Abstract
Introduction
Psilocybin-assisted therapy has shown significant promise in treating the cluster of mood and anxiety symptoms that comprise post-traumatic stress disorder (PTSD) but has yet to be tested specifically in this condition. Furthermore, current pharmacological and psychotherapeutic treatments for PTSD are difficult to tolerate and limited in efficacy, especially in the US Military Veteran (USMV) population. This open-label pilot study will examine the safety and efficacy of two psilocybin administration sessions (15 mg and 25 mg), combined with psychotherapy, among USMVs with severe, treatment resistant PTSD.
Methods and analysis
We will recruit 15 USMVs with severe, treatment resistant PTSD. Participants will receive one low dose (15 mg) and one moderate/high dose (25 mg) of psilocybin in conjunction with preparatory and post-psilocybin therapy sessions. The primary safety outcome will be the type, severity and frequency of adverse events and suicidal ideation/behaviour, as measured by the Columbia Suicide Severity Rating Scale. The primary outcome measure for PTSD will be the Clinician Administered PTSD Scale-5. The primary endpoint will be 1 month following the second psilocybin administration session, and the total follow-up time will be 6 months.
Ethics and dissemination
All participants will be required to provide written informed consent. The trial has been authorised by the Ohio State University Institutional Review Board (study number: 2022H0280). Dissemination of results will occur via a peer-reviewed publication and other relevant media.
Trial registration number
Keywords: PSYCHIATRY, Adult psychiatry, Clinical trials, MENTAL HEALTH, CLINICAL PHARMACOLOGY
STRENGTHS AND LIMITATIONS OF THIS STUDY.
A strength of the study design includes long-term follow-up of participants to assess the durability of the treatment over time.
Another strength is that primary outcomes will be assessed by self-report and structured interviews by clinicians not affiliated with the study.
A third strength is that the study includes clinician ratings after the preparatory psychotherapy and prior to the first psilocybin dosing to account for change that may be due solely to therapy.
The single-arm design comes with limitations, such as having no comparison group or blinding procedures.
Due to a small sample size, generalisation of findings will be limited.
Introduction
In recent years, there has been increasing interest in investigating the use of psilocybin, a classic hallucinogen, as an adjunct to psychotherapy for the treatment of various psychiatric conditions. Recently, completed trials have shown positive effects of this treatment among patients with depression and anxiety,1–3 obsessive compulsive disorder4 and substance use disorders.5–7 Results from these studies demonstrate that psilocybin-assisted therapy (PAT) is safe and has a minimal adverse event (AE) profile, with no documented serious AEs when provided in a context that includes substantial psychological support. In addition, data revealed robust short-term and long-term improvements in the constellation of mood, substance use and anxiety symptoms that represent the core aspects of post-traumatic stress disorder (PTSD).8
Current evidence supports the notion that PAT may be effective in treating PTSD, in part due to the high rates of comorbidity with depression and anxiety, and the overlap in symptomology in these conditions.9 Consistent with this hypothesis, a recent open-label trial of group PAT for demoralisation in long-term AIDS survivors showed a clinically meaningful change in PTSD symptom severity, as measured by the PTSD Checklist-5 (PCL-5), from baseline to 3-month follow-up with a large effect size.10 However, this study was not designed specifically to address symptoms of PTSD, nor did it focus on the broader population of people with this diagnosis. Furthermore, no study to date has focused on examining the safety and efficacy of PAT among US Military Veterans (USMV), a population with a substantial burden of mental health problems compared with the general population. For example, despite inherent resiliencies and specialised training, members of the armed forces are often exposed to several military deployments and intense combat incidents, which are associated with a higher risk of PTSD compared with the civilian population.11 12 In addition, there has been an alarming increase in the incidence of suicides among Veterans, set in the context of limited effective treatment methods for this unique population.12 13 Although the association between PTSD and suicide risk is complex and sometimes related to other factors, such as psychiatric comorbidity and demographic, social and psychological characteristics,14 15 the need to reduce the risk associated with suicidality in this population remains critical. Moreover, evidence suggests a general association between psychedelic administration and reduced suicidality in the general population and in PAT trials,16–18 suggesting this approach could be helpful regardless of the aetiology of suicidality.
Although several pharmacological and psychotherapy interventions have been developed to treat PTSD,19 20 these treatments are difficult to complete, challenging to access and are lacking in efficacy for many Veterans.21–23 Furthermore, there is emerging evidence that standard pharmacological treatments are not as effective in Veterans with PTSD as they are in civilians,21 24 and are consequently no longer recommended as a front-line treatment in this population.25 Although there is encouraging preliminary data on the safety and efficacy of PAT from a variety of patient populations, more research is needed to establish safety and efficacy of this treatment among USMV with PTSD. Not only could this novel treatment help ameliorate the burden of PTSD in this population, but it could also potentially reduce the risk of suicides among USMV and reduce the devastating impact of this mental health crisis in families and communities across the USA.
Therefore, the current study was designed as an open-label pilot study testing the following primary hypotheses in USMVs with severe, treatment resistant PTSD: (1) PAT is safe to administer among Veterans with PTSD (2) PAT will be associated with decreases in PTSD symptom severity at 1 month after final psilocybin session as measured by the Clinician Administered PTSD Scale-5 (CAPS-5) and the PCL-5.
Methods and analysis
Objectives
The primary objective of the study is to investigate the safety of PAT among USMVs with severe, treatment-resistant PTSD based on the type, severity and frequency of AEs and suicidal ideation/behaviour, as measured by the Columbia Suicide Severity Rating Scale (CSSR-S), from baseline to primary endpoint, 1-month post-second psilocybin administration.
The secondary objectives of the study are as follows:
Investigate the effect of PAT on clinician rated PTSD symptom severity through the CAPS-5 measured at baseline and 1-month, 3-month and 6-month post-second psilocybin administration.
Investigate the effect of PAT on participant rated PTSD symptom severity using PCL-5 measured at baseline and 1-month, 3-month and 6-month post-second psilocybin administration.
Setting
This study takes place in the Clinical Research Centre at the Davis Medical Research Center within the Wexner Medical Center at The Ohio State University (OSU) in Columbus, Ohio, USA.
Design
The study was designed to partially replicate the PAT intervention used in a trial for people with major depressive disorder1 by conducting an open-label pilot study of two psilocybin administration sessions combined with psychotherapy for PTSD among USMV with severe, treatment resistant PTSD (Trial Protocol Version #1). Because this study is a single group open-label pilot safety and feasibility study, there is no randomisation procedure or blinding to condition.
Participants and recruitment
Recruitment
We will consent up to 100 volunteers (some will not pass the in-person screening), to achieve 15 individuals who will be enrolled in the study and subsequently complete both psilocybin sessions and the final follow-up assessment. We will attempt to reflect recent data from the 2019 to 2020 National Health and Resilience in Veterans Study, which showed that 26.7% of US Veterans with past-month PTSD were female and 34.8% were non-white.26 Extrapolating from this analysis, we expect to recruit 11 male participants, 4 female participants, 10 white and 5 non-white participants. Recruitment will consist of electronic dissemination of study flyers, social media ads, provider network communications via in-person meetings/telephone/email, email networking and word of mouth.
Screening
Potential participants will be invited to prescreen for the study via word of mouth, clinician referrals, listserv postings, social media advertising and email distribution. Initial prescreening will occur via a secure online questionnaire to determine if major inclusion/exclusion criteria are met. Participants who meet all study criteria, and who are not currently taking any psychiatric medications, will be invited to the OSU Department of Psychiatry and Behavioral Health Davis Medical Research Center for an in-person screening. If a potential participant is currently taking psychiatric medications, they must taper off their medications under the direction and monitoring of their prescribing physician before they can be invited to an in-person screening. Written informed consent will be obtained by study staff during a scheduled meeting after participants have passed the initial online prescreening.
Inclusion/exclusion criteria
Inclusion:
Participants must be USMV.
Must have a Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) diagnosis of PTSD with symptom duration of at least 6 months with a CAPS-5 total severity score of ≥35 at baseline.
Have had at least 3 months of prior selective serotonin reuptake inhibitor (SSRI) or selective norepinephrine reuptake inhibitor (SNRI) treatment in addition to at least 6 months of any psychotherapy.
Have at least a high school level of education or equivalent (eg, General Education Development (GED) Certification).
No antidepressant medications for approximately five half-lives prior to baseline assessment and enrolment.
Be judged by study team clinicians to be at low risk for suicidality.
Be medically stable as determined by screening for medical problems via a personal interview, a medical questionnaire, a physical examination, an ECG, and routine medical blood and urinalysis laboratory tests.
Have limited lifetime use of hallucinogens (the following criteria are preferred: no use in the past 5 years; total hallucinogen use less than 10 times).
Exclusion:
Participants who were assigned female at birth who are pregnant (as indicated by a positive urine pregnancy test assessed at intake and before each drug session) or nursing; people who are of childbearing potential and sexually active who are not practising a highly effective means of birth control (ie, implants, injectables, combined oral contraceptives, progestin containing intrauterine device (IUD) or vasectomised partner).
Participants who were assigned male at birth with partners of childbearing potential who are sexually active and not practising a highly effective means of contraception (ie, condom with spermicidal foam/gel/film/cream/suppository).
Current medical condition incompatible with psilocybin administration (eg, coronary artery disease, uncontrolled hypertension).
Systolic blood pressure >139 mm Hg; diastolic blood pressure >89 mm Hg; heart rate (HR)>90 bpm.
Currently taking, on a regular (eg, daily) basis, any medication(s) having a primary centrally acting serotonergic effect, including monoamine oxidase inhibitors (MAOIs).
Current or history of meeting DSM-5 criteria for schizophrenia spectrum or other psychotic disorders (except substance/medication-induced or due to another medical condition), bipolar I or II disorder.
Current or history of (within 1 year of meeting DSM-5 criteria) a moderate or severe alcohol, tobacco or other drug use disorder (excluding caffeine).
Have a first-degree or second-degree relative with schizophrenia spectrum or other psychotic disorders (except substance/medication-induced or due to another medical condition) or bipolar I or II disorder.
Has a psychiatric condition that precludes the establishment of therapeutic rapport.
History of a medically significant suicide attempt.
Current antidepressant use.
Enrolment
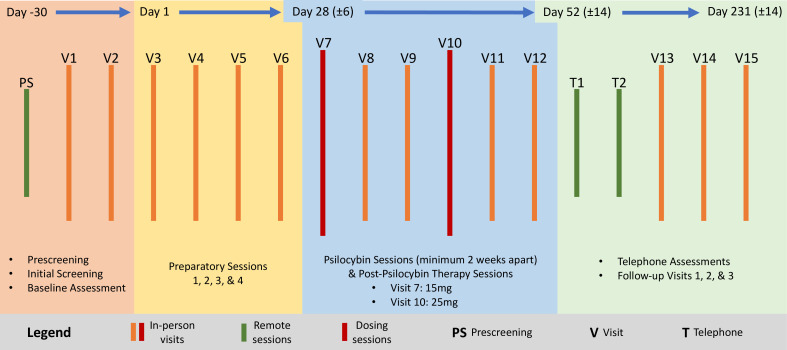
After enrolment, participants will receive preparatory psychotherapy followed by one low dose (15 mg) and one moderate/high dose (25 mg) of psilocybin, followed by post-psilocybin therapy sessions. Each psilocybin session will last approximately 8 hours and will be overseen by two trained session facilitators (session facilitators can vary due to scheduling or other treatment considerations). Before the first psilocybin session, participants will meet with one or both session facilitators for approximately 8 hours of contact time (or up to 4 meetings). Two post-psilocybin therapy session visits will follow psilocybin sessions 1 and 2. Psilocybin sessions 1 and 2 will occur at least 2 weeks apart. Follow-up visits will occur 1 and 2 week(s) and 1, 3 and 6 month(s) after the final psilocybin session, with additional contact hours scheduled as needed. Thus, the intervention and follow-up require 15 visits over a period of about 8–10 months. A schematic of trial activities is provided in figure 1. Protocol amendments will be reported to the Food and Drug Administration, institutional review board (IRB) and ClinicalTrials.gov.
Figure 1.
Trial schematic. PS, Prescreening (defined in the bottom right of the legend).
Preparation
Before the first psilocybin session, participants will meet with both session facilitators for approximately 8 hours before the first psilocybin session day. The main purpose of the preparation meetings is to develop rapport and trust, which helps minimise the risk of fear or anxiety reactions during the psilocybin sessions. This approach has been successful in previous studies conducted by the principal investigator, including in a recent psilocybin study of depression.1 Additional meetings and contact hours will be scheduled if it is judged necessary to establish sufficient rapport and trust prior to psilocybin administration. Consistent with previous psilocybin protocols authored and conducted by the lead investigator and others, the participant’s life history and current situation in life will be reviewed, and intentions and expectations for the psilocybin sessions will be discussed.27 28 In addition, during these sessions, the facilitators will explore the participant’s trauma history and experience of PTSD symptoms, as well as their goals, values, and perceived strengths and weaknesses. Lastly, an overview of common and uncommon psilocybin effects will be discussed, and skills for navigating such experiences will be explored and practised.
Psilocybin dosing sessions
Procedures for psilocybin administration and the conduct of the session will be similar to procedures used in previous studies with psilocybin among patients diagnosed with major depressive disorder.1 Psilocybin is provided by Usona Institute in opaque gelatin capsules and will be administered with approximately 100 mL water. At least one session monitor, under the supervision of the investigators, will be present in the room and available to respond to participants’ physical and emotional needs during the full course of psilocybin effects and for at least 8 hours. Typically, both session monitors are present unless one needs to step out of the session room to manage personal needs or to communicate with study team members. A physician on the study team will be immediately available via pager or mobile phone for at least 3 hours, or until the peak effects of psilocybin have subsided—whichever is longer. The physician will also be available for consultation by phone for up to 8 hours post-dosing.
During the psilocybin session, participants will be encouraged to lie on a couch, wear eyeshades and listen to a programme of music through headphones.1 The music playlist for this trial was designed by the research team specifically for this study and a detailed description of the methods used to develop this playlist will be available in a forthcoming manuscript. During the session, the participant will be encouraged to focus their attention inward. The eyeshades and music are intended to encourage this inward reflection. Vital signs and measures of the intensity of behaviours, signs and reported symptoms will be assessed throughout the day by the session monitors will be monitored at 30, 60, 90, 120, 180, 240, 300 and 360 min after capsule administration. Acute anxiety, agitation or panic will be handled with reassurance. In the unlikely event that these symptoms do not respond to reassurance, or in the unlikely event that the volunteer experiences unexpected psychosis, the clinical judgement of a study physician will determine the most appropriate medical treatment.
At the end of the experimental drug session, participants will complete paper or computer-based questionnaires (eg, mystical experience,29 30 psychological insight,31 challenging experience32) designed to assess acute subjective experiences associated with the psilocybin session. Participants will also be asked to write a narrative description of the experience of the psilocybin session before their next in-person meeting. Study facilitators will complete a safety assessment (C-SSRS), which will be completed at all visits throughout the study.
Participants will be released to the care of a significant other, who will pick them up at the end of the day and transport them to their residence or place of lodging. The pick-up person will remain with them overnight. At the permission of the participant, the pick-up person will be invited to the session room prior to leaving with the participant, where they will be instructed about procedures for care during the evening/night following the psilocybin session. In addition, at least one session monitor will be on-call via telephone for 24 hours after each psilocybin session.
Follow-up
The follow-up period is 6 months in total, with debriefing visits occurring immediately (1–3 days) following the psilocybin sessions, 1-week post-psilocybin sessions, and then at various time points following the final psilocybin dosing session. The follow-up sessions will include a discussion of the narrative description of the psilocybin session(s), an exploration of the participant’s experience of PTSD symptoms, as well as the ongoing integration of their therapy experience into their day-to-day lives. Additional clinician and monitor contact hours will be scheduled if it is judged that the participant would benefit from additional meetings to discuss experiences from their session(s) or prepare for the next session. During the visits immediately following a psilocybin session, participants will be asked to discuss a narrative description of their most recent psilocybin session and psychological support will be provided. The primary goal of these sessions is to offer support for the participant’s reflections on the psilocybin session. Study clinicians will support the participant’s narrative expression of their experience and use their experience as a foundation for discussion about how to move forward and integrate this experience into their lives.
Psilocybin session facilitators
Each dyad of psilocybin session facilitators for this study includes at least one independently licensed clinician (eg, clinical psychologist, psychiatrist, social worker, counsellor) with clinical training in PAT and in the treatment of trauma and PTSD. The secondary facilitator could be another licensed clinician or a licence-eligible person under the supervision of the primary facilitator. Training in PAT is provided to all facilitators via a 2-day workshop led by the principal investigator, as well as direct clinical supervision and training in the therapeutic approach during the study. To qualify as a primary facilitator, each facilitator who is eligible for this role will first serve as a secondary facilitator under the supervision of the principal investigator (serving as primary facilitator) or another approved primary facilitator for at least two participants before determining whether they can serve as a primary facilitator. The principal investigator will continue to monitor the facilitators in this study via regular meetings for the duration of the study.
Fidelity
Those involved in providing the intervention in this trial will complete checklists to ensure consistency across staff in the delivery of intervention content.
Participant retention
Retention activities will include: (1) obtaining various methods of contact (ie, email, primary phone number, home address, secondary phone number), (2) release of information to contact a primary physician or mental health provider and (3) release of information to contact a primary supportive friend/partner/spouse. Participants will be engaged in the trial on a weekly basis through the primary endpoint (1-month post-psilocybin session 2). During each weekly study contact, participants will receive therapeutic intervention to assess for safety and need for ongoing support as necessary. During safety assessments, any unforeseen challenges with study participation will be addressed. During long-term follow-up time periods (between primary endpoint at 1 month and follow-up at 3 months and 6 months), participants will receive a monthly contact from study personnel reminding them of upcoming visits and encouraging them to schedule a telephone or in-person visit should one be needed prior to the next scheduled study visit.
To aid in study retention, participants will also receive monetary compensation for the time they spend participating in the preparatory, psilocybin and post-psilocybin therapy session meetings and measurements taken at the Ohio State University Medical Center (OSUMC). Remuneration will consist of US$25 per study visit after enrolment, except for psilocybin session visits which will be compensated at the rate of US$100/session visit as they will spend more time at the clinic on these days. Total remuneration will be up to US$475 per participant.
Outcomes
Data collection
Primary safety and clinical outcome data are collected by clinician interview (eg, AEs, suicidal ideation and behaviour, PTSD symptoms via the CAPS-5) and self-report (eg, PTSD symptoms via the PCL-5). Data will be entered by study staff (into a secure data collection platform; REDCap), using a double data entry method. Data will be stored on secure cloud servers and accessed only by approved study personnel. There was no requirement for a data monitoring committee for this trial, and thus none will be used for this trial.
Confidentiality
Participant confidentiality and privacy is strictly held in trust by the participating investigators and their staff. This confidentiality is extended to the data being collected as part of this study. Data that could be used to identify a specific study participant will be held in strict confidence within the research team. No personally identifiable information from the study will be released to any unauthorised third party. All research activities will be conducted in as private a setting as possible. The study monitor, other authorised representatives of the sponsor or funding agency, representatives of the IRB, regulatory agencies or representatives from companies or organisations supplying the product, may inspect all documents and records required to be maintained by the investigator, including but not limited to, medical records (office, clinic or hospital) and pharmacy records for the participants in this study. The clinical study site will permit access to such records. The study participant’s contact information will be securely stored for internal use during the study. At the end of the study, all records will continue to be kept in a secure location for as long a period as dictated by the reviewing IRB, institutional policies, or sponsor requirements.
Study participant research data, which is for purposes of statistical analysis and scientific reporting, will be transmitted to and stored in a locked room. This will not include the participant’s contact or identifying information. Rather, individual participants and their research data will be identified by a unique study identification number. Some therapy sessions may occur via telephone or videoconferencing (eg, if a participant is unable to travel to our study site for an integration session or for a follow-up qualitative interview). Such sessions may be recorded using an encrypted audio/video format (eg, Zoom). The study data entry and study management systems used by research staff will be secured and password protected. At the end of the study, all study databases will be de-identified and archived. A Certificate of Confidentiality has also been obtained for this study.
Safety outcomes
The CSSR-S will be used to assess the severity of suicidal ideation during every study visit (in-person and virtual; https://cssrs.columbia.edu/).33 The C-SSRS is divided into four subscales based on (1) severity of ideation, (2) intensity of ideation, (3) suicidal behaviour and (4) lethality of attempt. Respondents are categorised as low, medium and high risk based on where their affirmative answers are in the various subscales as opposed to their total score.
In addition, the primary clinician/session facilitator for each participant will identify/record AEs (ie, the emergence of any untoward physical or psychological events or symptoms) and safety concerns at each study visit using a non-standardised form developed for use in prior studies.1 The type, severity and frequency of AEs will be collected to identify and characterise any safety concerns that may arise.
Clinical outcomes
PTSD symptom severity will be measured using the CAPS-5 and PCL-5. The CAPS-5 is an extensively validated, widely used 30-item structured interview that assesses PTSD diagnostic status and symptom severity. It is scored on a scale of 0–80 with moderate and severe PTSD ranges rationally derived and defined as 23–34 and ≥35, respectively.34 In addition to assessing the 20 DSM-5 PTSD symptoms, questions target the onset and duration of symptoms, subjective distress, impact of symptoms on social and occupational functioning, improvement in symptoms since a previous CAPS administration, overall response validity, overall PTSD severity, and specifications for the dissociative subtype (depersonalisation and derealisation). The CAPS-5 will be administered by independent raters (blinded to all other aspects of study participation) at baseline assessment, at the primary endpoint (1 month), and at 3 months and 6 months following psilocybin session 2. Independent raters will be composed of IRB-approved team members of this protocol and trained in the assessment of PTSD using the CAPS-5.
The PCL-5 is one of the most widely used self-report measures of PTSD, with scores ranging from 0 to 80, with higher scores indicating greater PTSD symptom severity.35 The PCL-5 will be self-administered at baseline as well as at additional time points (preparatory session 4, therapy session 1.2, 2.2, and follow-up 1, 2 and 3) to assess whether there is an immediate post-psilocybin effect on PTSD symptom severity. Other clinical outcomes (eg, depression and anxiety symptoms, psychological flexibility, personality) will be assessed in supplemental analyses separately from primary and secondary outcomes.
Statistical analysis
No prior investigations of the effects of psilocybin on PTSD symptoms have been published to date. Because this trial has a primary objective of establishing the safety of this treatment approach among this population, and based on effect sizes in prior laboratory studies of psilocybin for other clinical populations,1 2 we believe that a sample size of 15 will provide sufficient power to detect a moderate effect size of pre-psilocybin and post-psilocybin changes in PTSD symptoms. If the data suggest possible efficacy, these data should be sufficient to conduct a power analysis for a subsequent randomised controlled trial.
All participants enrolled in the trial and who complete both psilocybin sessions will be the evaluable population for analyses. Missing data will be managed through the use of imputation. For primary and secondary measures assessed at baseline and follow-up, repeated-measures analysis of variances (ANOVAs) with post-hoc comparisons will be conducted to test for differences between baseline and follow-up visits. Planned comparisons from baseline to 1-month post-final psilocybin session will be conducted for primary outcome measures. These longitudinal outcome measures will be regressed on measures of acute and persisting psilocybin effects to examine whether such effects predict clinically relevant outcomes.
Primary hypotheses/endpoints:
-
PAT will be safe to administer among USMV with PTSD as indicated by no statistically significant increases in mean ratings of suicidal ideation on the C-SSRS from Baseline through 1-month post-psilocybin session 2.
Ratings of suicidal ideation on the C-SSRS will be included from baseline, 1-day post-psilocybin session 1, 1-day post-psilocybin session 2 and 1-month post-psilocybin session 2. A repeated-measures ANOVA will be conducted with post-hoc mean pairwise comparisons to test for differences in ratings of suicidal ideation from baseline through 1-month post-psilocybin session 2.
Data will be presented with F-tests using an alpha (p value) cut-off of 0.05 for statistical significance. Partial eta squared effect sizes will be reported with 90% CIs.
-
PAT will be safe to administer among USMV with PTSD as indicated by no serious AE reporting related to the administration of psilocybin.
A descriptive analysis of AE reporting logs will be conducted to determine if any serious AEs have been reported related to psilocybin administration.
Secondary hypotheses/endpoints
-
We will find statistically significant decreases in PTSD symptom severity from baseline to 1 month after the final psilocybin session as measured by the CAPS-5.
Mean ratings of PTSD symptoms severity will be included from baseline, preparation visit 4 and 1-month post-psilocybin session 2. A repeated-measures ANOVA will be conducted with post-hoc mean pairwise comparisons to test for differences in ratings of PTSD symptoms severity from baseline to 1-month post-psilocybin session 2.
Data will be presented with F-tests using an alpha (p value) cut-off of 0.05 for statistical significance. Partial eta squared effect sizes will be reported with 90% CI.
-
We will find statistically significant decreases in PTSD symptom severity from baseline to 1 -month after the final psilocybin session as measured by the self-report PCL-5.
Mean ratings of PTSD symptoms severity will be included from baseline, preparation visit 4 and 1-month post-psilocybin session 2. A repeated-measures ANOVA will be conducted with post-hoc mean pairwise comparisons to test for differences in ratings of PTSD symptom severity from baseline through 1 month post-psilocybin session 2.
Data will be presented with F-tests using an alpha (p value) cut-off of 0.05 for statistical significance. Partial eta squared effect sizes will be reported with 90% CIs.
Ethics and dissemination
This study was granted an IND from the Food and Drug Administration (IND#162567). The study also is registered with the Drug Enforcement Administration. All participants will be required to provide written informed consent (See patient consent form). The trial has been authorised by the Ohio State University Institutional Review Board (study number: 2022H0280). Dissemination of results will occur via a peer-reviewed publication and other relevant media.
Patient and public involvement
Neither patients nor the public has been involved in the design and conduct of this study, the choice of outcome measures or recruitment. There are no plans to include patients/public in dissemination efforts. Research questions were informed by the professional experiences working with Veterans with PTSD.
Supplementary Material
Acknowledgments
The authors would like to thank Usona Institute for providing the study drug for this trial. We also thank Dr. Emil Coccaro for their work as medical monitor of this study, and Deanna Golden-Kreutz, Holly Bookless, and the nursing staff at the Clinical Research Center at Ohio State University for their support during the trial. We also thank the following Ohio State University administrators, faculty, and staff who have been instrumental in establishing the infrastructure for this study: Tom Gregoire, Bridget Friesthler, David Jenkins, Peter Mohler, Courtney Mankowski, Julia Behnfeldt, Hallie Barr, Kaitlyn Vitek, Ellen Patricia, Susan Garfinkel, Dana McTigue, Brooke McDaniel, Richard Gumina, Kent Kiffner, Erin Scott, Elysabeth Bonar-Bouton, and Grace Adams.
Footnotes
Contributors: AKD, principal investigator: project coordination, trial monitoring, manuscript writing. SBA: study coordinator, measurement selection, protocol development, manuscript writing. AWL: protocol development, manuscript writing, measurement selection, medical protocol development. RLL: contributed to protocol development, contributed to measure selection, analytical strategy, designed music playlist, led playlist validation process, made comments on the manuscript. PBN: protocol writing and development, medical protocol development, led music playlist validation process, manuscript review and editing, visualisation, provided feedback during playlist development.
Funding: This work was funded by the Center for Psychedelic Drug Research and Education (award number: NA).
Competing interests: AKD and RLL are board members of Source Research Foundation. This organisation was not involved in the design/execution of this study or the interpretation or communication of findings. AKD is a lead trainer at Fluence.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Davis AK, Barrett FS, May DG, et al. Effects of psilocybin-assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry 2021;78:481–9. 10.1001/jamapsychiatry.2020.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol 2016;30:1181–97. 10.1177/0269881116675513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grob CS, Danforth AL, Chopra GS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 2011;68:71–8. 10.1001/archgenpsychiatry.2010.116 [DOI] [PubMed] [Google Scholar]
- 4.Moreno FA, Wiegand CB, Taitano EK, et al. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry 2006;67:1735–40. 10.4088/jcp.v67n1110 [DOI] [PubMed] [Google Scholar]
- 5.Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol 2015;29:289–99. 10.1177/0269881114565144 [DOI] [PubMed] [Google Scholar]
- 6.Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 2014;28:983–92. 10.1177/0269881114548296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson MW, Garcia-Romeu A, Griffiths RR. Long-Term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse 2017;43:55–60. 10.3109/00952990.2016.1170135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin Neurosci 2015;17:141–50. 10.31887/DCNS.2015.17.2/jflory [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bird CIV, Modlin NL, Rucker JJH. Psilocybin and MDMA for the treatment of trauma-related psychopathology. Int Rev Psychiatry 2021;33:229–49. 10.1080/09540261.2021.1919062 [DOI] [PubMed] [Google Scholar]
- 10.Anderson BT, Danforth A, Daroff PR, et al. Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: an open-label safety and feasibility pilot study. EClinicalMedicine 2020;27:100538.:100538. 10.1016/j.eclinm.2020.100538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanwella R, de Silva V. Mental health of special forces personnel deployed in battle. Soc Psychiatry Psychiatr Epidemiol 2012;47:1343–51. 10.1007/s00127-011-0442-0 [DOI] [PubMed] [Google Scholar]
- 12.Hing M, Cabrera J, Barstow C, et al. Special operations forces and incidence of post-traumatic stress disorder symptoms. J Spec Oper Med 2012;12:23–35. 10.55460/663M-6L7P [DOI] [PubMed] [Google Scholar]
- 13.Rocklein Kemplin K, Paun O, Godbee DC, et al. Resilience and suicide in special operations forces: state of the science via integrative review. J Spec Oper Med 2019;19:57–66. 10.55460/BQES-AM8H [DOI] [PubMed] [Google Scholar]
- 14.Stanley IH, Chu C, Gildea SM, et al. Predicting suicide attempts among U.S. Army soldiers after leaving active duty using information available before leaving active duty: results from the study to assess risk and resilience in servicemembers-longitudinal study (starrs-ls). Mol Psychiatry 2022;27:1631–9. 10.1038/s41380-021-01423-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holliday R, Borges LM, Stearns-Yoder KA, et al. Posttraumatic stress disorder, suicidal ideation, and suicidal self-directed violence among U.S. military personnel and veterans: a systematic review of the literature from 2010 to 2018. Front Psychol 2020;11. 10.3389/fpsyg.2020.01998 Available: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.01998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones GM, Nock MK. Race and ethnicity moderate the associations between lifetime psychedelic use (MDMA and psilocybin) and psychological distress and suicidality. Sci Rep 2022;12:16976. 10.1038/s41598-022-18645-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones GM, Nock MK. MDMA/ecstasy use and psilocybin use are associated with lowered odds of psychological distress and suicidal thoughts in a sample of US adults. J Psychopharmacol 2022;36:46–56. 10.1177/02698811211058923 [DOI] [PubMed] [Google Scholar]
- 18.Ross S, Agin-Liebes G, Lo S, et al. Acute and sustained reductions in loss of meaning and suicidal ideation following psilocybin-assisted psychotherapy for psychiatric and existential distress in life-threatening cancer. ACS Pharmacol Transl Sci 2021;4:553–62. 10.1021/acsptsci.1c00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravindran LN, Stein MB. Pharmacotherapy of PTSD: premises, principles, and priorities. Brain Res 2009;1293:24–39. 10.1016/j.brainres.2009.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watkins LE, Sprang KR, Rothbaum BO. Treating PTSD: a review of evidence-based psychotherapy interventions. Front Behav Neurosci 2018;12. 10.3389/fnbeh.2018.00258 Available: https://www.frontiersin.org/articles/10.3389/fnbeh.2018.00258/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hertzberg MA, Feldman ME, Beckham JC, et al. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry 2000;12:101–5. 10.1023/a:1009076231175 [DOI] [PubMed] [Google Scholar]
- 22.Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD psychopharmacology Working group. Biol Psychiatry 2017;82:e51–9. 10.1016/j.biopsych.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 23.Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA 2015;314:489–500. 10.1001/jama.2015.8370 [DOI] [PubMed] [Google Scholar]
- 24.van der Kolk BA. The body keeps the score: brain, mind, and body in the healing of trauma. New York, NY, US: Viking, 2014: 443. [Google Scholar]
- 25.VA/DOD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. 2017:200. [DOI] [PMC free article] [PubMed]
- 26.Wisco BE, Nomamiukor FO, Marx BP, et al. Posttraumatic stress disorder in US military veterans: results from the 2019-2020 National health and resilience in veterans study. J Clin Psychiatry 2022;83. 10.4088/JCP.20m14029 [DOI] [PubMed] [Google Scholar]
- 27.Davis AK, Averill LA, Sepeda ND, et al. Psychedelic treatment for trauma-related psychological and cognitive impairment among US special operations forces veterans. Chronic Stress (Thousand Oaks) 2020;4. 10.1177/2470547020939564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol 2008;22:603–20. 10.1177/0269881108093587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett FS, Johnson MW, Griffiths RR. Validation of the revised mystical experience questionnaire in experimental sessions with psilocybin. J Psychopharmacol 2015;29:1182–90. 10.1177/0269881115609019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLean KA, Johnson MW, Griffiths RR. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol 2011;25:1453–61. 10.1177/0269881111420188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis AK, Barrett FS, So S, et al. Development of the psychological insight questionnaire among a sample of people who have consumed psilocybin or LSD. J Psychopharmacol 2021;35:437–46. 10.1177/0269881120967878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrett FS, Bradstreet MP, Leoutsakos J-MS, et al. The challenging experience questionnaire: characterization of challenging experiences with psilocybin mushrooms. J Psychopharmacol 2016;30:1279–95. 10.1177/0269881116678781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posner K, Brown GK, Stanley B, et al. The columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–77. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weathers FW, Bovin MJ, Lee DJ, et al. The clinician-administered PTSD scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess 2018;30:383–95. 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess 2016;28:1379–91. 10.1037/pas0000254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.