Abstract
We previously showed that in vitro susceptibility profiles of Staphylococcus aureus to thrombin-induced platelet microbicidal protein 1 (tPMP-1) impacted the outcome of vancomycin treatment in experimental infective endocarditis. In this same model, treatment with oxacillin (a more rapid staphylocidal agent than vancomycin) enhanced the clearance of both tPMP-1-susceptible and -resistant cells from vegetations. The extent of clearance was greater for tPMP-1-susceptible cells.
Platelets have received recent recognition for contributions to antimicrobial host defense, believed to be mediated in part through the secretion of cationic antimicrobial peptides, termed platelet microbicidal proteins (PMPs) (21). Thrombin-induced PMP-1 (tPMP-1) is released from rabbit platelets in vitro upon stimulation with thrombin, a molecule generated at sites of endovascular damage (11, 12, 21, 24, 25). In vitro, tPMP-1 exerts potent microbicidal activity against common blood-borne organisms, including Staphylococcus aureus (1, 9, 20, 22, 24). Moreover, S. aureus strains are synergistically killed in vitro by combinations of oxacillin or vancomycin and tPMP-1 (9, 23). Furthermore, sublethal concentrations of tPMP-1 induce prolonged growth inhibitory effects which are magnified by sequential exposures to tPMP-1 and then to vancomycin or oxacillin (23).
It was recently shown that the tPMP-1 susceptibility profiles of S. aureus strains in vitro impact significantly on the therapy of experimental infective endocarditis (IE) with vancomycin, an agent with slow in vitro and in vivo staphylocidal effects (9, 19). Thus, clearance of a tPMP-1-resistant (tPMP-1r) S. aureus strain from IE vegetations was significantly slower during vancomycin therapy than in IE caused by the isogenic tPMP-1-susceptible (tPMP-1s) counterpart strain (9). The present study was designed to evaluate the comparative impact of the intrinsic tPMP-1 susceptibility phenotypes in vitro on the treatment success and prophylactic efficacy in S. aureus IE of oxacillin, a more rapid bactericidal agent than vancomycin (19).
(This study was presented in part at the 37th Annual Meeting of the Infectious Disease Society of America, Philadelphia, Pa., November 1999 [V. K. Dhawan, A. S. Bayer, and M. R. Yeaman, 37th Annu. Meet. Infect. Dis. Soc. Am., abstr. 170, 1999].)
Strain ISP479R (tPMP-1r) was constructed by transposon mutagenesis as previously described (13). Strain ISP479C is the spontaneously plasmid-cured and tPMP-1s variant of ISP479. A detailed genotypic and phenotypic comparison of the ISP479C and ISP479R strains has been reported elsewhere (13) and revealed no substantive differences, other than susceptibility or resistance, respectively, to tPMP-1 in vitro. Staphylococci were stored, grown in medium, and prepared for animal inoculation as previously described (10).
Oxacillin was purchased from a commercial source (SmithKline Beecham, Bristol, Tenn.).
The oxacillin MICs for the S. aureus strains were determined by a broth microdilution method (9) in Trypticase soy broth at a final inoculum concentration of either ∼5 × 105 or ∼5 × 107 logarithmic-phase cells/ml (to encompass the vegetation densities observed for experimental IE [8–10]). MICs were read after 48 h of incubation at 37°C as the lowest oxacillin concentration yielding no visible growth.
Time-kill studies were performed for the two S. aureus strains, at a final inoculum concentration of ∼5 × 105 or ∼5 × 107 log-phase cells/ml, as previously described (9). The final oxacillin concentration utilized (20 μg/ml) represents a readily achievable level in serum at the dose regimens employed in the in vivo studies described below (5, 14).
tPMP-1 was prepared and standardized as previously described, following thrombin stimulation of washed rabbit platelets (24, 25).
We examined the synergistic potential of tPMP-1 and oxacillin against the study strains at a high bacterial inoculum concentration to simulate the conditions believed to exist within experimental vegetations during the therapy of established IE (9, 14). Mid-logarithmic-phase cells were suspended in minimal essential medium at a final inoculum concentration of 106 CFU/ml and a final oxacillin concentration of 0.5 μg/ml (sub-MIC) (34). tPMP-1 was added to achieve a range of final peptide concentrations from 0.38 to 3 μg/ml. The endpoint interpretations of this assay were as previously detailed (9, 15).
To examine the effect of in vitro tPMP-1 susceptibility phenotypes on therapeutic and prophylactic outcomes of IE, a previously described rabbit IE model was used (17). IE was induced by intravenous injection of 2 × 106 CFU of either strain at 24 h postcatheterization (8, 10).
At 24 h after induction of IE, animals were divided into two groups for each study strain: untreated (controls) and oxacillin treated (50 mg/kg of body weight intramuscularly [i.m.] three times a day for 2 days). This regimen reliably achieves supra-MIC levels in serum for each infecting S. aureus strain in experimental IE (5, 14). At the randomization point, controls were sacrificed to establish target tissue bacterial densities at the outset of oxacillin therapy (8–10). These tissue bacterial densities from untreated controls were used to confirm that the ISP479C and ISP479R strains achieved similar extents of infection within target tissues at the time of initiation of antibiotic therapy. For the purposes of statistical analyses, culture-negative tissues were assigned a normalized value based on the tissue sample weights (i.e., ≤2 log10 CFU/g of vegetation based on a mean weight of 0.1 g). Since the pharmacokinetics of oxacillin are well established for this model (5), serum drug levels were not obtained.
The impact of the in vitro tPMP-1 susceptibility phenotypes on the prophylactic efficacy of oxacillin against IE was also studied. At 24 h postcatheterization, animals received oxacillin (50 mg/kg, i.m.) 2 h prior to bacterial challenge (2 × 106 CFU) with either strain, followed by a second dose 6 h later. This two-dose regimen parallels that recommended for the prevention of human IE (7). Untreated animals served as controls. Animals were sacrificed 48 h postinoculation, and vegetations were removed and quantitatively cultured as described above. Prophylaxis was considered effective if vegetations were rendered culture negative.
Proportional differences in the frequencies of IE induction were compared by Fisher's exact test. Differences in median tissue bacterial densities were compared by the Mann-Whitney U test. P values of ≤0.05 were considered statistically significant.
The oxacillin MICs for both study strains were identical (1 and 2 μg/ml, at inoculum concentrations of 105 and 107 CFU/ml, respectively). In time-kill assays, the rates of killing of the study strains (inoculated at 105 and 107 CFU/ml) were equal (data not shown).
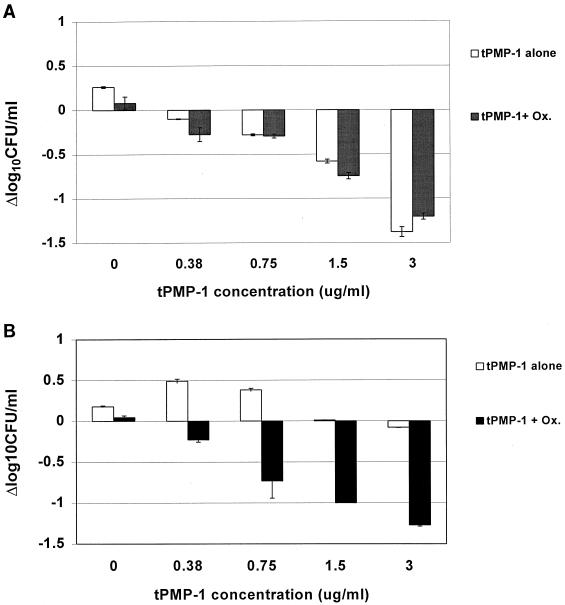
For ISP479C, tPMP-1 alone caused concentration-dependent killing, resulting in a mean count of ∼−1.4 Δlog10 CFU/ml/2 h at the highest peptide concentration tested. In contrast, tPMP-1 alone caused no significant reductions in the bacterial densities of the ISP479R strain.
The in vitro interaction characteristics of tPMP-1 with oxacillin differed substantially between the two study strains (Fig. 1). The addition of oxacillin to tPMP-1 caused no enhancement of bactericidal effects against ISP479C. The addition of oxacillin to tPMP-1 resulted in a peptide concentration-dependent killing of the tPMP-1r strain, at a magnitude similar to that seen for ISP479C. The degree of enhancement of the bactericidal effect against the tPMP-1r strain with the combination of tPMP-1 and oxacillin fell short of synergy (i.e., <2 Δlog10 CFU/ml/2 h) (15).
FIG. 1.
In vitro bactericidal interaction assays with the combination of sub-MIC oxacillin (Ox.) (0.5 μg/ml) and tPMP-1 at various concentrations against tPMP-1-susceptible strain ISP479C (A) or tPMP-1-resistant strain ISP479R (B). Oxacillin alone yielded no in vitro bactericidal effects over the 2-h incubation period for either strain.
Bacterial densities in the three target tissues (vegetations, kidney abscesses, and splenic abscesses) at 24 h postinduction did not differ significantly in control animals infected with either the ISP479C or ISP479R strain (Table 1). Compared to their respective untreated controls, oxacillin therapy significantly reduced bacterial densities in all target tissues, with all kidneys and spleens being rendered culture negative. Oxacillin therapy produced a greater overall reduction in the vegetation densities of ISP479C cells than in those of ISP479R cells; the mean decreases in vegetations infected with ISP479C or ISP479R cells were −3.97 and −3.0 log10 CFU/g, respectively (P = 0.065).
TABLE 1.
Oxacillin therapy of established experimental S. aureus endocarditis
| Strain and target tissue | Mean log10 CFU/g ± SD (no. of animals)
|
P value | |
|---|---|---|---|
| Untreated | Oxacillin treateda | ||
| ISP479C | |||
| Vegetations | 6.20 ± 1.00 (10) | 2.33 ± 0.8 (12) | <0.0001 |
| Kidneys | 3.90 ± 1.43 (10) | 2.00 ± 0.0 (12) | <0.0005 |
| Spleens | 4.16 ± 0.98 (10) | 2.00 ± 0.0 (12) | <0.0005 |
| ISP479R | |||
| Vegetations | 6.87 ± 0.50 (12) | 3.72 ± 1.8 (12) | <0.01 |
| Kidneys | 4.70 ± 0.60 (12) | 2.00 ± 0.0 (12) | <0.0005 |
| Spleens | 4.60 ± 0.53 (12) | 2.00 ± 0.0 (12) | <0.0005 |
Oxacillin was given at 50 mg/kg i.m. three times a day for 2 days.
In contrast to the successful treatment of established IE, no significant differences were observed in the efficacies of oxacillin prophylaxis of IE caused by either challenge strain. All control animals developed IE. The standard two-dose oxacillin prophylaxis was effective in 10 of 16 (63%) animals inoculated with ISP479C and 11 of 18 (61%) animals inoculated with ISP479R (P was not significant).
We previously used a genetically related S. aureus strain pair to study the impact of in vitro tPMP-1 susceptibility phenotypes on antistaphylococcal antibiotic treatment and prophylactic outcomes of experimental IE (9). We initially used vancomycin in this context since this agent exerts relatively slow in vitro and in vivo staphylocidal effects (19), thus increasing the potential for identifying synergistic bactericidal interactions in vivo (9). The present study was aimed at comparing those observations with the effect of the tPMP-1 susceptibility phenotypes in vitro on the treatment and prophylaxis of IE using oxacillin, a much more rapid staphylocidal agent than vancomycin.
Similar to our prior study utilizing vancomycin (9), oxacillin therapy of established IE produced a greater extent of clearance of ISP479C cells than of ISP479R cells from vegetations, compared to their respective untreated controls. However, in contrast to our prior study, in which vancomycin did not substantially reduce the densities of ISP479R (9), oxacillin substantially reduced vegetation densities of strain ISP479R, albeit to a lesser extent than that observed for strain ISP479C.
This difference in microbiologic outcome between the vancomycin and oxacillin regimens is likely to be multifactorial. For example, the rapid staphylocidal activity of oxacillin for both S. aureus strains compared to that of vancomycin may account for the reduction in vegetation bacterial densities of the ISP479R strain. Further, the more extensive and uniform penetration of β-lactam agents (such as oxacillin) into cardiac vegetations than that of glycopeptide antibiotics (such as vancomycin) may have also contributed to our findings (6). Moreover, our in vitro data clearly showed that the combination of oxacillin with tPMP-1 substantially increased the bactericidal effects against the intrinsically tPMP-1r strain, ISP479R, compared to tPMP-1 alone. The mechanism of the latter in vitro phenomenon is not known. However, it has been well documented that cell wall-active agents (such as oxacillin) can promote the facilitated uptake of cationic antimicrobial agents (such as aminoglycosides) in antibiotic-resistant bacteria (e.g., enterococci [16]). Thus, it is conceivable that in the presence of oxacillin the intracellular uptake of the cationic peptide tPMP-1 into tPMP-1r ISP479R cells may also be facilitated. Further study is needed to address this hypothesis.
In contrast to bacterial clearance from vegetations, oxacillin therapy rendered all kidneys and spleens culture negative, irrespective of the infecting strain. This difference in treatment outcomes in distinct target tissues was also noted in our previous study utilizing vancomycin (9).
As opposed to the findings with treatment of well-established IE, similar degrees of prophylactic efficacy were obtained with oxacillin in catheterized rabbits challenged with either the tPMP-1s or tPMP-1r S. aureus strain, compared to untreated controls. Equivalent results were noted in our previous prophylaxis studies with vancomycin (9). The differences observed between the treatment and the prophylaxis outcomes with respect to tPMP-1 susceptibility phenotypes may be explained by differential determinants of therapeutic versus prophylactic efficacy in IE. While the microbicidal effects of antibiotics, such as oxacillin, appear to best correlate with the successful outcome of therapy of established IE (4, 18), current paradigms emphasize that prophylactic efficacy is primarily dependent on nonmicrobicidal effects, such as prolonged growth inhibition and antivegetation adhesion (2, 3, 13). It has been suggested that the platelet, a pivotal component of the vegetation in IE, might well be responsible for this function via secretion of PMPs (21). Our present data underscore the probable importance of local tPMP-1 secretion by platelets in modifying the course and response to therapy of endovascular infections.
Acknowledgments
We thank Nimee Bhat and Yin Li Chai for their assistance in the in vitro and animal studies. We thank Ambrose Cheung (Dartmouth School of Medicine, Hanover, N.H.) for assistance in the genotypic and phenotypic characterization of the study strains.
This research was supported in part by the following grants from the National Institutes of Health: RCMI G12 RR03026-09 (to V.K.D.), AI39108 (to A.S.B.), and AI39001 (to M.R.Y.).
REFERENCES
- 1.Bayer A S, Cheng D, Yeaman M R, Corey G R, McClelland R S, Harrel L J, Fowler V G., Jr In vitro resistance to thrombin-induced platelet microbicidal protein among clinical bacteremic isolates of Staphylococcus aureus correlates with an endovascular infectious source. Antimicrob Agents Chemother. 1998;42:3169–3172. doi: 10.1128/aac.42.12.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer A S, Tu J. Chemoprophylactic efficacy against experimental endocarditis caused by β-lactamase-producing, aminoglycoside-resistant enterococci is associated with prolonged serum inhibitory activity. Antimicrob Agents Chemother. 1990;34:1068–1074. doi: 10.1128/aac.34.6.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berney P, Francioli P. Successful prophylaxis of experimental streptococcal endocarditis with single-dose amoxicillin administered after bacterial challenge. J Infect Dis. 1990;161:281–285. doi: 10.1093/infdis/161.2.281. [DOI] [PubMed] [Google Scholar]
- 4.Brennan R O, Durack D T. Therapeutic significance of penicillin tolerance in experimental streptococcal endocarditis. Antimicrob Agents Chemother. 1983;23:273–277. doi: 10.1128/aac.23.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers H F, Sachdeva M, Kennedy S. Binding affinity for penicillin-binding protein 2a correlates with in vivo activity of β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990;162:705–710. doi: 10.1093/infdis/162.3.705. [DOI] [PubMed] [Google Scholar]
- 6.Cremieux A-C, Maziere B, Vallois J-M, Ottaviani M, Azancot A, Raffoul H, Bouvet A, Pocidalo J-J, Carbon C. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J Infect Dis. 1989;159:938–944. doi: 10.1093/infdis/159.5.938. [DOI] [PubMed] [Google Scholar]
- 7.Dajani A S, Taubert K A, Wilson W, Bolger A F, Bayer A S, Ferrieri P. Prevention of bacterial endocarditis: recommendations by the American Heart Association. JAMA. 1997;277:1794–1801. [PubMed] [Google Scholar]
- 8.Dhawan V K, Bayer A S, Yeaman M R. In vitro resistance to thrombin-induced platelet microbicidal protein is associated with enhanced progression and hematogenous dissemination in experimental Staphylococcus aureus infective endocarditis. Infect Immun. 1998;66:3476–3479. doi: 10.1128/iai.66.7.3476-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhawan V K, Yeaman M R, Bayer A S. Influence of in vitro susceptibility phenotype against thrombin-induced platelet microbicidal protein on treatment and prophylaxis outcomes of experimental Staphylococcus aureus endocarditis. J Infect Dis. 1999;180:1561–1568. doi: 10.1086/315063. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan V K, Yeaman M R, Cheung A L, Kim E, Sullam P M, Bayer A S. Phenotypic resistance to thrombin-induced platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect Immun. 1997;65:3293–3299. doi: 10.1128/iai.65.8.3293-3299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake T A, Pang M. Effects of interleukin-1, lipopolysaccharide, and streptococci on procoagulant activity of cultured human cardiac valve endothelial and stromal cells. Infect Immun. 1989;57:507–512. doi: 10.1128/iai.57.2.507-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drake T A, Pang M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J Infect Dis. 1988;157:749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- 13.Fluckiger U, Francioli P, Blaser J, Glauser M P, Moreillon P. Role of amoxicillin serum levels for successful prophylaxis of experimental endocarditis due to tolerant streptococci. J Infect Dis. 1994;169:397–400. doi: 10.1093/infdis/169.6.1397. [DOI] [PubMed] [Google Scholar]
- 14.Hirano L, Bayer A S. β-Lactam–β-lactamase-inhibitor combinations are active in experimental endocarditis caused by β-lactamase-producing oxacillin-resistant staphylococci. Antimicrob Agents Chemother. 1991;35:685–690. doi: 10.1128/aac.35.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moellering R C., Jr Antimicrobial synergism—an elusive concept. J Infect Dis. 1979;140:639–641. doi: 10.1093/infdis/140.4.639. [DOI] [PubMed] [Google Scholar]
- 16.Moellering R C, Jr, Wennersten C B G, Weinberg A N. Synergy of penicillin and gentamicin against enterococci. J Infect Dis. 1971;124:207–209. doi: 10.1093/infdis/124.supplement_1.s207. [DOI] [PubMed] [Google Scholar]
- 17.Perlman B B, Freedman L S. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971;42:394–410. [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos M C, Grayson M L, Eliopoulos G M, Bayer A S. Comparison of daptomycin, vancomycin, and ampicillin-gentamicin for treatment of experimental endocarditis caused by penicillin-resistant enterococci. Antimicrob Agents Chemother. 1992;36:1864–1869. doi: 10.1128/aac.36.9.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small P M, Chambers H F. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34:1227–1231. doi: 10.1128/aac.34.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu T, Yeaman M R, Bayer A S. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among bacteremic staphylococcal and streptococcal isolates. Antimicrob Agents Chemother. 1994;38:729–732. doi: 10.1128/aac.38.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman M R. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–970. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 22.Yeaman M R, Ibrahim A S, Edwards J E, Jr, Bayer A S, Ghannoum M A. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1993;37:546–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeaman M R, Norman D C, Bayer A S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeaman M R, Puentes S M, Norman D C, Bayer A S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992;60:1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeaman M R, Tang Y-Q, Shen A J, Bayer A S, Selsted M E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun. 1997;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]