Abstract
Objectives
This study aimed to evaluate the performance of the triglyceride glucose (TyG) index and its related markers in predicting metabolic-associated fatty liver disease (MAFLD) in healthy Chinese participants.
Design
This was a cross-sectional study.
Setting
The study was conducted at Health Management Department of the Affiliated Hospital of Xuzhou Medical University.
Participants
A total of 20 922 asymptomatic Chinese participants (56% men) were enrolled.
Outcome measures
Hepatic ultrasonography was performed to diagnose MAFLD based on the latest diagnostic criteria. The TyG, TyG-body mass (TyG-BMI) and TyG-waist circumference indices were calculated and analysed.
Results
Compared with the lowest quartile of the TyG-BMI, the adjusted ORs and 95% CIs for MAFLD were 20.76 (14.54 to 29.65), 92.33 (64.61 to 131.95) and 380.87 (263.25 to 551.05) in the second, third and fourth quartiles, respectively. According to the subgroup analysis, the TyG-BMI in the female and the lean groups (BMI<23 kg/m2) showed the strongest predictive value, with optimal cut-off values for MAFLD of 162.05 and 156.31, respectively. The areas under the receiver operating characteristic curves in female and lean groups were 0.933 (95% CI 0.927 to 0.938) and 0.928 (95% CI 0.914 to 0.943), respectively, with 90.7% sensitivity and 81.2% specificity in female participants with MAFLD and 87.2% sensitivity and 87.1% specificity in lean participants with MAFLD. The TyG-BMI index demonstrated superior predictive ability for MAFLD compared with other markers.
Conclusions
The TyG-BMI is an effective, simple and promising tool for predicting MAFLD, especially in lean and female participants.
Keywords: Other metabolic, e.g. iron, porphyria; Adult gastroenterology; Risk management
STRENGTHS AND LIMITATIONS OF THIS STUDY
To our knowledge, this is the first study to comprehensively evaluate the predictive performance of the triglyceride glucose index and its related markers for metabolic-associated fatty liver disease (MAFLD) in healthy Chinese participants.
A limitation was that the diagnosis of MAFLD was based primarily on ultrasonography, which may have underestimated the true prevalence of MAFLD.
Another limitation was the lack of liver biopsy data and the controlled attenuation parameter and liver stiffness measurement from the FibroScan Test.
Results should be interpreted carefully due to the study’s observational design and further studies are warranted to validate our findings in larger and more diverse populations.
Introduction
The global prevalence of metabolic-associated fatty liver disease (MAFLD), formerly known as non-alcoholic fatty liver disease (NAFLD), has dramatically increased to up to 25%.1 Furthermore, studies have associated MAFLD with a variety of adverse clinical sequelae that may eventually result in increased mortality, including severe liver inflammation and fibrosis, metabolic and cardiovascular diseases and extra-hepatic cancer such as bladder cancer.2–5 Early identification of MAFLD is therefore critical. However, a simple, effective, non-invasive tool for MAFLD screening is unavailable.
MAFLD develops through complex interactions between obesity and insulin resistance (IR).6 Traditional obesity indicators, including body mass index (BMI) and waist circumference (WC) are strongly associated with fatty liver and metabolic disorders.7 8 However, some studies have shown that 5%–26% of patients with MAFLD have a BMI within the normal range.9 Thus, these individuals and those who exhibit pre-MAFLD are often disregarded during MALFD screening. Moreover, relying solely on BMI and WC as a comprehensive reflection of MAFLD is unreliable due to their omission of IR. The triglyceride glucose (TyG) index is a newly proposed index that is simpler and more reliable for evaluating IR than the homeostasis model assessment of IR index. Furthermore, Gastaldelli et al found that the TyG index was well correlated with hepatic fat content in the San Antonio Metabolism study, indicating the potential significance of this index.10
The TyG index, combined with obesity markers such as the TyG-BMI and TyG-WC index, captures both obesity and IR, thereby more accurately reflecting these complex pathophysiological features. Several studies have demonstrated that TyG-related indices outperform single indicators in identifying metabolic and cardiovascular diseases.11–13 Therefore, we speculated that the TyG-related indices were promising markers in predicting MAFLD. In the present study, we investigated the effectiveness of TyG-related markers in distinguishing MAFLD in healthy participants and established a better prediction model for MAFLD.
Participants and methods
Study design and populations
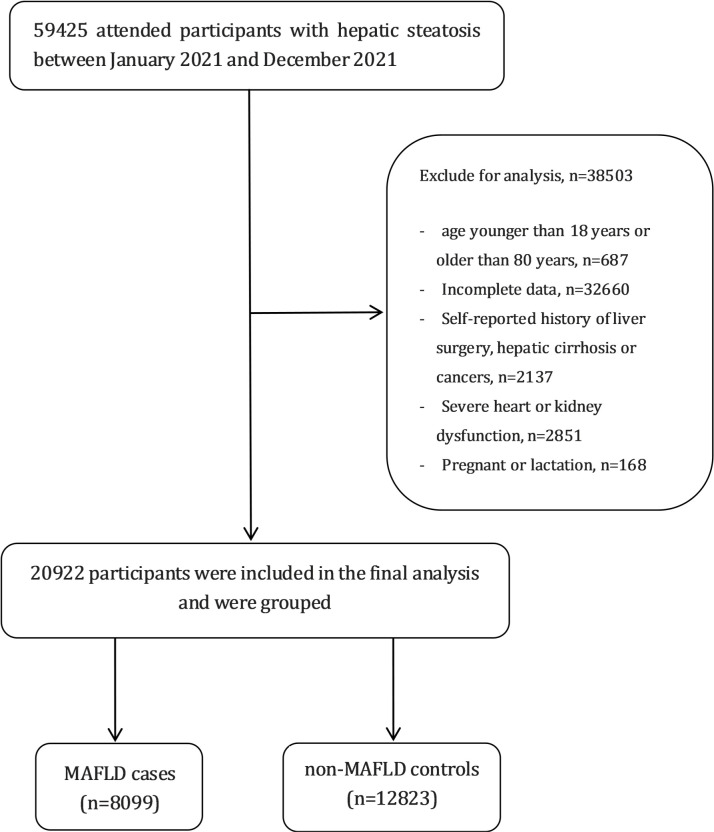
This cross-sectional study used data obtained from an urban population in eastern China who underwent a health examination at the Affiliated Hospital of Xuzhou Medical University between January 2021 and December 2021. The inclusion criteria were as follows: age between 18–80 years; and hepatic steatosis diagnosed through abdominal ultrasound. The exclusion criteria were as follows: incomplete data; age <18 years or >80 years; cirrhosis, hepatocellular carcinoma or history of liver surgery; history of malignant tumours; New York Heart Association class III or IV heart failure; chronic kidney disease with an estimated glomerular filtration rate of <60 mL/min/1.73m2; and pregnancy or lactation. Participants with missing outcome measures or lost clinical and biochemical records were also excluded. Figure 1 provides the flowchart of the study design. This study followed the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis reporting guidelines.14 To avoid duplication of information, we included only the initial physical examination data of participants who underwent multiple physical examinations throughout the year, thereby ensuring that each participant contributed only one set of data to the study.
Figure 1.
Flowchart of the study design. MAFLD, metabolic-associated fatty liver disease.
Health survey examinations and laboratory measurements
BMI, WC and blood pressure were measured by trained examiners, and the following laboratory data were obtained during the health examinations: fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate transaminase (AST), γ‐glutamyltransferase (GGT), blood urea nitrogen (BUN), creatinine (Cr) and uric acid (UA) levels. The TyG-related parameters were calculated using the following formulae:15 16
Patient and public involvement
The research question, design and outcome measures of the study were determined without patient involvement, and patient contribution was limited to study participation. Furthermore, there are no plans to involve patients in the dissemination of study findings.
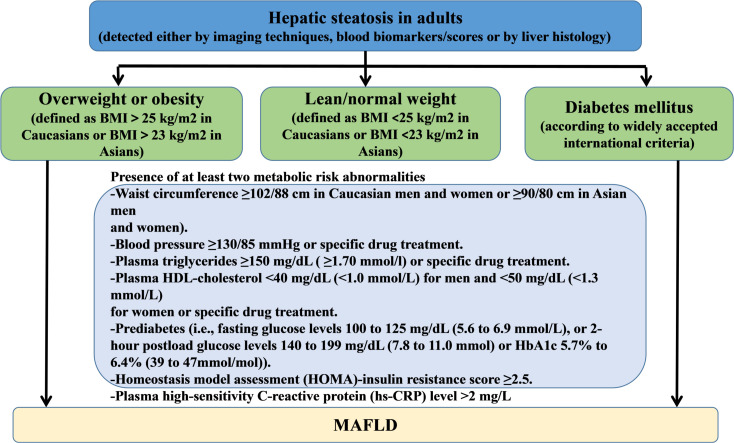
Diagnosis of MAFLD
In this study, we used novel and positive criteria to diagnose MAFLD irrespective of other concomitant liver diseases or alcohol consumption.17 The diagnosis of MAFLD was based on ultrasonically diagnosed hepatic steatosis with the presence of at least one of the following three criteria: overweight or obesity, type 2 diabetes mellitus or clinical evidence of metabolic dysfunction. The latter was defined by the presence of at least two metabolic risk abnormalities, listed in figure 2.18 The diagnosis of steatosis was based on the following ultrasonographic patterns: liver parenchymal brightness, increased echo contrast between hepatic and renal parenchyma and vascular blurring or poor visualisation of diaphragm.19
Figure 2.
Flowchart of diagnostic criteria for MAFLD. BMI, body mass index; HDL, high-density lipoprotein; MAFLD, metabolic-associated fatty liver disease.
Statistical analysis
Statistical analysis was conducted using SPSS V.22.0 (IBM Corp) and MedCalc V.16.2 (MedCalc Software, Ostend, Belgium). Descriptive statistics are presented as mean±SD or medians IQRs for continuous variables and frequencies or percentage (%) for categorical variables. The differences between individuals with MAFLD and non-MAFLD were assessed using the Student’s t-test or the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. Multiple logistic regression models were constructed to explore correlations between indicators and MAFLD after adjusting for sociodemographic and laboratory data, including age, sex, blood pressure, fasting glucose serum lipid levels and liver and kidney function. The targeted parameters were categorised into quartiles to further explore these relationships. The predictive value of TyG-related indices for MAFLD was assessed using a receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC). The subgroup analyses were performed according to sex and BMI, and the AUC differences of TyG-related indices were compared with the non-parametric DeLong test. A two-tailed p value <0.05 was considered statistically significant.
Results
Clinical and biochemical characteristics of the participants
In total, 20 922 participants were included in the final analysis. The baseline characteristics of the study participants are shown in table 1. Among the 20 922 participants, 8099 (38.71%) were diagnosed with MAFLD while there were 12 823 non-MAFLD controls. The prevalence of MAFLD was significantly higher in men (n=6152, 75.96%) than in women (n=1947, 24.04%) (p<0.0001). In all three BMI subgroups, the incidence of MAFLD gradually increased with BMI, with increases of 3.5%, 33.3% and 71.4%, respectively. Compared with those in the non-MAFLD group, individuals in the MAFLD group were significantly older, and had higher blood pressure, and levels of ALT, AST, GGT, BUN, UA, FPG, TC, TG and LDL-C (all p<0.0001). Notably, the BMI, WC and TyG-related indices were significantly higher in the MAFLD participants than in the non-MAFLD participants (all p<0.0001). In addition, we also found that men had significantly higher WC and TyG-WC values than women in both the MAFLD and non-MAFLD groups (p<0.0001).
Table 1.
Clinical and biochemical characteristics of the MAFLD and non-MAFLD groups
| MAFLD | Non-MAFLD | P value | |
| N (%) | 8099 (38.71%) | 12 823 (61.29%) | <0.0001 |
| Male (%) | 6152 (75.96%) | 6191 (48.29%) | <0.0001 |
| Age (years) | 46.91±12.57 | 42.16±12.65 | <0.0001 |
| SBP (mm Hg) | 131.97±17.47 | 120.11±16.60 | <0.0001 |
| DBP (mm Hg) | 81.53±11.90 | 73.51±10.97 | <0.0001 |
| BMI (kg/m2) | 27.14±2.90 | 22.69±2.65 | <0.0001 |
| BMI<23 (%) | 258 (3.50%) | 7119 (96.50%) | <0.0001 |
| 23≤BMI<25 (%) | 1598 (33.30%) | 3201 (66.70%) | <0.0001 |
| BMI≥25 (%) | 6244 (71.40%) | 2502 (28.60%) | <0.0001 |
| WC (cm) | 90.44±8.46 | 77.16±9.06 | <0.0001 |
| WCmale | 92.38±7.73 | 82.84±7.46 | <0.0001 |
| WCfemale | 84.06±7.62 | 71.85±6.98 | <0.0001 |
| TyG | 7.44±0.61 | 6.77±0.53 | <0.0001 |
| TyG-BMI | 202.04±28.85 | 154.16±24.91 | <0.0001 |
| TyG-WC | 673.57±90.67 | 524.59±87.49 | <0.0001 |
| TyG-WCmale | 692.52±86.67 | 577.04±77.69 | <0.0001 |
| TyG-WCfemale | 612.49±76.61 | 475.47±64.79 | <0.0001 |
| ALT (U/L) | 26 (18,38) | 15 (11,21) | <0.0001 |
| AST (U/L) | 22 (18,27) | 18 (16,22) | <0.0001 |
| GGT (U/L) | 32 (22,49) | 17 (13,25) | <0.0001 |
| BUN (mmol/l) | 5.15±1.24 | 4.84±1.26 | <0.0001 |
| Cr (µmol/l) | 66.43±13.07 | 66.49±13.07 | 0.731 |
| UA (µmol/l) | 354.36±84.81 | 290.96±76.54 | <0.0001 |
| FPG (mmol/l) | 5.29 (4.93,5.78) | 4.98 (4.71,5.29) | <0.0001 |
| TG (mmol/l) | 4.78±0.96 | 4.45±0.87 | <0.0001 |
| TC (mmol/l) | 1.83 (1.31,2.63) | 1.03 (0.76,1.43) | <0.0001 |
| HDL-C (mmol/l) | 1.19±0.26 | 1.38±0.30 | <0.0001 |
| LDL-C (mmol/l) | 3.13±0.73 | 2.84±0.69 | <0.0001 |
Data are expressed as mean±SD or medians (IQRs) for skewed variables or numbers (proportions) for categorical variables.
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GGT, γ‐glutamyltransferase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MAFLD, metabolic-associated fatty liver disease; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; TyG, triglyceride glucose; UA, uric acid; WC, waist circumference.
Relationships between different indicators and MAFLD
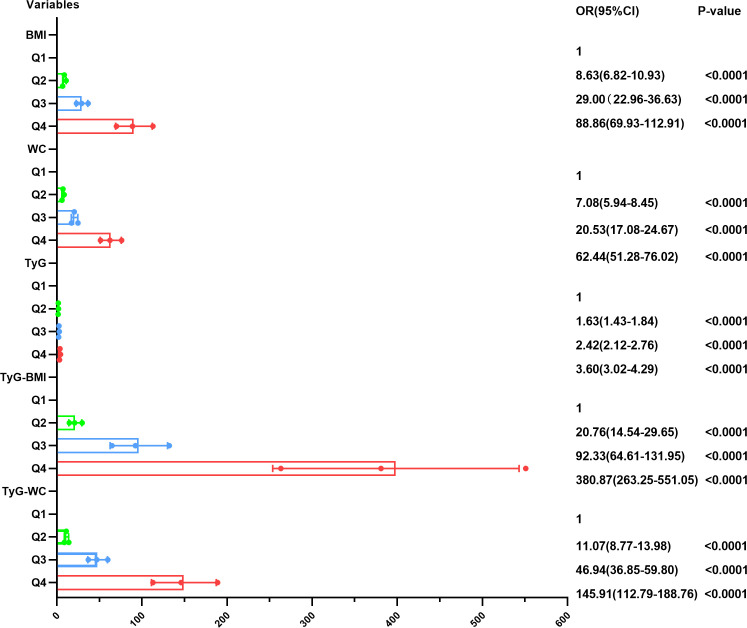
Our findings indicated that elevated BMI, WC, TyG, TyG-BMI and TyG-WC were all independent predictors of MAFLD even after adjustment (all p<0.0001) (table 2). Furthermore, after categorising the parameters into quartiles, we observed a dose–response relationship between all the parameters and the risk of MAFLD (all p<0.0001) (figure 3).
Table 2.
Binary logistic regression analysis of five markers for predicting metabolic-associated fatty liver disease
| Variable | Unadjusted | Model 1 | Model 2 | |||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| BMI | 1.867 (1.835 to 1.899) | <0.0001 | 1.831 (1.799 to 1.864) | <0.0001 | 1.668 (1.636 to 1.700) | <0.0001 |
| WC | 1.184 (1.178 to 1.189) | <0.0001 | 1.209 (1.202 to 1.216) | <0.0001 | 1.164 (1.156 to 1.171) | <0.0001 |
| TyG | 8.270 (7.750 to 8.826) | <0.0001 | 6.789 (6.349 to 7.261) | <0.0001 | 4.366 (3.827 to 4.981) | <0.0001 |
| TyG-BMI | 1.074 (1.072 to 1.076) | <0.0001 | 1.074 (1.072 to 1.076) | <0.0001 | 1.073 (1.070 to 1.075) | <0.0001 |
| TyG-WC | 1.019 (1.018 to 1.019) | <0.0001 | 1.021 (1.021 to 1.022) | <0.0001 | 1.020 (1.020 to 1.021) | <0.0001 |
Model 1: adjusted for age and sex; model 2: adjusted for age, sex, blood pressure, fasting glucose, blood lipids and liver and kidney function.
BMI, body mass index; TyG, triglyceride glucose; WC, waist circumference.
Figure 3.
Metabolic-associated fatty liver disease ORs and CIs according to the quartiles of BMI, WC, TyG, TyG-BMI and TyG-WC in the total population. BMI, body mass index; TyG, triglyceride glucose; WC, waist circumference.
The ORs for MAFLD increased with higher quartiles of the parameters and was particularly more pronounced for the TyG-BMI. The adjusted ORs and 95% CIs for MAFLD were 20.76 (14.54 to 29.65), 92.33 (64.61 to 131.95) and 380.87 (263.25 to 551.05) in the second, third and fourth quartiles of the TyG-BMI, respectively, compared with that in the first quartile. The multivariable-adjusted ORs (95% CIs) for the fourth quartile compared with the first quartile of the BMI, WC, TyG and TyG-WC were 88.86 (69.93 to 112.91), 62.44 (51.28 to 76.02), 3.60 (3.02 to 4.29) and 145.91 (112.79 to 188.76), respectively.
Predictive values of different indicators for MAFLD according to subgroup analyses
Predictive values of different indicators for MAFLD according to sex
As shown in table 3 and figure 4, the highest AUC was demonstrated by the TyG-BMI in both men and women (AUC=0.870 and 0.933, respectively). The TyG-BMI had significantly higher AUC values than the traditional metabolic parameters (BMI and WC) and other TyG-related indices (all p<0.0001). A TyG-BMI cut-off of 162.05 in females showed the best overall test performance, with a sensitivity of 90.7% and a specificity of 81.2%. However, the TyG index showed the worst performance both in men and women among different indicators (AUC=0.753 and 0.830, respectively) (table 2 and figure 4).
Table 3.
Cut-off values and AUCs (95% CI) of each parameter for predicting metabolic-associated fatty liver disease according to sex
| AUC (95% CI) | Cut-off value | Sensitivity (%) | Specificity (%) | |
| Male (n=12 343) | ||||
| BMI | 0.844 (0.837 to 0.851) | 25.35 | 75.4 | 75.7 |
| WC | 0.818 (0.810 to 0.825) | 87.50 | 73.7 | 73.5 |
| TyG | 0.753 (0.744 to 0.761) | 7.10 | 73.3 | 64.4 |
| TyG-BMI | 0.870 (0.864 to 0.876) | 181.22 | 79.9 | 76.3 |
| TyG-WC | 0.847 (0.841 to 0.854) | 625.58 | 78.0 | 74.5 |
| Female (n=8579) | ||||
| BMI | 0.900 (0.893 to 0.907) | 23.05 | 92.2 | 73.1 |
| WC | 0.890 (0.883 to 0.897) | 76.50 | 84.9 | 76.8 |
| TyG | 0.830 (0.820 to 0.841) | 6.86 | 77.6 | 73.8 |
| TyG-BMI | 0.933 (0.927 to 0.938) | 162.05 | 90.7 | 81.2 |
| TyG-WC | 0.922 (0.915 to 0.928) | 529.41 | 87.9 | 80.9 |
AUC, area under the receiver operating characteristic curve; BMI, body mass index; TyG, triglyceride glucose; WC, waist circumference.
Figure 4.
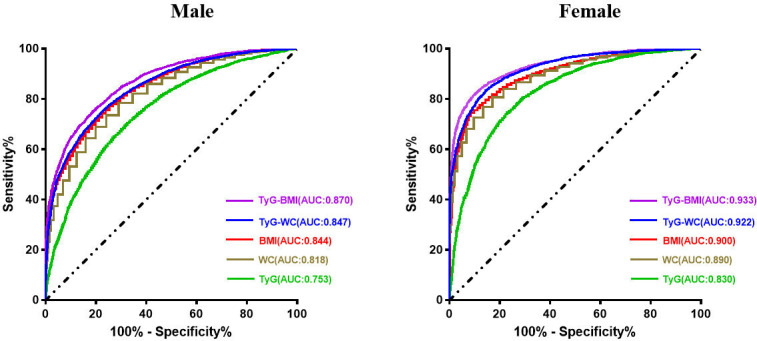
Receiver operating characteristic curve of each parameter for predicting metabolic-associated fatty liver disease according to sex. AUC, area under the receiver operating characteristic curve; BMI, body mass index; TyG, triglyceride glucose; WC, waist circumference.
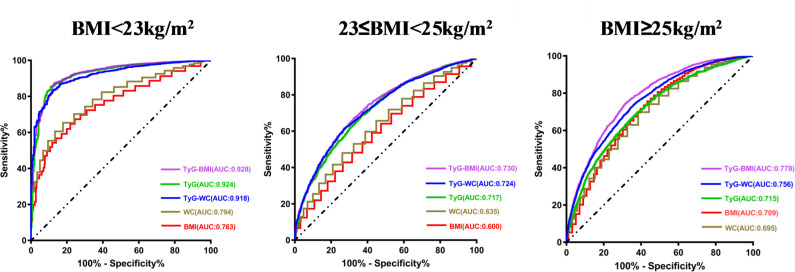
Predictive values of different indicators for MAFLD according to BMI
As shown in table 4 and figure 5, the performance of the TyG-BMI was particularly noteworthy in the lean group (BMI<23 kg/m2; AUC of 0.928), followed by the performance of TyG (AUC of 0.924) and TyG-WC (AUC of 0.918) indices. A TyG-BMI cut-off value of 156.31 in the lean group showed the best overall performance, with a sensitivity of 87.2% and a specificity of 87.1%. In contrast to the previous analyses, BMI and WC exhibited the worst performances across all three groups (AUC (BMI), 0.763, 0.600, 0.709; AUC (WC), 0.794, 0.635, 0.695, respectively).
Table 4.
Cut-off values and AUCs (95% CI) of each parameter for predicting metabolic-associated fatty liver disease in different BMI subgroups
| AUC (95% CI) | Cut-off value | Sensitivity (%) | Specificity (%) | |
| BMI<23 (n=7377) | ||||
| BMI | 0.763 (0.739 to 0.788) | 21.65 | 77.5 | 64.7 |
| WC | 0.794 (0.771 to 0.817) | 74.50 | 79.8 | 65.4 |
| TyG | 0.924 (0.908 to 0.940) | 7.11 | 89.1 | 85.2 |
| TyG-BMI | 0.928 (0.914 to 0.943) | 156.31 | 87.2 | 87.1 |
| TyG-WC | 0.918 (0.905 to 0.931) | 541.99 | 88.0 | 83.0 |
| 23≤BMI<25 (n=4799) | ||||
| BMI | 0.600 (0.583 to 0.616) | 24.05 | 55.3 | 59.1 |
| WC | 0.635 (0.618 to 0.651) | 80.50 | 70.7 | 48.0 |
| TyG | 0.717 (0.702 to 0.732) | 7.10 | 63.7 | 68.3 |
| TyG-BMI | 0.730 (0.716 to 0.745) | 169.67 | 67.7 | 66.9 |
| TyG-WC | 0.724 (0.709 to 0.739) | 572.91 | 73.1 | 60.9 |
| BMI≥25 (n=8746) | ||||
| BMI | 0.709 (0.698 to 0.720) | 27.25 | 55.7 | 75.7 |
| WC | 0.695 (0.683 to 0.707) | 90.50 | 58.3 | 69.8 |
| TyG | 0.715 (0.703 to 0.726) | 7.19 | 65.6 | 66.0 |
| TyG-BMI | 0.778 (0.767 to 0.788) | 194.83 | 69.2 | 73.5 |
| TyG-WC | 0.756 (0.745 to 0.767) | 652.43 | 65.4 | 72.4 |
AUC, area under the receiver operating characteristic curve; BMI, body mass index; TyG, triglyceride glucose; WC, waist circumference.
Figure 5.
Receiver operating characteristic curve of each parameter for predicting metabolic-associated fatty liver disease in different BMI subgroups. AUC, area under the receiver operating characteristic curve; BMI, body mass index; TyG, triglyceride glucose; WC, waist circumference.
Discussion
In this cross-sectional study, we identified the relationships between TyG-related indices and the risk of MAFLD. We discovered that individuals with higher values of TyG-related indices were more likely to have MAFLD. Furthermore, these parameters followed a dose–response relationship across the quartiles even after adjustment. In particular, the TyG-BMI exhibited the strongest predictive performance among the indices, and participants in the highest TyG-BMI quartile group were 380.87 times more likely to have MAFLD than those in the lowest quartile group. Subgroup analysis further verified the validity of the TyG-BMI for detecting MAFLD in healthy participants. Therefore, the TyG-BMI may be the most reliable indicator for MAFLD among other traditional parameters, as evidenced by its high discriminatory power in both the sex and BMI subgroups. Notably, this index performed exceptionally in the lean and female subgroups. Although the TyG and TyG-WC indices also presented some predictive value for MAFLD, we observed that they were not quite stable and fluctuated in different subgroups. The above-mentioned study findings support the adoption of the TyG-BMI as an alternative screening instrument for MAFLD.
To date, there have only been a few investigations on the diagnostic effectiveness of TyG-related indices for MAFLD.20–22 Taheri et al first evaluated the association between the TyG index and MAFLD risk in an Iranian population. Among those in the highest, relative to the lowest TyG tertile, the multivariable-adjusted ORs (95% CI) were 12.01 (9.03 to 15.98) and 10.89 (7.66 to 15.48), respectively. Their results demonstrated that a TyG index cut-off of 8.62 had 81.66% sensitivity and 75.36% specificity.20 However, that study used the fatty liver index to define MAFLD rather than ultrasonography or liver biopsies, and it did not assess the performance of the TyG-BMI or the TyG-WC index. Similarly, a Chinese study, while reporting results consistent with Taheri’s findings, found that a combination of TyG, BMI and ALT improved the diagnostic capability for MAFLD. The combined model demonstrated an AUC of 0.985 (95% CI 0.973 to 0.998) which outperformed the TyG alone (AUC=0.943; 95% CI 0.912 to 0.973) and TyG-BMI (AUC=0.956; 95% CI 0.933 to 0.980). This study exhibited a higher diagnostic accuracy than that of the present study; however, it included a small sample size of 229 patients.21 Xue et al provided evidence for TyG-related indices as better predictive indicators for MAFLD than NAFLD. The TyG-WC index had the strongest performance, with an AUC (95% CI) of 0.815 (0.796 to 0.833) for predicting NAFLD and 0.832 (0.814 to 0.850) for predicting MAFLD.22 However, unlike previous studies, our study provided a comprehensive assessment of the TyG-related indices, including TyG, TyG-BMI and TyG-WC, for their ability to screen for and identify MAFLD in healthy Chinese participants.
Interestingly, the present study revealed that the predictive accuracies of TyG-related indices varied among different subgroups. When we stratified MAFLD individuals by BMI profile, we found that the TyG-BMI performed the strongest in the lean population. It is noteworthy that the incidence of MAFLD has been observed to increase in tandem with the escalating prevalence of obesity. However, it should be emphasised that individuals with a lean body composition may also be susceptible to the condition. A recent study in China found that among the non-obese population, the prevalence of MAFLD was 11.5% (males: 16.4%, females: 6.9%), which was consistent with Vilarinho et al’s findings.23 24 Importantly, MAFLD in lean participants was not benign or stable, contrary to what was initially believed. Numerous studies have even suggested that compared with those with obese MAFLD, lean individuals with MAFLD have an increased risk of diabetes mellitus and cardiovascular and all-cause mortality.25 26 BMI is widely used to evaluate obesity, but fails to evaluate regional fat distribution. The contribution of visceral fat to MAFLD has been found to be more important than that of total body fat.27 Although Asians have a lower absolute BMI than Westerners, Asians are more vulnerable to visceral fat accumulation and IR.28 Thus, reduced BMI levels are not necessarily representative of a metabolically healthy state. Based on the formula of the TyG-BMI,16 we could reasonably infer that the higher an individual’s BMI, the higher the TyG-BMI. From this perspective alone, the TyG-BMI does not appear to be an ideal predictor for MAFLD. However, our study observed that increased TyG-BMI values were positively correlated with the risk of MAFLD in lean individuals. Thus, the lack of attention to the dynamic changes of various metabolic states may be a reason why the predictive ability of TyG has often been overlooked. In lean individuals with MAFLD, the impact of TyG increase may outweigh that of BMI decrease. That is to say, IR induced by excessive accumulation of visceral fat may have a more pronounced role in MAFLD development in lean individuals.9 Chen et al revealed that incidence of metabolic disorders in non-obese individuals with MAFLD were significantly higher than that in non-obese individuals without MAFLD.23 Therefore, relying solely on decreased BMI or increased TyG may not be adequate for predicting lean MAFLD. A comprehensive consideration of the TyG-BMI is essential for a better understanding of its predictive value in lean MAFLD.
The predictive value of TyG-related indices differed depending on sex classification. Significantly, while the TyG-BMI demonstrated superior performance in both men and women, it was more accurate in predicting MAFLD in women in the present study. Moreover, the current study and a previous study23 came to the same conclusion that MAFLD has a higher prevalence in men than in women (p<0.0001). In addition, Chen et al further described the age-related prevalence of MAFLD, with men being more susceptible at younger ages and after which it increased only gradually through middle age, while women showed a slow rise in susceptibility until the age of 45, after which it accelerated sharply.23 This finding suggests that a decrease in oestrogen may be the primary cause of the sudden increase in MAFLD prevalence in older women and thus low oestrogen levels during the postmenopausal period may be an important risk factor for MAFLD in women.29 Several studies have found that decreased oestrogen levels are associated with many metabolic disorders, including dyslipidaemia and IR. The lack of oestrogen availability also decreases hepatic insulin clearance and allows the development of diet-induced IR.30 31 Notably, in the current study, we observed that increased TyG-BMI values were closely related to the risk of MAFLD in female individuals. However, the specific mechanisms underlying this phenomenon remain to be elucidated.
Our study had several limitations. First, the diagnosis of MAFLD was based on ultrasonography, which may have showed decreased sensitivity when liver steatosis is below 30%.32 Therefore, using ultrasound to screen for MAFLD may have underestimated the true prevalence of MAFLD. Second, certain data were not available from the health examination, such as the liver biopsy data or the controlled attenuation parameter and liver stiffness measurement from the FibroScan Test. Hence, further studies on the relationships between TyG-related indices and the severity of MAFLD are needed. Third, we included asymptomatic individuals from a single centre; thus, selection bias to a certain extent was inevitable. In addition, we noticed that the 95% CIs of the quartile analysis were relatively wide, especially the fourth quartile of the TyG-BMI (263.25 to 551.05), which may be related to the insufficient sample size. Therefore, multicentre and prospective studies with larger and more diverse populations are required to validate our findings. Our study had several notable strengths. First and foremost, we provide novel evidence regarding the utility of the TyG-BMI in predicting MAFLD in lean and female individuals. Moreover, we enrolled participants from diverse occupations and backgrounds and collected extensive clinical data to ensure statistical reliability and to validate our findings from multiple perspectives. In addition, our study has important clinical implications, as it is the first to demonstrate that the assessment of the TyG-BMI could be helpful in identifying individuals with high risk of MAFLD, especially among those who are lean and female.
In conclusion, the present study suggested that the TyG-BMI was a promising predictor for MAFLD. Individuals with BMI values within the normal range but high TyG-BMI levels should undergo a more detailed assessment for MAFLD. Our findings extended previous investigations by demonstrating that the TyG-BMI may be an ideal predictor for the presence of MAFLD in lean and female individuals.
Supplementary Material
Footnotes
Contributors: MC and GS conceived of and designed the study. MC and ZS coordinated data collection and performed the analyses. MC wrote the manuscript. All authors have read and approved the final version of the manuscript. MC is the article guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (approval number: XYFY2023-KL086-01). The requirement for written informed consent was waived due to the retrospective nature of the study.
References
- 1. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nature reviews. Gastroenterology & Hepatology 2018;15:11–20. 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 2. Huang S-C, Su H-J, Kao J-H, et al. Clinical and histologic features of patients with biopsy-proven metabolic dysfunction-associated fatty liver disease. Gut and liver 2021;15:451–8. 10.5009/gnl20218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guerreiro GTS, Longo L, Fonseca MA, et al. Does the risk of cardiovascular events differ between biopsy-proven NAFLD and MAFLD Hepatology International 2021;15:380–91. 10.1007/s12072-021-10157-y [DOI] [PubMed] [Google Scholar]
- 4. Kim D, Konyn P, Sandhu KK, et al. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. Journal of Hepatology 2021;75:1284–91. 10.1016/j.jhep.2021.07.035 [DOI] [PubMed] [Google Scholar]
- 5. Tarantino G, Crocetto F, Di Vito C, et al. Association of NAFLD and insulin resistance with non metastatic bladder cancer patients: A cross-sectional retrospective study. Journal of clinical medicine 2021;10:346. 10.3390/jcm10020346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xian YX, Weng JP, Xu F. MAFLD vs. NAFLD: Shared features and potential changes in epidemiology, pathophysiology, diagnosis, and Pharmacotherapy. Chinese medical Journal 2021;134:8–19. 10.1097/CM9.0000000000001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abe M, Fujii H, Funakoshi S, et al. Comparison of body mass index and waist circumference in the prediction of diabetes: A retrospective longitudinal study. Diabetes therapy : Research, treatment and education of diabetes and related disorders 2021;12:2663–76. 10.1007/s13300-021-01138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aizawa M, Inagaki S, Moriyama M, et al. Modeling the natural history of fatty liver using lifestyle-related risk factors: Effects of body mass index (BMI) on the life-course of fatty liver. Plos one 2019;14:e0226059. 10.1371/journal.pone.0226059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eslam M, El-Serag HB, Francque S, et al. Metabolic (Dysfunction)-Associated fatty liver disease in individuals of normal weight. Nature reviews. Gastroenterology & Hepatology 2022;19:638–51. 10.1038/s41575-022-00635-5 [DOI] [PubMed] [Google Scholar]
- 10. Gastaldelli A, Folli F, Defronzo RA. The product of triglycerides and glucose as index of insulin resistance. Validation in the SAM study. J Clin Endocrinol Metab 2010;95. [Google Scholar]
- 11. Khamseh ME, Malek M, Abbasi R, et al. Triglyceride glucose index and related parameters (Triglyceride glucose-body mass index and Triglyceride glucose-waist circumference) identify Nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metabolic syndrome and related disorders 2021;19:167–73. 10.1089/met.2020.0109 [DOI] [PubMed] [Google Scholar]
- 12. Cho YK, Lee J, Kim HS, et al. Triglyceride glucose-waist circumference better predicts coronary calcium progression compared with other indices of insulin resistance: A longitudinal observational study. Journal of clinical medicine 2020;10:92. 10.3390/jcm10010092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raimi TH, Dele-Ojo BF, Dada SA, et al. Triglyceride-glucose index and related parameters predicted metabolic syndrome in Nigerians. Metabolic syndrome and related disorders 2021;19:76–82. 10.1089/met.2020.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as Surrogate for identifying insulin resistance in apparently healthy subjects. Metabolic syndrome and related disorders 2008;6:299–304. 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 15. Er L-K, Wu S, Chou H-H, et al. Triglyceride glucose-body mass index is a simple and clinically useful Surrogate marker for insulin resistance in nondiabetic individuals. Plos one 2016;11:e0149731. 10.1371/journal.pone.0149731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. The TRIPOD group. circulation 2015;131:211–9. 10.1161/CIRCULATIONAHA.114.014508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eslam M, Sanyal AJ, George J, et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999–2014.S0016-5085(20)30171-2. 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 18. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. Journal of Hepatology 2020;73:202–9. 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 19. Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. Journal of Hepatology 2009;50:204–10. 10.1016/j.jhep.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 20. Taheri E, Pourhoseingholi MA, Moslem A, et al. The Triglyceride-glucose index as a clinical useful marker for metabolic associated fatty liver disease (MAFLD): A population-based study among Iranian adults. Journal of diabetes and metabolic disorders 2022;21:97–107. 10.1007/s40200-021-00941-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Z, He H, Dai Y, et al. Comparison of the diagnostic value between Triglyceride-glucose index and Triglyceride to high-density lipoprotein cholesterol ratio in metabolic-associated fatty liver disease patients: A retrospective cross-sectional study. Lipids in health and disease 2022;21:55. 10.1186/s12944-022-01661-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xue Y, Xu J, Li M, et al. n.d. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: Triglyceride glucose index-related parameters. Frontiers in Endocrinology;13. 10.3389/fendo.2022.951689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Li H, Li S, et al. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: A cross-sectional comparative study. BMC Gastroenterology 2021;21:212. 10.1186/s12876-021-01782-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vilarinho S, Ajmera V, Zheng M, et al. Emerging role of Genomic analysis in clinical evaluation of lean individuals with NAFLD. Hepatology (Baltimore, Md.) 2021;74:2241–50. 10.1002/hep.32047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng R-N, Du S-S, Wang C, et al. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World Journal of Gastroenterology 2014;20:17932–40. 10.3748/wjg.v20.i47.17932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. The Lancet. Gastroenterology & Hepatology 2020;5:739–52. 10.1016/S2468-1253(20)30077-7 [DOI] [PubMed] [Google Scholar]
- 27. Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological molecular mechanisms of obesity: A link between MAFLD and NASH with cardiovascular diseases. International Journal of molecular sciences 2021;22:11629. 10.3390/ijms222111629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chan JCN, Malik V, Jia W, et al. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. Jama 2009;301:2129–40. 10.1001/jama.2009.726 [DOI] [PubMed] [Google Scholar]
- 29. Della Torre S. n.d. Non-alcoholic fatty liver disease as a Canonical example of metabolic inflammatory-based liver disease showing a sex-specific prevalence: Relevance of estrogen signaling. Frontiers in Endocrinology;11. 10.3389/fendo.2020.572490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alemany M. Estrogens and the regulation of glucose metabolism. World Journal of diabetes 2021;12:1622–54. 10.4239/wjd.v12.i10.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Advances in experimental medicine and biology 2017;1043:227–56. 10.1007/978-3-319-70178-3_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of Ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology (Baltimore, Md.) 2011;54:1082–90. 10.1002/hep.24452 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.