B). While there was some overlap in positives for each specimen type, all specimens contributed unique positives, with the greatest number of unique positives contributed by saliva and serology. A total of 56 among 109 participants (51%) had RSV detected from NP swabs, while 53 participants had RSV diagnosed only by other specimen types (i.e., negative by NP swab), corresponding to 49% of positives missed by NP swab testing alone. Table 1 depicts specimen collection frequency, percent RSV diagnosis by sample type for the entire study population, as well as test sensitivity estimates. Although sputum and serology were not available in all participants, these samples yielded the highest percent of RSV diagnoses when a sample was available (sputum n = 41 of 606 participants, 6.8%, and serology n = 23 of 367 specimens, 6.3%). The percent detection of saliva and NP swabs was less, but the overall number of positives greater: saliva (n = 75 of 1740 specimens, 4.3%), and NP swabs (n = 56 of 1766, 3.2%). In the overall study population, saliva detected the most positives (75/109, 69%), followed by NP swab (56/109, 51%), sputum (41/109, 38%), and serology (23/109, 21%). We examined each test’s sensitivity by limiting to those with that result type, and sensitivity ranged from 51% for NP swab to 79% for serology.
Fig. 2.
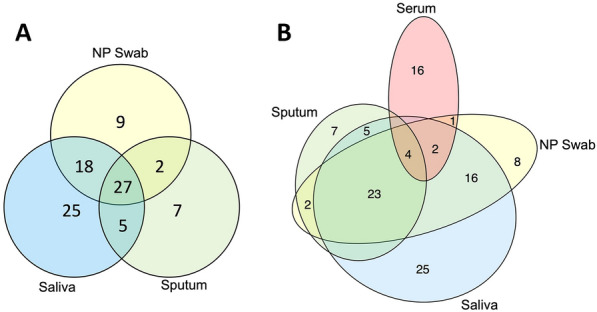
Respiratory syncytial virus (RSV) diagnosis by specimen type: all specimen types contribute unique positives. A (left) Venn diagram of nasopharyngeal (NP) swab, saliva, and sputum specimens detecting RSV from RT–PCR diagnostic testing. B (right) Euler diagram of NP swab, saliva, sputum, and serology specimens diagnosing RSV
RSV Diagnosis Rates by Sample Type Combinations
For the entire study population, regardless of specific results available, the percent increase in RSV diagnosis when adding results from additional sample types, relative to using NP swab alone (reference value), is depicted in Fig. 3 for each combination of sample types. As noted, NP swab alone diagnosed RSV in 56 (3.2% of participants). Adding results from other specimen types, RSV diagnoses increased to 109 (6.2% of participants), corresponding to a 1.95-fold increase (95% CI 1.62, 2.34-fold increase) in detection, over NP swab results alone. The number of participants with each of the various combinations of specimen types tested and the percent increases in RSV diagnosis for each combination of sample types, relative to using NP swab alone (reference value), limiting to those participants with the specific results available in the comparison, are depicted in Table 2. When limiting analysis to the 150 participants with all four specimen types available, RSV was diagnosed in 5 participants (3.3%) by NP swab alone and in 13 participants (8.67%) when all results were used. A 3.20-fold increase in detection (95% CI 1.83, 5.78) was seen adding saliva and serum results to NP among 363 participants with those three specimen types tested. For the 580 subjects with available NP swab, saliva, and sputum samples, there was a 1.63-fold increase in detection (95% CI 1.31, 2.04) when adding the saliva and sputum results.
Fig. 3.
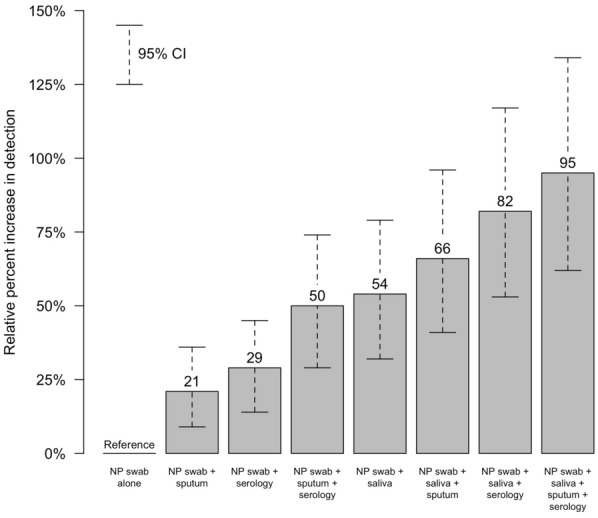
The percent increase in respiratory syncytial virus (RSV) diagnosis when adding additional specimen types in the analysis, over using nasopharyngeal (NP) specimens alone
Table 2.
Increase in RSV detection associated with testing additional specimen types, beyond NP swab, for populations with specific sample results available
| Groups by available specimens | N | Count of patients by NP swab positive |
Detection rate with NP swab positive (per 100 patients) |
Count of patients by any listed specimen positive |
Detection rate with any listed specimen positive (per 100 patients) |
Detection rate ratio (any listed/NP swab) |
95% CI of detection rate ratio |
|---|---|---|---|---|---|---|---|
| All subjects | |||||||
| All four specimens | 150 | 5 | 3.33 | 13 | 8.67 | 2.60 | 1.31, 5.17 |
| Three specimens | |||||||
| NP swab/saliva/sputum | 580 | 30 | 5.17 | 49 | 8.45 | 1.63 | 1.31, 2.04 |
| NP swab/saliva/serum | 363 | 8 | 2.20 | 26 | 7.16 | 3.25 | 1.83, 5.78 |
| NP swab/sputum/serum | 154 | 5 | 3.25 | 14 | 9.09 | 2.80 | 1.39, 5.65 |
| Two specimens | |||||||
| NP swab/saliva | 1740 | 55 | 3.16 | 85 | 4.89 | 1.55 | 1.32, 1.81 |
| NP swab/sputum | 606 | 31 | 5.12 | 43 | 7.10 | 1.39 | 1.15, 1.67 |
| NP swab/serum | 367 | 8 | 2.18 | 24 | 6.54 | 3.00 | 1.70, 5.28 |
Participant Characteristics by Specimen Type Positive
Characteristics for RSV positive participants by specimen type positive are depicted in Table 2. Overall, participants with RSV identified by NP swab (regardless of other results) had a similar time from symptom onset to specimen collection than subjects exclusively positive by other non-NP respiratory specimen types (median of 4 days versus 3 days, respectively), but subjects that were NP negative and serology positive had a longer median duration of 6 days for symptom onset. Thirty percent of RSV-diagnosed subjects were immunocompromised, 23% of participants with RSV identified by NP swab were immunocompromised compared to 38% of subjects detected by non-NP swab specimen types.
Discussion
Our study indicates that the inclusion of saliva, sputum, and serology to RT–PCR of NP swab increased the diagnostic yield for RSV by two-fold or more in adult patients hospitalized with ARI. Even though RT–PCR of NP swab is the most commonly used test to detect RSV in hospitalized patients, our study indicates that it will miss a substantial percentage of patients hospitalized with RSV-associated ARI. Prior literature has reported increased detection associated with adding sputum or serology to NP swab [9], but this is the first study utilizing a wide variety of specimen types, including saliva, and assessing their synergistic effects for RSV diagnosis.
Our data indicate that a more accurate burden of RSV disease in future studies can be achieved by testing multiple specimen types, or adjusting for underestimation associated with use of limited specimen types. Furthermore, our study suggests that vaccine studies evaluating efficacy or effectiveness of an RSV vaccine should include multiple specimens for diagnosis of disease. In a Centers for Disease Control and Prevention (CDC) meeting of experts for the purpose of identifying gaps in the epidemiology of RSV, the experts noted a need to document potential underestimation of disease burden due to testing behaviors [18]. In a recent metaanalysis of RSV incidence among older adults in the USA, an adjustment factor of 1.5 was included to account for diagnostic testing under-ascertainment when only RT–PCR was used [9]. This correction factor was based on pairwise comparisons of different specimen type results from the literature that did not account for synergistic effects of multiple specimen use. Our results indicate that a correction factor greater than 2 may be more appropriate, as indicated by the 2.6-fold increase in yield among those with all four specimen types.
Notably, saliva specimens yielded the highest number of RSV detections among respiratory specimens if used alone (n = 75) and added an additional 30 unique RSV cases to the 56 diagnosed with NP samples. Since saliva is readily obtained from most subjects, as shown in our data, the simple addition of this sample to NP swabs may provide much more accurate estimates of RSV incidence in this population. There are several potential reasons for this finding. RSV may replicate in the primary salivary glands such as parotid, submandibular, and sublingual glands, producing a constant flow of the virus or viral genetic material into the saliva. In addition, saliva may also serve as reservoir of pooled secretions from the nasopharynx. Saliva has emerged as a sensitive and reliable specimen type for SARS-CoV-2 testing, with one study finding that saliva has higher viral titers than NP swab and is a more consistent specimen, such that no instances were seen of a negative result followed by a positive result [13]. We did not attempt to measure viral load in the saliva in comparison to NP swabs. In our study, it is notable that saliva (or normal saline mouth wash) was available in nearly all study subjects. It is possible that saliva may be a more desirable diagnostic sample for the diagnosis of respiratory viruses, both for better yield and tolerability to patients.
Among hospitalized adults, material captured with NP or nasal swabs can be limited by the difficulty of taking a sufficient sample and nasal dryness, potentially from nasal oxygen use [7], diuretic administration, or dry indoor air. The lower positivity rate of NP swab testing may also be due in part to a prolonged time from symptom onset to hospitalization and swab collection, such that at the time of hospitalization, the viral titers in nasal secretion may have dropped and RSV may no longer be detectable in the nasopharynx [19]. Nasal swabs are more likely to be positive in persons that still have upper respiratory symptoms [19]. Patients with RSV detected by serology specimens only, had a longer duration of symptoms at the time of sampling (median 6 days versus 4 days), but further study is needed to better characterize the differences in the cases detected by each specimen type.
In patients with a productive cough, sputum was a useful specimen for RSV identification. Sputum has been shown to have higher RSV titers than nasal swabs [12], allowing for increased detection of RSV when this specimen is available [11], which is consistent with results from other respiratory viruses such as influenza and SARS-CoV-2 [13, 19–21]. In our study, we found seven patients with RSV that were NP swab negative and diagnosed by sputum alone (Fig. 1), all but one of whom had lower respiratory tract illness diagnosis. This corresponds to a 39% increase in RSV detection over NP swab alone among subjects with both specimen types, comparable to published paired assessments of adding sputum to NP swab testing [pooled percent increase from recent metaanalysis: 52% (95% CI 15, 101)] [11].
Serology testing does not impact the clinical management of a hospitalized patient; however, it represents an important epidemiological tool to define the burden of disease and can be used in vaccine efficacy studies to augment RSV diagnosis end points when feasible. A recent metaanalysis reported a 42% increase in detection (95% CI 19, 70) over NP/nasal RT–PCR swab alone. Analyses limited to older adults (more comparable to our study population) reported a detection increase of 50% to 64% [11]. This higher detection rate by serology among older adults may be due to their higher serum IgG responses following RSV infection compared to younger adults, possibly related to their higher RSV nasal titers and longer viral shedding [12]. This longer viral shedding correlates with persistent secretion of antibody by plasma cells and is presumed due to diminished cellular immunity associated with immune-senescence [22]. One potential limitation of serology is being certain that the rise in IgG clearly brackets an identifiable illness. It is possible that a rise is RSV specific IgG could be related to an illness that occurred after hospital discharge. To mitigate this, we collected NP swabs at convalescent visits from anyone with intercurrent ARI symptoms; we did not have any positive results suggesting intercurrent RSV was not an important contributor to infections, identified by a four-fold rise in serology.
One strength of our study was that all respiratory specimens were collected on the same day, at time of enrollment. Additionally, all collected specimens had RT–PCR tests performed on the same platform. Furthermore, we collected sputum in 95% of the 646 patients producing sputum in our study population.
The primary limitation of our study was the low number of subjects with serology results available, thus diminishing the number of subjects with all 4 sample types for analysis. Consequently, our estimate of the increase in detection of RSV may be too conservative. Another limitation of our study is that with only 109 patients with RSV detected, we were unable to perform analysis in subgroups such as the immunocompromised patients. Further study is required to improve precision regarding the level of RSV underestimation within specific subgroups. Lastly, another potential limitation is that we did not assess if nasal swab or oropharyngeal swab may increase RSV detection when compared with RT–PCR of NP swab alone, because of its dominance in RSV incidence studies [9, 13]. Nasal and oropharyngeal swab likely have substantial overlap regarding material collected with other specimens included in the study, namely NP swab and saliva, respectively. Finally, RT–PCR positive results may uncommonly reflect a prior infection with residual viral RNA in the nasopharynx, particularly among immunocompromised individuals.
In conclusion, our study found that RSV detection increases several-fold with the addition of testing from other specimen types besides NP swab, especially saliva. Future studies assessing the RSV burden should consider additional testing of saliva, sputum, and serology to adequately detect RSV-positive patients. Burden of disease estimates based solely on NP swab RT–PCR should be adjusted for underestimation, as should metaanalyses of existing RSV incidence estimates [23].
Acknowledgements
We would like to thank the NIDI RSV study group (see supplementary materials) for performing the study. Additionally, we would like to thank the nurses at Norton Healthcare for their collaboration with the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by Pfizer Inc., including supporting the journal’s rapid service fee. This study was conducted as a collaboration between Norton Infectious Diseases Institute, Norton Healthcare, and Pfizer. Norton Infectious Diseases Institute, Norton Healthcare is the study sponsor.
Author Contributions
Study concept and design was carried out by Elizabeth Begier, Robin Hubler, Julio Ramirez, Ruth Carrico, Ashley Wilde, Paula Peyrani, Ann R. Falsey, Edward Walsh, Luis Jodar, and Bradford D. Gessner. Data acquisition was performed by Julio Ramirez, Ruth Carrico, Ashley Wilde, Alan Junkins, Stephen Furmanek, Thomas Chandler, and Warren V Kalina. Data interpretation and analysis was performed by all authors. Writing the first draft was performed by Julio Ramirez and Stephen Furmanek. Participation in drafting or revision involved all authors.
Disclosures
Julio Ramirez, Ruth Carrico, Ashley Wilde, Alan Junkins, Stephen Furmanek, Thomas Chandler, and Paul Schulz are employees of Norton, which received fees from Pfizer in relation to this study. Robin Hubler, Paula Peyrani, Qing Liu, Sonali Trivedi, Sonal Uppal, Warren V Kalina, Kari Yacisin, Luis Jodar, Bradford D. Gessner, and Elizabeth Begier are employees of Pfizer and may hold Pfizer stock and/or stock options. Ann R. Falsey has research grants from Pfizer, Janssen, CyanVac, and BioFire Diagnostics, served on the Data and Safety Monitoring Board for Novavax, and consulted for Arrowhead Pharmaceuticals. Edward E. Walsh has research grants from Merck and Pfizer and non paid consulting for Moderna and Pfizer.
Compliance with Ethics Guidelines
This study was approved (#21-N0325) by WCG IRB. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All specimen and data collection were performed after informed consent was obtained.
Data Availability
Data access may be requested from Pfizer data request portal: https://www.pfizer.com/science/clinical-trials/trial-data-and-results/data-requests
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ivey KS, Edwards KM, Talbot HK. Respiratory syncytial virus and associations with cardiovascular disease in adults. J Am Coll Cardiol. 2018;71(14):1574–1583. doi: 10.1016/j.jacc.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Falsey AR, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Chatzis O, Darbre S, Pasquier J, et al. Burden of severe RSV disease among immunocompromised children and adults: a 10 year retrospective study. BMC Infect Dis. 2018;18(1):1–9. doi: 10.1186/s12879-018-3002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackerson B, Tseng HF, Sy LS, et al. Severe morbidity and mortality associated with respiratory syncytial virus versus influenza infection in hospitalized older adults. Clin Infect Dis. 2019;69(2):197–203. doi: 10.1093/cid/ciy991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branche AR, Saiman L, Walsh EE et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis. 2020; [DOI] [PubMed]
- 6.Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging. 2015;32(4):261–269. doi: 10.1007/s40266-015-0258-9. [DOI] [PubMed] [Google Scholar]
- 7.Talbot HK, Falsey AR. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis. 2010;50(5):747–751. doi: 10.1086/650486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havers F. Epidemiology and Burden of Respiratory Syncytial Virus in Older Adults in the US. Accessed September 3, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/04-RSV-Havers-508.pdf
- 9.McLaughlin JM, Khan F, Begier E, et al. Rates of Medically Attended RSV Among US Adults: A Systematic Review and Meta-analysis. Open Forum Infect Dis. 2022 Jun 17;9(7):ofac300. [DOI] [PMC free article] [PubMed]
- 10.Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222(Suppl 7):S577–S583. doi: 10.1093/infdis/jiz059. [DOI] [PubMed] [Google Scholar]
- 11.Onwuchekwa C, Moreo LM, Menon S et al. Under-ascertainment of Respiratory Syncytial Virus infection in adults due to diagnostic testing limitations: a systematic literature review and meta-analysis. Poster Presentation. International RSV Symposium, September 2022, Belfast, UK [The Journal of Infectious Diseases. 2023; in Press.] [DOI] [PMC free article] [PubMed]
- 12.Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, Falsey AR. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J Infect Dis. 2013;207:1424–1432. doi: 10.1093/infdis/jit038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyllie AL, Fournier J, Casanovas-Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383(13):1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luminex Corporation. ARIES system operation manual, revision E. May 2022.
- 15.Cambridge Research Biochemicals. Chambridge Research Biochemicals—Discovery. Accessed September 3, 2022. https://crbdiscovery.com.
- 16.Southern Biotech. Antibodies For Research. Accessed September 3, 2022. https://www.southernbiotech.com.
- 17.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021; https://www.R-project.org/.
- 18.Kim L, Rha B, Abramson JS, et al. Identifying gaps in respiratory syncytial virus disease epidemiology in the United States prior to the introduction of vaccines. Clin Infect Dis. 2017;65(6):1020–1025. doi: 10.1093/cid/cix432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falsey AR, Formica MA, Walsh EE. Yield of sputum for viral detection by reverse transcriptase PCR in adults hospitalized with respiratory illness. J Clin Microbiol. 2012 Jan;50(1):21–4. doi: 10.1128/JCM.05841-11Epub 2011 Nov 16. PMID: 22090400; PMCID: PMC3256730. [DOI] [PMC free article] [PubMed]
- 20.Jeong JH, Kim KH, Jeong SH, Park JW, Lee SM, Seo YH. Comparison of sputum and nasopharyngeal swabs for detection of respiratory viruses. J Med Virol. 2014 Dec;86(12):2122–7. 10.1002/jmv.23937Epub 2014 May 6. PMID: 24797344; PMCID: PMC7166652. [DOI] [PMC free article] [PubMed]
- 21.Song M, Wilde AM, Moore SE, et al. False-negative SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) is an important consideration for patient management and infection prevention: a case report from the louisville COVID-19 epidemiology study. The Univ Louisville J Respiratory Infect. 2020;4(1):43. [Google Scholar]
- 22.Lee F Eun-Hyung, Halliley JL, Sanz I, Falsey AR, Walsh EE. Circulating Antibody Secreting Cells during Acute Respiratory Syncytial Virus infection in Adults. J Infect Dis 2010;202(11):1659–66. [DOI] [PMC free article] [PubMed]
- 23.Li Y, Kulkarni D, Begier E, Wahi-Singh P, Wahi-Singh B, Gessner B, Nair H. Adjusting for case under-ascertainment in estimating RSV hospitalisation burden of older adults in high-income countries: a systematic review and modelling study. Infect Dis Ther. 2023;20:1–13. doi: 10.1007/s40121-023-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data access may be requested from Pfizer data request portal: https://www.pfizer.com/science/clinical-trials/trial-data-and-results/data-requests


