Abstract
Objective
Postoperative infection is a common but costly complication. The neutrophil-lymphocyte ratio is a promising marker for the identification of postsurgical infectious events. We aimed to perform this meta-analysis to assessed the accuracy of the neutrophil-lymphocyte ratio for the prediction of postsurgical infection.
Methods
We searched PubMed, Embase, Web of Science, and Cochrane Library without language restriction from their inceptions to April 2022, and checked reference lists of included studies. Studies were included if they assessed predictive accuracy of neutrophil-lymphocyte ratio for postsurgical infection. We estimated its predictive value and explored the source of heterogeneity. The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to assess methodological quality and the Deeks’ test to evaluate publication bias. The bivariate model and hierarchical summary receiver operating characteristic (HSROC) curve were used for meta-analysis and generated a summary receiver operating characteristic space (ROC) curve.
Results
Our search returned 379 reports, of which 12 fulfilled the inclusion criteria, accounting for 4375 cases. The bivariate analysis yielded a pooled sensitivity of 0.77 (95%C.I.: 0.65–0.85) and specificity of 0.78 (95%C.I.: 0.67–0.86). Pooled positive LR and negative LR were 3.48 (95%C.I.: 2.26–5.36) and 0.30 (95%C.I.: 0.20–0.46), respectively. A negative LR of 0.30 reduces the post-test probability to 2% for a negative test result. The area under of receiver operating characteristic curve was 0.84 (95%C.I.: 0.80–0.87). Subgroups comparisons revealed difference by study design, surgical site, presentence of implant, time of sampling, type of infection event and prevalence of infection. The Deeks’ test showed no publication bias. The sensitivity analysis showed no study affected the robustness of combined results.
Conclusions
Low-certainty evidence suggests that the neutrophil-lymphocyte ratio is a helpful marker for predicting postoperative infectious complication. The negative predictive value of the neutrophil-lymphocyte ratio enables for reliable exclusion of postoperative infection.
Trial registration
PROSPERO registration number CRD42022321197. Registered on 27 April 2022.
Keywords: Neutrophil-lymphocyte ratio, Postoperative complication, Infection, Prediction, Meta-analysis
Highlights
-
•
The neutrophil-lymphocyte ratio is a helpful marker for predicting postoperative infectious complication.
-
•
The neutrophil-lymphocyte ratio had a powerful prognostic value for postsurgical infection in patients with foreign implants.
-
•
The negative predictive value of the neutrophil-lymphocyte ratio enables for reliable exclusion of postoperative infection.
-
•
No publication bias existed in the analysis and no studies affected the robustness of the combined result.
1. Introduction
Postoperative infectious complications are rising with surgical procedures increasing [1]. An observational study about postoperative complications over 7 years showed the respective prevalence of sepsis, superficial surgical site infection (SSI), organ/space SSI, deep SSI, urinary tract infection and pneumonia ranged from 0.5% to 1.6%, and the trend for organ/space SSI still increased from 1.1 to 1.5% [2]. These infectious complications lengthen hospital stays, aggravate financial burden and threaten patient lives [1]. However, there lacks a golden standard for evidence of postsurgical infection. Pathogen culture takes a long growth time but makes a low positive rate. Clinical signs of infection appear too late to avoid adverse outcomes. Therefore, a reliable and easily available marker is needed to early risk stratification based on the likelihood of acquiring postoperative infection complications which allows a window of time when interventions might be able to prevent.
Some studies used acute-phase related C-reactive protein (CRP) and white blood cell count to monitor and detect postsurgical infectious complications, which were testified to be unoptimistic in clinical practice [3,4]. Meanwhile, neutrophil-lymphocyte ratio (NLR), a simpler marker obtained by dividing the number of neutrophils (count/mL) by the number of lymphocytes (count/mL), had been proven to have significant predictive value in bacterial infection in an emergency care setting, and can be integrated into daily practice without extra costs [5]. In 2017, Bolat et al. found that NLR could well predict early penile prosthesis implant infection after penile prosthesis implantation with a high area under the curve (AUC) of 0.91 [6]. Two years later, Inose and Shen CJ validated the predictive value of NLR for postoperative infections in patients after spinal surgery in Japan and China, respectively, and noticed a limited value of NLR with an AUC less than 0.7 [7,8]. NLR is an easy-applicable, rapid and cost-effective parameter, hence it will bring good news for the perioperative management if it can be proved to reflect the postoperative inflammatory stress. Unfortunately, their results are conflicting, but no meta-analysis has surveyed the accuracy of NLR for the prediction of postoperative infectious complications. The three studies were limited by their chosen participants, so they did not include different surgical populations. And the heterogeneous sampling time, preoperative or postoperative, among the above studies may bring bias to some extent. In addition, many other studies regarding NLR in diverse surgical circumstances have been published since 2019 [[9], [10], [11], [12], [13], [14]].
Therefore, we performed the first meta-analysis to figure out the ability of NLR to predict infection in postoperative patients and explore the effect of patient heterogeneity and individual covariates.
2. Methods
We performed this systematic review and meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [15]. The protocol was registered on 27 April 2022 with the PROSPERO database (registration number CRD42022321197).
2.1. Data sources and search strategy
Two analysts (BSQ and RYG) individually searched PubMed, Embase, Web of Science, and Cochrane Library from inception to April 2022 for relevant studies without language restrictions using text words or medical subject headings enclosing “neutrophil-lymphocyte ratio”, “neutrophil to lymphocyte ratio”, “NLR”, “surgical”, “surgery”, and “infection”. Full details of the search strategy are provided at Supplementary appendix S1. In addition, we confirmed additional studies when reviewing reference lists.
2.2. Study selection criteria
We screened the title and abstract, then reviewed full text for potential eligible records. Two investigators (BSQ and RYG) independently assessed eligibility, with discrepancies solved by a third author (HMJ). Studies were included if they: were observational studies with a prospective or respective cohort or case-control design (cross sectional studies were ineligible because the order of occurrence of operative procedure and infectious event is usually difficult to determine); were original clinical studies with patients over 18 years old undergoing operative therapy in the study period; reported at least one postoperative infectious event including special or general infection; collected blood NLR to predict postoperative infectious complications. Studies without original data, such as case reports, reviews, comments and editorials were excluded. If there were repeated reports based on the same cohorts, we chose for analysis the study with the most detailed information. If necessary, potential papers in non-English were translated with the help of translators or translation software. We calculated κ statistics to assess the agreement for study inclusion between the two investigators. EndNote software was used for recording decisions.
2.3. Data extraction and outcome assessments
Two authors (BSQ and RYG) separately extracted data from individual reports and recorded data in a prepared excel sheets. Extracted data included first author, year of publication, study design, country, surgery setting, sample size, the sampling time of blood routine tests, and the number of infectious and non-infectious, prevalence of infections, the AUC and optimal cutoff of NLR. Moreover, infectious characteristics such as antibiotic prophylaxis, infection site, infection definition, and microorganisms were also tabulated. If multiple time points of NLR were detected for postoperative infection prognosis in the original study, we extracted the data at the point close to the remaining studies to ensure homogeneity. If there was any disagreement between the two reviewers in the process of data extraction, it was resolved by a third party (HMJ). For publications lacking sufficient information on predictive accuracy to calculate the 2 × 2 contingency tables, we asked the corresponding authors for help via email first then excluded those studies if received no response after sending a second email.
The primary endpoints were defined as any infectious events during the follow-up period including surgical site infection (SSI), periprosthetic joint infection, prosthesis infection, bacterial infection, and general infection such as pneumonia, urinary tract infection and endometritis. SSI was diagnosed according to the Center for Disease Control Guideline for the Prevention of Surgical Site Infection (CDC Guideline) [16], which was classified as incisional (superficial or deep) SSI or organ/space SSI. Periprosthetic joint infection was diagnosed according to the criteria recently proposed by the Musculoskeletal Infection Society [17]. A bacterial infection was diagnosed according to the Centers for Disease Control and Prevention's National Healthcare Safety Network surveillance definitions [18]. General infections were defined by clinical signs, such as postoperative hospitalization fever or re-admission for fever.
2.4. Quality assessment
The methodological quality of enrolled studies was assessed by two investigators (BSQ and RYG) independently using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool [19]. This tool focused on the risk of bias and concerns about applicability by surveying four key domains: patient selection, index test, reference standard, and flow and timing. Green, yellow, and red indicate a low, moderate, and high risk of bias, respectively. The result was presented by Review Manager (version 5.3) software. A senior reviewer (HMJ) was responsible for resolving discrepancies.
2.5. Statistical analysis
We tabulated true positive, false positive, false negative and true negative in patients with infection, stratified by study. We calculated sensitivity and specificity and a corresponding confidence interval (CI) using the numbers for each study. A two-sided p < 0.05 was considered statistically significant.
Hierarchical models are recommended as standard methods for the meta-analysis of diagnostic test data by the Cochrane Collaboration [20]. This model considers correlation between sensitivity and specificity and accounts for both within- and between-study heterogeneity. Therefore, the hierarchical summary receiver operating characteristic (HSROC) curve was computed to generate a summary receiver operating characteristic space (ROC) curve, and the bivariate mixed-effects regression model was used to determine the summary estimates of the sensitivity, specificity, positive likelihood ratio (LR), and negative LR.
The heterogeneity is a well-recognized problem in meta-analysis of diagnostic test accuracy according to the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Heterogeneity caused by threshold was calculated by testing Spearman correlation, and p < 0.05 represented significant threshold effects. To explore non-threshold heterogeneity, we carried out subgroup analyses based on study design (cohort vs. case-control), surgical site (spine vs. non-spine), presence of implant (implant vs. non-implant), type of infection event (only SSI vs. general infection), time of sampling (preoperative vs. postoperative) and prevalence of infection (>7% vs. ≥ 7%). Additionally, we constructed funnel plots with the Deeks’ test to assess publication bias [21] and introduced sensitivity analyses to examine the stability of results by omitting studies one by one. All statistical analyses were performed using STATA (version 15.1) software with MIDAS module and Review Manager (version 5.3) software. We assessed the certainty of evidence according to GRADE for diagnostic test studies and generate a summary of findings table.
3. Results
3.1. Study selection and characteristics
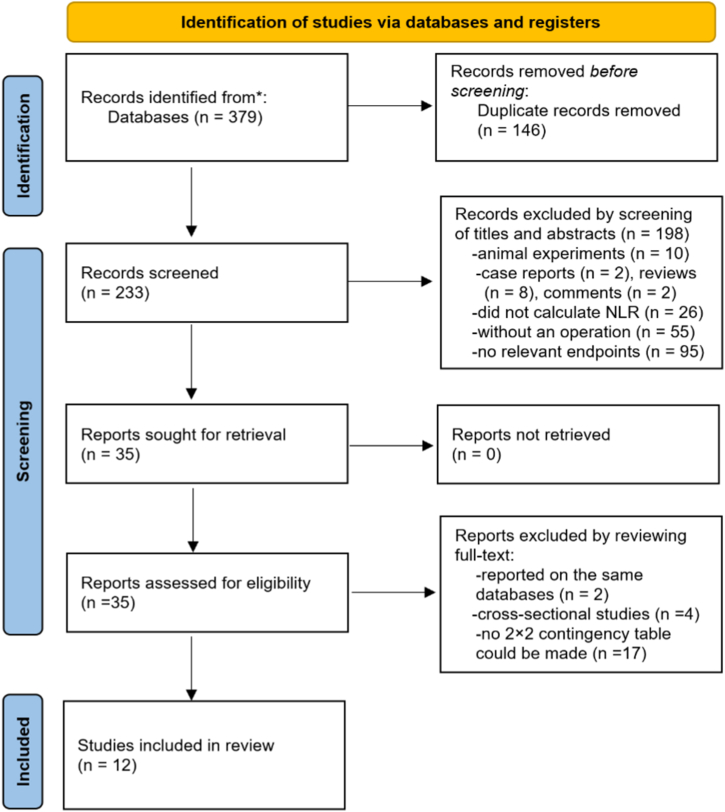
As the study selection process (Fig. 1) shows, a total of 379 potential articles were initially obtained from the databases. After removing 146 duplicates, 233 records were screened by title and abstract. After excluding 198 records for some reasons, 35 records were rescreened by full text. We further excluded 23 studies for no specific data, leaving 12 studies [[6], [7], [8], [9], [10], [11], [12],14,[22], [23], [24], [25]] included in this meta-analysis. The inter-rater reliability for assessment of study inclusion was 0.82, which meant very good agreement between the two investigators.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the study selection process. NLR neutrophil-lymphocyte ratio.
Table 1 shows the main characteristics of individual studies. One prospective and eleven retrospective studies were published from 2017 to 2022, with sample sizes between 104 and 1177. This meta-analysis included 4375 postoperative patients, of whom 314 (7.8%) had infectious events and 4061 (92.8%) had non-infection events. The prevalence of postoperative infectious complications among studies ranged from 2.8% to 33.5%. Four studies focused on spinal surgeries, and the rest aimed at varied surgical settings. Only five studies collected preoperative data, whereas seven studies investigated postoperative data. Table S1 in supplementary material indicates that infectious events in nine studies were diagnosed according to the definition of the CDC Guideline. The pathogens were mainly staphylococcus.
Table 1.
Details of the included studies (n = 12).
| Author (year) | Design | Country | Surgical setting | N | Infection (n) | Sampling times | Prevalence (%) |
|---|---|---|---|---|---|---|---|
| Bolat (2017) | RC | Turkey | penile prosthesis implantation | 153 | 18 | Pre 1–7days | 11.80% |
| Inose (2019) | RC | Japan | spinal instrumentation surgery | 242 | 10 | Post 6–7 days | 4.13% |
| Liu YC (2019) | RC | China | radical resection for rectal cancer | 298 | 20 | Pre | 6.71% |
| Shen CJ (2019) | CC | China | posterior lumbar spinal surgery | 293 | 13 | Post 7 days | 4.43% |
| Hani SM (2020) | RC | Indonesia | primary surgery for ovarian cancer | 183 | 20 | Pre | 12.20% |
| Inose (2020) | RC | Japan | spinal decompression surgery | 254 | 7 | Post 6–7 days | 2.80% |
| Rotem (2020) | CC | Israel | cesarean sections | 337 | 113 | Post 6–24h | 33.50% |
| Yao S (2020) | PC | Japan | hepato-biliary-pancreatic surgery | 105 | 15 | Post 3–14 days | 14.29% |
| Zhao G (2020) | CC | China | total joint arthroplasty | 104 | 26 | Post 2–4 weeks | 25.00% |
| Imabayashi (2021) | RC | Japan | spine surgery | 329 | 9 | Post 7 days | 2.74% |
| Zhuo YY (2021) | RC | China | mesh repair of groin hernia | 1177 | 39 | Pre | 3.20% |
| Lu K (2022) | RC | China | open reduction and internal fixation | 900 | 24 | Pre at admission | 2.70% |
RC retrospective cohort, CC case-control, PC prospective cohort, Pre preoperative, Post postoperative.
Table 2 lists predictive value data of eligible studies covering sensitivities, specificities, AUCs, and cutoffs. In studies concentrating on postoperative markers, two were measured at multiple time points. We selected one at about 7 days to reduce heterogeneity because the other three studies were investigated at 6–7 days. AUCs fluctuated widely, from 0.66 to 0.93 in these studies, and the studies by Hani [14]and Lu K [9] lacked the AUC values. The cut-off values for NLR varied substantially in these studies (Median 4.19, interquartile range 2.88–6.35).
Table 2.
Predictive value of NLR for postoperative infectious complications in all studies.
| Author (year) | TP | FP | FN | TN | Sensitivity 95% (CI) |
Specificity (95% CI) | AUC | Cut-off value |
|---|---|---|---|---|---|---|---|---|
| Bolat 2017 [6] | 12 | 1 | 6 | 134 | 0.67 (0.41–0.87) | 0.99 (0.96–1.00) | 0.91 | 6.20 |
| Inose 2019 [7] | 7 | 62 | 3 | 170 | 0.70 (0.35–0.93) | 0.73 (0.67–0.79) | 0.69 | 3.87 |
| Liu YC 2019 [26] | 19 | 135 | 1 | 143 | 0.95 (0.75–1.00) | 0.51 (0.45–0.57) | 0.71 | 2.13 |
| Shen CJ 2019 [8] | 9 | 104 | 4 | 176 | 0.69 (0.39–0.91) | 0.63 (0.57–0.69) | 0.66 | 3.85 |
| Hani SM 2020 [14] | 14 | 70 | 6 | 93 | 0.70 (0.46–0.88) | 0.57 (0.49–0.65) | NR | 4.56 |
| Inose 2020 [27] | 6 | 55 | 1 | 192 | 0.86 (0.42–1.00) | 0.78 (0.72–0.83) | 0.80 | 3.21 |
| Rotem 2020 [11] | 81 | 85 | 32 | 139 | 0.72 (0.62–0.80) | 0.62 (0.55–0.68) | 0.67 | 6.80 |
| Yao S 2020 [12] | 10 | 13 | 5 | 77 | 0.67 (0.38–0.88) | 0.85 (0.77–0.92) | 0.80 | 11.10 |
| Zhao G 2020 [28] | 22 | 8 | 4 | 70 | 0.85 (0.65–0.96) | 0.90 (0.81–0.95) | 0.93 | 2.77 |
| Imabayashi 2021 [29] | 6 | 48 | 3 | 272 | 0.67 (0.30–0.93) | 0.85 (0.81–0.89) | 0.77 | 4.50 |
| Zhuo YY 2021 [10] | 38 | 338 | 1 | 800 | 0.97 (0.87–1.00) | 0.70 (0.68–0.73) | 0.88 | 2.44 |
| Lu K 2022 [9] | 10 | 180 | 14 | 696 | 0.42 (0.22–0.63) | 0.79 (0.77–0.82) | NR | 6.40 |
NLR neutrophil-lymphocyte ratio, TP True-positive, FP False-positive, FN False-negative, TP True-negative, AUC the area under curve, NR not reported.
3.2. Quality assessment and publication bias
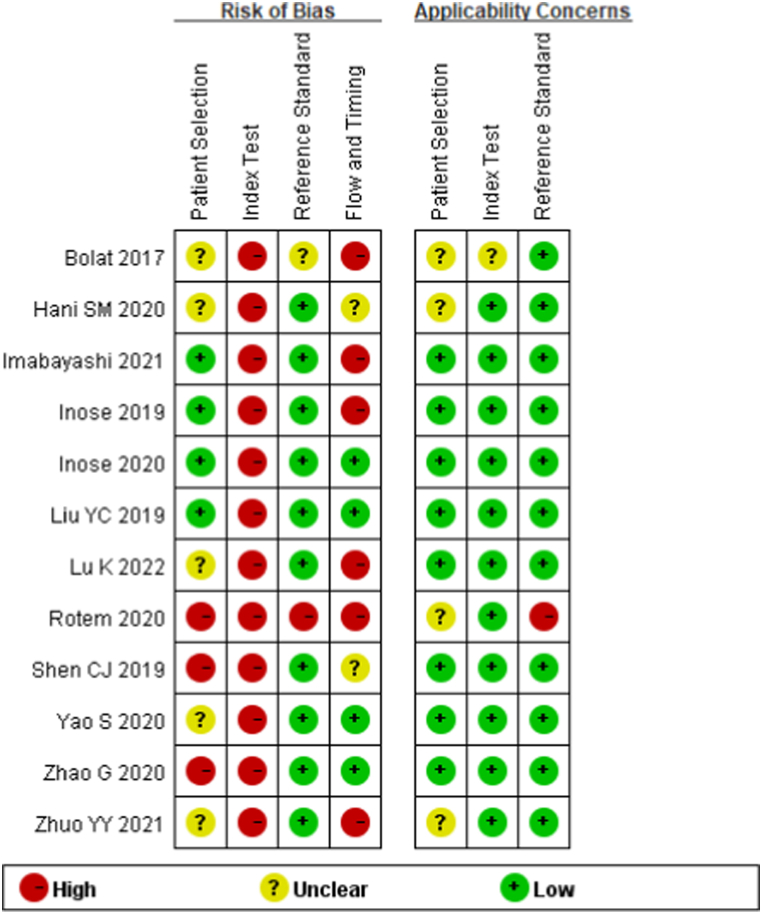
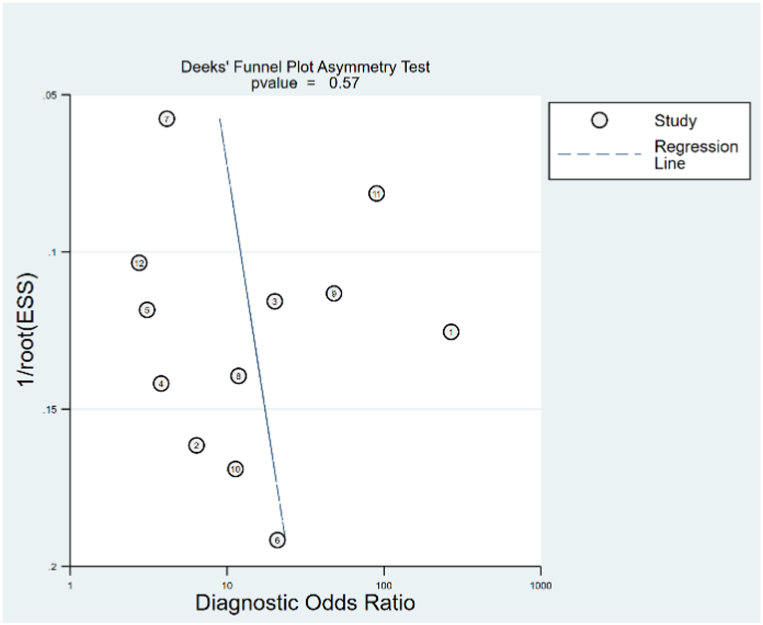
Fig. 2 depicts the study quality of each domain for individual studies. Seven studies did not indicate whether patients were enrolled consecutively or randomly, and three studies adopted case-control design. Therefore, for patient selection, we noticed high risk of bias in 25% of studies. The thresholds in all twelve studies were obtained by ROC curves rather than predefined, as a result, all studies indicated a high risk of bias for the index test. Only one study did not explicitly describe reference standard, which made 8% of studies as high risk of bias for reference standard. We noted longer or unclear time intervals between index and reference standard in six studies, hence 50% of studies were regarded as having high risk of bias for flow and timing. The Deeks’ test (Fig. 3) demonstrates no publication bias in included studies (p = 0.57).
Fig. 2.
Quality assessment summary in each domain for individual studies.
Fig. 3.
Deeks' funnel plot symmetry test for publication bias.
3.3. Data synthesis
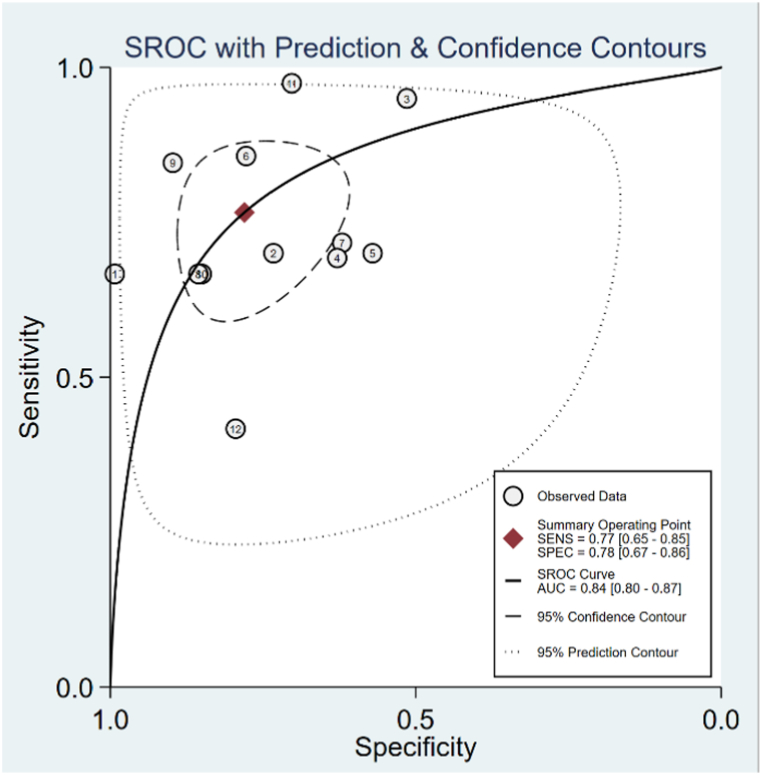
The HSROC curve for all included studies is presented in Fig. 4, and the AUC was 0.84 (95%C.I.: 0.80–0.87). The pooled sensitivity was 0.77 (95%C.I.: 0.65–0.85), and the pooled specificity was 0.78 (95%C.I.: 0.67–0.86). The pooled positive LR was 3.48 (95%C.I.: 2.26–5.36), and the pooled negative LR was 0.30 (95%C.I.: 0.20–0.46). The prediction region around the point of HSROC curve visually provided the existence of heterogeneity, while the threshold analysis p value was 0.10, giving no evidence of a threshold effect.
Fig. 4.
Summary receiver operating characteristic curve of neutrophil-lymphocyte ratio for predicting postoperative infection.
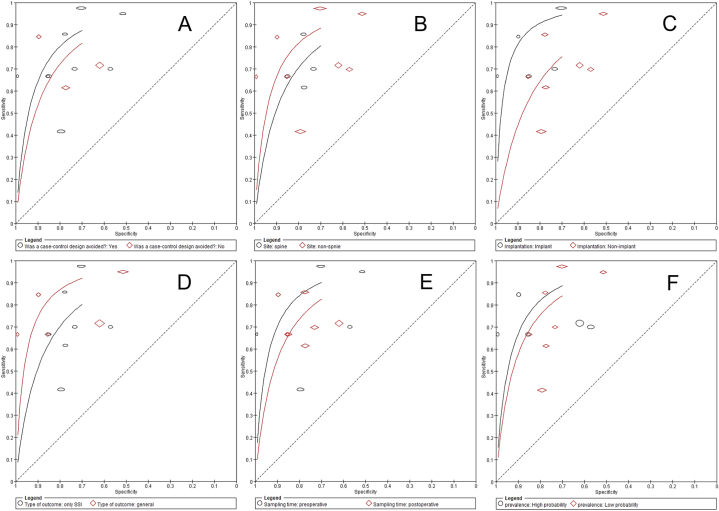
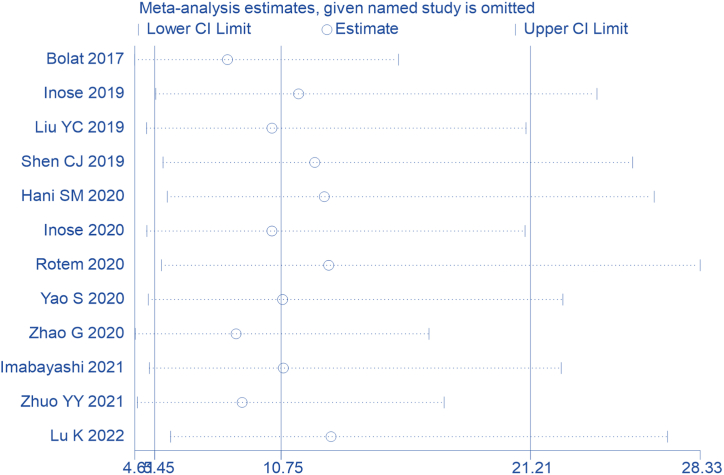
We then performed subgroup analyses to determine the source of heterogeneity. Subgroup analyses were based on study design, surgical site, presence of implant, type of infection event, time of sampling and prevalence of infection. Subgroup analysis by study design showed that cohort study (9 studies) was better compared to case-control study (3 studies), with the curve more towards the left upper corner of the ROC space (Fig. 5A). Subgroup analysis by surgical site (spine vs. non-spine) showed better accuracy with non-spine (Fig. 5B). The subgroup analysis by presence of implant (implant vs. non-implant) showed better accuracy with implant (Fig. 5C). Subgroup analysis by type of infection event (only SSI vs. general infection) showed better accuracy with general infection (Fig. 5D). The subgroup analysis of sampling time (preoperative and postoperative) showed sampling preoperative had better predictive accuracy (Fig. 5E). The subgroup analysis by prevalence of infection (>7% vs. ≥ 7%) showed better accuracy with high probability (Fig. 5F). The sensitivity analysis showed that no study affected the robustness of combined results (Fig. 6). Certainty of evidence for outcome was downgraded to low due to inconsistency (variability in the estimated sensitivities and specificities among studies) and imprecision (wide 95% confidence intervals of the summary estimates of sensitivity and specificity) (Table 3).
Fig. 5.
Subgroup comparisons. A: subgroup by study design. B: subgroup by surgical site. C: subgroup by presence of implant. D: subgroup by type of infection event. E: subgroup by sampling time. F: subgroup by prevalence of infection.
Fig. 6.
The sensitivity analysis to assess the influence of the studies on the combined results.
Table 3.
Summary of findings table of GRADE for meta-analysis.
| Factors that may decrease certainty of evidence |
Test accuracy CoE | |||||
|---|---|---|---|---|---|---|
| Risk of bias | Indirectness | Inconsistency | Imprecision | Publication bias | ||
| GRADE rating of certainty of evidence | not serious | not serious | serious | serious | none | Low |
CoE: certainty of evidence.
4. Discussion
To our knowledge, this is the first meta-analysis of NLR as a predictive marker for postoperative infectious complications. Our meta-analysis involved 12 studies, and the findings confirmed that NLR has the potential to forecast the development of postoperative infectious complications. However, there are considerable variations in the cut-offs in each study, possibly due to differences in surgical situation, which limited recommendation of a specific threshold value of NLR for prognosis of postoperative infectious complications.
Several studies on postoperative complications have previously explored the predictive value of other “star” markers in infection, such as C-reactive protein (CRP) and procalcitonin. But their predictive performance did not surpass that of NLR. A meta-analysis of CRP [4] involving 7 cohort studies demonstrated a lower AUC (0.76) for predicting postsurgical infection complications when compared to NLR (0.84) in our meta-analysis. The AUC in the most recent study of CRP by Winsen M and colleagues was only 0.765 [30]. As for procalcitonin, the levels were insignificantly different between the infection and non-infection groups in spinal instrumentation surgery [7]. And in radical gastrectomy, a study that consecutively included 552 adults from two large centers, the power of procalcitonin to detect infection early was also not strong, with an AUC of 0.678 [26]. More importantly, in the same study comparing multiple markers in hepato-biliary-pancreatic surgery, NLR had better AUC, sensitivity and specificity than procalcitonin [12]. This may be because procalcitonin concentration is affected by the surgery itself. It increases to a peak on the first postoperative day (POD 1) and then returns to normal levels until the third postoperative day (POD 3) according to Aouifi et al. [27]. Although neutrophils and lymphocytes also responded to major surgery stresses (neutrophils increased and lymphocytes decreased), their changes were simultaneous in opposite directions [28]. NLR was a ratio that reflected the dynamic relationships between the neutrophil and lymphocyte. So, the characteristic of relative change of the ratio may weaken the effect of surgery on individual indicators. The last, both CRP and procalcitonin are costly to monitor as perioperative indicators. However, NLR has a clear advantage as a cheap, simple, and easily accessible parameter derived from the blood route test.
Subgroup analysis found that study design, surgical site, presence of implant, type of infection event, time of sampling and prevalence of infection can explain some heterogeneity and hence generalizability is limited. The existence of implant and surgical sites were key attributes of heterogeneity. NLR had a better accuracy in patients with foreign implants. Surgical patients are more likely to develop inflammatory complications due to the implants in their bodies. On the one hand, a study by James and colleagues proved the “dose” of contaminating bacteria required to cause infection was lower in the presence of vitro material [29]. On the other hand, a foreign object probably provides an avascular surface for the bacteria to escape immune attack and help microbial growth [31,32]. Luckily, Pengfei Wei et al. developed a microsphere loaded with vancomycin and strontium three years ago, and the invention has the potential to treat infected bone defects [33]. Integrating the microsphere into implants may also help prevent postsurgical infections in the future.
The surgical site also influenced the predictive value of NLR. A better accuracy was observed in the non-spine subgroup than in the spine subgroup. The major reason could be that the disc of the spine is one of the tissues devoid of vessels [34], which can hide from the human immune system, whereas major abdominal surgery is dominant in the non-spine subgroup, which involves rich vascular tissue. At the same time, neutrophils and lymphocytes are both immune cells that do not fully respond to vascular-less regions, making the average neutrophil-lymphocyte ratio lower than that in major abdominal surgery. We may be able to appropriately lower the cut-off value in the spine subgroup to increase sensitivity. More research is needed to verify the kinetics of NLR in spinal surgery patients.
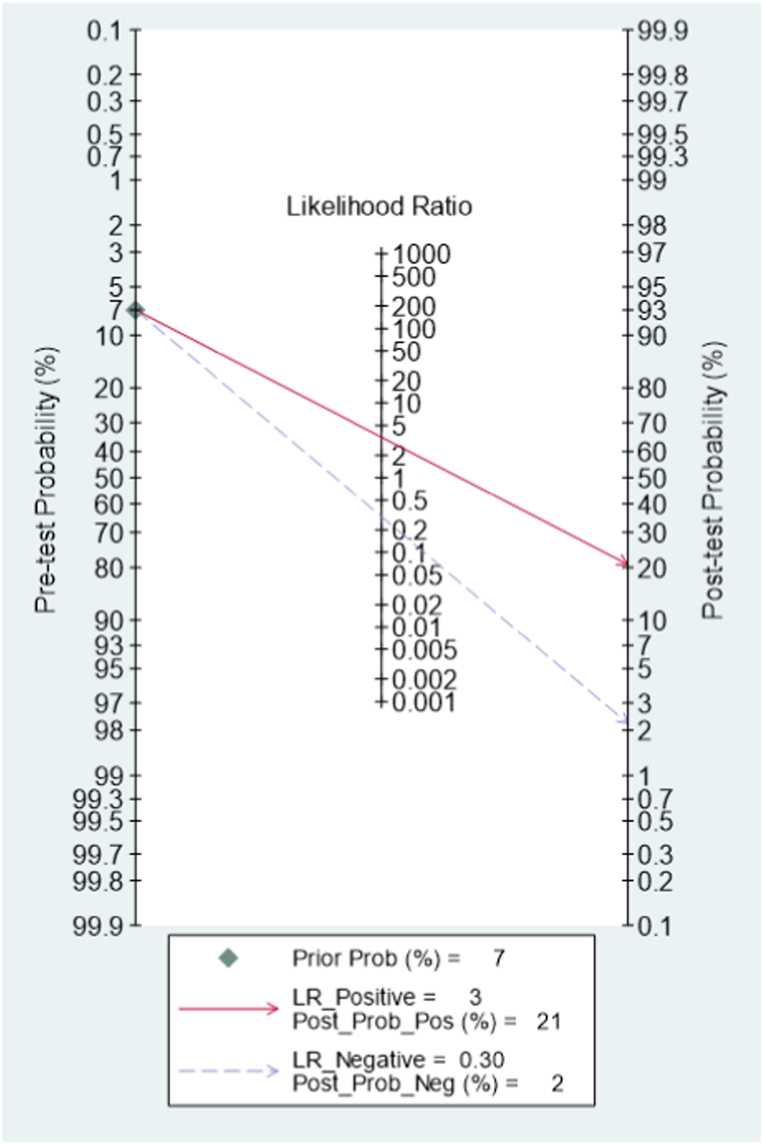
The most important feature of a biomarker is its potential to influence the clinical decision. The median cutoff of the studies included was 4.19 (interquartile range 2.88–6.35). Likelihood ratios and post-test probabilities are also relevant for clinicians, providing information on whether a patient with a positive or negative test has a postoperative infection. We structured a Fagan's nomogram to evaluate the utility of prediction of postoperative infectious complication. In our study, both likelihood ratio and post-test probability were moderate. A positive likelihood ratio of 3 implies that a person with infection is three times more likely to have a positive test result than a non-infection person. Given a pretest probability of 7%, the post-test probability for a positive test result is 21%. Likewise, a negative likelihood ratio of 0.30 reduces the post-test probability to 2% for a negative test result, which almost can exclude the occurrence of postoperative infection (Fig. 7). Those patients may be consider discharging earlier to enjoy a good rest at home and promote postoperative recovery. In domestic primary hospitals and in developing countries with limited medical resources, attention to NLR in perioperative monitoring is a promising option without extra costs.
Fig. 7.
Fagan's nomogram showing the posttest probability of postoperative infectious complications.
Several limitations existed in our meta-analysis. First, unlike meta-analyses of intervention trials, heterogeneity is to be expected meta-analyses of diagnostic test accuracy. Unfortunately, the number of eligible studies was less than five to ten times to the number of factors explored, so we could not use meta regression to explore the covariates how to affect summary sensitivity and specificity. Second, eligible studies mainly focused on Asians, but plenty of direct proofs cited in a review suggested ethnic differences in NLR [28]. Third, we observed gender differences in the spectrum of surgery in our included studies. For example, penile surgeries were performed only in men and cesarean sections only in women. Since we were unable to obtain detailed information from respective studies, we were unable to explore the effect of gender factors on heterogeneity.
As our results show, the neutrophil-lymphocyte ratio is not a perfect predictor of postoperative infection, but an ideal predictor does not exist. Nevertheless, the neutrophil-lymphocyte ratio is a simple but promising parameter. A composite scoring system that includes specific NLR values and clinical parameters such as age, serum glucose level, or glycosylated hemoglobin may improve predictive accuracy and generalizability, but needs evaluation.
5. Conclusions
Low-certainty evidence suggests that NLR is a helpful marker for predicting postoperative infectious complications. The negative predictive value of the neutrophil-lymphocyte ratio enables for reliable exclusion of postoperative infection. Blood routine tests are necessary items for perioperative monitoring. We can pay more attention to the neutrophil-lymphocyte ratio when monitoring blood routine tests.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at [URL].
Acknowledgments
The authors are grateful to the relevant fund for financial help.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15586.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cullen Ka H.M., Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl. Health Stat. Rep. 2009;(11):1–25. [PubMed] [Google Scholar]
- 2.Dencker E.E., Bonde A., Troelsen A., Varadarajan K.M., Sillesen M. Postoperative complications: an observational study of trends in the United States from 2012 to 2018. BMC Surg. 2021;21(1):393. doi: 10.1186/s12893-021-01392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi J., Shono Y., Hirabayashi H., Kamimura M., Nakagawa H., Ebara S., et al. Usefulness of white blood cell differential for early diagnosis of surgical wound infection following spinal instrumentation surgery. Spine. 2006;31(9):1020–1025. doi: 10.1097/01.brs.0000214895.67956.60. [DOI] [PubMed] [Google Scholar]
- 4.Adamina M., Steffen T., Tarantino I., Beutner U., Schmied B.M., Warschkow R. Meta-analysis of the predictive value of C-reactive protein for infectious complications in abdominal surgery. Br. J. Surg. 2015;102(6):590–598. doi: 10.1002/bjs.9756. [DOI] [PubMed] [Google Scholar]
- 5.de Jager C.P., van Wijk P.T., Mathoera R.B., de Jongh-Leuvenink J., van der Poll T., Wever P.C. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit. Care. 2010;14(5):R192. doi: 10.1186/cc9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolat D., Topcu Y.K., Aydogdu O., Minareci S., Dincel C. Neutrophil to lymphocyte ratio as a predictor of early penile prosthesis implant infection. Int. Urol. Nephrol. 2017;49(6):947–953. doi: 10.1007/s11255-017-1569-z. [DOI] [PubMed] [Google Scholar]
- 7.Inose H., Kobayashi Y., Yuasa M., Hirai T., Yoshii T., Okawa A. Procalcitonin and neutrophil lymphocyte ratio after spinal instrumentation surgery. Spine. 2019;44(23):E1356–e1361. doi: 10.1097/brs.0000000000003157. [DOI] [PubMed] [Google Scholar]
- 8.Shen C.J., Miao T., Wang Z.F., Li Z.F., Huang L.Q., Chen T.T., et al. Predictive value of post-operative neutrophil/lymphocyte count ratio for surgical site infection in patients following posterior lumbar spinal surgery. Int. Immunopharm. 2019;74 doi: 10.1016/j.intimp.2019.105705. [DOI] [PubMed] [Google Scholar]
- 9.Lu K., Ma T., Yang C., Qu Q., Liu H. Risk prediction model for deep surgical site infection (DSSI) following open reduction and internal fixation of displaced intra-articular calcaneal fracture. Int. Wound J. 2022;19(3):656–665. doi: 10.1111/iwj.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuo Y., Cai D., Chen J., Zhang Q., Li X. Pre-surgical peripheral blood inflammation markers predict surgical site infection following mesh repair of groin hernia. Medicine. 2021;100(9) doi: 10.1097/md.0000000000025007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rotem R., Erenberg M., Rottenstreich M., Segal D., Yohay Z., Idan I., et al. Early prediction of post cesarean section infection using simple hematological biomarkers: a case control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;245:84–88. doi: 10.1016/j.ejogrb.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Yao S., Kaido T., Uozumi R., Hirata M., Iwamura S., Miyachi Y., et al. Diagnostic potential of presepsin in bacterial infection following hepato-biliary-pancreatic surgery: a prospective observational study. J. Hepatobiliary Pancreat. Sci. 2020;27(10):756–766. doi: 10.1002/jhbp.802. [DOI] [PubMed] [Google Scholar]
- 13.Sigmund I.K., Dudareva M., Watts D., Morgenstern M., Athanasou N.A., McNally M.A. Limited diagnostic value of serum inflammatory biomarkers in the diagnosis of fracture-related infections. Bone Joint Lett. J. 2020;102-b(7):904–911. doi: 10.1302/0301-620x.102b7.Bjj-2019-1739.R1. [DOI] [PubMed] [Google Scholar]
- 14.Hani S.M., Fahmi M.N., Dewi F.S.T. Neutrophil-lymphocyte ratio as an independent preoperative risk factor of surgical site infection in ovarian cancer patients undergoing primary surgery. J. Obstet. Gynaecol. Res. 2020;46(SUPPL 1):131. doi: 10.1111/jog.14462. [DOI] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangram A.J., Horan T.C., Pearson M.L., Silver L.C., Jarvis W.R. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect. Control Hosp. Epidemiol. 1999;20(4) doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 17.Parvizi J., Zmistowski B., Berbari E.F., Bauer T.W., Springer B.D., Della Valle C.J., et al. New definition for periprosthetic joint infection: from the workgroup of the musculoskeletal infection society. Clin. Orthop. Relat. Res. 2011;469(11):2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Lee J., Kim K.W., Choi S.H., Huh J., Park S.H. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-Part II. Statistical methods of meta-analysis. Korean J. Radiol. 2015;16(6):1188–1196. doi: 10.3348/kjr.2015.16.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Enst W.A., Ochodo E., Scholten R.J., Hooft L., Leeflang M.M. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med. Res. Methodol. 2014;14:70. doi: 10.1186/1471-2288-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y.C., Li X.R., Du X.H., Li S.Y., Hu S.D., Yang Y., et al. Predictive value of preoperative neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in surgical site infection following radical resection for rectal cancer. Med. J. Chin. Peoples Lib. Army. 2019;44(3):243–247. doi: 10.11855/j.issn.0577-7402.2019.03.10. [DOI] [Google Scholar]
- 23.Inose H., Kobayashi Y., Yuasa M., Hirai T., Yoshii T., Okawa A. Postoperative lymphocyte percentage and neutrophil-lymphocyte ratio are useful markers for the early prediction of surgical site infection in spinal decompression surgery. J. Orthop. Surg. 2020;28(2) doi: 10.1177/2309499020918402. [DOI] [PubMed] [Google Scholar]
- 24.Zhao G., Chen J., Wang J., Wang S., Xia J., Wei Y., et al. Predictive values of the postoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio for the diagnosis of early periprosthetic joint infections: a preliminary study. J. Orthop. Surg. Res. 2020;15(1):571. doi: 10.1186/s13018-020-02107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imabayashi H., Miyake A., Chiba K. Establishment of a suitable combination of serological markers to diagnose surgical site infection following spine surgery: a novel surgical site infection scoring system. J. Orthop. Sci. 2021 doi: 10.1016/j.jos.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Xiao H., Zhang P., Xiao Y., Xiao H., Ma M., Lin C., et al. Diagnostic accuracy of procalcitonin as an early predictor of infection after radical gastrectomy for gastric cancer: a prospective bicenter Cohort study. Int. J. Surg. 2020;75:3–10. doi: 10.1016/j.ijsu.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Aouifi A., Piriou V., Bastien O., Blanc P., Bouvier H., Evans R., et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit. Care Med. 2000;28(9):3171–3176. doi: 10.1097/00003246-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy. 2021;122(7):474–488. doi: 10.4149/bll_2021_078. [DOI] [PubMed] [Google Scholar]
- 29.James R.C., Macleod C.J. Induction of staphylococcal infections in mice with small inocula introduced on sutures. Br. J. Exp. Pathol. 1961;42:266–277. [PMC free article] [PubMed] [Google Scholar]
- 30.van Winsen M., McSorley S.T., McLeod R., MacDonald A., Forshaw M.J., Shaw M., et al. Postoperative C-reactive protein concentrations to predict infective complications following gastrectomy for cancer. J. Surg. Oncol. 2021;124(7):1060–1069. doi: 10.1002/jso.26613. [DOI] [PubMed] [Google Scholar]
- 31.Smith J.S., Shaffrey C.I., Sansur C.A., Berven S.H., Fu K.-M.G., Broadstone P.A., et al. Rates of infection after spine surgery based on 108,419 procedures: a report from the scoliosis research society morbidity and mortality committee. Spine. 2011;36(7):556–563. doi: 10.1097/BRS.0b013e3181eadd41. [DOI] [PubMed] [Google Scholar]
- 32.Chahoud J., Kanafani Z., Kanj S. Surgical site infections following spine surgery: eliminating the controversies in the diagnosis. Front. Med. 2014;1 doi: 10.3389/fmed.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei P., Jing W., Yuan Z., Huang Y., Guan B., Zhang W., et al. Vancomycin- and strontium-loaded microspheres with multifunctional activities against bacteria, in angiogenesis, and in oteogenesis for enhancing infected bone regeneration. ACS Appl. Mater. Interfaces. 2019;11(34):30596–30609. doi: 10.1021/acsami.9b10219. [DOI] [PubMed] [Google Scholar]
- 34.Scholz B., Kinzelmann C., Benz K., Mollenhauer J., Wurst H., Schlosshauer B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur. Cell. Mater. 2010;20:24–36. doi: 10.22203/ecm.v020a03. ; discussion 36-27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.