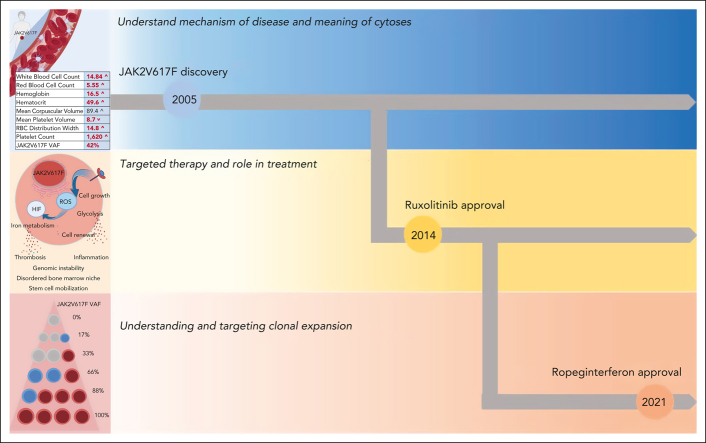
Visual Abstract
Abstract
Polycythemia vera (PV) is a hematopoietic stem cell neoplasm defined by activating somatic mutations in the JAK2 gene and characterized clinically by overproduction of red blood cells, platelets, and neutrophils; a significant burden of disease-specific symptoms; high rates of vascular events; and evolution to a myelofibrosis phase or acute leukemia. The JAK2V617F variant allele frequency (VAF) is a key determinant of outcomes in PV, including thrombosis and myelofibrotic progression. Here, we critically review the dynamic role of JAK2V617F mutation burden in the pathogenesis and natural history of PV, the suitability of JAK2V617F VAF as a diagnostic and prognostic biomarker, and the utility of JAK2V617F VAF reduction in PV treatment.
Our knowledge about the biology of myeloproliferative neoplasms (MPNs) has exploded in the last 20 years, and this increased knowledge has led to advances in therapy. Introduced by Associate Editor Mario Cazzola, this Review Series brings readers up to date on our understanding of the natural history of the classical MPNs—polycythemia vera, essential thrombocythemia, and myelofibrosis—and the approaches to diagnosis, prognostication, and treatment for patients with these conditions.
Introduction
Polycythemia vera (PV) is a hematopoietic stem cell (HSC) neoplasm defined by activating somatic mutations in the JAK2 gene, characterized clinically by overproduction of red blood cells, platelets, and neutrophils. PV results in varying burdens of disease-specific symptoms, high rates of vascular events, and evolution to a myelofibrosis phase or acute leukemia. The pivotal discovery in 2005 that JAK2V617F underlies nearly all PV transformed our understanding of the disease pathobiology and formed the basis for disease classification criteria and targeted therapeutics.1, 2, 3 Despite this, contemporary management of PV largely remains focused on normalization of peripheral blood cell counts with little regard to the clonal expansion of JAK2 mutant cells over time. Recent data from prospective clinical trials for PV have demonstrated the ability of some therapies to significantly decrease not only peripheral blood cell counts but also JAK2V617F mutant allele burden. This review focuses on the role of JAK2V617F clonal burden in PV outcomes, highlighting the prospective clinical trials where JAK2V617F burden is measured.
JAK2V617F and PV
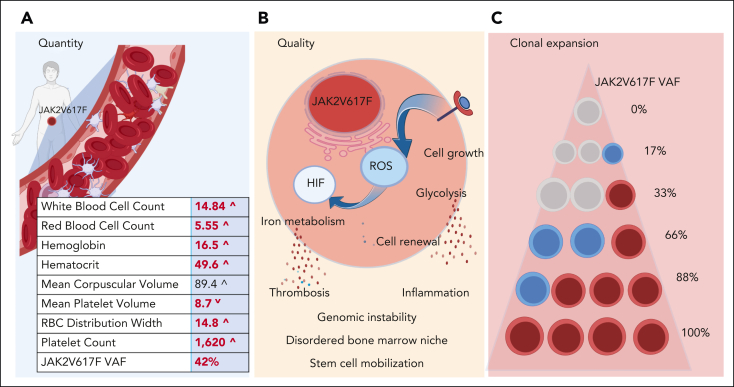
JAK2 functions as a signal transducer of growth and differentiation in the HSC compartment for cytokine receptors that do not have intrinsic kinase activity, including the erythropoietin receptor, the thrombopoietin receptor, and the granulocyte colony–stimulating factor receptor. The activating JAK2 mutation, JAK2V617F, results in increased signal transduction, which translates to panmyelosis and increased numbers of red cells, neutrophils, and platelets. (Figure 1A). Mutations in JAK2 are highly associated with mitotic recombination events, which render cells homozygous for JAK2V617F, such that unmutated heterozygous and homozygous JAK2V617F stem cells and progenitors may populate the hematopoietic compartment in varying and dynamic combinations.4, 5, 6, 7 JAK2V617F heterozygous vs homozygous cells differ in their biological behavior as gene dosage influences the degree of enhanced JAK2V617F signal transduction (Figure 1B). The variability in JAK2V617F genotypes and their clonal expansion results in quantitative measures of JAK2V617F that range from as low as a fraction of a percent to as high as 100%, expressed clinically as variant allele fraction (VAF) (Figure 1C).8 As quantitative JAK2V617F testing has become sensitive to VAFs of <1%, virtually all patients with a clinical phenotype of PV harbor JAK2V617F (97%) or mutations in exon 12 of JAK2.8, 9, 10, 11, 12 The effect of JAK2V617F gene dosage to a PV phenotype is recapitulated in murine models of JAK2V617F PV, where stages of PV phenotype depended both upon the quantity of JAK2V617F expressed, and whether JAK2V617F was expressed at the HSC or progenitor level.13, 14, 15 Genome-wide association studies in JAK2V617F-positive clonal hematopoiesis (CH) or myeloproliferative neoplasm (MPN) show highly significant associations with loci in JAK2, TERT, SH2B3, TET2, ATM, CHEK2, PINT, and GFIB, with nearly 18% of heritable risk attributed to these and other loci.16, 17, 18 Inflammatory states and/or genetic predisposition to inflammation are risk factors for both the acquisition and expansion of JAK2V617F clones, as is male sex.10,19, 20, 21, 22, 23, 24
Figure 1.
Quantitative, qualitative, and clonal burden in PV. (A) JAK2V617F increases cell quantity by transmitting an excessive growth signal that induces a blood stem cell with the mutation to over produce red blood cells, white blood cells, and platelets. (B) JAK2V617F excessive signaling in cells turns on ROS and HIF, resulting in activation of pathways within the HSC cell, the bone marrow niche, and beyond. (C) JAK2V617F can be present in a single cell as a single copy (heterozygote, blue circle) or as a double copy (homozygote, red circle). Measured JAK2V617F VAF in PV may range from 1% to 100%, depending on relative quantities of the various JAK2V617F genotypes. HIF, hypoxia-inducible factor; ROS, reactive oxygen species.
JAK2V617F VAF in HSC and terminally differentiated blood cell pathophysiology
JAK2V617F increases signal transduction and drives quantitative and qualitative alterations in HSC biology. JAK2V617F-mutant HSCs elaborate excess cytokines and reactive oxygen species, which alter the bone marrow niche, reinforce the growth advantage of the clonal cells, and drive genomic instability, myelofibrosis, and osteosclerosis.14,25, 26, 27, 28, 29, 30, 31 Mechanistically, excessive JAK2 signaling in the HSC setting results in a pseudohypoxia state owing to hypoxia-inducible factor (HIF) stabilization by reactive oxygen species. Increased HIF leads to a switch from aerobic metabolism to glycolysis and enhances HSC self-renewal via the downregulation of LKB1/ STK11.29,32 The disordered niche in addition allows for increased egress of CD34+ cells, which lodge in spleen and other tissue that begets extramedullary hematopoiesis (Figure 1B).33,34 Indeed, JAK2V617F VAF in PV positively correlates with circulating CD34+ cell levels, bone marrow cellularity, and splenomegaly (a haven for extramedullary hematopoiesis) (Table 1).11,12,38
Table 1.
JAK2V617F associations in PV and CH
| PV JAK2V617F VAF |
CH JAK2V617F VAF |
|||
|---|---|---|---|---|
| <50% | >50% | <1% | >1% | |
| Quantitative | ||||
| WBC, ×109/L | 9.335 | 12.435 | 7.823 | 8.723 |
| Hg, g/dL | 14.635 | 15.935 | 14.223 | 14.323 |
| Platelets, ×109/L | 57135 | 49035 | 26423 | 36123 |
| Qualitative | ||||
| Venous thrombosis risk ratio | 1.035,∗ | 2.9735,∗ | 1.023† | 3.023† |
| CRP, mg/L | 27% > 336 | 63% > 336 | 2.223 | 2.823 |
| LDH, U/L | 30412 | 48012 | ||
| PRV-1 (CD177) expression, fold upregulation | 2012 | 57612 | ||
| Clonal expansion | ||||
| Splenomegaly prevalence | 12%12 | 91%12 | ||
| Red cell mass (% of normal) | 150%37‡ | 180%37‡ | ||
| CD34 circulation, ×106/L | 312 | 612 | ||
| 15-year myelofibrosis free survival | 100%11 | 40%11 | ||
CH, clonal hematopoiesis; CRP, C-reactive protein; LDH, lactate dehydrogenase; PRV-1, polycythemia rubra vera gene 1; WBC, white blood cell counts.
Subhazard ratio in adjusted model.
Odds ratio in adjusted model; values in bold demonstrated statistical significance.
Estimate made based on highly significant linear correlation.
The growth trajectories of mutant HSCs are varied and reflect the stem cell fitness of mutation-bearing clones that are both mutation-specific and age-specific.39, 40, 41 In MPN, high peripheral blood VAF may overestimate the concomitant HSC burden, given the advantage of JAK2-mutated progenitors to terminally differentiate.4,5 The development of clonal dominance at the HSC and progenitor level occurs variably in the MPN and is a manifestation of stem cell fitness. Stem cell fitness in the MPN, and particularly PV, is correlated with JAK2V617F VAF in HSC and progenitor compartments, with higher VAF in these compartments associating with higher fitness levels.5, 6, 7 The highest fitness levels associated with worse event-free survival and poorer clinical response and predicted those outcomes better than peripheral blood VAF alone. Thus, peripheral VAF is an inadequate surrogate for evaluating stem cell fitness in the MPN in general.7 However, clinically in PV, a high peripheral VAF is a marker for developing clonal expansion of HSC and is an established risk factor for progression to post-PV myelofibrosis (MF).4,5,8,11,38
JAK2V617F VAF is also a critical determinant of both quantitative and qualitative aspects of terminally differentiated cells in PV (Figure 1A-B). Peripheral blood JAK2V617F VAFs greater than 50% correlate with a higher leukocyte count, a higher absolute neutrophil count, a higher hematocrit (HCT), and a lower platelet count than VAFs <50% (Table 1).11,12,38 When considering JAK2V617F VAF in PV as a continuous variable, WBC and HCT tightly positively correlate with VAF, whereas mean corpuscular volume and platelet count negatively correlate with VAF.8 Further, higher JAK2V617F VAF is associated with increased red cell mass, decreased serum iron, decreased transferrin saturation, and lower serum erythropoietin (Table 1).8,37,38,42 Excess HIF may be the basis of the reduced hepcidin, increased erythroferrone, and altered iron metabolism observed in patients with PV, although additional mechanisms may be at play.43 PV neutrophils have increased HIF-mediated gene expression as well as increased expression of tissue factor, F3, the primary initiator of the extrinsic pathway of coagulation, which again correlate with JAK2V617F VAF (Figure 1B)32,43, 44, 45, 46 Further, clonal neutrophils in PV exhibited enhanced ability to release extracellular traps, activate integrin signaling, and enhance adhesion.47, 48, 49
JAK2V617F and thrombotic risk in PV
JAK2V617F and its VAF influence both quantity and quality of blood cells, critical factors associated with the high prevalence of thrombotic events before, at, and after the diagnosis of PV.50,51 Several studies have identified an independent signal of JAK2V617F VAF in thrombosis risk, showing that in PV, VAF ≥50% exerts risk for venous thrombosis (VTE), even after adjusting for prior events, white blood cell count, and age.35,38,52 Focusing on 576 patients with PV, Guglielmelli confirmed that JAK2V617F VAF >50% independently associated with a higher VTE risk and noted that this was evident in both conventionally low-risk and high-risk patients with PV.38 Moreover, JAK2 signaling interacts with traditional vascular risk factors, and in PV, JAK2V617F VAF associated with a higher neutrophil to lymphocyte ratio and carotid plaque burden, and higher elevations of C-reactive protein (Table 1).36,53
The JAK2V617F VAF dosage effect on both cytoses and thrombotic risk observed in PV is recapitulated in clonal hematopoiesis (CH) studies in non-MPN populations. Individuals with JAK2V617F CH had significantly higher white blood cell counts, platelet counts, and hemoglobin concentrations in addition to higher rates of arterial and venous thrombotic events compared with those without CH.54, 55, 56, 57 In 2019, Cordua et al explored the association between JAK2V617F-positive CH and VAF in 19 958 participants in the Danish General Suburban Population, using an assay sensitive to 0.009% VAF. JAK2V617F-positive CH was detected in 3.1% with a median VAF of 2.1%. Individuals with JAK2V617F CH and higher VAFs had higher blood cell counts and a greater risk for developing venous thrombosis, pulmonary embolism, or a cerebrovascular event, demonstrating the increase in thrombotic events associated with JAK2V617F positivity and with increasing clonal burden (Table 1).23
JAK2V617F VAF increase over time and progression risk in CH and PV
Clonal expansion, as evidenced by increasing JAK2V617F VAF over time, has consequences in both CH and PV. In the general population, JAK2V617F CH progresses to overt MPN and is associated with increased JAK2V617F VAF.56,57 Cordua and colleagues enrolled 41 JAK2V617F mutation-positive individuals from the Danish Suburban Population study to undergo re-evaluation on average 7 years after their initial exam. Individuals were classified as no MPN, possible MPN, or MPN based on bone marrow evaluation and peripheral blood counts. JAK2V617F VAF baseline and increase from baseline were higher (5.9 and 9.4, respectively) in the MPN group than those measures in the no MPN group (.2 and .39, respectively).24 Like in CH, in PV, JAK2V617F VAF is a dynamic, time-dependent factor. Although the diagnosis of PV occurs most commonly in the sixth decade of life, acquisition of JAK2V617F occurs in an individual years to decades before clinical presentation.58,59 A 2010 study estimated an increase in neutrophil JAK2V617F VAF over the first 10 years after PV diagnosis to be 1.4% per year, whereas a 2022 analysis of the hydroxyurea (HU)–treated arm of the PROUD-PV study showed an average VAF increase of 7% over 5 years (1.4% per year).22,60 Increasing JAK2V617F VAF in PV is associated with longer disease duration, higher risk of developing post-PV MF, higher lactate dehydrogenase (LDH), and more prevalent splenomegaly (Figure 1C; Table 1).8,11,12,38 Indeed, the mean JAK2V617F VAF in recently diagnosed patients with low-risk PV enrolled in the LOW-PV trial was 35%, whereas the mean VAF in patients with high-risk PV enrolled in the RESPONSE-1 trial was 75% (Table 2).61
Table 2.
JAK2V617F VAF in prospective PV clinical trials
| Conventional risk | Age (y) | Disease duration (y) | Previous cytoreductive (%) |
Thrombosis (%) |
WBC, ×109/L |
Median JAK2V617F VAF (%) | |
|---|---|---|---|---|---|---|---|
| LOW-PV61 | Low | 50 | <2 | 0 | 0 | 10 | 35 |
| PROUD-PV62 | Low and high | 60 | <2 | 30 | 20 | 10 | 42 |
| RESPONSE-263 | High | 65 | 6 | 100 | 26 | 12 | 53 |
| RESPONSE-13 | High | 61 | 8 | 100 | 30 | 18 | 76 |
WBC, white blood cell count.
PV treatment and JAK2V617F VAF
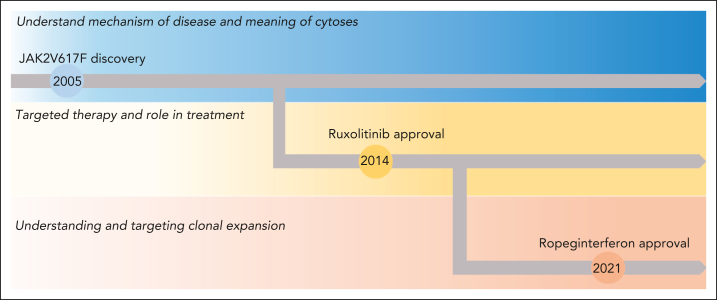
The study of JAK2V617F VAF has reinforced our understanding of the latency of clonal dynamics in PV and explains the decades-long courses of individuals with PV. The treatment of PV is therefore on decades-long terms, with the goals of preventing thromboses, improving PV-associated symptoms, and preventing the progression of the disease to MF or acute myeloid leukemia (AML) (Figure 2). The strategy to achieve these goals since the time of Dameshek had been to consider patients with PV as “generally normal” and to target the production of excess circulating blood cells.64 However, we have learned that PV is fundamentally abnormal, with overactivation of the JAK2 signaling pathway resulting in a thrombo-inflammatory state and a disordered HSC niche that propagates disease progression to MF or AML (Figure 1B-C). Accordingly, clinical trials in PV must be carried out over at least a 5-year period to assess outcomes and their relationship to clonal dynamics.
Figure 2.
PV clinical and research goals. The 2005 JAK2V617F discovery opened new windows for the understanding and treatment of PV. Hydroxyurea and phlebotomy have been used in the past and at present to manage cytoses. In 2014, the first targeted therapy, ruxolitinib, was approved for PV. More recently the focus has shifted to understanding disease latency and targeting clonal expansion, and ropeginterferon was approved in 2021.
Targeting blood counts, thrombosis risks, and disease progression in PV
The cornerstone of therapy in PV is controlling HCT (Figure 2; Table 3). The importance of HCT control was validated in CYTO-PV, where cardiovascular events and deaths were reduced by fourfold when hematocrit was targeted to below 45% vs 50%, with treatments to achieve these targets primarily phlebotomy and/or HU.72 Beyond HCT, control of white cell or platelet count and beneficial outcomes in PV have been more difficult to define, given the strong influence of HCT control on thrombosis outcomes. Problematically, real-world analyses in PV, where the primary therapies were phlebotomy and HU, failed to demonstrate the benefit of normalizing blood cell counts in reducing the risk of thrombosis, even when applying ELN response criteria to the analyses.73,74 Control of HCT was a primary end point of the RESPONSE-1 and RESPONSE-2 trials, where ruxolitinib was tested against the best available therapy (BAT; majority HU) in individuals requiring phlebotomy and with an enlarged spleen (RESPONSE-1) or without an enlarged spleen (RESPONSE-2).3,63 Ruxolitinib arms in both trials demonstrated superior HCT control, and a higher probability of normalization of blood counts, in both the original and 5 year extension trials.68,69 The PROUD-PV and CONTINUATION-PV trials, where patients with PV were randomized to either ropeginterferon or HU, showed equivalent complete hematologic responses at 12 months but significantly higher complete hematologic responses in the ropeginterferon arms at 24, 36, and 60 months .60,62
Table 3.
Effects of therapy on JAK2V617F cell quantity, quality, and clonal expansion
| Phlebotomy | Hydroxyurea | Ruxolitinib | Ropeginterferon | |
|---|---|---|---|---|
| Quantity | + | ++ | +++ | +++ |
| Comments | Rapid reduction of red cells and blood volume | Control of WBC, RBC, and platelets | Control of WBC, RBC, and platelets. Most reliable hematocrit control | Control of WBC, RBC, and platelets, sustained with molecular responses |
| Quality | – | – | + | + |
| Comments | Reinforces iron deficiency, activates HIF42,46 | Selection stress on bone marrow HSC65 | Reduced intracellular signaling47 | Targets and extinguishes JAK2V617F-positive HSC66 |
| Clonal suppression | – | – | + | +++ |
| Comments | VAF increases ∼1% per year61 | Transient reduction in first year, then rebounds VAF increases ∼1% per year60,67 | Mild to moderate, but highly variable VAF reduction3,68,69 | Consistent reduction in VAF VAF decreases ∼1% per month over 36 months60,61,70,71 |
EMH, extramedullary hematopoiesis; RBC, red blood cell; WBC, white blood cell.
Superior blood count control does translate to reduced thrombotic risk in prospective trials, although many years of follow-up are required to observe a significant signal. Thrombosis rates per 100 patient years in the 5-year follow-up studies of the RESPONSE-1 (1.2%) and −2 (1.5%) trials were lower in the ruxolitinib arms than those in BAT, although this did not achieve statistical significance. In contrast, MAJIC-PV, a randomized prospective trial testing upfront treatment with ruxolitinib vs BAT in high-risk PV, showed significantly improved thrombosis-free, progression-free, and overall survival in ruxolitinib-treated patients.75 The PROUD-PV and CONTINUATION-PV trials showed low but very similar thrombotic event rates at 3- and 5-year follow-up in the ropeginterferon arm compared with HU; however, by 6 years of follow-up, event-free survival (disease progression, death, and thromboembolic events) was significantly higher among ropeginterferon alfa-2b–treated patients.76
Evaluating JAK2V617F VAF in prospective clinical trials
Prospective PV trials where VAF was collected show distinct dynamics in both the HU- and phlebotomy-only treatment arms. In the DAHLIA, PROUD-PV and Myeloproliferative Disorders Research Consortium 112, HU was shown to reduce JAK2V617F VAF in the first year but then rebound and continue to rise after 2 years, such that the VAF increase over 5 years was roughly 1.2% per year (Table 3).60,67,77 Similarly, the VAF rise in the BAT arm in RESPONSE-1 was 1.2% at week 32, and in RESPONSE-2, was 1.2% per year over the 5-year trial.3,69 In the LOW-PV study, where phlebotomy alone was compared with phlebotomy plus low-dose ropeginterferon, the increase in JAK2V617F VAF in the phlebotomy arm was 1% over 12 months (Table 3).61
In contrast, both ruxolitinib and ropeginterferon induced sustained reductions in JAK2V617F VAF. In RESPONSE-1, the mean baseline VAF of 75% fell to 47% at 5 years in the ruxolitinib arm, whereas in RESPONSE-2, the median baseline VAF of 53% fell to 38% at 5 years.68,69,78 Lower JAK2V617F VAF nadirs were achieved by treatment with pegylated interferons. In a phase 2 trial of peginterferon alfa-2a in PV, VAF dropped from 45% to 3% by 3 years, with nearly identical dynamics in other phase 2 trials of either peginterferon alfa-2a or ropeginterferon, whether used alone or in combination with ruxolitinib (Table 4 ).70,80,81 In the randomized trials of ropeginterferon vs phlebotomy alone (LOW-PV) or HU (PROUD-PV), VAF reductions in the ropeginterferon arms were nearly identical between the 2 studies at 12 and 24 months (Table 4).60,61,79 Over the first 24 months of ropeginterferon treatment, JAK2V617F VAF on average declined by 1% per month in the PROUD-PV study, and at a very similar rate in 4 additional trials of this biologic in PV.60,79,82 In PROUD-PV/CONTINUATION-PV, 60 months of ropeginterferon reduced the mean baseline JAK2V617F VAF from 37% to 8%, and 20% of individuals achieved a VAF below 1%.60,76 (Table 4)
Table 4.
JAK2V617F VAF dynamics in prospective PV clinical trials
| Treatment | Baseline JAK2V617F VAF (%) | 12-month JAK2V617F VAF (%) | 24-month JAK2V617F VAF (%) | 60-month JAK2V617F VAF (%) | |
|---|---|---|---|---|---|
| LOW-PV61,79 | Phlebotomy | 35 | 36 | ||
| PROUD-PV62 | BAT, HU | 42 | 18 | 25 | 44 |
| RESPONSE-263 | BAT, HU | 66 | 72 | ||
| RESPONSE-13 | BAT, HU | 76 | 77 | ||
| LOW-PV61,79 | Ropeginterferon | 35 | 25 | 12 | |
| PROUD-PV62 | Ropeginterferon | 37 | 24 | 14 | 8 |
| Pegasys in PV80 | Peginterferon alfa-2a | 45 | 22 | 5 | |
| COMBI71 | Peginterferon alfa-2a or peginterferon alfa-2b and ruxolitinib | 47 | 12 | ||
| COMBI-II81 | Peginterferon alfa-2a and ruxolitinib | 47 | 6 |
BAT, best available therapy.
JAK2V617F VAF reductions were more commonly associated with superior complete hematologic responses and spleen responses in both the RESPONSE-1 and PROUD-PV trials.3,62 In analyses of >5-year follow-up of patients with PV treated with ropeginterferon, JAK2V617F VAF was reduced compared with those treated with HU, and was associated with complete hematologic responses and reduced disease progression.60 Although ruxolitinib has a more modest effect on VAF in PV when used in the high-risk setting, ruxolitinib was associated with deeper molecular responses compared with BAT, and those with deeper molecular responses (<2% VAF) had a greater likelihood of complete hematologic responses and improved progression-free survival.75,83
Ruxolitinib and interferons have different impacts on the molecular response in PV, potentially explained by the differential ability of the agents to target the mutant long-term stem cell pool, but this interpretation is confounded by the fact that ruxolitinib trials treated higher-risk (and higher median VAF) patients with PV.66 JAK2V617F VAF sensitivity testing was <0.01% in many of the recent trials, and thus could detect 3 log reductions. The average depth of molecular response achieved by interferon therapies in the reported studies are 1 to 2-log reduction, although some patients may achieve more (Tables 2 and 4).84 Moreover, in the interferon context, deeper molecular responses associate with longer disease remissions after discontinuation of therapy.85,86 It remains to be seen whether deeper molecular responses in PV exert a more profound impact on disease progression, apropos of chronic myeloid leukemia treatment. This is an area of active study, with recent investigations reported on the clinical basis of variation in interferon response and pathways that potentiate the susceptibility of JAK2V617F-mutant stem cells to interferon therapy, and trials that target PV earlier in the disease course. (Figure 2; Table 3).66,71,87, 88, 89 Outstanding questions also center on the degree of VAF reduction to mitigate thrombosis risk, how closely peripheral VAF suppression reflects clonal suppression of JAK2-mutated stem cells, when to initiate therapy, and the durability of clonal suppression after achieving VAF reductions.
Conclusions
The JAK2V617F discovery has elucidated the basis of the quantity of blood cell production, the unique qualities of JAK2V617F cells, and the clonal expansion dynamics of JAK2V617F HSCs in PV. Physicians and their patients can apply the knowledge of JAK2V617F VAF to understand that risk is defined not by blood counts alone but on the intrinsic effects of JAK2V617F VAF that impart risk to both thrombosis and disease progression (Table 5). The meaning of VAF reduction in PV is emerging through the analysis of prospective randomized trials where VAF was correlated with response and outcomes, and proof is mounting that reduction of VAF is tied to not only blood count control but also thrombosis risk reduction and disease progression reduction. Taken together, PV treatments that do not address clonal expansion of JAK2V617F and therefore do not reduce JAK2V617F VAF do not optimally address thrombosis or disease progression risk and represent missed opportunities for our patients to receive disease-modifying therapies (Table 5).
Table 5.
Challenges and opportunities in PV
| Challenge | Opportunity | |
|---|---|---|
| Quantity | Educate patients and doctors about latency and dynamic nature of disease | Understand the role of JAK2V617F VAF as a diagnostic and prognostic marker |
| Meaningful cytoreduction without toxicity | ||
| Quality | Understand mechanism in which JAK2V617F alters cell function | Design and use medications that will reform cell function and reduce thrombosis risk |
| Understand methods of thrombosis risk reduction | ||
| Clonal expansion | Scientific underpinning of latent and dynamic disease owing to the clonal nature of JAK2V617F | Design trials and drugs to address clonal expansion |
| Study mechanisms underlying both latency and clonal expansion |
PV therapy, like chronic myeloid leukemia therapy, requires years of exposure to achieve molecular responses, and thus off-target effects, tolerability, immune suppression, and cancer development are additional major concerns and challenges (Table 5). Long-term exposure to agents that place JAK2V617F HSCs under a selection stress without ultimate clonal suppression, translate to selection for mutational events that drive progression or AML (Tables 4 and 5).65 The study of JAK2V617F pathophysiology has yielded many new candidates to target clonal expansion in PV. Therapies that upregulate hepcidin,90 suppress key inflammatory signaling pathways,26,91,92 activate p53,93,94 inhibit HIF,95 target DNA repair pathways,30,96 or facilitate JAK2V617F stem cell extinction97,98 are exciting developments for PV and will complement JAK2 inhibitor and interferon alfa–based therapies. Our clinical and research goals should be to incorporate these new targets in the treatment of PV, to use the knowledge of JAK2V617F VAF to better prognosticate and monitor clonal dynamics, and to aspire to provide reliable, safe, and timely clonal suppression (Table 5).
Conflict-of-interest disclosure: A.R.M. received consulting fees from PharmaEssentia and Protagonist. B.N.R. has received consulting fees from PharmaEssentia, CTI BioPharma, and Incyte. H.K. declares no competing financial interests.
Acknowledgments
The authors are honored to prepare this review and thank the global community of patients, clinicians, and scientists for participating in PV research.
A.R.M. receives research support through the MPN Research Foundation and National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) grant 1R44HL162386-01A1. H.K. is supported by NIH, NHLBI grant T32HL007525 and the Nancy and Lou Grasmick Scholarship Fund. B.N.R. is supported by an HTRS Mentored Research Award (supported by an educational grant from CSL Behring) and NIH, National Center for Advancing Translational Sciences grant UL1TR002489.
The authors dedicate this work to Richard Silver, Jerry Spivak, and Josef Prchal, who, through their devotion to intellectual curiosity, mentorship, and science, have inspired generations of MPN physician-scientists.
Authorship
Contribution: A.R.M. and B.N.R. designed the research and wrote and edited the manuscript; and H.K. designed the research, edited the manuscript, and created the figures.
References
- 1.Arber DA, Orazi A, Hasserjian RP, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200–1228. doi: 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerds AT, Gotlib J, Ali H, et al. Myeloproliferative Neoplasms, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(9):1033–1062. doi: 10.6004/jnccn.2022.0046. [DOI] [PubMed] [Google Scholar]
- 3.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(17):1670–1671. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James C, Mazurier F, Dupont S, et al. The hematopoietic stem cell compartment of JAK2V617F-positive myeloproliferative disorders is a reflection of disease heterogeneity. Blood. 2008;112(6):2429–2438. doi: 10.1182/blood-2008-02-137877. [DOI] [PubMed] [Google Scholar]
- 5.Moliterno AR, Williams DM, Rogers O, Isaacs MA, Spivak JL. Phenotypic variability within the JAK2 V617F-positive MPD: roles of progenitor cell and neutrophil allele burdens. Exp Hematol. 2008;36(11):1480–1486. doi: 10.1016/j.exphem.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godfrey AL, Chen E, Pagano F, et al. JAK2V617F homozygosity arises commonly and recurrently in PV and ET, but PV is characterized by expansion of a dominant homozygous subclone. Blood. 2012;120(13):2704–2707. doi: 10.1182/blood-2012-05-431791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Zeinah G, Di Giandomenico S, Choi D, et al. Hematopoietic fitness of JAK2V617F myeloproliferative neoplasms is linked to clinical outcome. Blood Adv. 2022;6(18):5477–5481. doi: 10.1182/bloodadvances.2022007128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vannucchi AM, Antonioli E, Guglielmelli P, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21(9):1952–1959. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Zeinah G, Krichevsky S, Cruz T, et al. Interferon-alpha for treating polycythemia vera yields improved myelofibrosis-free and overall survival. Leukemia. 2021;35(9):2592–2601. doi: 10.1038/s41375-021-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karantanos T, Chaturvedi S, Braunstein EM, et al. Sex determines the presentation and outcomes in MPN and is related to sex-specific differences in the mutational burden. Blood Adv. 2020;4(12):2567–2576. doi: 10.1182/bloodadvances.2019001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24(9):1574–1579. doi: 10.1038/leu.2010.148. [DOI] [PubMed] [Google Scholar]
- 12.Larsen TS, Pallisgaard N, Møller MB, Hasselbalch HC. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis--impact on disease phenotype. Eur J Haematol. 2007;79(6):508–515. doi: 10.1111/j.1600-0609.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 13.Benlabiod C, Dagher T, Marty C, Villeval JL. Lessons from mouse models of MPN. Int Rev Cell Mol Biol. 2022;366:125–185. doi: 10.1016/bs.ircmb.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Mullally A, Poveromo L, Schneider RK, Al-Shahrour F, Lane SW, Ebert BL. Distinct roles for long-term hematopoietic stem cells and erythroid precursor cells in a murine model of Jak2V617F-mediated polycythemia vera. Blood. 2012;120(1):166–172. doi: 10.1182/blood-2012-01-402396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansier O, Kilani B, Guitart AV, et al. Description of a knock-in mouse model of JAK2V617F MPN emerging from a minority of mutated hematopoietic stem cells. Blood. 2019;134(26):2383–2387. doi: 10.1182/blood.2019001163. [DOI] [PubMed] [Google Scholar]
- 16.Hinds DA, Barnholt KE, Mesa RA, et al. Germ line variants predispose to both JAK2 V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood. 2016;128(8):1121–1128. doi: 10.1182/blood-2015-06-652941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao EL, Nandakumar SK, Liao X, et al. Inherited myeloproliferative neoplasm risk affects haematopoietic stem cells. Nature. 2020;586(7831):769–775. doi: 10.1038/s41586-020-2786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler MD, Damask A, O'Keeffe S, et al. Common and rare variant associations with clonal haematopoiesis phenotypes. Nature. 2022;612(7939):301–309. doi: 10.1038/s41586-022-05448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen KM, Çolak Y, Ellervik C, Hasselbalch HC, Bojesen SE, Nordestgaard BG. Loss-of-function polymorphism in IL6R reduces risk of JAK2V617F somatic mutation and myeloproliferative neoplasm: a Mendelian randomization study. EClinicalMedicine. 2020;21 doi: 10.1016/j.eclinm.2020.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moliterno AR, Braunstein EM. The roles of sex and genetics in the MPN. Int Rev Cell Mol Biol. 2022;366:1–24. doi: 10.1016/bs.ircmb.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Sobas M, Kiladjian JJ, Beauverd Y, et al. Real world study of children and young adults with myeloproliferative neoplasms identifying risks and unmet needs. Blood Adv. 2022;6(17):5171–5183. doi: 10.1182/bloodadvances.2022007201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein BL, Williams DM, Wang NY, et al. Sex differences in the JAK2 V617F allele burden in chronic myeloproliferative disorders. Haematologica. 2010;95(7):1090–1097. doi: 10.3324/haematol.2009.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cordua S, Kjaer L, Skov V, Pallisgaard N, Hasselbalch HC, Ellervik C. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood. 2019;134(5):469–479. doi: 10.1182/blood.2019001113. [DOI] [PubMed] [Google Scholar]
- 24.Cordua S, Kjaer L, Skov V, et al. Early detection of myeloproliferative neoplasms in a Danish general population study. Leukemia. 2021;35(9):2706–2709. doi: 10.1038/s41375-021-01159-8. [DOI] [PubMed] [Google Scholar]
- 25.Arranz L, Sánchez-Aguilera A, Martín-Pérez D, et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]
- 26.Rai S, Grockowiak E, Hansen N, et al. Inhibition of interleukin-1β reduces myelofibrosis and osteosclerosis in mice with JAK2-V617F driven myeloproliferative neoplasm. Nat Commun. 2022;13(1):5346. doi: 10.1038/s41467-022-32927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagoya Y, Yoshimi A, Tsuruta-Kishino T, et al. JAK2V617F+ myeloproliferative neoplasm clones evoke paracrine DNA damage to adjacent normal cells through secretion of lipocalin-2. Blood. 2014;124(19):2996–3006. doi: 10.1182/blood-2014-04-570572. [DOI] [PubMed] [Google Scholar]
- 28.Stetka J, Vyhlidalova P, Lanikova L, et al. Addiction to DUSP1 protects JAK2V617F-driven polycythemia vera progenitors against inflammatory stress and DNA damage, allowing chronic proliferation. Oncogene. 2019;38(28):5627–5642. doi: 10.1038/s41388-019-0813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumeister J, Chatain N, Hubrich A, et al. Hypoxia-inducible factor 1 (HIF-1) is a new therapeutic target in JAK2V617F-positive myeloproliferative neoplasms. Leukemia. 2020;34(4):1062–1074. doi: 10.1038/s41375-019-0629-z. [DOI] [PubMed] [Google Scholar]
- 30.Nieborowska-Skorska M, Maifrede S, Dasgupta Y, et al. Ruxolitinib-induced defects in DNA repair cause sensitivity to PARP inhibitors in myeloproliferative neoplasms. Blood. 2017;130(26):2848–2859. doi: 10.1182/blood-2017-05-784942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marty C, Lacout C, Droin N, et al. A role for reactive oxygen species in JAK2 V617F myeloproliferative neoplasm progression. Leukemia. 2013;27(11):2187–2195. doi: 10.1038/leu.2013.102. [DOI] [PubMed] [Google Scholar]
- 32.Marinaccio C, Suraneni P, Celik H, et al. LKB1/STK11 Is a tumor suppressor in the progression of myeloproliferative neoplasms. Cancer Discov. 2021;11(6):1398–1410. doi: 10.1158/2159-8290.CD-20-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andréasson B, Swolin B, Kutti J. Increase of CD34 positive cells in polycythaemia vera. Eur J Haematol. 1997;59(3):171–176. doi: 10.1111/j.1600-0609.1997.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 34.Casu C, Liu A, De Rosa G, et al. Tmprss6-ASO as a tool for the treatment of polycythemia vera mice. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0251995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soudet S, Le Roy G, Cadet E, et al. JAK2 allele burden is correlated with a risk of venous but not arterial thrombosis. Thromb Res. 2022;211:1–5. doi: 10.1016/j.thromres.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Barbui T, Carobbio A, Finazzi G, et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica. 2011;96(2):315–318. doi: 10.3324/haematol.2010.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maslah N, Ravdan O, Drevon L, et al. Revisiting Diagnostic performances of serum erythropoïetin level and JAK2 mutation for polycythemias: analysis of a cohort of 1090 patients with red cell mass measurement. Br J Haematol. 2022;196(3):676–680. doi: 10.1111/bjh.17848. [DOI] [PubMed] [Google Scholar]
- 38.Guglielmelli P, Loscocco GG, Mannarelli C, et al. JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis. Blood Cancer J. 2021;11(12):199. doi: 10.1038/s41408-021-00581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabre MA, de Almeida JG, Fiorillo E, et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606(7913):335–342. doi: 10.1038/s41586-022-04785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson CJ, Papula AL, Poon GYP, et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science. 2020;367(6485):1449–1454. doi: 10.1126/science.aay9333. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen C, Bojesen SE, Nordestgaard BG, Kofoed KF, Birgens HS. JAK2V617F somatic mutation in the general population: myeloproliferative neoplasm development and progression rate. Haematologica. 2014;99(9):1448–1455. doi: 10.3324/haematol.2014.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu D, Xu Z, Zhang P, et al. Iron deficiency in JAK2 exon12 and JAK2-V617F mutated polycythemia vera. Blood Cancer J. 2021;11(9):154. doi: 10.1038/s41408-021-00552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ginzburg YZ, Feola M, Zimran E, Varkonyi J, Ganz T, Hoffman R. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia. 2018;32(10):2105–2116. doi: 10.1038/s41375-018-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gangaraju R, Song J, Kim SJ, et al. Thrombotic, inflammatory, and HIF-regulated genes and thrombosis risk in polycythemia vera and essential thrombocythemia. Blood Adv. 2020;4(6):1115–1130. doi: 10.1182/bloodadvances.2019001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casu C, Oikonomidou PR, Chen H, et al. Minihepcidin peptides as disease modifiers in mice affected by β-thalassemia and polycythemia vera. Blood. 2016;128(2):265–276. doi: 10.1182/blood-2015-10-676742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jimenez K, Khare V, Evstatiev R, et al. Increased expression of HIF2α during iron deficiency-associated megakaryocytic differentiation. J Thromb Haemost. 2015;13(6):1113–1127. doi: 10.1111/jth.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolach O, Sellar RS, Martinod K, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10(436) doi: 10.1126/scitranslmed.aan8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves BN, Kim SJ, Song J, et al. Tissue factor activity is increased in neutrophils from JAK2 V617F-mutated essential thrombocythemia and polycythemia vera patients. Am J Hematol. 2022;97(2):E37–E40. doi: 10.1002/ajh.26402. [DOI] [PubMed] [Google Scholar]
- 49.Edelmann B, Gupta N, Schnoeder TM, et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J Clin Invest. 2018;128(10):4359–4371. doi: 10.1172/JCI90312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enblom A, Lindskog E, Hasselbalch H, et al. High rate of abnormal blood values and vascular complications before diagnosis of myeloproliferative neoplasms. Eur J Intern Med. 2015;26(5):344–347. doi: 10.1016/j.ejim.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Moliterno AR, Ginzburg YZ, Hoffman R. Clinical insights into the origins of thrombosis in myeloproliferative neoplasms. Blood. 2021;137(9):1145–1153. doi: 10.1182/blood.2020008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Zhou Y, Wang Y, et al. Thrombosis among 1537 patients with JAK2(V617F) -mutated myeloproliferative neoplasms: risk factors and development of a predictive model. Cancer Med. 2020;9(6):2096–2105. doi: 10.1002/cam4.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon SS, Yoon SY, Jeong SY, et al. Neutrophil-lymphocyte ratio and carotid plaque burden in patients with essential thrombocythemia and polycythemia vera. Nutr Metab Cardiovasc Dis. 2022;32(8):1913–1916. doi: 10.1016/j.numecd.2022.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Xu X, Zhang Q, Luo J, et al. JAK2(V617F): prevalence in a large Chinese hospital population. Blood. 2007;109(1):339–342. doi: 10.1182/blood-2006-03-009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen C, Birgens HS, Nordestgaard BG, Kjaer L, Bojesen SE. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011;96(3):450–453. doi: 10.3324/haematol.2010.033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen C, Birgens HS, Nordestgaard BG, Bojesen SE. Diagnostic value of JAK2 V617F somatic mutation for myeloproliferative cancer in 49 488 individuals from the general population. Br J Haematol. 2013;160(1):70–79. doi: 10.1111/bjh.12099. [DOI] [PubMed] [Google Scholar]
- 58.Van Egeren D, Escabi J, Nguyen M, et al. Reconstructing the lineage histories and differentiation trajectories of individual cancer cells in myeloproliferative neoplasms. Cell Stem Cell. 2021;28(3):514–523.e9. doi: 10.1016/j.stem.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams N, Lee J, Mitchell E, et al. Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature. 2022;602(7895):162–168. doi: 10.1038/s41586-021-04312-6. [DOI] [PubMed] [Google Scholar]
- 60.Kiladjian JJ, Klade C, Georgiev P, et al. Long-term outcomes of polycythemia vera patients treated with ropeginterferon Alfa-2b. Leukemia. 2022;36(5):1408–1411. doi: 10.1038/s41375-022-01528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barbui T, Vannucchi AM, De Stefano V, et al. Ropeginterferon alfa-2b versus phlebotomy in low-risk patients with polycythaemia vera (Low-PV study): a multicentre, randomised phase 2 trial. Lancet Haematol. 2021;8(3):e175–e184. doi: 10.1016/S2352-3026(20)30373-2. [DOI] [PubMed] [Google Scholar]
- 62.Gisslinger H, Klade C, Georgiev P, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7(3):e196–e208. doi: 10.1016/S2352-3026(19)30236-4. [DOI] [PubMed] [Google Scholar]
- 63.Passamonti F, Griesshammer M, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): a randomised, open-label, phase 3b study. Lancet Oncol. 2017;18(1):88–99. doi: 10.1016/S1470-2045(16)30558-7. [DOI] [PubMed] [Google Scholar]
- 64.Dameshek W. Physiopathology and course of polycythemia vera as related to therapy. J Am Med Assoc. 1950;142(11):790–797. doi: 10.1001/jama.1950.02910290018005. [DOI] [PubMed] [Google Scholar]
- 65.Spivak JL. Advances in polycythemia vera and lessons for acute leukemia. Best Pract Res Clin Haematol. 2021;34(4) doi: 10.1016/j.beha.2021.101330. [DOI] [PubMed] [Google Scholar]
- 66.Mosca M, Hermange G, Tisserand A, et al. Inferring the dynamics of mutated hematopoietic stem and progenitor cells induced by IFNα in myeloproliferative neoplasms. Blood. 2021;138(22):2231–2243. doi: 10.1182/blood.2021010986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mascarenhas J, Kosiorek HE, Prchal JT, et al. A randomized phase 3 trial of interferon-α vs hydroxyurea in polycythemia vera and essential thrombocythemia. Blood. 2022;139(19):2931–2941. doi: 10.1182/blood.2021012743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kiladjian JJ, Zachee P, Hino M, et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020;7(3):e226–e237. doi: 10.1016/S2352-3026(19)30207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Passamonti F, Palandri F, Saydam G, et al. Ruxolitinib versus best available therapy in inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): 5-year follow up of a randomised, phase 3b study. Lancet Haematol. 2022;9(7):e480–e492. doi: 10.1016/S2352-3026(22)00102-8. [DOI] [PubMed] [Google Scholar]
- 70.Them NCC, Bagienski K, Berg T, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol. 2015;90(4):288–294. doi: 10.1002/ajh.23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sørensen AL, Mikkelsen SU, Knudsen TA, et al. Ruxolitinib and interferon-α2 combination therapy for patients with polycythemia vera or myelofibrosis: a phase II study. Haematologica. 2020;105(9):2262–2272. doi: 10.3324/haematol.2019.235648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- 73.Ronner L, Podoltsev N, Gotlib J, et al. Persistent leukocytosis in polycythemia vera is associated with disease evolution but not thrombosis. Blood. 2020;135(19):1696–1703. doi: 10.1182/blood.2019003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tremblay D, Srisuwananukorn A, Ronner L, et al. European LeukemiaNet response predicts disease progression but not thrombosis in polycythemia vera. Hemasphere. 2022;6(6) doi: 10.1097/HS9.0000000000000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harrison C, Nangalia J, Boucher RH, et al. Ruxolitinib versus best available therapy for PV intolerant or resistant to hydroxycarbamide in a randomized trial [abstract] Blood. 2022;140(suppl 1) Abstract 739. [Google Scholar]
- 76.Gisslinger H, Klade C, Georgiev P, et al. S196: ropeginterferon alfa-2B achieves patient-specific treatment goals in polycythemia vera: final results from the PROUD-PV/CONTINUATION-PV studies. HemaSphere. 2022;6:97–98. [Google Scholar]
- 77.Dam MJB, Pedersen RK, Knudsen TA, et al. A novel integrated biomarker index for the assessment of hematological responses in MPNs during treatment with hydroxyurea and interferon-alpha2. Cancer Med. 2022 doi: 10.1002/cam4.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Griesshammer M, Saydam G, Palandri F, et al. Ruxolitinib for the treatment of inadequately controlled polycythemia vera without splenomegaly: 80-week follow-up from the RESPONSE-2 trial. Ann Hematol. 2018;97(9):1591–1600. doi: 10.1007/s00277-018-3365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barbui T, Vannucchi AM, De Stefano V, et al. Ropeginterferon Alfa-2b versus standard therapy for low-risk patients with polycythemia vera. Final results of low-PV randomized phase II trial [abstract] Blood. 2022;140(suppl 1) Abstract 744. [Google Scholar]
- 80.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–3072. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- 81.Sørensen ALL, Skov V, Kjær L, et al. Combination therapy with ruxolitinib and interferon in newly diagnosed patients with polycythemia vera [abstract] Blood. 2022;140(suppl 1) Abstract 3029. [Google Scholar]
- 82.Edahiro Y, Ohishi K, Gotoh A, et al. Efficacy and safety of ropeginterferon alfa-2b in Japanese patients with polycythemia vera: an open-label, single-arm, phase 2 study. Int J Hematol. 2022;116:215–227. doi: 10.1007/s12185-022-03341-9. [DOI] [PubMed] [Google Scholar]
- 83.Guglielmelli P, Mora B, Gesullo F, et al. JAK2V617F molecular response to ruxolitinib in patients with PV and ET is associated with lower risk of progression to secondary myelofibrosis [abstract] Blood. 2022;140(suppl 1) Abstract 741. [Google Scholar]
- 84.Hasselbalch HC, Holmström MO. Perspectives on interferon-alpha in the treatment of polycythemia vera and related myeloproliferative neoplasms: minimal residual disease and cure? Semin Immunopathol. 2019;41(1):5–19. doi: 10.1007/s00281-018-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Daltro De Oliveira R, Soret-Dulphy J, Zhao L-P, et al. Interferon-alpha (IFN) therapy discontinuation is feasible in myeloproliferative neoplasm (MPN) patients with complete hematological remission [abstract] Blood. 2020;136(suppl 1):35–36. [Google Scholar]
- 86.Utke Rank C, Weis Bjerrum O, Larsen TS, et al. Minimal residual disease after long-term interferon-alpha2 treatment: a report on hematological, molecular and histomorphological response patterns in 10 patients with essential thrombocythemia and polycythemia vera. Leuk Lymphoma. 2016;57(2):348–354. doi: 10.3109/10428194.2015.1049171. [DOI] [PubMed] [Google Scholar]
- 87.Saleiro D, Wen JQ, Kosciuczuk EM, et al. Discovery of a signaling feedback circuit that defines interferon responses in myeloproliferative neoplasms. Nat Commun. 2022;13(1):1750. doi: 10.1038/s41467-022-29381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knudsen TA, Skov V, Stevenson KE, et al. Genomic profiling of a randomized trial of interferon-α versus hydroxyurea in MPN reveals mutation-specific responses. Blood Adv. 2022;6(7):2107–2119. doi: 10.1182/bloodadvances.2021004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Skov V, Thomassen M, Kjær L, et al. Interferon-alpha2 treatment of patients with polycythemia vera and related neoplasms favorably impacts deregulation of oxidative stress genes and antioxidative defense mechanisms. PLoS One. 2022;17(6) doi: 10.1371/journal.pone.0270669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffman R, Ginzburg Y, Kremyanskaya M, et al. American Society of Clinical Oncology; 2022. Rusfertide (PTG-300) treatment in phlebotomy-dependent polycythemia vera patients. [Google Scholar]
- 91.Rahman MFU, Yang Y, Le BT, et al. Interleukin-1 contributes to clonal expansion and progression of bone marrow fibrosis in JAK2V617F-induced myeloproliferative neoplasm. Nat Commun. 2022;13(1):5347. doi: 10.1038/s41467-022-32928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li L, Kim JH, Lu W, et al. HMGA1 chromatin regulators induce transcriptional networks involved in GATA2 and proliferation during MPN progression. Blood. 2022;139(18):2797–2815. doi: 10.1182/blood.2021013925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gotlib J, Gabrail N, O'Connell CL, et al. A randomized, open-label, multicenter, phase 2 study to evaluate the efficacy, safety, and pharmacokinetics of KRT-232 compared with ruxolitinib in patients with phlebotomydependent polycythemia vera [abstract] Blood. 2019;134(suppl 1) Abstract 4168. [Google Scholar]
- 94.Mascarenhas J, Passamonti F, Burbury K, et al. The MDM2 antagonist idasanutlin in patients with polycythemia vera: results from a single-arm phase 2 study. Blood Adv. 2022;6(4):1162–1174. doi: 10.1182/bloodadvances.2021006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghosh MC, Zhang DL, Ollivierre WH, et al. Therapeutic inhibition of HIF2α reverses polycythemia and pulmonary hypertension in murine models of two human diseases. Blood. 2021;137(18):2509–2519. doi: 10.1182/blood.2020009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li B, An W, Wang H, et al. BMP2/SMAD pathway activation in JAK2/p53-mutant megakaryocyte/erythroid progenitors promotes leukemic transformation. Blood. 2022;139(25):3630–3646. doi: 10.1182/blood.2021014465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dagher T, Maslah N, Edmond V, et al. JAK2V617F myeloproliferative neoplasm eradication by a novel interferon/arsenic therapy involves PML. J Exp Med. 2021;218(2) doi: 10.1084/jem.20201268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pawinwongchai J, Jangprasert P, Nilsri N, Israsena N, Rojnuckarin P. Mutated JAK2 signal transduction in human induced pluripotent stem cell (iPSC)-derived megakaryocytes. Platelets. 2022;33(5):700–708. doi: 10.1080/09537104.2021.1981850. [DOI] [PubMed] [Google Scholar]