Abstract
Cystic lesions of the adrenal glands are relatively uncommon and most of them are clinically silent. Though rarely associated with malignant changes, they may carry clinically detrimental consequences if misdiagnosed. Cystic adrenal lesions exhibit a broad histomorphological spectrum, ranging from pseudocysts, endothelial cysts, epithelial cysts and parasitic cysts. Here we present the case of a young woman with left-sided abdominal pain and contrast-enhanced CT showing a 10.4×7.7×7.8 cm fluid-filled left suprarenal lesion. The patient underwent exploratory laparotomy with cyst excision, and the histopathological examination of the specimen revealed a pseudocyst of the left adrenal gland. Despite being rare, usually benign and asymptomatic, the diagnosis and management of these cystic lesions of the adrenal glands are often unclear. Any functional lesion, potentially malignant lesion or lesion more than 5 cm deserves surgical management, whereas others can be managed conservatively.
Keywords: Adrenal disorders, Endocrine cancer, Surgical oncology, Urological surgery
Background
Cystic lesions of the adrenal glands are relatively uncommon, with only a handful of case series and case reports published to date. The true incidence of these cysts may be higher, as corroborated by various autopsy series (0.064%–0.18%), as most of them are clinically silent.1 2 They often present with non-specific clinical and radiological findings and are thus usually incidentally diagnosed. When large, they may present with mass effects resulting in gastrointestinal symptoms. Though rarely associated with malignant changes, they may carry clinically detrimental consequences if misdiagnosed. Cystic adrenal lesions exhibit a broad histomorphological spectrum, ranging from pseudocysts, endothelial cysts, epithelial cysts and parasitic cysts. Pseudocysts are the most frequently identified cystic lesions in surgical series, and endothelial cysts account for the most in autopsy series, up to 45%.3 Here we discuss the case of a left-sided adrenal pseudocyst in a young woman, presenting to our department.
Case presentation
A young woman in her 20s, with no known comorbidities, presented to our department with left-sided vague abdominal pain on and off over a period of 3 months. The pain was associated with intermittent episodes of nausea and generalised weakness. There was no history of fever, abdominal distension, weight loss, diarrhoea, constipation, jaundice, haematemesis or melaena. The patient never had similar symptoms in the past, and there was no evidence of similar symptoms in any other members of the family. On clinical examination, she was stable haemodynamically with no palpable organomegaly.
Investigations
Laboratory investigations revealed a haemoglobin level of 133 g/L, total leucocyte count of 7.0 x 1099/L and platelet counts of 147 x 109/L. Her viral markers were all negativend her coagulation profile was within normal limits. Liver function tests, renal function tests, C reactive protein, plasma and urinary fractionated free metanephrines, anti-citrullinated antibody, rheumatoid factor assay, anti-p-ANCA and anti-c-ANCA were all within the normal range. The patient underwent contrast-enhanced CT (CECT) of the abdomen, which showed a well-defined suprarenal fluid-attenuating lesion (figure 1) measuring 7.7×7.8 cm in the axial plane and 10.4 cm in the craniocaudal extent, with thin marginal calcification at places. The lesion was superior to the upper pole of the left kidney just abutting the left adrenal gland and posterior to the tail of the pancreas. There was no evidence of any obvious enhancing solid component or any regional lymphadenopathy. Upper and lower gastrointestinal endoscopy was done, which showed no abnormality.
Figure 1.
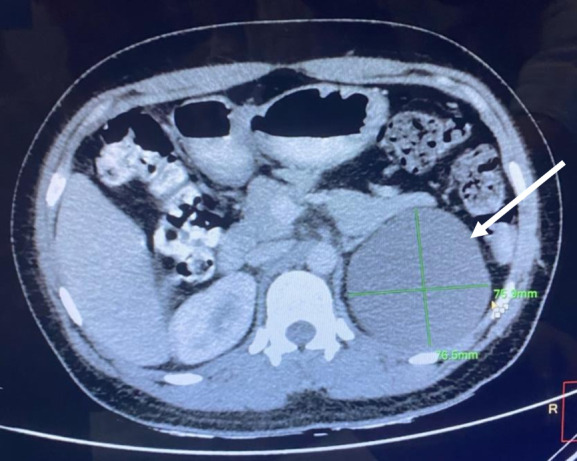
A well-defined suprarenal fluid-attenuating lesion (white arrow) measuring 7.7×7.6 cm in the axial plane and 10.4 cm in the craniocaudal extent, located superior to the upper pole of the left kidney just abutting the left adrenal gland and posterior to the left pancreas.
Treatment
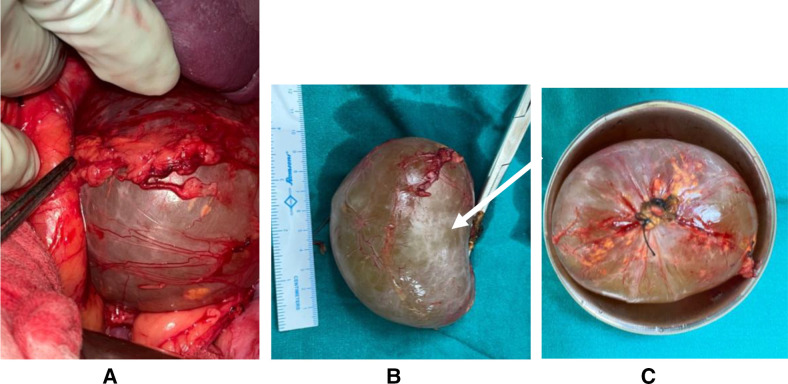
The patient was then provisionally diagnosed as a case of exophytic cystic lesion of the adrenal glands and was planned for exploratory laparotomy with excision of the cyst. The splenic flexure was taken down, and a 10×8 cm cystic lesion was noted in the left suprarenal region (figure 2), arising from the inferolateral aspect of the left adrenal gland, and was free from the upper pole of the left kidney. The cyst was excised along with a partial left adrenalectomy, and the specimen was sent for histopathological evaluation.
Figure 2.
(A) The intraoperative image of the cystic lesion, with adherent adrenal tissue (B) and (C) the excised specimen measuring about 10×8 cm.
Outcome and follow-up
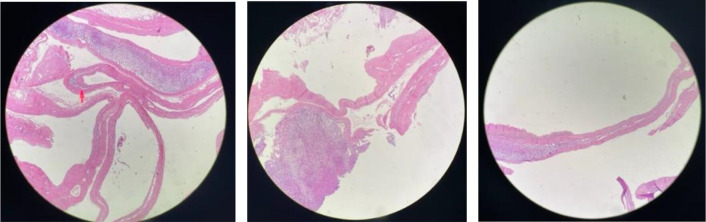
The postoperative course was uneventful and the patient was discharged by the fourth postoperative day. On macroscopic histopathological examination of the specimen, the specimen had a smooth outer surface, with a focally attached adrenal gland and cut surface showed a unilocular cyst. On microscopic examination (figure 3), normal adrenal tissue was seen along with a fibrous cyst wall having focal areas of dystrophic calcification. Some areas showed small foci of large round polygonal cells projecting into the lumen and adherent to the cyst wall. The cells showed abundant cytoplasm with small pyknotic nuclei and condensed chromatin. Also, haemosiderin pigment was identified and no evidence of atypia or malignancy was seen. Further immunohistochemistry evaluation showed polygonal cells to be positive for CD68 and negative for inhibin and synaptophysin. Hence, the diagnosis of adrenal pseudocyst was made. The patient is currently asymptomatic at 9 months of follow-up.
Figure 3.
Multiple sections with normal adrenal tissue along with a fibrous cyst wall having focal areas of dystrophic calcification.
Discussion
A cystic lesion of the adrenal glands was first described in 1670 by Greselius, an Austrian anatomist when it caused death in a patient following the rupture of more than 4 kg of blood and fluid content of the cyst.4 Since then, multiple case series and reports have described these lesions with an incidence ranging from 5% to 6% in surgical adrenalectomy specimens, which was lower than in other autopsy series. Adrenal ‘incidentalomas’ identified on imaging may be seen in 5%–9% of the general population, of which an estimated 4%–22% are adrenal cysts.5 6 Most of the adrenal cysts are unilateral but around 8%–15% can present bilaterally.2 They are most common in the third to fifth decade with a female preponderance (2–3:1) and a size ranging from a few millimetres to as large as 50 cm in some case reports.7 8 The larger lesions are traditionally associated with symptoms of a mass effect, displacing the adjacent abdominal organs. They often present with a palpable flank mass, gastrointestinal symptoms, and more commonly, vague abdominal pain as was seen in our case. Larger lesions also have the risk of massive haemorrhage, rupture, cyst infection or arterial hypertension despite negative hormonal status, which is due to distortion of renal vessels and neuroendocrine/vasoactive stimulations.2
Cystic adrenal lesions are often associated with a variety of syndromes, including polycystic renal disease,9 Klippel-Trenaunay-Weber syndrome,10 Beckwith-Wiedemann syndrome,11 schwannomas, aortic aneurysms, abdominal neuroblastomas and rarely with pregnancy.12 Infrequently, metastasis from other organs like the breast, pancreas, lungs and colon can present as cystic adrenal lesions, which need to be evaluated accordingly.13
Cystic lesions of the adrenal glands have a wide morphological spectrum, as a result of which, multiple classifications have been attempted. The earliest of these was in 1906 by Terrier and Lecene,14 which included haemorrhagic cysts, endothelial cysts, congenital retention cysts, cystic adenomas and parasitic cysts. Further modifications by Abeshouse et al3 in 1959 and subsequently by Foster15 in 1966 from an autopsy series have delineated four different subtypes: pseudocysts (39%), endothelial cysts (45%), epithelial cysts (9%) and parasitic cysts (7%). However, when compared with a surgical series of 41 patients by the Mayo Clinic, pseudocysts (78%) were the most frequently identified cystic adrenal lesions.16 Both pseudocysts and endothelial cysts are types of vascular-origin cysts, whereas the other two are non-vascular. Also, there exists controversy regarding the classification of the cystic neoplasms arising from the cortex or medulla as some authors argue that they should be classified as pseudocysts owing to the inner cyst wall being devoid of any lining, whereas other authors believed them to have epithelial/neuroectodermal derivation.
Pseudocysts are the second most common subtype of adrenal cysts as per autopsy series (39%), but many surgical series describe them to be the most common.3 They are complex cysts, resulting from haemorrhage into the adrenal parenchyma due to trauma, toxins or infections. Other causes include uraemia, pregnancy, Waterhouse-Friderichsen syndrome, acute trauma, burns, adrenal vein thrombosis, syphilis, leukaemia and incompatible blood transfusions.15 There may be anomalous vascular and lymphatic channels surrounding them. They are usually unilocular, encapsulated and devoid of inner wall lining, as seen in our case. Sixty per cent of the cases may show calcification within the fibrous capsules. The cyst cavity usually contains blood clots and proteinaceous materials, and no necrotic debris is usually present. A series by Foster describes around 7% association of pseudocyst with neoplastic lesions including adenomas, haemangiomas, pheochromocytomas or malignant haemangioendothelioma.15 Other smaller series have estimated it to be as high as 18%–44%.17 The pseudocyst usually results from degenerative necrosis and haemorrhage within these tumours. The appearance on imaging in these lesions can vary with the stage of disease progression and is difficult to differentiate from tumours, metastases or abscesses.18 There may be septations, blood or a soft tissue component, but these organise with time and evolve into a homogeneous thin-walled collection with eventually an absence of solid components. MRI can also be used in cases with active intracystic haemorrhage, with acute bleeds seen to be isointense and subacute bleeds hyperintense on T1—both being low intensity on T2.19
Endothelial adrenal cysts account for 45% of all the cysts as per autopsy series, but the incidence drops to 2%–24% in surgical series, signifying its more incidental nature.3 16 They are simple cysts, usually less than 2 cm in diameter, and are thought to arise from pre-existing vascular malformations or ectatic lymphatic channels subsequent to some obstruction.3 20 Accordingly, they are subclassified as hamartomatous, angiomatous and lymphangiomatous subtypes. These lesions have a smooth, flattened and non-proliferating endothelial lining, with clear fluid content (usually in lymphangiomatous cases), and are rarely associated with malignancy. Angiomatous cysts are rarer, with only four case reports to date.1 On imaging, they are unilateral thin-walled lesions with smooth borders. Lymphangiomatous subtypes may usually have internal septations. Any rim enhancement on CECT may be due to the peripheral compressed adrenal tissue.8 19 MRI shows findings similar to simple cysts of other organs, with homogeneous lesions having low T1/high T2 intensity.5
Epithelial cysts are true cysts with a reported incidence of around 6%–9%.21 They are subdivided into (1) glandular or retention cysts due to the presence of displaced urogenital tissue, (2) cystic adenomas and (3) embryonal cysts.17 22 They are thought to have a mesothelial origin due to positive mesothelial immunostaining. They are differentiated from other simple cysts by the true epithelium lining the flattened wall. Imaging is similar to simple cysts and it is rare to find calcification or enhancement in the walls.8
Parasitic cysts are usually echinococcal in origin, and rarely associated with leishmaniasis.7 They are commonly associated with hydatid cystic lesions in other organs too. The cysts are thick walled and may or may not have wall calcification, depending on the stage of the lesion. The usual complications seen with liver or spleen hydatid lesions are also seen with adrenal hydatid lesions. The imaging is also typical with the appearance of echo-free cysts, hydatid sands, floating membranes or daughter cysts.8
Functional cystic adrenal lesions have also been described in the cortex as well as medulla, and owing to severe implications with pheochromocytoma, a thorough evaluation is a must. About 15% of incidentalomas are functional, with an even lesser number of cystic incidentalomas being functional.6 A cystic pheochromocytoma can have negative hormonal screening but CECT shows tumour rim enhancement with a central cystic mass. Also, MRI shows hyperintense lesions in T2 imaging, also called the ‘light bulb sign’.5 In cases with a diagnostic dilemma, metaiodobenzylguanidine scintigraphy has 95% specificity for pheochromocytomas.13 18
The cysts with solid components can harbour neoplastic lesions ranging from benign adenomas to malignant carcinomas. The risk of malignancy is less than 6% in lesions less than 6 cm in size,13 and there is a reported incidence of around 7% typically within pseudocysts.1 Most malignant cysts are usually metastasis (95%), followed by pheochromocytoma and adrenal cortical lesions.23 CECT is the preferred modality for differentiating these lesions from simple cysts, with MRI beneficial in adrenal adenoma cases.5 Hyperattenuating lesions with wall thickness >5 mm, wall enhancement, thick rim or central stippled calcification may suggest malignancy.8 18 The 18-fluorodeoxyglucose positron emission tomography can also help in distinguishing adenomas from malignancy.13 Table 1 describes the radiological and histological characteristics of common differentials of cystic adrenal lesions.
Table 1.
Comparison of various radiological and histological characteristics of cystic adrenal lesions
| Common differentials of cystic adrenal lesions | ||
| Radiological features | Histopathological features | |
| Pseudocyst | CT—well-demarcated round or oval mass with fluid density. Similarity to complex cyst makes it difficult to differentiate from necrosis, metastasis or abscess MRI is better as it detects intracystic haemorrhage (hyperintense on T1/T2) |
Thick walled Dense hyalinised connective tissue with focal calcifications entrapped cortical cells in cyst wall. No endothelial lining. Haemorrhage and haemosiderin common |
| Endothelial cysts | CT—low density (<20 HU) masses with smooth borders and thin walls | Smooth flattened endothelial lining, filled with clear/milky fluid. Absent proliferating endothelium |
| Epithelial cysts | CT—similar to endothelial cysts | Smooth, flattened wall lined with true epithelium |
| Parasitic cysts | CT—hydatid sand, floating membranes, daughter cysts, septal/mural calcifications | Wall and cyst contain eosinophils Calcified parasite may be found in the cyst |
| Adenomas | CT— <10 HU on non contrast CT; <30 HU on CECT; 10-minute delayed CT washout >50%31 MRI—high intracellular lipid leads to drop in signal relative to spleen/liver on chemical shift imaging in MRI. Adrenal:spleen ratio of <0.70 is diagnostic 32 33 |
Cells are larger with different foamy cytoplasm and distinct cell borders Balloon cells with enlarged lipid-rich cytoplasm may be seen34 |
| Adrenocortical tumours | CT—wall thickness >5 mm with wall enhancement, thick rim and stippled central calcification35 | Encapsulated tumour with variably sized nests, large sheets and trabeculae. Large cells with clear to eosinophilic granular cytoplasm present. IGF2 overexpression seen36 |
| Pheochromocytoma | CT—tumour rim enhancement associated with central cystic mass37 High signal intensity on T2 MRI—‘light bulb sign’ MIBG has 95%–100% specificity |
Nested/trabecular/solid arrangement of large polygonal vacuolated cells38 Chromogranin A, synaptophysin and S100 positive |
| Metastasis | CT—ill-defined heterogeneous echotexture with thick enhancing rim on contrast MRI—low T1/high T2 signal. Does not drop signal on opposed-phase MRI (unlike adenoma)39 |
Morphologically similar to the primary tumour |
| Lipoma/myolipoma | Gross fat on CT/MRI Can demonstrate flow on Doppler Pseudo-capsule present |
Mixture of mature adipocytes and extramedullary haematopoietic cells with marked increase in megakaryocytes40 (No haematopoietic cells in lipoma) |
CECT, contrast-enhanced CT; MIBG, metaiodobenzylguanidine.
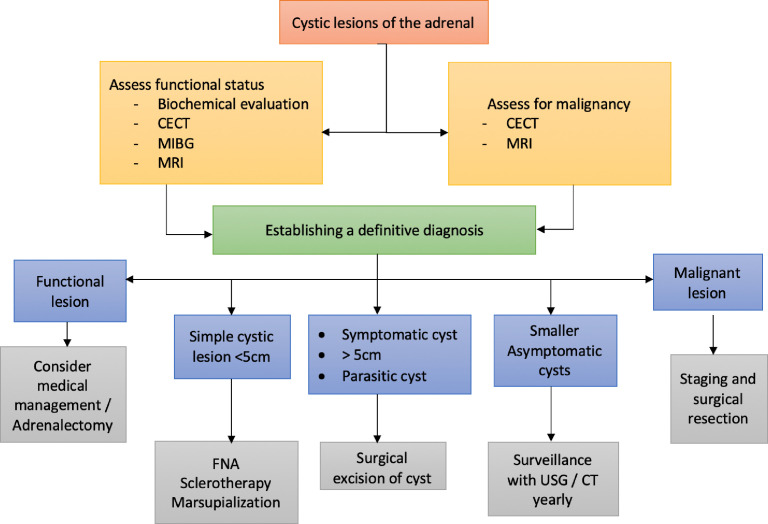
Owing to the relatively low incidence of cystic adrenal lesions and the usual lack of preoperative pathological diagnosis, ambiguity exists regarding the preferred management for these. Various modalities have been tried, including fine-needle aspiration (FNA), sclerotherapy, surgical resection or cyst marsupialisation.1 7 The management includes ruling out the functional status, evaluation for the risk of malignancy by imaging and weighing the benefits of conservative management against potential complications, especially in large cysts.23 24 A general agreement exists that cysts larger than 5 cm should be resected due to the potential risk of haemorrhage and secondary complications. Studies recommend surgical resection for lesions, which are (1) symptomatic, (2) functional, (3) greater than 5 cm, (4) heterogeneous in nature suggestive of malignancy, (5) parasitic cysts and (6) cysts associated with other anomalies like superior vena cava syndrome.2 7 Other small asymptomatic lesions can be followed conservatively with imaging by CECT, MRI or ultrasound, but no consensus exists regarding the surveillance protocol.7
Percutaneous FNA has a sensitivity of around 85% for detecting malignancy in adrenal cysts. Haemorrhagic aspirate, positive cytology or irregular cyst lining on examination should prompt surgical excision of the lesion. Some lesions might regress completely following aspiration and require only surveillance, but the fluid may reaccumulate in about 30%–50% of cases even after sclerotherapy.1 2 Decortication or marsupialisation of the cyst can be done with open or minimally invasive techniques, similar to those done in renal cysts.25 There is a small theoretical risk of malignant spread or seeding, but it has not been corroborated in any study. Surgical excision can be done with simple enucleation of the cyst, which is the procedure of choice for simple cystic lesions.21 In some cases, partial or total adrenalectomy may also be required. Minimally invasive cyst excision can be done laparoscopically or robotically, and have low morbidity and with less blood loss, short hospital stay and better cosmesis.26–30 Resection can also provide a specimen for pathological examination, giving a definitive diagnosis and guiding further follow-up. A flow chart summarising the management of the cystic adrenal lesions is shown in figure 4.
Figure 4.
Summary of management of cystic adrenal lesions. CECT, contrast-enhanced CT; FNA, fine-needle aspiration; MIBG, metaiodobenzylguanidine; USG, ultrasonography.
To summarise, adrenal cysts are an uncommon group of lesions that are usually benign and asymptomatic. However, diagnosis and management can be challenging due to their heterogeneous appearance and difficulty in ascertaining the pathological subtype on routine imaging. It is essential to rule out malignancy and functional status when managing these lesions. Any functional lesion, potentially malignant lesion or lesion more than 5 cm should be surgically managed, while other cases can be managed conservatively. Owing to advances in surgical techniques and minimally invasive options, the morbidity and mortality associated with these lesions have been significantly reduced, even when detecting malignancy by imaging is difficult.
Learning points.
Uncommon group of lesions which are clinically silent in most cases.
Exhibit a broad histomorphological spectrum, ranging from pseudocysts, endothelial cysts, epithelial cysts and parasitic cysts.
Ruling out malignancy and functional status is vital to the management.
Have a heterogeneous appearance based on the pathological subtype, which can be difficult to ascertain on routine imaging.
Any functional lesion, potentially malignant lesion or lesion more than 5 cm deserves surgical management, whereas others can be managed conservatively.
Footnotes
Twitter: @NundySamiran
Contributors: SS—writing the manuscript and collecting data. SB—histopathology images. SN—correction of the draft and supervision. NNM—writing the manuscript, correction of the draft and supervision.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Neri LM, Nance FC. Management of adrenal cysts. The American surgeon 1999;65:151–63. [PubMed] [Google Scholar]
- 2.Bellantone R, Ferrante A, Raffaelli M, et al. Adrenal cystic lesions: Report of 12 surgically treated cases and review of the literature. Journal of endocrinological investigation 1998;21:109–14. 10.1007/BF03350324 [DOI] [PubMed] [Google Scholar]
- 3.ABESHOUSE GA, GOLDSTEIN RB, ABESHOUSE BS. Abeshouse BS: Adrenal cysts: Review of the literature and report of three cases. The Journal of Urology 1959;81:711–9. 10.1016/S0022-5347(17)66099-3 [DOI] [PubMed] [Google Scholar]
- 4.Doran AH. Cystic tumour of the Suprarenal body successfully removed by operation, with notes on cases previously published. British medical Journal 1908;1:1558–63. 10.1136/bmj.1.2478.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsayes KM, Mukundan G, Narra VR, et al. Adrenal masses: MR imaging features with pathologic correlation. Radiographics : A review publication of the radiological society of North America, Inc 2004;24(suppl_1):S73–86. 10.1148/rg.24si045514 [DOI] [PubMed] [Google Scholar]
- 6.Turner DJ, Miskulin J. Management of adrenal lesions. Current opinion in oncology 2009;21:34–40. 10.1097/CCO.0b013e32831d2aa9 [DOI] [PubMed] [Google Scholar]
- 7.Lal TG, Kaulback KR, Bombonati A, et al. Surgical management of adrenal cysts. The American surgeon 2003;69:812–4. [PubMed] [Google Scholar]
- 8.Sanal HT, Kocaoglu M, Yildirim D, et al. Imaging features of benign adrenal cysts. European Journal of Radiology 2006;60:465–9. 10.1016/j.ejrad.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 9.Bastide C, Boyer L, Djellouli N, et al. Bilateral adrenal cysts and Hepatorenal Polycystic disease. La Presse Medicale 1997;26:711–2. [PubMed] [Google Scholar]
- 10.Young SA, Shapiro B. Klippel-Trenaunay-Weber syndrome with adrenal Pseudocyst: Characterization by blood pool and Adrenocortical Iodocholesterol scintigraphy. Clinical nuclear medicine 1998;23:528–31. 10.1097/00003072-199808000-00008 [DOI] [PubMed] [Google Scholar]
- 11.Ciftci AO, Salman AB, Tanyel FC, et al. Bilateral multiple adrenal Pseudocysts associated with incomplete Beckwith-Wiedemann syndrome. J Pediatr Surg 1997;32:1388–90. 10.1016/s0022-3468(97)90332-8 [DOI] [PubMed] [Google Scholar]
- 12.Karaman K, Teke Z, Dalgic T, et al. Giant hemorrhagic adrenal Pseudocyst in a Primiparous pregnancy: Report of a case. Surg today 2011;41:153–8. 10.1007/s00595-009-4207-2 [DOI] [PubMed] [Google Scholar]
- 13.Kuruba R, Gallagher SF. Current management of adrenal tumors. Curr Opin Oncol 2008;20:34–46. 10.1097/CCO.0b013e3282f301fd [DOI] [PubMed] [Google Scholar]
- 14.Terrier F, Lecène P. Les Grands Kystes de la capsule SurréNale. Rev de Chir Paris 1906;34:321. [Google Scholar]
- 15.Foster DG. Adrenal cysts. review of literature and report of case. Arch Surg 1966;92:131–43. 10.1001/archsurg.1966.01320190133032 [DOI] [PubMed] [Google Scholar]
- 16.Erickson LA, Lloyd RV, Hartman R, et al. Cystic adrenal Neoplasms. Cancer 2004;101:1537–44. 10.1002/cncr.20555 [DOI] [PubMed] [Google Scholar]
- 17.Chien H-P, Chang Y-S, Hsu P-S, et al. Adrenal cystic lesions: A Clinicopathological analysis of 25 cases with proposed Histogenesis and review of the literature. Endocr Pathol 2008;19:274–81. 10.1007/s12022-008-9046-y [DOI] [PubMed] [Google Scholar]
- 18.Lockhart ME, Smith JK, Kenney PJ. Imaging of adrenal masses. Eur J Radiol 2002;41:95–112. 10.1016/s0720-048x(01)00444-2 [DOI] [PubMed] [Google Scholar]
- 19.Guo Y-K, Yang Z-G, Li Y, et al. Uncommon adrenal masses: CT and MRI features with histopathologic correlation. Eur J Radiol 2007;62:359–70. 10.1016/j.ejrad.2006.12.011 [DOI] [PubMed] [Google Scholar]
- 20.Torres C, Ro JY, Batt MA, et al. Vascular adrenal cysts: A Clinicopathologic and immunohistochemical study of six cases and a review of the literature. Mod Pathol 1997;10:530–6. [PubMed] [Google Scholar]
- 21.Tagge DU, Baron PL. Giant adrenal Cyst: Management and review of the literature. The American surgeon 1997;63:744–6. [PubMed] [Google Scholar]
- 22.Medeiros LJ, Weiss LM, Vickery AL. Epithelial-lined (true) Cyst of the adrenal gland: A case report. Hum Pathol 1989;20:491–2. 10.1016/0046-8177(89)90017-8 [DOI] [PubMed] [Google Scholar]
- 23.Stimac G, Katusic J, Sucic M, et al. A giant hemorrhagic adrenal Pseudocyst: Case report. Med Princ Pract 2008;17:419–21. 10.1159/000141509 [DOI] [PubMed] [Google Scholar]
- 24.Pradeep PV, Mishra AK, Aggarwal V, et al. Adrenal cysts: An institutional experience. World J Surg 2006;30:1817–20. 10.1007/s00268-005-0307-3 [DOI] [PubMed] [Google Scholar]
- 25.Yoder BM, Wolf JS. Long-term outcome of Laparoscopic Decortication of peripheral and Peripelvic renal and adrenal cysts. J Urol 2004;171(2 Pt 1):583–7. 10.1097/01.ju.0000103642.29044.71 [DOI] [PubMed] [Google Scholar]
- 26.El-Hefnawy AS, El Garba M, Osman Y, et al. Surgical management of adrenal cysts: Single-institution experience. BJU International 2009;104:847–50. 10.1111/j.1464-410X.2009.08537.x [DOI] [PubMed] [Google Scholar]
- 27.Mishra AK, Agarwal G, Agarwal A, et al. Laparoscopic Adrenalectomy of large cystic pheochromocytoma. Surg Endosc 2001;15:220. 10.1007/s004640040036 [DOI] [PubMed] [Google Scholar]
- 28.Disick GIS, Munver R. Adrenal-preserving minimally invasive surgery: Update on the current status of Laparoscopic partial Adrenalectomy. Current Urology reports 2008;9:67–72. 10.1007/s11934-008-0013-4 [DOI] [PubMed] [Google Scholar]
- 29.Ramacciato G, Paolo M, Pietromaria A, et al. Ten years of Laparoscopic Adrenalectomy: Lesson learned from 104 procedures. Am Surg 2005;71:321–5. [PubMed] [Google Scholar]
- 30.Lee J, El-Tamer M, Schifftner T, et al. Open and Laparoscopic Adrenalectomy: Analysis of the National surgical quality improvement program. J Am Coll Surg 2008;206:953–9. 10.1016/j.jamcollsurg.2008.01.018 [DOI] [PubMed] [Google Scholar]
- 31.Szolar DH, Kammerhuber F. Quantitative CT evaluation of adrenal gland masses: A step forward in the differentiation between adenomas and Nonadenomas Radiology 1997;202:517–21. 10.1148/radiology.202.2.9015083 [DOI] [PubMed] [Google Scholar]
- 32.Outwater EK, Siegelman ES, Huang AB, et al. Adrenal masses: Correlation between CT Attenuation value and chemical shift ratio at MR imaging with in-phase and opposed-phase sequences. Radiology 1996;200:749–52. 10.1148/radiology.200.3.8756926 [DOI] [PubMed] [Google Scholar]
- 33.McNicholas MM, Lee MJ, Mayo-Smith WW, et al. An imaging algorithm for the differential diagnosis of adrenal adenomas and metastases. Am J Roentgenol 1995;165:1453–9. 10.2214/ajr.165.6.7484585 [DOI] [PubMed] [Google Scholar]
- 34.Stelow EB, Debol SM, Stanley MW, et al. Sampling of the adrenal glands by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol 2005;33:26–30. 10.1002/dc.20273 [DOI] [PubMed] [Google Scholar]
- 35.Krebs TL, Wagner BJ. MR imaging of the adrenal gland: Radiologic-pathologic correlation. Radiographics 1998;18:1425–40. 10.1148/radiographics.18.6.9821192 [DOI] [PubMed] [Google Scholar]
- 36.Erickson LA. Challenges in surgical pathology of Adrenocortical tumours. Histopathology 2018;72:82–96. 10.1111/his.13255 [DOI] [PubMed] [Google Scholar]
- 37.Ctvrtlik F, Tudos Z, Szasz P, et al. Characteristic CT features of Pheochromocytomas - probability model calculation tool based on a Multicentric study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2019;163:212–9. 10.5507/bp.2019.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson LDR. Pheochromocytoma of the adrenal gland scaled score (PASS) to separate benign from malignant Neoplasms: A Clinicopathologic and Immunophenotypic study of 100 cases. Am J Surg Pathol 2002;26:551–66. 10.1097/00000478-200205000-00002 [DOI] [PubMed] [Google Scholar]
- 39.Korobkin M, Dunnick NR. Characterization of adrenal masses. AJR Am J Roentgenol 1995;164:643–4. 10.2214/ajr.164.3.7863886 [DOI] [PubMed] [Google Scholar]
- 40.Bishop E, Eble JN, Cheng L, et al. Adrenal Myelolipomas show Nonrandom X-Chromosome inactivation in hematopoietic elements and fat: Support for a Clonal origin of Myelolipomas. Am J Surg Pathol 2006;30:838–43. 10.1097/01.pas.0000202044.05333.17 [DOI] [PubMed] [Google Scholar]