Abstract
Objective
To establish the prevalence of hyperuricaemia in an elderly Finnish cohort and to assess its association with comorbidities and mortality.
Design
Prospective cohort study.
Setting
Good Ageing in Lahti Region study, Finland 2002–2012 (mortality data analysed until 2018).
Participants
2673 participants (mean age 64 years; 47% men).
Primary and secondary outcome measures
Prevalence of hyperuricaemia in the study population was detected. Associations between hyperuricaemia and mortality were assessed using multivariable adjusted Cox proportional hazards models.
Methods
Data from a prospective, population-based study of elderly people (52–76 years) in the Lahti region, Finland, were used. Information on serum uric acid (SUA) levels as well as several other laboratory variables, comorbidities, lifestyle habits and socioeconomic factors was collected, and the association between SUA level and mortality in a 15-year follow-up period was analysed.
Results
Of 2673 elderly Finnish persons included in the study 1197 (48%) were hyperuricaemic. Hyperuricaemia was extremely prevalent in men (60%). There was an association between elevated SUA and mortality which remained after adjustment for potential confounding factors (age, gender, education, smoking status, body mass index, hypertension and dyslipidaemia). The adjusted HR for all-cause mortality among clearly hyperuricaemic individuals with SUA≥420 µmol/L compared with normouricaemic individuals (SUA<360 µmol/L) was 1.32 (95% CI 1.05 to 1.60) in women and 1.29 (95% CI 1.05 to 1.60) in men. In slightly hyperuricaemic individuals (SUA 360–420 µmol/L) the corresponding HRs were 1.03 (95% CI 0.78 to 1.35) and 1.11 (95% CI 0.89 to 1.39).
Conclusions
Hyperuricaemia is very prevalent in the elderly Finnish population and is independently associated with increased mortality.
Keywords: epidemiology, rheumatology, rheumatology, cardiac epidemiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study is population based and includes a large number of hyperuricaemic elderly individuals.
Abundant information available on many covariables made it possible to adjust the main finding of our study—the association of hyperuricaemia with all-cause and cardiovascular mortality—for confounding factors and the finding was not affected after the adjustment.
The long follow-up period of 15 years enabled to detect that mortality of patients with hyperuricaemia starts to significantly rise in comparison to patients with normouricaemia after the 7th year of follow-up in women and after the 11th in men.
The most ill and disabled persons of the age groups studied were probably under-represented due to lower response rate to the invitations.
Even though we know that a history of urate-lowering therapy was rare during the active follow-up period (years 2002–2012), we have only limited information on the diagnoses of gout in the study population.
Introduction
A high uric acid level in blood (hyperuricaemia) is common and affects as much as 20%–25% of the general population.1 The serum uric acid (SUA) level used to define hyperuricaemia varies somewhat in different studies, but the most widely used threshold is 360 µmol/L (approximately 6 mg/dL), which is the precipitation threshold of monosodium urate in the peripheral joints. The prevalence of hyperuricaemia differs across geographical areas2 and has increased significantly in the recent decades, particularly in developed, high-income countries.2–6
High SUA levels have been identified as an independent risk factor for all-cause mortality as well as for cardiovascular, renal and respiratory mortality.7–16 Several studies indicate that the association between SUA and mortality is U shaped, suggesting that extremely low SUA levels are unfavourable, as well.9–13 The cut-off point for high SUA associated with mortality varies rather widely—from 238 µmol/L (4 mg/dL) to 506 µmol/L (8.5 mg/dL) and it tends to be higher in men than in women. A significant number of the studies have been done in Asian populations and to our knowledge there have been only a few studies done in northern Europe on the association between hyperuricaemia and mortality and the evidence for causality is still insufficient.17–19
There is insufficient evidence in favour for pharmacological urate-lowering treatment (ULT) for patients with hyperuricaemia who have not had gout attacks. Indeed, in current guidelines pharmacological ULT is generally not suggested.20–22
We conducted a population-based study in a senior population of the Lahti region in Finland to establish the prevalence of elevated SUA levels and the association—if any—between hyperuricaemia and mortality over a follow-up period of 15 years.
Subjects, materials and methods
Study population
The data for this research were retrieved from the Good Ageing in Lahti Region (GOAL) study. GOAL is a prospective, population-based study of elderly people that started with baseline visits in 2002. Follow-up visits were conducted in 2005, 2008 and 2012. Mortality data are available up until the end of 2018. Three age cohorts (52–76 years) were included—individuals born in 1926–1930, 1936–1940 and 1946–1950. A total of 4272 subjects from the catchment area of the Päijät-Häme Central Hospital (located in Lahti) were invited and 2815 (66%) responded to the invitation. The SUA level of the study subjects was measured at baseline and at each follow-up visit. The data on the study subjects included socioeconomic status, psychosocial background, education, income, lifestyle habits (smoking, alcohol consumption, exercise), previously diagnosed medical conditions (hypertension, diabetes, coronary heart disease, stroke, cancer) and hospitalisation 12 months prior to the baseline visit. The blood pressure (BP) of the study subjects was measured at baseline three times and the average was documented. Height and weight of the study subjects were measured and the body mass index (BMI) calculated. The waist circumference was measured at a level mid-way between the lowest rib and the iliac crest. In addition to the SUA, the following laboratory tests were made from the blood samples taken on each visit: creatinine, cystatin C, blood glucose, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, C-reactive protein (CRP), high-sensitivity CRP (hsCRP) and 25-hydroxyvitamin D. The use of medication was also documented.
Data on mortality were provided by Statistics Finland. Causes of death were classified according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision. The follow-up of each subject started at the time of the first study visit (February to August 2002) and ended on 31 December 2018.
Uric acid
We present the demographics and the mortality in three SUA groups (<360, 360–419 and ≥420 µmol/L). The cut-off point of 360 μmol/L (approximately 6 mg/dL) was chosen because it is a precipitation threshold of monosodium urate in the peripheral joints and it is used as a definition of hyperuricaemia in numerous sources.23–25 Other sources use cut-off point of 420 μmol/L (approximately 6 mg/dL) to define hyperuricaemia26 27 and some use cut-off point of 360 μmol/L in women and 420 μmol/L in men.28 29 We decided to investigate the associations with mortality separately in group with SUA of ≥420 µmol/L (clearly hyperuricaemic individuals) and group with SUA of 360–419 µmol/L (slightly hyperuricaemic individuals) to detect if slight hyperuricaemia poses a risk for mortality and does differ from the risk associated with clear hyperuricaemia.
Statistical methods
Data are presented as means with SD or as counts (n) with percentages (%). Statistical significances for the unadjusted hypothesis of linearity across categories of the SUA levels were evaluated by using the Cochran–Armitage test, analysis of variance or logistic models with an appropriate contrast. In the case of violation of the assumptions (eg, non-normality), a bootstrap type of test was used. The Kaplan-Meier method was applied to estimate all-cause mortality and the log-rank test was used to test the trend of the mortality function across three SUA levels.
Cox proportional hazards regression was used to estimate the adjusted HRs and their 95% CIs. Age, gender, education, current smoking, BMI, hypertension and dyslipidaemia were used as covariates in these models. The proportional hazards assumption was evaluated by Schoenfeld residuals and log-log plots. A possible non-linear relationship between mortality and SUA was assessed by using 4-knot restricted cubic spline Cox regression models. The length of the distribution of knots was located at the 5th, 35th, 65th and 95th percentiles; knot locations are based on Harrell’s recommended percentiles.30 The ratio of observed to expected number of deaths (ie, the standardised mortality ratio, SMR) for all-cause deaths was calculated using subject-years methods with 95% CIs. The expected number of deaths was calculated on the basis of gender, age and calendar period-specific mortality rates in the Finnish population (Official Statistics of Finland). The normality of the distribution of variables was evaluated graphically and by using the Shapiro-Wilk W test. Stata V.17.0 (StataCorp, College Station, Texas, USA) was used for the statistical analyses.
The study followed the guidelines of the Declaration of Helsinki. All participants gave their written informed consent prior to data collection.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Of the 2815 individuals who responded to the invitation, the SUA level was available from 2673 (1404 women, 1269 men (47%)) representing 95% of all invited. If these data were lacking at the baseline visit, the individual was excluded from the study. The mean age of the participants was 64 years (range 52–76 years). The baseline characteristics of the study subjects by SUA levels (<360, 360–419 and ≥420 µmol/L) are presented in table 1.
Table 1.
Baseline characteristics of the study subjects according to SUA levels (μmol/L)
| Women | Men | |||||||
| <360 n=968 |
360–419 n=255 |
≥420 n=181 |
P value* | <360 n=508 |
360–419 n=345 |
≥420 n=416 |
P value* | |
| Demographics | ||||||||
| Age, years, n (%) | 0.001 | 0.024 | ||||||
| 52–57 | 345 (36) | 87 (34) | 43 (24) | 177 (35) | 85 (25) | 121 (29) | ||
| 62–67 | 345 (36) | 89 (35) | 66 (36) | 179 (35) | 148 (43) | 147 (35) | ||
| 72–77 | 278 (29) | 79 (31) | 72 (40) | 152 (30) | 112 (32) | 148 (36) | ||
| Education years, mean (SD) | 9.7 (3.3) | 9.4 (3.4) | 8.7 (2.9) | <0.001 | 9.4 (3.4) | 9.3 (3.3) | 9.6 (3.4) | 0.30 |
| Retired, n (%) | 591 (61) | 160 (63) | 129 (71) | 0.015 | 311 (61) | 230 (67) | 282 (68) | 0.082 |
| Cohabiting, n (%) | 617 (64) | 154 (60) | 103 (57) | 0.062 | 423 (83) | 280 (81) | 326 (78) | 0.059 |
| Health behaviours | ||||||||
| Current smokers, n (%) | 113 (12) | 31 (12) | 23 (13) | 0.68 | 127 (25) | 73 (21) | 71 (17) | 0.003 |
| AUDIT score C8, n (%) | 2.2 (1.9) | 2.4 (2.1) | 2.5 (2.3) | 0.30 | 4.0 (2.6) | 4.5 (2.5) | 4.5 (2.8) | 0.009 |
| LTPA, n (%) | <0.001 | 0.060 | ||||||
| Low | 192 (20) | 51 (20) | 60 (33) | 144 (29) | 88 (26) | 129 (32) | ||
| Moderate | 407 (43) | 114 (46) | 71 (39) | 198 (40) | 138 (40) | 178 (44) | ||
| High | 353 (37) | 84 (34) | 49 (27) | 157 (31) | 116 (34) | 98 (24) | ||
| Clinical | ||||||||
| Body mass index | 27.1 (4.6) | 29.6 (4.7) | 31.5 (5.9) | <0.001 | 26.5 (3.7) | 27.5 (3.5) | 29.0 (4.3) | <0.001 |
| Waist | 89.1 (12.3) | 95.7 (12.1) | 100.7 (12.4) | <0.001 | 96.6 (10.5) | 99.7 (9.6) | 103.9 (11.3) | <0.001 |
| Fasting glucose (mmol/L), mean (SD) | 5.46 (1.20) | 5.63 (1.17) | 5.99 (1.25) | <0.001 | 5.94 (1.66) | 5.84 (0.98) | 6.10 (1.42) | <0.001 |
| Total cholesterol (mmol/L), mean (SD) | 5.88 (1.03) | 5.90 (1.04) | 5.88 (1.20) | 0.92 | 5.63 (1.11) | 5.58 (1.07) | 5.65 (1.16) | 0.79 |
| HDL cholesterol (mmol/L), mean (SD) | 1.70 (0.46) | 1.55 (0.41) | 1.46 (0.41) | <0.001 | 1.42 (0.39) | 1.36 (0.35) | 1.30 (0.36) | <0.001 |
| LDL cholesterol (mmol/L), mean (SD) | 3.57 (0.93) | 3.57 (0.95) | 3.53 (1.05) | 0.70 | 3.58 (0.97) | 3.53 (0.96) | 3.52 (1.03) | 0.34 |
| Triglycerides (mmol/L), mean (SD) | 1.33 (0.63) | 1.69 (0.84) | 1.95 (1.14) | <0.001 | 1.40 (0.75) | 1.54 (0.81) | 1.93 (1.83) | <0.001 |
| hsCRP | 2.39 (4.77) | 2.99 (3.23) | 4.06 (4.94) | <0.001 | 3.03 (6.91) | 2.28 (3.30) | 3.40 (6.03) | <0.001 |
| Blood pressure, mean (SD) | ||||||||
| Systolic | 145 (20) | 146 (19) | 150 (19) | 0.002 | 145 (19) | 145 (18) | 149 (19) | 0.003 |
| Diastolic | 84 (9) | 86 (9) | 86 (9) | 0.001 | 87 (10) | 88 (10) | 88 (10) | 0.031 |
| Comorbidities | ||||||||
| DM | 45 (5) | 16 (6) | 16 (9) | 0.019 | 45 (9) | 26 (8) | 42 (10) | 0.55 |
| Hypertension | 302 (31) | 103 (40) | 97 (54) | <0.001 | 118 (23) | 119 (34) | 179 (43) | <0.001 |
| CVD | 60 (6) | 20 (8) | 19 (10) | 0.033 | 48 (9) | 45 (13) | 67 (16) | 0.002 |
| Respiratory diseases | 87 (9) | 17 (7) | 21 (12) | 0.59 | 21 (4) | 19 (6) | 35 (8) | 0.007 |
| Mental health disorders | 66 (7) | 15 (6) | 6 (3) | 0.080 | 23 (5) | 16 (5) | 17 (4) | 0.76 |
| Cancer | 34 (4) | 13 (5) | 6 (3) | 0.74 | 27 (5) | 14 (4) | 19 (5) | 0.57 |
| Neurological disorders | 39 (4) | 7 (3) | 7 (4) | 0.65 | 24 (5) | 22 (6) | 28 (7) | 0.19 |
| Musculoskeletal disorders | 360 (37) | 102 (40) | 68 (38) | 0.69 | 153 (30) | 104 (30) | 151 (36) | 0.051 |
| Medication | ||||||||
| Urate lowering† | 21 (2) | 10 (4) | 26 (14) | <0.001 | 16 (3) | 17 (5) | 102 (25) | <0.001 |
| Antidiabetic‡ | 35 (4) | 12 (5) | 13 (7) | 0.031 | 37 (7) | 22 (6) | 32 (8) | 0.84 |
| Antihypertensive‡ | 241 (25) | 95 (37) | 97 (54) | <0.001 | 100 (20) | 104 (30) | 159 (38) | <0.001 |
| CVD‡ | 100 (10) | 31 (12) | 40 (22) | <0.001 | 59 (12) | 59 (17) | 95 (23) | <0.001 |
| Lipid lowering‡ | 126 (13) | 44 (17) | 27 (15) | 0.22 | 60 (12) | 77 (22) | 94 (23) | <0.001 |
| Psychiatric‡ | 56 (6) | 11 (4) | 4 (2) | 0.036 | 16 (3) | 8 (2) | 9 (2) | 0.34 |
*P for linearity
†Number of patients with a history of a urate-lowering drug purchase in the active follow-up period of the study (years 2002–2012).
‡Number of patients who used medication at baseline.
AUDIT, Alcohol Use Disorders Identification Test; CVD, cardiovascular disease; DM, diabetes mellitus; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; LTPA, leisure-time physical activity, graded as low (exercise less than once a week), moderate (from one to two times a week) or high (at least three times a week); SUA, serum uric acid.
We found hyperuricaemia to be very prevalent in the study population. SUA ≥360 µmol/L at the baseline visit was present in 436 (31%, 95% CI 29% to 33%) of the female subjects and 761 (60%, 95% CI 57% to 63%) of the male subjects, p<0.001. The mean (SD) SUA level of the whole study group was 357 μmol/L (86); in the female population it was 330 (78) and in the male population 387 (86). The distribution of urate levels in women and men is shown in figure 1.
Figure 1.
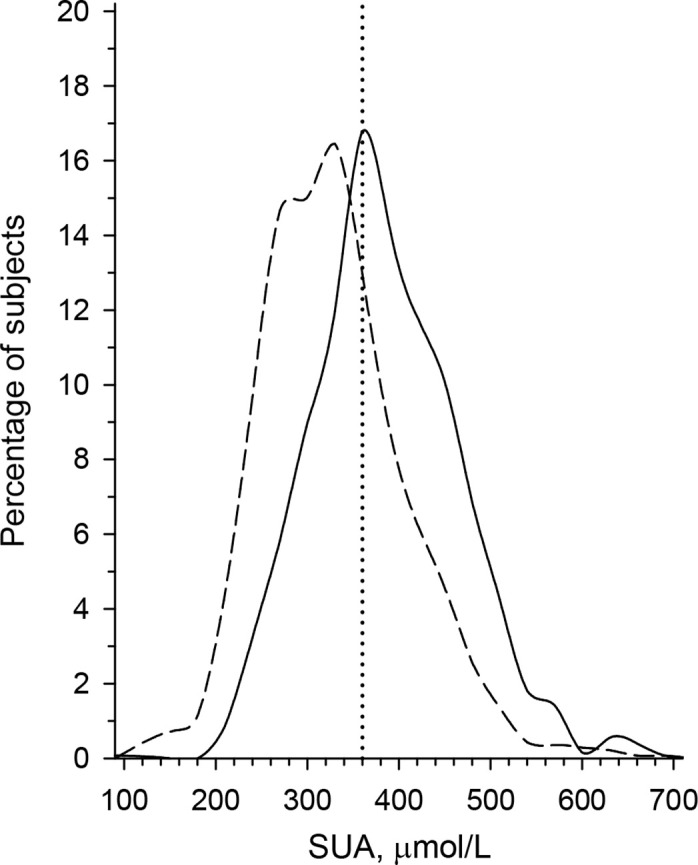
The distribution of urate levels (µmol/L) in women and men. The distributions are based on the kernel estimate. SUA, serum uric acid.
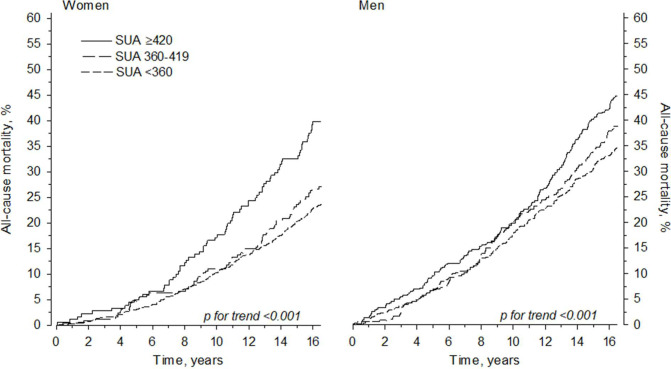
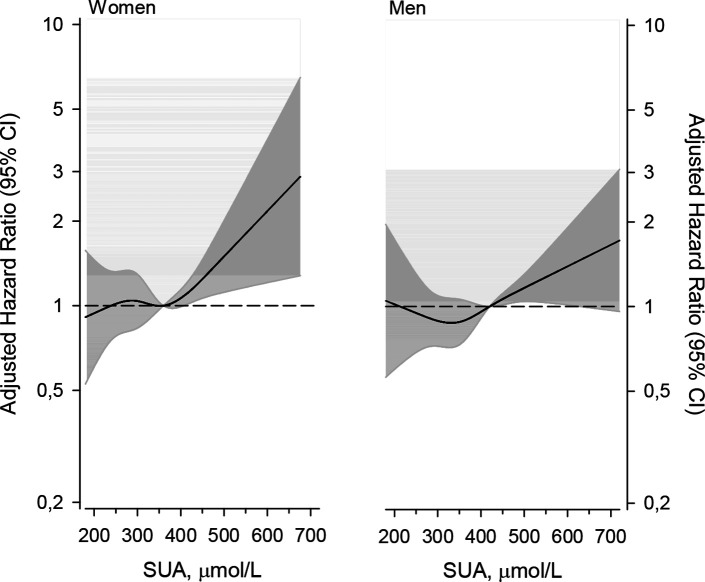
Adjusted HRs (adjusted for age, gender, education, current smoking status, BMI, hypertension (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg) or antihypertensive medication) and dyslipidaemia (total cholesterol ≥5.0 mmol/L or medication for lipid disorders) for all-cause mortality and cardiovascular mortality are shown in table 2. Also, the median age at mortality and time from enrolment in the study to death in both men and women are presented in table 2. Figure 2 demonstrates the cumulative all-cause mortality by SUA levels during the follow-up period. The change in the adjusted mortality HRs by SUA level is shown in figure 3. The SMRs by SUA level groups are presented in table 3.
Table 2.
Adjusted HRs for all-cause and cardiovascular mortality
| All cause | CVD | |||
| All CVD | IHD | CeVD | ||
| Women, n | 381 | 153 | 63 | 33 |
| Age at mortality, years, median (IQR) | 81 (76, 86) | 83 (79, 87) | 83 (79, 87) | 82 (77, 87) |
| Time to mortality*, years, median (IQR) | 11 (8, 14) | 11 (8, 14) | 11 (7, 15) | 11 (7, 14) |
| Adjusted HR (95% CI)† | ||||
| SUA <360 µmol/L | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SUA 360–419 µmol/L | 1.03 (0.78 to 1.35) | 0.79 (0.50 to 1.25) | 1.37 (0.72 to 2.61) | 0.25 (0.06 to 1.08) |
| SUA ≥420 µmol/L | 1.32 (1.01 to 1.74) | 1.31 (0.87 to 1.99) | 1.96 (1.05 to 3.65) | 0.82 (0.30 to 2.22) |
| P for trend | 0.045 | 0.56 | 0.039 | 0.17 |
| Men, n | 506 | 221 | 138 | 33 |
| Age at mortality, years, median (IQR) | 80 (74, 84) | 80 (76, 85) | 79 (74, 84) | 82 (79, 87) |
| Time to mortality*, years, median (IQR) | 10 (6, 14) | 10 (7, 14) | 11 (6, 13) | 10 (7, 14) |
| Adjusted HR (95% CI)† | ||||
| SUA <360 µmol/L | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SUA 360–419 µmol/L | 1.11 (0.89 to 1.39) | 1.18 (0.83 to 1.67) | 1.23 (0.79 to 1.91) | 1.53 (0.65 to 3.60) |
| SUA ≥420 µmol/L | 1.29 (1.05 to 1.60) | 1.41 (1.01 to 1.95) | 1.49 (0.99 to 2.45) | 1.12 (0.47 to 2.66) |
| P for trend | 0.018 | 0.040 | 0.042 | 0.97 |
*Time from enrolment in the study to death.
†Adjusted for age, gender, education, smoking status, body mass index (BMI), hypertension and dyslipidaemia.
CeVD, cerebrovascular disease; CVD, cardiovascular disease; IHD, ischaemic heart disease; SUA, serum uric acid.
Figure 2.
All-cause mortality in men and women during the 15-year follow-up period. SUA, serum uric acid.
Figure 3.
The change in adjusted HR according to serum uric acid (SUA) level.
Table 3.
Standardised mortality ratios in study subjects according to SUA levels (μmol/L)
| Standardised mortality ratio (SMR) | |
| Women | |
| SUA <360 µmol/L | 0,74 (0.65 to 0.84) |
| SUA 360–419 µmol/L | 0.83 (0.65 to 1.05) |
| SUA ≥420 µmol/L | 1.10 (0.87 to 1.39) |
| P for trend | 0.007 |
| Men | |
| SUA <360 µmol/L | 0.69 (0.59 to 0.80) |
| SUA 360–419 µmol/L | 0.74 (0.62 to 0.87) |
| SUA ≥420 µmol/L | 0.87 (0.75 to 1.00) |
| P for trend | 0.026 |
SUA, serum uric acid.
Discussion
The present study is one of the few studies that report the prevalence of hyperuricaemia in the elderly population of a Nordic country. It shows that elevated plasma urate levels are very prevalent in the ageing Finnish population and is, in general, consistent with previous studies.3 31–33 In the current study population the rate of hyperuricaemia appeared to be even higher than in other developed countries and it could be explained by the fact that the population of our study included only elderly persons.
The most important finding is the identification of hyperuricaemia as an independent risk factor for all-cause mortality and cardiovascular mortality. The higher unadjusted mortality in the hyperuricaemic population is easily explained by well-known confounding risk factors for cardiovascular diseases and mortality. Importantly, however, the mortality of hyperuricaemic individuals in the present study remained statistically significantly higher than of normouricaemic individuals after adjustment for age, gender, education, smoking, BMI, hypertension and dyslipidaemia.
The pathophysiology of the association between hyperuricaemia and cardiovascular disease and premature death is unknown. Some possible mechanisms have been proposed. Uric acid has both pro-oxidative and antioxidative properties, and the pro-oxidative properties are thought to be linked to cardiovascular disease.34 Another mechanism contributing to cardiovascular disease and mortality might be chronic low-level inflammation induced by high levels of intracellular uric acid, which is known to promote the expression of inflammatory markers.35 In our study, we found a statistically significant association between elevated levels of the well-known inflammatory marker hsCRP and hyperuricaemia in both genders. A similar association has been demonstrated in a handful of Chinese studies36–38 and in a Nepalese study,39 but has not previously been unequivocally demonstrated in Western populations. High level of hsCRP is a predictor of myocardial infarction, stroke, peripheral arterial disease and sudden cardiac death in healthy individuals without a history of cardiovascular disease and of recurrent events and death in patients with acute or stable coronary syndromes.40
Hyperuricaemia is linked with insulin resistance and diabetes and this might partially explain the association between high SUA levels and mortality (both cardiovascular and all cause).41 Hyperinsulinaemia caused by insulin resistance might be the driver of a process of rising urate levels, since high levels of insulin in the blood enhance the activity and protein expression of urate transporter 1 (URAT1). This, in turn, leads to reabsorption of uric acid in the renal proximal tubules increasing urate levels in the serum.42
Also, hyperuricaemia-induced endothelial dysfunction and endoplasmic reticulum stress may increase cardiovascular morbidity.43
Similar findings of hyperuricaemia-associated mortality increases have been reported in several studies in other populations.7–14 This raises a need for discussing the potential usefulness of treating hyperuricaemia also in asymptomatic individuals (ie, individuals with no urate crystal deposition disorder).44–46 There is already quite strong evidence that ULT in patients with gout improves cardiovascular outcomes.47–49 The evidence favouring benefits of ULT in asymptomatic individuals has, however, thus far been generally regarded as inconclusive and guidelines, at least in Western countries, do not recommend ULT in such cases. In a recent large randomised controlled trial of allopurinol therapy in elderly asymptomatic patients with hyperuricaemia with ischaemic heart disease, the rates of cardiovascular events and cardiovascular mortality were similar in the group treated with allopurinol and in the usual care group.50 The guidelines published by the Polish Society of Hypertension, however, recommend striving for serum urate levels of 5.0 mg/dL (approximately 300 μmol/L) or less in all patients with hypertension with high cardiovascular risk.51 Treatment of asymptomatic hyperuricaemia has been advised in the Japanese guidelines for over a decade, and a recommendation to treat asymptomatic SUA of ≥9.0 mg/dL without complication or asymptomatic SUA of ≥8.0 mg/dL with complication (kidney disease, cardiovascular disease, diabetes mellitus, metabolic syndrome) has been included also in the most recent version of the Japanese guidelines.27
Some remarks are in order before ULT for asymptomatic patients with hyperuricaemia is initiated. The evidence for ULT reducing mortality and cardiovascular morbidity is scarce, and some studies have not distinguished between patients with gout and asymptomatic patients with hyperuricaemia. The results of the studies have been somewhat inconsistent. A SUA threshold for initiating ULT has not been established.
In our study, we found hyperuricaemia (defined as SUA ≥360 µmol/L) to be very prevalent and treating all patients with hyperuricaemia would mean prescribing urate-lowering medication for 60% of the elderly male population and to almost one-third of the elderly female population. It would be irrational to recommend ULT to such a substantial part of the elderly population and the risks of it would very likely outweigh any benefits due to adverse events. Although urate-lowering drugs are generally well tolerated, rare severe adverse events do occur and the number of them would markedly increase if there would be a surge of patients using ULT.
A higher threshold for considering ULT in asymptomatic patients would be more reasonable. In our study, mortality was considerably higher in the clearly hyperuricaemic group (SUA ≥420 µmol/L) and that SUA level or probably even a higher one (as recommended in the Japanese guidelines) may be a feasible threshold for considering ULT for asymptomatic patients. A further uncertain issue is the target of treatment in asymptomatic hyperuricaemia. In patients with gout, we strive to lower the SUA level below 360 μmol/L, which is the precipitation threshold of uric acid in peripheral joints. In asymptomatic patients with hyperuricaemia it might be reasonable to accept slight hyperuricaemia (SUA 360–420 µmol/L), but in cases of higher SUA values it might be prudent to consider ULT, if the patient has other risk factors for cardiovascular diseases.
More studies are clearly necessary to provide us with conclusive evidence on the importance of uric acid as an independent risk factor for all-cause mortality and cardiovascular mortality and on the usefulness of ULT in asymptomatic patients with hyperuricaemia.
Our study has several important strengths. It is population based and includes a large number of elderly individuals. In this population, the prevalence of hyperuricaemia is high. There was an abundance of information available on lifestyle factors, comorbidities and laboratory parameters of each study subject and this made it possible to adjust the results for many potential confounding factors. The main finding—that is, that hyperuricaemia is associated with mortality—was not affected after adjustments for confounding factors. Thus, hyperuricaemia was demonstrated to be an independent risk factor for mortality. Another substantial strength of our study is the long follow-up period of 15 years. We identified the difference in mortality between hyperuricaemic and normouricaemic elderly subjects, and found that mortality increases most substantially after the 7th year of follow-up in women and after the 11th year in men. Thus, a long follow-up time is needed to evaluate the mortality risks associated with hyperuricaemia.
There are some limitations in our study. First, invitations were sent to a randomly selected cohort of subjects of defined age groups in the Lahti region, and although the participation rate was quite good (66%), there was still a substantial proportion of persons who did not respond, and thus the study might not represent the entire population of this age equally. It would be reasonable to assume that the most ill and disabled persons were less likely to respond to the invitation, possibly leading to under-representation of that group. The invitees were instructed to visit a local study centre. Data were not collected from inpatients in hospitals or from homes for the aged.
The fact that our series does not include the most ill population probably attenuates the findings. The invitees who did not take part in the study would probably have higher mortality rates and SUA levels than the average population of this study. This is reflected by the finding that people with high SUA levels did have a significantly increased mortality risk compared with those with normal SUA levels, but still their mortality risk was on the level of the general population of similar age.
Second, we have only limited information on the diagnoses of gout in this population. We know, however, that a history of ULT was rare during the active follow-up period (years 2002–2012), but we do not have this information about the second part of the follow-up (2013–2018) and we do not have exact information on the doses and duration of the urate-lowering drugs in those who used them.
Conclusions
In this large population-based cohort study we found hyperuricaemia to be very prevalent in the elderly Finnish population. There is an independent association between hyperuricaemia and all-cause mortality and cardiovascular mortality. The findings imply that reducing the SUA levels in persons with no history of gout attacks might be beneficial from a cardiovascular perspective and reduce mortality. Meticulous further research is required to shed light on the effectiveness of interventions to reduce serum urate levels and to maintain a scientific discussion on the subject is important, since the outcome of more information may carry substantial health benefits.
Supplementary Material
Acknowledgments
We thank all the participants in the GOAL project.
Footnotes
Contributors: All authors (JT, JEK, AMK, TML, HK and MJK) were involved in critical review of the manuscript. JT, HK and MJK designed and conceived the study. JT, JEK, AMK, TML, HK and MJK contributed substantially to interpretation of data. HK conducted the statistical analyses. JT conducted the drafting of the manuscript. JT, JEK, AMK, TML, HK and MJK reviewed the manuscript, tables and figures and contributed in writing to the interpretation and discussion of data. JT is the guarantor of the study. The study was supervised by MJK.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved in 2002 by the Ethics Committee of Päijät-Häme Central Hospital, Lahti, Finland (ID number: PHSP 2/2002/Q11 § 87). Participants gave informed consent to participate in the study before taking part.
References
- 1.Li L, Zhang Y, Zeng C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am J Transl Res 2020;12:3167–81. [PMC free article] [PubMed] [Google Scholar]
- 2.Roman YM, Daniel K. The Daniel K. inouye College of pharmacy scripts: perspectives on the epidemiology of gout and hyperuricemia. Hawaii J Med Public Health 2019;78:71–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A. U. A, Browne LD, Li X, et al. Temporal trends in hyperuricaemia in the Irish health system from 2006-2014: a cohort study. PLoS ONE 2018;13:e0198197. 10.1371/journal.pone.0198197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace KL, Riedel AA, Joseph-Ridge N, et al. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 2004;31:1582–7. [PubMed] [Google Scholar]
- 5.Trifirò G, Morabito P, Cavagna L, et al. Epidemiology of gout and hyperuricaemia in Italy during the years 2005–2009: a nationwide population-based study. Ann Rheum Dis 2013;72:694–700. 10.1136/annrheumdis-2011-201254 [DOI] [PubMed] [Google Scholar]
- 6.Chen-Xu M, Yokose C, Rai SK, et al. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National health and nutrition examination survey, 2007-2016. Arthritis Rheumatol 2019;71:991–9. 10.1002/art.40807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juraschek SP, Tunstall-Pedoe H, Woodward M. Serum uric acid and the risk of mortality during 23 years follow-up in the Scottish heart health extended cohort study. Atherosclerosis 2014;233:623–9. 10.1016/j.atherosclerosis.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi A, Kawamoto R, Ninomiya D, et al. Hyperuricemia is associated with all-cause mortality among males and females: findings from a study on Japanese community-dwelling individuals. Metabol Open 2022;14:100186. 10.1016/j.metop.2022.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu L, Hu G, Xu BP, et al. U-Shaped association of serum uric acid with all-cause and cause-specific mortality in US adults: a cohort study. J Clin Endocrinol Metab 2020;105:dgz068. 10.1210/clinem/dgz068 [DOI] [PubMed] [Google Scholar]
- 10.Cho SK, Chang Y, Kim I, et al. U-Shaped association between serum uric acid level and risk of mortality: a cohort study. Arthritis Rheumatol 2018;70:1122–32. 10.1002/art.40472 [DOI] [PubMed] [Google Scholar]
- 11.Tseng W-C, Chen Y-T, Ou S-M, et al. U-shaped association between serum uric acid levels with cardiovascular and all-cause mortality in the elderly: the role of malnourishment. J Am Heart Assoc 2018;7:e007523. 10.1161/JAHA.117.007523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim K, Go S, Son HE, et al. Association between serum uric acid level and ESRD or death in a Korean population. J Korean Med Sci 2020;35:e254. 10.3346/jkms.2020.35.e254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cang Y, Xu S, Zhang J, et al. Serum uric acid revealed a U-shaped relationship with all-cause mortality and cardiovascular mortality in high atherosclerosis risk patients: the assure study. Front Cardiovasc Med 2021;8:641513. 10.3389/fcvm.2021.641513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virdis A, Masi S, Casiglia E, et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension 2020;75:302–8. 10.1161/HYPERTENSIONAHA.119.13643 [DOI] [PubMed] [Google Scholar]
- 15.Masulli M, D’Elia L, Angeli F, et al. Serum uric acid levels threshold for mortality in diabetic individuals: the uric acid right for heart health (URRAH) project. Nutr Metab Cardiovasc Dis 2022;32:1245–52. 10.1016/j.numecd.2022.01.028 [DOI] [PubMed] [Google Scholar]
- 16.Ungar A, Rivasi G, Di Bari M, et al. The association of uric acid with mortality modifies at old age: data from the uric acid right for heart health (urrah) study. J Hypertens 2022;40:704–11. 10.1097/HJH.0000000000003068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niskanen LK, Laaksonen DE, Nyyssönen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med 2004;164:1546–51. 10.1001/archinte.164.14.1546 [DOI] [PubMed] [Google Scholar]
- 18.Reunanen A, Takkunen H, Knekt P, et al. Hyperuricemia as a risk factor for cardiovascular mortality. Acta Med Scand Suppl 1982;668:49–59. 10.1111/j.0954-6820.1982.tb08521.x [DOI] [PubMed] [Google Scholar]
- 19.Bengtsson C, Lapidus L, Stendahl C, et al. Hyperuricaemia and risk of cardiovascular disease and overall death. A 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Acta Med Scand 1988;224:549–55. [PubMed] [Google Scholar]
- 20.FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken) 2020;72:744–60. 10.1002/acr.24180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui M, Carr A, Cameron S, et al. The British Society for rheumatology guideline for the management of gout. Rheumatology (Oxford) 2017;56:1246. 10.1093/rheumatology/kex250 [DOI] [PubMed] [Google Scholar]
- 22.Pascart T, Latourte A, Flipo R-M, et al. 2020 recommendations from the French Society of rheumatology for the management of gout: urate-lowering therapy. Joint Bone Spine 2020;87:395–404. 10.1016/j.jbspin.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 23.Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol 2014;26:186–91. 10.1097/BOR.0000000000000028 [DOI] [PubMed] [Google Scholar]
- 24.Desideri G, Castaldo G, Lombardi A, et al. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci 2014;18:1295–306. [PubMed] [Google Scholar]
- 25.Bardin T. Hyperuricemia starts at 360 micromoles (6 mg/dl). Joint Bone Spine 2015;82:141–3. 10.1016/j.jbspin.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Huang H, Chen Y, et al. Association between circulating cystatin C and hyperuricemia: a cross-sectional study. Clin Rheumatol 2022;41:2143–51. 10.1007/s10067-022-06139-6 [DOI] [PubMed] [Google Scholar]
- 27.Hisatome I, Ichida K, Mineo I, et al. Japanese Society of gout and uric & nucleic acids 2019 guidelines for management of hyperuricemia and gout. Gout and Uric & Nucleic Acids 2020;44:sp1–sp-40. [Google Scholar]
- 28.Yang Y, Zhou W, Wang Y, et al. Gender-Specific association between uric acid level and chronic kidney disease in the elderly health checkup population in China. Ren Fail 2019;41:197–203. 10.1080/0886022X.2019.1591994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akashi N, Kuwabara M, Matoba T, et al. Hyperuricemia predicts increased cardiovascular events in patients with chronic coronary syndrome after percutaneous coronary intervention: a nationwide cohort study from Japan. Front Cardiovasc Med 2022;9:1062894. 10.3389/fcvm.2022.1062894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer-Verlag, 2001. 10.1007/978-1-4757-3462-1 [DOI] [Google Scholar]
- 31.Winder M, Owczarek AJ, Mossakowska M, et al. Prevalence of hyperuricemia and the use of allopurinol in older poles-results from a population-based polsenior study. Int J Environ Res Public Health 2021;18:387. 10.3390/ijerph18020387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burke BT, Köttgen A, Law A, et al. Physical function, hyperuricemia, and gout in older adults. Arthritis Care Res (Hoboken) 2015;67:1730–8. 10.1002/acr.22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guasch-Ferré M, Bulló M, Babio N, et al. Mediterranean diet and risk of hyperuricemia in elderly participants at high cardiovascular risk. J Gerontol A Biol Sci Med Sci 2013;68:1263–70. 10.1093/gerona/glt028 [DOI] [PubMed] [Google Scholar]
- 34.Kang D-H, Ha S-K. Uric acid puzzle: dual role as anti-oxidantand pro-oxidant. Electrolyte Blood Press 2014;12:1–6. 10.5049/EBP.2014.12.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura Y, Tsukui D, Kono H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int J Mol Sci 2021;22:12394. 10.3390/ijms222212394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu XZ, Tian YJ, Fan J, et al. Inverted J-shaped association of high-sensitivity C-reactive protein with the levels of serum uric acid: cross-sectional and longitudinal analyses. J Inflamm Res 2022;15:341–4. 10.2147/JIR.S350057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai H-X, Zhao Z-Y, Xia Y, et al. Higher levels of high-sensitivity C-reactive protein is positively associated with the incidence of hyperuricemia in Chinese population: a report from the China health and retirement longitudinal study. Mediators Inflamm 2020;2020:3854982. 10.1155/2020/3854982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang T, Ding X, Wang Y-L, et al. Association between high-sensitivity C-reactive protein and hyperuricemia. Rheumatol Int 2016;36:561–6. 10.1007/s00296-016-3429-z [DOI] [PubMed] [Google Scholar]
- 39.Sah SK, Khatiwada S, Pandey S, et al. Association of high-sensitivity C-reactive protein and uric acid with the metabolic syndrome components. Springerplus 2016;5:269. 10.1186/s40064-016-1933-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassuk SS, Rifai N, Ridker PM. High-Sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol 2004;29:439–93. [PubMed] [Google Scholar]
- 41.Rathmann W, Funkhouser E, Dyer AR, et al. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the cardia study. Coronary artery risk development in young adults. Ann Epidemiol 1998;8:250–61. 10.1016/s1047-2797(97)00204-4 [DOI] [PubMed] [Google Scholar]
- 42.Toyoki D, Shibata S, Kuribayashi-Okuma E, et al. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol 2017;313:F826–34. 10.1152/ajprenal.00012.2017 [DOI] [PubMed] [Google Scholar]
- 43.Yu W, Cheng J-D. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharmacol 2020;11:582680. 10.3389/fphar.2020.582680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petreski T, Ekart R, Hojs R, et al. Hyperuricemia, the heart, and the kidneys-to treat or not to treat? Ren Fail 2020;42:978–86. 10.1080/0886022X.2020.1822185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chalès G. How should we manage asymptomatic hyperuricemia? Joint Bone Spine 2019;86:437–43. 10.1016/j.jbspin.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 46.Yip K, Cohen RE, Pillinger MH. Asymptomatic hyperuricemia: is it really asymptomatic? Curr Opin Rheumatol 2020;32:71–9. 10.1097/BOR.0000000000000679 [DOI] [PubMed] [Google Scholar]
- 47.Szwejkowski BR, Gandy SJ, Rekhraj S, et al. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol 2013;62:2284–93. 10.1016/j.jacc.2013.07.074 [DOI] [PubMed] [Google Scholar]
- 48.Rekhraj S, Gandy SJ, Szwejkowski BR, et al. High-Dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol 2013;61:926–32. 10.1016/j.jacc.2012.09.066 [DOI] [PubMed] [Google Scholar]
- 49.Chen J-H, Lan J-L, Cheng C-F, et al. Effect of urate-lowering therapy on the risk of cardiovascular disease and all-cause mortality in patients with gout: a case-matched cohort study. J Rheumatol 2015;42:1694–701. 10.3899/jrheum.141542 [DOI] [PubMed] [Google Scholar]
- 50.Mackenzie IS, Hawkey CJ, Ford I, et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022;400:1195–205. 10.1016/S0140-6736(22)01657-9 [DOI] [PubMed] [Google Scholar]
- 51.Tykarski A, Filipiak KJ, Januszewicz A, et al. Zasady postępowania W nadcişnieniu tętniczym—2019 ROK. Nadciśnienie Tętnicze w Praktyce 2019;5:1–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.