Abstract
Foot and mouth disease (FMD) is consistently ranked as the most economically significant viral disease and one of the top five livestock diseases in Ethiopia. Although FMD is endemic in Ethiopia, the epidemiology and the farmers' knowledge, attitudes, and practices regarding FMD were poorly quantified. Thus, a cross-sectional study was conducted from November 2021 to April 2022 to estimate the seroprevalence, identify the FMD serotypes, and assess the farmers' knowledge, attitudes, and practices on FMD in Addis Ababa city and Sebeta special zone, central Ethiopia. A total of 384 serum samples were collected from cattle and tested using a 3ABC enzyme-linked immunosorbent assay (ELISA). In this study, an overall 56% seroprevalence was recorded. Two types of FMD serotypes were detected in which serotype O was the dominant serotype (75.5%) followed by serotype A (45.5%). A significantly higher seroprevalence (P = 0.00) was recorded in Addis Ababa (85%) compared to Sebeta (28.7%). Seropositivity in older and semi-intensively managed cattle was 2.9 (95% CI: 1.36–6.50; P = 0.006) and 2.1 (95% CI: 1.34–3.26; P = 0.001) times higher compared to young and intensively managed cattle, respectively. A survey on knowledge, attitude, and practice of 103 farmers revealed that 90.2% knew of FMD and the majority of them can recognize its clinical pictures. However, 12.7% of farmers who knew FMD didn't practice any prevention methods. Additionally, 70% of the farmers responded that their cattle roamed outside of their farms for communal grazing, watering, breeding purposes, and vaccination which might put them more at risk of FMD. The current study demonstrated that the majority of farmers have gaps in biosecurity practices and vaccination of cattle against FMD. Therefore, educating farmers on FMD prevention measures is necessary for successful disease control programs.
Keywords: Central Ethiopia, FMD, Knowledge and practices, Seroprevalence, Serotypes, Vaccination
1. Introduction
Cattle production plays an important role in the economies of farmers and pastoralists and the country at large. Ethiopia is attaining significant outcomes from the export of livestock products and has been contributing a crucial role in the development of the nation's economy. The Ethiopian livestock contribution to the national economy is estimated at 19% of the total gross domestic product [1]. Although there is substantial international demand for live animals and animal products, animal diseases are a significant factor that hampers the trade of animals and animal products [2,3]. The exports of livestock have suffered from trade bans due to importing countries' concerns over transboundary animal diseases. Among the transboundary animal diseases, foot and mouth disease (FMD) is the most important livestock disease that has a significant socio-economic impact on Ethiopia. In Ethiopia, FMD is consistently ranked as the most economically important viral disease, and among the top five important livestock diseases [4,5].
FMD is a highly contagious disease affecting multiple species of susceptible wild and domestic clove-hoofed animals and caused by the genus Alphavirus of the family Picornavirdae. This disease is clinically identified by fever, hypersalivation, lack of appetite, and vesicular eruptions on the hoof, mouth, and teats. The morbidity rate reaches up to 100%, however, the mortality rate is 5% in adult animals [6,7].
FMDV has seven immunologically diverse serotypes with varying global distributions. These are serotypes A, O, C, Asian 1, and three strains predominantly circulating in Sub-Saharan Africa, South African Territory (SAT) 1, 2, and 3 [8]. Eradication of this disease is hindered by the high infection rate of the virus, stability of the virus in aerosols and droplets of infected animals, and lack of cross-immunity between serotypes [9,10].
Successful livestock disease control programs, for example through mass vaccination, depend not only on technical and economic feasibilities but also the motivation of the farming community to fully participate in the implementation of the control program [11]. Besides, for effective disease control in livestock, holistic approaches are necessary. Previous studies in Ethiopia mainly focused on the epidemiology of the disease without integrating many aspects such as cultural or indigenous knowledge, attitude, and practices of livestock keepers about FMD and its management. Livestock owners might have good knowledge about the health of their animals and this is an opportunity for integrating their knowledge with the veterinary service delivery system to solve prevailing livestock health problems. Understanding livestock keepers’ knowledge and practice about the FMD and their motivation to apply control measures is important in designing effective disease control programs. Hence, there is a need to assess the knowledge and beliefs of livestock keepers regarding FMD before designing possible control programs.
Currently, the Ethiopian government is working on FMD prevention to reduce its effect on the livestock sector. To implement this strategy, baseline information on the epidemiology of the disease is required. Therefore, this study was designed with the objectives of estimating the seroprevalence and the associated risk factors with FMD, identifying FMD serotypes circulating in the study areas, and assessing the knowledge, attitude, and practice of the livestock owners about FMD and its diseases management practices.
2. Materials and methods
2.1. Study area and study population
The study was conducted from November 2021 to April 2022in the Addis Ababa city administration and Sebeta special zone in central Ethiopia. Addis Ababa is located at latitude of 8.9806° N and a longitude of 38.7578° E, while Sebeta is located at 8.9112° N latitude and 38.6268° E longitude. The climate in central Ethiopia is humid subtropical with moderate seasonality and mild dry winters and rainy summers [12]. Samples were collected from Alemgena, Dima, Daleti, and Sebeta kebeles of Sebeta special zone. In Addis Abeba City Administration, samples were collected from the sub-cities of Nifas Silk-Lafto, Bole, Akaki Kality, and Kolfe-Keranyo. These areas were purposefully chosen based on the relative abundance of dairy farms and the long tradition of keeping improved dairy cattle for milk production. Besides, the previous status of FMD in the cattle found in these study areas was not documented.
2.2. Study design, sampling, and sample size determination
A cross-sectional study design was used to address the objectives of the study in the dairy cattle population in the Addis Ababa city administration and Sebeta special zone. Samples were collected using simple random sampling techniques. Samples were drawn from smallholder dairy farmers to large-sized commercial farms with intensive and semi-intensive management systems.
The biodata of the individual animal from which the sample was obtained was recorded. Age was divided into two categories: young (<18 months) and adults (≥18 months). The management system used to keep the animals was classified as intensive and semi-intensive. Breeds of cattle were classified as local, exotic, and crossbreed. The herd size was categorized as small (<15 cattle), medium (15–30 cattle), and large (>30 cattle) size. A pretested semi-structured questionnaire survey was employed to assess dairy farmers' knowledge, attitudes, and practices regarding FMD.
The sample size determination was done according to Thrusfield [13]. The expected prevalence of 72.1% [14] was taken from a previous study to determine the sample size with a 95% confidence interval and 5% desired absolute precision.
where n = sample size; p = Expected prevalence; d = Desired level of precision (5%)
Accordingly, the sample size resulted in 308. However, to increase the precision, 384 sera sample was taken for serological examination.
2.3. Serum sample collection
About 8 ml of blood was collected from the jugular vein of cattle using plain vacutainer tubes. The sample was labeled with pertinent animal information and transported to the Animal Health Institute laboratory, Sebeta. Then, allowed to clot at room temperature for 24 h and collected into a sterile cryovial, and stored at −20 °C until its serological laboratory test. During sampling, data such as age and breed of animals, management system, and herd size were also recorded.
2.4. Questionnaire survey
To assess dairy farmers' knowledge, practices, and attitude toward FMD, a semi-structured questionnaire was administered as an interview. They were asked if they had observed any of the following clinical signs in their livestock: hypersalivation, lesions in the mouth, teats, feet, lameness, and death in young and adult cattle. Farmers were also asked about the vaccination history of their livestock against FMDV. Before administering the questionnaire, the farmer's consent was obtained verbally. The consent explained the confidentiality conditions and the right to refuse.
2.5. Laboratory diagnosis
The serum samples were tested for the presence antibody against FMDV using competition 3ABC ELISA (ID Screen®, ID Vet, Grabels, France) at the Animal Health Institute, Sebeta, Ethiopia. This test detects only antibodies produced against non-structural proteins of the virus and can thus distinguish vaccinated animals from infected. The tests were performed according to the manufacturer's instructions. A spectrophotometer was used to measure the optical density (OD with a wavelength of 450 nm). The test was validated if the mean value of the Negative Control OD was greater than 0.7 and the mean value of the positive control OD was less than 30% of the ODNC. The interpretation for each sample was based on the competition percentage (S/N%):
Samples presenting S/N % less than or equal to 50% were considered positive and those greater than 50% were considered negative.
2.6. FMDV serotyping
Serum samples exhibiting strong antibodies against FMDV non-structural protein using 3ABC ELISA were selected for FMD serotyping. A total of 44 strong positive serum samples were selected and subjected to antigen-capturing sandwich ELISA (IZSLER, Brescia Italy). This assay uses selected neutralizing anti-FMDV monoclonal antibodies specific for FMDV serotypes to measure antibodies against serotypes. The test was performed according to the manufacturer's manual. The optical density (OD) reading was recorded using a spectrophotometer at a wavelength of 450 nm. Results are calculated by the percentage inhibition of positive control and by test sera:
% inhibition = 100-(serum OD/Reference OD*) 100.
Reference OD = mean OD of four wells processed with negative control.
The test was valid if spectrophotometer readings must be ≥ 1 OD in wells of the negative control. The positive control serum was expected to give ≥90% inhibition at 1/10 dilution and >50% inhibition at the second dilution (1/30). Interpretation for test sera considered positive when producing an inhibition ≥70% at 1/10 dilution and considered negative when producing an inhibition <70% at 1/10 dilution.
2.7. Data analysis
The sample data and the questionnaire survey were entered and coded into Microsoft Excel Spreadsheet and analyzed using STATA software version 16.0 Windows (Stata Corp. College Station, TX, USA). Descriptive analyses were used to assess seroprevalence and knowledge, practice, and attitude of farmers. The association of risk factors to the seropositivity was conducted by Pearson's Chi-square test with a 95% confidence interval and a significance level of P < 0.05. The level of association between FMD seropositivity and categorical independent variables was determined using odds ratio (OR) and multivariable logistic regression analyses.
3. Results
3.1. Seroprevalence of FMD
In the current study, from the total of 384 blood samples tested, FMD antibodies were detected with an overall prevalence of 56% (217/384). A significantly higher (P = 0.001) prevalence was observed in Addis Ababa city administration (85%) compared to Sebeta special zone (28.7%) as shown in Table 1.
Table 1.
Overall seroprevalence and associated putative risk factors of FMD.
| Variable | No. of tested | No. of positive (%) | P-value | χ2 |
|---|---|---|---|---|
| Study area | ||||
| Sebeta | 195 | 56 (28.7%) | 124.5 | |
| Addis Ababa | 189 | 161 (85.1%) | 0.00 | |
| Age | ||||
| Young (<18 months) | 31 | 10 (32%) | 8.07 | |
| Adult (≥18 months) | 353 | 207 (58.6%) | 0.006 | |
| Sex | ||||
| Female | 344 | 192 (55.8%) | 0.65 | |
| Male | 40 | 25 (85.1%) | 0.42 | |
| Breed | ||||
| Exotic | 337 | 191 (56%) | 6.36 | |
| Local | 23 | 17 (73.9%) | 0.113 | |
| Cross | 24 | 9 (37.5%) | 0.074 | |
| Management system | ||||
| Intensive | 255 | 129 (50.5%) | 10.8 | |
| Semi-intensive | 129 | 88 (68%) | 0.001 | |
| Herd size | ||||
| Small | 162 | 80 (49.3%) | 21.7 | |
| Medium | 101 | 77 (76.2%) | 0.00 | |
| Large | 121 | 60 (49.5%) | 0.97 | |
3.2. Factors associated with FMD seropositivity
In this study, significantly higher (P = 0.001) seroprevalence was recorded in cattle managed in semi-intensive (68%) compared to intensive (50.5%) management systems. Although not statistically significant (P > 0.05), higher seroprevalence was found in males (85.1%) than in females (55.8%). A significantly higher (P = 0.006) prevalence was obtained in adults (58.6%) compared to young age groups (Table 2). The seroprevalence was higher in the local breed (73.9%) followed by exotic (56%) and crossbreed (37.5%), however, the variation was not statistically significant (P > 0.05).
Table 2.
Logistic regression analysis for the associated risk factors.
| Variables | No. of positive (%) | OR | P-value | 95%CI |
|---|---|---|---|---|
| Study area | ||||
| Sebeta | 56 (28.7) | 1 | ||
| Addis Ababa | 161 (85.1) | 14.27 | 0.00 | 8.59–23.7 |
| Age | ||||
| Young (<18 months) | 10 (32) | 1 | ||
| Adult (≥18 months) | 207 (58.6) | 2.97 | 0.006 | 1.36–6.5 |
| Management system | ||||
| Intensive | 129 (50.5) | 1 | ||
| Semi-intensive | 88 (68) | 2.10 | 0.001 | 1.34–3.26 |
3.3. Serotypes of FMDV in the study areas
This study revealed that out of 44 strong antibody-positive serum samples subjected to FMDV serotype-specific antigen capture ELISA, serotypes O and A were identified. Serotype O was the dominant serotype which accounted for 75.5% followed by serotype A with 45.5%.
3.4. Knowledge, attitude, and practice of farmers
To assess the knowledge, practices, and attitude of FMD, 103 dairy farmers were selected randomly; 36 participants from Addis Ababa city and 68 participants from Sebeta special zone as shown in Table 3. Most of the dairy farmers owned small farms (72.8%), whereas medium and large farm owners accounted for 22.3% and 4.8%, respectively. The vast majority of respondents owned exotic breeds (47.5%), followed by local breeds (39.8%), and 14.5% own more than one type of breed. The majority of the farms (93.2%) were adjacent to other farms and 57.2% of the farmers used communal watering. Zero-grazing, grazing outside and within the farm were 38.8%, 57.2%, and 3.8%, respectively.
Table 3.
Information on the total number of farmers who partook in the questionnaire.
| Variables | Response/total (%) |
|---|---|
| Study area | |
| Addis Ababa | 36/103 (34.9%) |
| Sebeta | 68/103 (66%) |
| Farm size | |
| Small farms | 75/103 (72.8%) |
| Medium farms | 23/103 (22.3%) |
| Large farms | 5/103 (4.8%) |
| Types of breeds owned | |
| Exotic | 49/103 (47.5%) |
| Local | 39/103 (37.8%) |
| More than one type of breed | 15/103 (14.5%) |
| Grazing methods | |
| Zero grazing | 40/103 (38.8%) |
| Grazing outside | 59/103 (57.2%) |
| Grazing within farms | 4/103 (3.8%) |
| Do you use communal watering? | |
| No | 44/103 (42.7%) |
| Yes | 59/103 (57.2%) |
| Do you share equipment from surrounding farms? | |
| No | 30/103 (29.1%) |
| Yes | 73/103 (70%) |
| Do you share workers from surrounding farms? | |
| No | 29/103 (28.7%) |
| Yes | 74/103 (71.8%) |
| Breeding method used | |
| AI | 41/103 (39.8%) |
| Share bull from surrounding farms | 44/103 (42.7%) |
| From own bull | 15/103 (14.5%) |
| Use both AI and shared bull | 30/103 (29.1%) |
Of the 103 dairy farmers interviewed, 70.8% share equipment, and 71.8% share workers from neighboring farms. The majority of these farmers (42.7%) share bulls from neighboring farms as a breeding method, with 39.9% using artificial insemination (AI). Furthermore, 29% use both breeding methods, while the remaining 14.5% use their bulls.
3.5. Knowledge of dairy farmers toward FMD
In the current study, from 103 dairy farmers interviewed, the majority (93/103 [90.2%]) of the respondents knew FMD. Participants in both study areas had an almost similar level of knowledge; Sebeta (60/67 [89%]) and Addis Ababa city (33/36 [91%]). Farmers who knew FMD were asked if they could describe the clinical signs. The most commonly described clinical signs were hypersalivation and mouth lesions (58%), followed by hoof lesions (47.3%) and lameness (35.4%). Furthermore, depression (18.2%) and a decrease in milk production (15%) were reported. Only 6.7% identified the presence of young mortality, and none identified adult mortality as a clinical sign of FMD as shown in Fig. 1.
Fig. 1.
Dairy farmers' knowledge of the clinical signs of FMD among respondents.
3.6. Knowledge of prevention measures
FMD prevention measures were asked for 93 dairy farmers who were aware of the disease. Vaccination was the most widely known preventive measure (68.9%) (Fig. 2). In this study, 9.6% and 6.3% of dairy farmers responded that keeping cattle within the farm compound and preventing other cattle from entering the farms, respectively. Only 4.2%, 3.1%, and 2.1% of dairy farmers mentioned do not share equipment, do not bring new cattle, and do not allow visitors to their farms, respectively. However, 12.7% of farmers who are aware of FMD didn't practice disease prevention.
Fig. 2.
Farmers' knowledge and practice on prevention method of FMD.
3.7. Vaccination practices of FMD
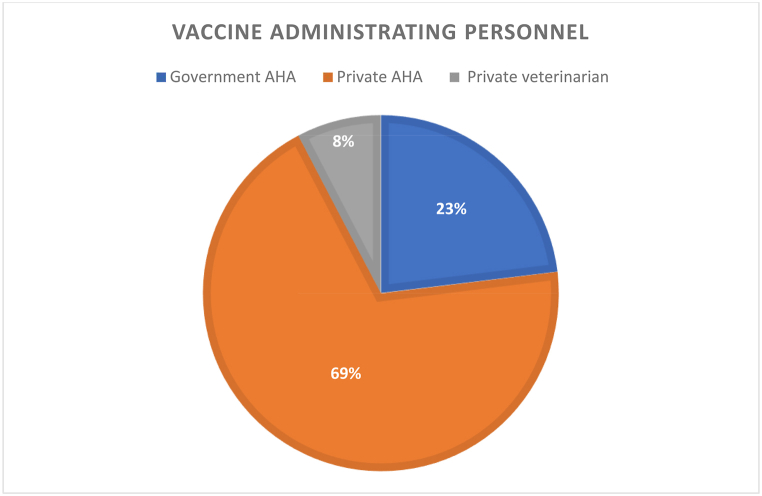
Of the 93 respondents who knew about FMD, only 27.9% (26/93) practiced vaccination for FMD. Vaccinating all their cattle was only recorded in 23.9% (6/26) of farmers. Among the 76.9% (20/26) farmers who were not vaccinating all their cattle, 55% (11/20) and 45% (9/20) do not vaccinate their pregnant and young cattle, respectively. These dairy farmers were also asked when they last vaccinated their cattle against FMD. About 7.6% (2/26) vaccinated their cattle in the past 4 months. About 19.2% (5/26) of farmers vaccinated their cattle in the past 5–6 months, and 7.6% (2/26) were vaccinated in the last 6–12 months. However, the majority of the farmers (11/26 [43.6%]) didn't report the vaccination date. The remaining 23.8% (6/26) of the farmers reported vaccinating their cattle more than a year ago (Table 4). From the 26 farmers who vaccinated their cattle against FMD, they were asked who vaccinates their cattle and 69.2% (18/26) of farmers' responses were private animal health assistants (AHA), and 23% (6/26) answered government AHA. Only 7.6% (2/26) of farmers vaccinate their cattle with a private veterinarian as shown in Fig. 3.
Table 4.
Vaccination practice of FMD of farmers that had ever vaccinated their cattle.
| Vaccination practice of FMD | Response/total (%) |
|---|---|
| Vaccination of FMD | 26/93 (27.8%) |
| Vaccinated ≤ 4 months ago | 2/26 (7.6%) |
| Vaccinated 5–6 months ago | 5/26 (19.2%) |
| Vaccinated 6–12 months ago | 2/26 (7.6%) |
| Vaccinated > 1 year ago | 6/26 (23.8%) |
| No vaccination date reported | 11/26 (43.6%) |
| Vaccinating all cattle | 6/26 (23.8%) |
| Pregnant cattle not vaccinated | 11/20 (55%) |
| Young calves not vaccinated | 9/20 (45%)c |
| Vaccination at the farm compound | 9/26 (34.6%)b |
| Vaccination off-farm | 17/26 (65.3%)b |
Fig. 3.
Individuals who vaccinate the cattle against FMD.
4. Discussion
This serosurvey indicated that FMD is a significant disease with an overall prevalence of 56% in the Addis Ababa city administration and Sebeta special zone, central Ethiopia. This study agreed with the previous report from the Borana zone 53.6% [15] and 52.8% in Dire district [16]. However, the current study estimated prevalence was higher than previous reports in different parts of the country; 41.5% in the eastern Tigray region [17], 8.9% in South Omo Zone [18], 14.3% in the Amhara region [19], 21.4% in Kellem Wollega zone [20], and 26.8% in Adama [21]. These variations in prevalence reports could be due to differences in agro-climatic conditions, animal management systems as well as disease prevention practices.
In the present study a significant difference in FMD seroprevalence between the study areas (Addis Ababa city and Sebeta special zone). These findings are consistent with the report of Ahmed et al. [22], Abunna et al. [23], and Dubie and Negash [24]. Participants in both study areas had almost similar levels of knowledge about FMD and its prevention. Thus, the difference in prevalence between the study areas could be associated with trade-related animal movements. In Ethiopia, animal prices are significantly higher in urban centers, the largest of which is Addis Ababa city, and thus, livestock usually moves toward the center from other parts of the country. In addition, these cattle are exotic pure and cross breeds densely populated, and kept on a small plot of land due to the city's scarcity of land [[25], [26], [27]].
In the current study, a significantly higher prevalence was recorded in adults (58.6%) compared to young age groups (32%). This result is in line with the study of Rufael et al. [28], Abdulahi et al. [29], Bayissa et al. [30], and Zerabruk et al. [31]. Adult cattle were 2.97 more likely to contract FMD than young ones. This effect might be the result of aged animals having acquired infection from different serotypes of FMD throughout their lifetime and the presence of antibodies in the serum for a prolonged time. And during this study farmers reared their calves in a separate pen which decreases their exposure to the virus [25,32].
In the present study, males (85.1%) had a higher prevalence than females (55.8%) but the association was statistically insignificant as in the reports of Esayas et al. [33]and Kebede et al. [34]. The study of Chowdhury et al. [35]and Mazengia et al. [36] reported the opposite. This might be due to the unproportional allocated sample size between males and females in this study, where the sample size of female cattle was greater than males.
The current study showed a significant variation between cattle management systems (P = 0.001) in which semi-intensively managed cattle have 2.09 odds of being exposed to FMDV compared to intensively managed cattle. This study was similar to the findings of Sulayeman et al. [21]. At times cattle feeding outside of the farm will have more exposure to the virus as a result of contact with infected cattle and communal grazing and watering [37]. There was a higher prevalence of FMD in local breeds (73.9%) compared to exotic (56%) and crossbreeds (36.5%). These findings are in line with Mazengia et al. [36]. The higher prevalence in local breeds might be associated with their free movement as well as management.
Data on dairy farmers' knowledge, attitudes, and practices are significantly important in planning, implementing, and evaluating FMD control strategies. The questionnaire survey of this study showed that dairy farmers had adequate knowledge of FMD. The clinical signs were well described among the farmers. The most commonly described clinical signs were hypersalivation and mouth lesions. It was also noticed that these signs were the primarily detected clinical signs by the majority of farm workers. This suggested that recognition of FMD infection by observation of profuse salivation and lesions on the oral cavity is late to optimally prevent the spread of the FMD virus. The ability of the farm workers to recognize the clinical signs varied with their level of attention to the animals as well as their knowledge about FMD.
Young mortality was also reported but none of them reported adult mortality. This report agrees with the report done in Addis Ababa by Negusssie et al. [38] where the mortality and case fatality rates were relatively higher in calves than in other age groups. This validates the point that FMD is one of the livestock's primary endemic diseases that affect farmers' livelihoods.
The majority of the respondents (59/103 [57%]) practiced communal watering and grazing. The study done by Kebede et al. [34] stated free animal movement in search of feed and water is an associated risk factor for the occurrence of FMD. Moreover, the study by Mesfine et al. [19] reported that 78% of the farmers’ perception of FMD exposure was due to communal watering points and grazing lands. Moreover, the vast majority of the farmers share equipment (70%) and workers (71%). Unrestricted cattle movement and sharing of equipment and workers increase the transmission of FMDV [39]. Thus, this suggested that effective biosecurity measures should be implemented.
The breeding methods of farmers in the study areas were primarily dependent on AI (39.8%) and shared bulls from the surrounding farms (42.7%). However, an additional 29% of the farmers used both AI and shared bulls. Although the major mode of FMD transmission is through direct contact, it can also be transmitted through the semen of infected animals [40]. The study conducted in neighboring Kenya by Nyaguthii et al. [41] demonstrated a higher risk of FMD transmission in farms using shared bulls for breeding compared to other methods. Besides, the majority of farmers (65.3%) employ vaccination of cattle in the off-farm compounds. These might increase direct contact exposure to other FMD-infected animals.
Vaccination was the most frequently mentioned prevention measure, followed by keeping cattle within farm compounds. Surprisingly, despite knowing about FMD, 12.7% of farmers don't practice any preventive measures. This could be due to a lack of knowledge, the perception that preventive measures are difficult to implement, or an underestimation of the disease risk. This suggests that farmers have to be educated on FMD preventive measures, especially on biosecurity as well as vaccination practices.
According to the findings of this survey, out of the 93 respondents who were aware of FMD, only 23 (27.9%) of them vaccinate their cattle against FMD. This clearly shows the presence of gaps in practicing one of the essential prevention methods of FMD. Furthermore, even though 23 of the farmers vaccinate their cattle, only 6 of them vaccinate all of their cattle. The remaining dairy farmers do not immunize their pregnant and young cattle. This could be due to farmers' fear of abortion and premature calving of their pregnant cattle. Jemberu et al. [42] stated that vaccines have been used sparingly to control the disease due to the perceived high cost of FMD vaccine as well as the vaccine's limited market availability. The date of vaccination reported varies among the farmers. About 23.8% of them vaccinated their animals more than a year ago and the majority of them (43.6%) didn't report the vaccination date. The FMD vaccines available in Ethiopia are administered 2 injections in 6-month intervals and with revaccination one year after the second injection and afterward administered annually according to the vaccine manufacturer (National Veterinary Institute of Ethiopia) instructions. The inability to keep track of vaccination dates impedes the effectiveness of these vaccines.
According to the current survey, 69% of FMD vaccine was administered by private AHA, followed by government AHA (23%), and private veterinarians (8%). This demonstrates the vaccination delivery services variation between private and public sectors. This study also highlighted that the private sectors are playing a key role in immunization services delivery in Ethiopia. Thus, close collaboration between the private and public sectors is essential to achieve the national vaccination target. Mass vaccination could greatly reduce the possibility of a major epidemic while targeting high-risk farms increases efficiency. Vaccination of cattle at different times may reduce vaccine effectiveness [43].
In this study, serotypes O and A were identified with a variable proportion in which serotype O was the dominant serotype identified, followed by serotype A. This is in agreement with reports of Awel et al. [14], Negusssie et al. [38], and Ayelet et al. [33]. Gizaw et al. [44] also reported that O and A FMD serotypes were widely distributed in Ethiopia specifically in the central part of Ethiopia and accounted for outbreaks occurring from the years 2008–2019.
5. Conclusion
This study demonstrated an overall FMD seroprevalence of 56% in cattle residing in the study areas. Two FMDV types were identified; serotype O was the dominant serotype followed by serotype A. Factors such as the study areas, age of cattle, and the management system was identified as risk factors for FMD seropositivity. This study concluded that the majority of cattle owners knew about FMD, however, major gaps were identified in biosecurity practices and vaccination of cattle against FMD. Thus, it is important to promote farmers’ awareness on FMD preventive and control measures.
Ethical considerations
Ethical approval for this study was granted by the animal research ethical review committee of the College of Veterinary Medicine and Agriculture of Addis Ababa University. Before conducting the research, animal owners were informed of the objectives and the benefits of the study and they gave consent for their animal's inclusion in the study. The consent obtained from animal owners was verbal because they are unable to write and read. These consents were taken in the presence of a third independent party.
Funding
This study was supported by the Addis Ababa University thematic research fund (RD/LT-408/2021), Ethiopia. The funder had no role in the conception, design of the study, data collection, analysis, and interpretation of the data reported in this manuscript.
Author contribution statement
Kalkidan Seifu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ayelech Muluneh: Performed the experiments.
Yitbarek Getachew; Yasmin Jibril: Analyzed and interpreted the data.
Haileleul Negussie: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Additional information
Supplementary content related to this article has been published online at [URL].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the Animal Health Institute (AHI) and Addis Ababa University, Ethiopia, for the provision of laboratory facilities and financial support. We also acknowledge the dairy animal owners for their willingness and commitment to the success of this research work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15771.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shapiro B.I., Gebru G., Desta S., Negassa A., Nigussie K., Aboset G., et al. International Livestock Research Institute (ILRI); Nairobi, Kenya: 2017. Ethiopia Livestock Sector Analysis. ILRI Project Report. [Google Scholar]
- 2.Shapiro B., Gebru G., Desta S., Negassa A., Nigussie K., Aboset G., et al. 2015. Ethiopia Livestock Master Plan Roadmaps for Growth and Transformation. Nairobi, Kenya. [Google Scholar]
- 3.Jemberu W.T., Mourits M.C.M., Sahle M., Siraw B., Vernooij J.C.M., Hogeveen H. Epidemiology of foot and mouth disease in Ethiopia: a retrospective analysis of district level outbreaks, 2007–2012. Transbound Emerg. Dis. 2016;63:e246–e259. doi: 10.1111/tbed.12338. [DOI] [PubMed] [Google Scholar]
- 4.Shiferaw T.J., Moses K., Manyahilishal K.E. Participatory appraisal of foot and mouth disease in the Afar pastoral area, northeast Ethiopia : implications for understanding disease ecology and control strategy. Trop. Anim. Health Prod. 2010;42:193–201. doi: 10.1007/s11250-009-9405-9. [DOI] [PubMed] [Google Scholar]
- 5.Jibat T., Admassu B., Rufael T., Baumann M.P.O., Pötzsch C.J. Impacts of foot-and-mouth disease on livelihoods in the Borena Plateau of Ethiopia. Pastor. Res. Policy Pract. 2013;3:1–11. [Google Scholar]
- 6.Yoo H.S. Foot and mouth disease: etiology, epidemiology and control measures. Infect. Chemother. 2011;43:178–185. [Google Scholar]
- 7.Donaldson A. Foot and Mouth Disease. CRC Press; 2019. Clinical signs of foot-and-mouth disease; pp. 93–102. [Google Scholar]
- 8.Knight-Jones T.J.D., Robinson L., Charleston B., Rodriguez L.L., Gay C.G., Sumption K.J., et al. Global foot‐and‐mouth disease research update and gap analysis: 2–epidemiology, wildlife and economics. Transbound Emerg. Dis. 2016;63:14–29. doi: 10.1111/tbed.12522. [DOI] [PubMed] [Google Scholar]
- 9.OIE . OIE; Paris: 2014. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals.www.oie.int/manual-of-diagnostic-tests-and-vaccines-for terrestrial animals/ (Chapter 2).1.5. Foot-and-mouth disease. Available at: 2014. [Google Scholar]
- 10.Ferrari G., Paton D., Duffy S., Bartels C., Knight T., Sus J. In: Metwally Samia, Münstermann Susanne., editors. 2016. Foot and Mouth Disease Vaccination and Post-vaccination Monitoring Guidelines. [Google Scholar]
- 11.Jemberu W., Mourits M.C.M., Hogeveen H. Farmers' intentions to implement foot and mouth disease control measures in Ethiopia. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0138363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CSA . 2020. Agricultural Sample Survey. Report on Livestock and Livestock Characteristics (Private Peasant Holdings). The Federal Democratic Republic of Ethiopia. [Google Scholar]
- 13.Thrusfield M.V. second ed. Black Well Science; 2005. Veterinary Epidemiology. [Google Scholar]
- 14.Awel S.M., Dilba G.M., Abraha B., Zewde D., Wakjira B.S., Aliy A. Seroprevalence and molecular detection of foot and mouth disease virus in dairy cattle around Addis Ababa, Central Ethiopia. Vet. Med. Res. Rep. 2021;12:187. doi: 10.2147/VMRR.S317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mekonen H., Beyene D., Rufael T., Feyisa A., Abunna F. Study on the prevalence of foot and mouth disease in Borana and Guji zones, southern Ethiopia. Vet. World. 2011;4:293–296. [Google Scholar]
- 16.Tesfaye A., Sehale M., Abebe A., Muluneh A., Gizaw D. Sero-prevalence of foot and mouth disease in cattle in Borena Zone, Oromia regional state, Ethiopia. Ethiop. Vet. J. 2016;20:55–66. [Google Scholar]
- 17.Ayelet G., Gelaye E., Negussie H., Asmare K. Study on the epidemiology of foot and mouth disease in Ethiopia. Rev. Sci. Tech. 2012;31:789–798. doi: 10.20506/rst.31.3.2153. [DOI] [PubMed] [Google Scholar]
- 18.Molla B., Ayelet G., Asfaw Y., Jibril Y., Ganga G., Gelaye E. Epidemiological study on foot‐and‐mouth disease in cattle: seroprevalence and risk factor assessment in South Omo zone, south‐western Ethiopia. Transbound Emerg. Dis. 2010;57:340–347. doi: 10.1111/j.1865-1682.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- 19.Mesfine M., Nigatu S., Belayneh N., Jemberu W.T. Sero-epidemiology of foot and mouth disease in domestic ruminants in Amhara Region, Ethiopia. Front. Vet. Sci. 2019;6:130. doi: 10.3389/fvets.2019.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanta D., Desalgn T., Bedaso M., Tesfaye R. Epidemiological study on foot and mouth disease in cattle: seroprevalence and risk factor assessment in Kellem Wollega Zone, West Ethiopia. Afr. J. Agric. Res. 2014;9:1391–1395. [Google Scholar]
- 21.Sulayeman M., Dawo F., Mammo B., Gizaw D., Shegu D. Isolation, molecular characterization and sero-prevalence study of foot-and-mouth disease virus circulating in central Ethiopia. BMC Vet. Res. 2018;14:1–10. doi: 10.1186/s12917-018-1429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed B., Megersa L., Mulatu G., Siraj M., Boneya G. Seroprevalence and associated risk factors of foot and mouth disease in cattle in West Shewa Zone, Ethiopia. Vet. Med. Int. 2020:2020. doi: 10.1155/2020/6821809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abunna F., Fikru S., Rufael T. Seroprevalence of foot and mouth disease (FMD) at Dire dawa and its surroundings, eastern Ethiopia. Global Vet. 2013;11:575–578. [Google Scholar]
- 24.Dubie T., Negash W. Seroprevalence of bovine foot and mouth disease (FMD) and its associated risk factors in selected districts of Afar region, Ethiopia. Vet. Med. Sci. 2021;7:1678–1687. doi: 10.1002/vms3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexandersen S., Mowat N. Foot-and-mouth disease: host range and pathogenesis. Foot-and-mouth Dis virus. 2005:9–42. doi: 10.1007/3-540-27109-0_2. [DOI] [PubMed] [Google Scholar]
- 26.Getabalew M., Alemneh T., Zewdie D. The milk processing: status, challenges and opportunities in Ethiopia. Int. J. Vet. Sci. Res. 2020;6:52–57. [Google Scholar]
- 27.Shmeiger Z., Miculitzki M., Gelman B., Vaxman I., Goshen T. The effect of foot and mouth disease morbidity influencing periparturient diseases and culling on Nir Yitzhak dairy cattle farm. Isr. J. Vet. Med. 2021;76:27. [Google Scholar]
- 28.Rufael T., Catley A., Bogale A., Sahle M., Shiferaw Y. Foot and mouth disease in the Borana pastoral system, southern Ethiopia and implications for livelihoods and international trade. Trop. Anim. Health Prod. 2008;40:29–38. doi: 10.1007/s11250-007-9049-6. [DOI] [PubMed] [Google Scholar]
- 29.Abdulahi M., Esaya T., Hailu D. Seroprevalence of bovine foot and mouth disease (FMD) in awbere and babille districts of Jijiga zone, Somalia regional state, eastern Ethiopia. Afr. J. Microbiol. Res. 2011;5:3559–3563. [Google Scholar]
- 30.Bayissa B., Ayelet G., Kyule M., Jibril Y., Gelaye E. Study on seroprevalence, risk factors, and economic impact of foot-and-mouth disease in Borena pastoral and agro-pastoral system, southern Ethiopia. Trop. Anim. Health Prod. 2011;43:759–766. doi: 10.1007/s11250-010-9728-6. [DOI] [PubMed] [Google Scholar]
- 31.Zerabruk G., Romha G., Rufael T. Sero-epidemiological investigation of foot and mouth disease in cattle managed under extensive husbandry system in Tigray, northern Ethiopia. Global Vet. 2014;13:112–116. [Google Scholar]
- 32.Sangare O., Bastos A.D.S., Marquardt O., Venter E.H., Vosloo W., Thomson G.R. Molecular epidemiology of serotype O foot-and-mouth disease virus with emphasis on West and South Africa. Virus Gene. 2001;22:345–351. doi: 10.1023/a:1011178626292. [DOI] [PubMed] [Google Scholar]
- 33.Esayas G., Gelagay A., Tsegalem A., Kassahun A. Seroprevalence of foot and mouth disease in Bench Maji zone, Southwestern Ethiopia. J. Vet. Med. Anim. Heal. 2009;1:5–10. [Google Scholar]
- 34.Kebede S.H., Tesfaye R.C., Enquibaher K. A study on sero prevalence of foot and mouth diseases in West and South West Shoa zones of Oromia regional state, central Ethiopia. J. Vet. Med. Anim. Heal. 2018;10:21–27. [Google Scholar]
- 35.Chowdhury M.S.R., Ahsan M.I., Khan M.J., Rahman M.M., Hossain M.M., Harun-Al-Rashid A., et al. Data on prevalence, distribution and risk factors for Foot and Mouth Disease in grazing cattle in haor areas of Bangladesh. Data Brief. 2020;28 doi: 10.1016/j.dib.2019.104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazengia H., Taye M., Negussie H., Alemu S., Tassew A. Incidence of foot and mouth disease and its effect on milk yield in dairy cattle at Andassa dairy farm, Northwest Ethiopia. Agric. Biol. J. N. Am. 2010;1:969–973. [Google Scholar]
- 37.Tadesse B., Molla W., Mengsitu A., Jemberu W.T. Transmission dynamics of foot and mouth disease in selected outbreak areas of northwest Ethiopia. Epidemiol. Infect. 2019:147. doi: 10.1017/S0950268819000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negusssie H., Kyule M.N., Yami M., Ayelet G., Jenberie T.S. Outbreak investigations and genetic characterization of foot-and-mouth disease virus in Ethiopia in 2008/2009. Trop. Anim. Health Prod. 2011;43:235–243. doi: 10.1007/s11250-010-9683-2. [DOI] [PubMed] [Google Scholar]
- 39.Muroga N., Kobayashi S., Nishida T., Hayama Y., Kawano T., Yamamoto T., et al. Risk factors for the transmission of foot-and-mouth disease during the 2010 outbreak in Japan: a case–control study. BMC Vet. Res. 2013;9:1–9. doi: 10.1186/1746-6148-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma G.K., Subramaniam S., De A., Das B., Bihari Dash B., Sanyal A., et al. Detection of foot-and-mouth disease virus in semen of infected cattle bulls. Indian J. Anim. Sci. 2012;82:1472. [Google Scholar]
- 41.Nyaguthii D.M., Armson B., Kitala P.M., Sanz-Bernardo B., Di Nardo A., Lyons N.A. Knowledge and risk factors for foot-and-mouth disease among small-scale dairy farmers in an endemic setting. Vet. Res. 2019;50:1–12. doi: 10.1186/s13567-019-0652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jemberu W.T., Molla W., Dagnew T., Rushton J., Hogeveen H. Farmers' willingness to pay for foot and mouth disease vaccine in different cattle production systems in Amhara region of Ethiopia. PLoS One. 2020;15(10 October):1–12. doi: 10.1371/journal.pone.0239829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keeling M.J., Woolhouse M.E.J., May R.M., Davies G., Grenfell B.T. Modelling vaccination strategies against foot-and-mouth disease. Nature. 2003;421:136–142. doi: 10.1038/nature01343. [DOI] [PubMed] [Google Scholar]
- 44.Gizaw D., Tesfaye Y., Wood B.A., Di Nardo A., Shegu D., Muluneh A., et al. Molecular characterization of foot and mouth disease viruses circulating in Ethiopia between 2008 and 2019. Transbound Emerg. Dis. 2020;67:2983–2992. doi: 10.1111/tbed.13675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.