Abstract
Background
Alcohol use and alcohol-related health problems are on the rise in developing countries. This meta-analysis was conducted to determine the effects of alcohol consumption on human male reproductive function through semen parameters, antioxidants in semen, sperm DNA fragmentation, and sex hormones.
Methods
Studies regarding the effects of alcohol consumption on male reproductive function were searched on databases. Based on the random-effects model, STATA software was used to analyze and synthesize the selected studies. Alcoholics, moderate alcoholics, heavy alcoholics, and no alcoholics values were compared using the standard mean difference. Publications were assessed for publication bias by the Egger test.
Result
Forty studies were selected from databases examining the effect of alcohol consumption on male reproductive health in 23,258 people on five continents of the world. The meta-analysis revealed that alcohol intake reduced semen volume during each ejaculation (SMD = −0.51; 95% CI −0.77, −0.25). However, there were no significant associations with other semen indicators such as density, mobility, and normal and abnormal sperm count from this analysis. In addition, drinking alcohol lowered antioxidant enzymes in semen (SMD = −7.93; 95% CI −12.59, −3.28) but had no effect on sperm DNA fragmentation. Finally, the results showed a decrease in general testosterone levels (SMD = −1.60; 95% CI −2.05, −1.15), Follicle Stimulating Hormone (SMD = −0.47; 95% CI −0.88, −0.05), Luteinizing Hormone (SMD = −1.35; 95% CI −1.86, −0.83), but no effect in other sex hormones named as estradiol, Inhibin B and Sex Hormone-Binding Globulin. Furthermore, when analyzing subgroups at different drinking levels, the results showed that the moderate alcoholic group (less than 7 units/week) had no change in the semen index. Meanwhile, the group of heavy alcoholics (more than 7 units/week) harmed the semen index and sex hormones, especially by increasing estradiol.
Conclusion
There is evidence that alcohol consumption affected semen volume and antioxidant, reproductive hormones thus negatively affecting male reproductive function. This study might be necessary to make recommendations regarding alcohol consumption for men.
Keywords: Alcohol consumption, Heavy drinkers, Sperm parameters, Testosterone, Antioxidant, Sex hormones, Sperm DNA fragmentation
1. Introduction
The use of alcoholic beverages in daily life is considered a cultural feature of many countries in the world. They were used in several religions because they bring excitement and addiction to consumers [1]. According to a recent WHO study, one of the alarming current issues is that while alcohol use is declining in developed countries, it is increasing in developing countries [[2], [3], [4]]. From 1993 to 2000, alcohol consumption per capita among Vietnamese adults increased by roughly 2.5 times [5]. Excessive or long-term alcohol consumption has negative consequences not only for the person who consumes it but also for the family and society [[6], [7], [8], [9]]. Reports suggest that unhealthy alcohol use affects nearly every organ system [[10], [11], [12], [13], [14]].
Alcohol consumption affects the entire hypothalamic-pituitary-gonadal region of the male reproductive system and disrupted the production of Gonadotropin-Releasing Hormone (GnRH), Follicle Stimulating Hormone (FSH), and Luteinizing Hormone (LH), the natural oestradiol levels due to changes in free testosterone, impaired function of Leydig and Sertoli cells, resulting in reduced sperm quality to normal morphology and sperm maturation [[15], [16], [17]]. Consumption of alcohol with a high concentration and frequency has led to negative effects on the concentration of sperm; normal morphology of spermatozoa; total sperm count, reduced testosterone and SHBG, and increased serum testosterone levels [18,19]. Scientific evidence shows that it causes impotence, infertility, and spermatogenic arrest (Pajarinen JT and Karhunen 1994, [[20], [21], [22], [23], [24], [25]]. Many studies showed negative impacts based on evidence that drinking alcohol leads to an increase in enzymatic antioxidants, sperm DNA damage [26], and high levels of DNA fragmentation [27]. However, several reports give the opposite findings. Barratt et al. have shown that moderate alcohol consumption did not affect semen quality despite higher testosterone levels [28]. The reports of Teijon et al. and Hansen et al. show no statistically significant difference in semen parameters between the alcoholics and no-alcoholics [29] and no association between alcohol consumption and sperm quality [15]. Moreover, a meta-analytical conducted by Ying Li et al. found drinking alcohol did not affect sperm parameters [20].
For a more general and accurate assessment of existing information, we conducted a meta-analysis of published literature that assessed the effects of alcohol intake and male reproductive function. This report shows a general opinion on drinking alcohol and parameters related to male reproductive quality and provides recommendations on alcohol consumption with concentrations and levels defined.
2. Materials and methods
2.1. Search strategy and identification of relevant studies
Research subjects are scientific publications on databases that study the associations between alcohol consumption and male reproductive health parameters such as semen parameters, antioxidant enzymes, sperm DNA fragmentation, and sex hormone levels. The studies were searched from MEDLINE, EMBASE databases, and scientific websites through keywords: “alcohol consumption”, “DNA fragmentation”, “semen quality”, “semen parameter”, “normal morphology”, “reproductive hormones”, “alcohol”, “motility”, “male fertility”, “testosterone”, “estradiol”, “inhibin B”, “oxidative damage”, “semen quality”, “sperm abnormalities”, “alcohol abuse”, “male fertility”, “enzymatic antioxidant activity”.
The selection criteria include articles in English, having full text, and providing data of interest. Exclusion criteria reviewed articles that provided no data or only in the form of graphs, cell, and animal studies. Studies were selected when they: (a) evaluated the association between alcohol consumption and male reproductive health parameters; (b) defined drinking levels; (c) effect of alcohol consumption on sex hormones; (d) represented original data; (e) used a cohort study design, and (f) were written in English. Papers were excluded when they: (a) described a review, case report, or conference abstract; (b) did not contain original data; (c) did not provide data.
2.2. Collect data for analysis (Data extraction)
The studies extracted data regarding the effect of alcohol on indicators of male reproductive function such as semen volume (mL), concentration (106 sperm/mL), ability semen motility (%), normal morphology, abnormal morphology, and sperm defects, sperm DNA fragmentation (SDF) (%), testosterone (ng/mL), β-estradiol (pg/mL), Inhibin B (pg/mL), sex hormone-binding globulin (SHBG) (nmol/L), Follicle Stimulating Hormone (FSH) (mUI/mL), Luteinizing Hormone (LH) (mUI/mL). Metric values were described as mean and standard deviation or median and IQR. If data are provided as median and IQR, they were converted to mean and standard deviation before proceeding with the meta-analysis [[30], [31], [32]].
2.3. Statistical analysis
A meta-analysis of the studies was done using the statistical analysis software STATA 15 based on the random effects model (Random Effects Model). Heterogeneity between studies was assessed through the heterogeneity index I-squared. The values groups were compared using the standard mean difference (SMD). Publications are evaluated for publication bias using the Egger test.
2.4. Subgroup analysis
We employed subgroup analysis by drinking levels definition (according to alcohol concentration and frequency consumption). For that there are three groups of people based on their drinking habits: no-alcoholics if they drink less than 1 unit of alcohol a week; moderate alcoholics if they use at least 1 unit/week to 7 units/week; and heavy alcoholics when they take more than 7 units of alcohol/week. One unit of alcohol is equivalent to 7.9 g of ethanol or 10 mL of ethanol contained in an oral solution [33]. Thus, 1 unit of alcohol is equivalent to 3/4 bottles/cans of 330 mL beer (5% alcohol); 1 cup of draft beer 330 mL; 1 glass of wine 100 mL (13.5% alcohol); or 1 cup of spirits 30 mL (40% alcohol).
3. Results
3.1. Characterization of eligible studies
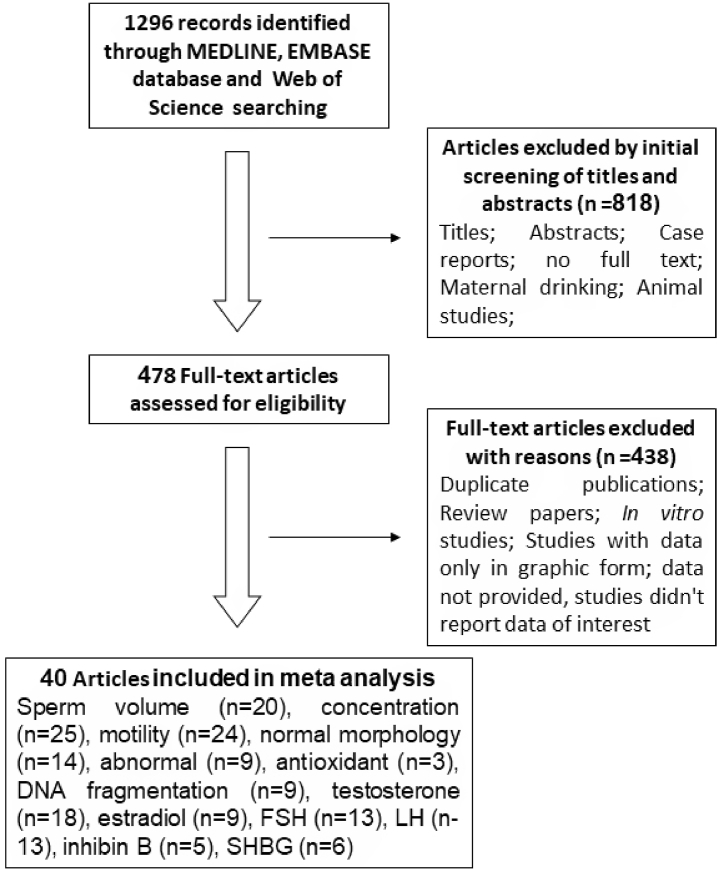
Studies were searched on scientific databases MEDLINE, EMBASE, and scientific websites. The process of searching and filtering the studies for the meta-analysis is shown in Fig. 1. The results of 1296 studies were found, of which 818 were omitted because the title and abstract did not match the selection criteria to conduct the review. The remaining 478 full-text articles continued to be evaluated. Of these, 438 articles were excluded for reasons such as duplicate content, some reviews, animal studies, in vitro studies, studies with graphical data only, and articles that had not provided complete data. In the end, we obtained 40 studies that were eligible for inclusion in the systematic review, of which 20 reported on alcohol consumption and semen volume (mL), 25 articles included data on sperm density (million/mL), 24 articles had information on sperm motility of (percentage of motile sperm), 14 articles show results on sperm morphology. The remaining studies involved alcohol use and other indicators such as DNA fragmentation (9 studies), testosterone (18 studies), estradiol (9 studies), FSH (13 studies), LH (13 studies), inhibit B (5 studies), SHBG (6 studies) [15,18,26,[34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70]] (Fig. 1 and Table 1).
Fig. 1.
The selection process of papers for the meta-analysis study.
Table 1.
Characteristics of included studies assessing an association between alcohol intake and male reproductive function.
| Study | Year | Country | Age | Participation | Sample size |
Research index | Main outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Drinking Levels Defined |
||||||||||
| No-alcoholics | Moderate alcoholics | Heavy alcoholics | Alcoholics | ||||||||
| Anifandis | 2014 | Greece | 37.43 ± 0.3 | Men who were seeking semen analysis for fertility purposes in IVF Unit | 207 | 124 | 58 | 25 | 83 | V, C, M, DF | Heavy alcohol consumption may impair sperm volume, concentration, and sperm DNA fragmentation |
| Chia | 1998 | Singapore | 33.2 ± 5.3 | Fertile men | 243 | 146 | 97 | V, C, M, NM, | Social alcoholic consumption did not appear to affect sperm quality | ||
| Condorelli | 2014 | Italy | 34.0 ± 6.0 31.0 ± 8.0 |
Fertile and infertile men | 76 | 0 | 40 | 36 | 76 | V, C, M, NM, T, E, FSH, LH | ‘Daily drinkers’ have semen quality, testicular pathology, and hormonal characteristics significantly worse |
| Eskenazi | 2003 | USA | 44.5 | Non-smoking men without known fertility problems | 97 | 34 | 63 | V, C, M, | Semen volume and sperm motility decreased continuously | ||
| Goverde | 1995 | Netherlands | Men with poor semen quality | 47 | 20 | 27 | V, C, M, NM, | Alcohol consumption may further decrease an already low percentage of sperm with normal morphology | |||
| Hansen | 2012 | Denmark | Young Danish men | 345 | 54 | 84 | 109 | 291 | V, C, M, NM, DF, T, E, F, L, I, S | Alcohol intake and a hormonal shift towards a higher estradiol/testosterone ratio | |
| Hart | 2015 | Australia | 20 ± 0.5 | Fertile men | 887 | 120 | 463 | 304 | 767 | C, T, E, F, L, I, | Alcohol intake was not associated with any significant semen variables or circulating reproductive hormones |
| Jensen (a) | 2014 | Denmark | 18–28 | Young Danish men attending military service from 2008 to 2012 | 1206 | 176 | 680 | 92 | 1030 | V, C, M, NM, T, E, F, L, I, S | Alcohol consumption was also linked to changes in testosterone and SHBG levels. |
| Jensen (b1) | 2014 | Europe | 18–28 | Fertile young men | 6472 | 1133 | 2872 | 5339 | V, C, M, NM, T, F, LH, I, S | Alcohol consumption is not related to semen quality | |
| Jensen (b2) | 2014 | Europe and USA | 18–45 | Fertile men | 1872 | 560 | 429 | 1312 | V, C, M, NM, T, F, LH, I, S | Alcohol consumption is not related to semen quality | |
| Joo | 2012 | Korea | 39.5 ± 2.6 35.4 ± 1.2 |
Fertile men and nonsmokers | 13 | 8 | 5 | 13 | V,C,M, NM, | Alcohol consumption was associated with increased numbers of morphologically abnormal sperm. | |
| Kumar | 2014 | India | 31.91 ± 0.37 | Oligozoospermia | 63 | 54 | 9 | C, M, NM, | Alcohol use might be attributed to the risk of declining semen quality | ||
| Lopez Teijon | 2007 | Spain | 32.6 ± 6.0 | Volunteers from the province of Barcelona | 967 | 440 | 527 | C, M, NM | The consumption of alcohol did not have a significant effect on semen quality | ||
| Martini | 2004 | Argentina | Semen samples (one per patient) with toxic habits | 3430 | 3194 | 236 | V, C, M, NM | Alcohol consumption did not alter the seminal parameters | |||
| Muthusami | 2005 | India | 36.6 ± 5.7 35.0 ± 6.1 |
Nonsmoking alcoholics - normal healthy persons | 96 | 30 | 66 | V, C, M, NM, AM, T, E, F, Lh, I, S, | Alcohol consumption has a detrimental effect on male reproductive hormones and semen quality | ||
| Kucheria | 1985 | India | 25–42 | Alcohol Dependence Syndrome – control case | 30 | 10 | 20 | V, C, M, AM, T, F, L, | Alcohol consumption for a considerable period affects spermatogenesis, spermiogenesis and causes oligozoospermia | ||
| Brzek | 1987 | Czechoslovakia | 35,7–33.1 | Alcoholics and control group | 235 | 135 | 100 | V, C, M | Patients with alcohol abuse had defective stereograms | ||
| Komiya | 2014 | Japan | 40,7 ± 4,1 | Male Japanese patients with infertility | 54 | 27 | 27 | DF, | Chronic alcohol use increased the SDF | ||
| Keskin | 2016 | Turkey | 33.0 ± 5.43 | Infertile men who use alcohol | 356 | 256 | 81 | 19 | 100 | V, C, M, NM, AM | There was no significant difference in any of the parameters |
| Wdowiak | 2016 | Poland | 35.0 ± 4.82 | Couples who had been diagnosed with infertility | 499 | 345 | 154 | DF, | The percentage of sperm cells with nuclear DNA strand breaks was significantly higher in men with risky alcohol consumption | ||
| Ricci | 2018 | Italy | 39.3 ± 5.2 | Couples who had been diagnosed with infertility | 323 | 31 | 195 | 97 | 292 | V, C, M | Moderate alcohol intake appears positively associated with semen quality in male partners of infertile couples undergoing ARTs |
| Aboulmaouahib | 2018 | Morocco | 39.23 ± 8.87 37.95 ± 7.83 |
Infertile couples | 59 | 36 | 23 | V, C, M, NM, DF, AO | Alcohol intake had detrimental effects on DNA integrity with the potential adverse effects of OS on fertility. | ||
| Schmid | 2007 | USA | 41.8 ± 14.9 47.5 ± 14.5 |
Non-smokers | 80 | 30 | 50 | DF | Alcohol use affects sperm DNA fragmentation | ||
| Varshini | 2012 | India | 21–40 | Men who visited the University infertility clinic between 2006 and 2010 | 504 | 445 | 59 | DF | Alcoholics had a significantly higher median distribution of TUNEL-positive spermatozoa | ||
| Schmid | 2012 | USA | 46.4 | Healthy male volunteers | 79 | 31 | 48 | DF | Men with higher dietary and supplement intake of certain micronutrients may produce sperm with less DNA damage | ||
| Marshburn | 1989 | USA | Men couples are seen at the University of North Carolina infertility clinic between 1978 and 1982. | 446 | 338 | 108 | V, C, M, AM, | Ethanol could produce addictive effects on semen parameters. | |||
| Wogatzky | 2012 | Austria | 40.4 ± 5.9 | Men with undergoing ART | 1573 | 282 | 1127 | 164 | 1291 | V, C, M | Using alcohol does not affect sperm |
| Gautam | 2015 | India | 29.5 ± 5.4 28.5 ± 4.3 |
Fathers of children with retinoblastoma and controls | 56 | 31 | 25 | DF | ROS and DFI levels in alcohol users were higher as compared to alcohol non-users. | ||
| Maneesh | 2006 | India | 29.6 ± 4.2 | Male alcohol abusers- healthy male volunteers | 101 | 55 | 46 | T, F, L, AO | Ethanol caused low plasma testosterone in men accompanied by low LH and FSH | ||
| Shiels | 2009 | USA | 41.5 ± 0.7 | Men C20 years old who participated in the Third National Health and Nutrition Examination Survey | 1275 | 466 | 809 | T, E, S, | Alcohol consumption activity may be associated with concentrations of sex steroid hormones among adult men. | ||
| Frias | 2002 | Spain | 20–27 | Men with acute alcohol intoxication | 23 | 11 | 12 | T, F, L, | Effect of alcohol on pituitary-gonadal axis hormones in humans | ||
| Handa | 1997 | Japan | 50–54 | Men who received a preretirement health examination at the Self-Defence Forces Fukuoka Hospital | 303 | 59 | 224 | T, F, | Drinking alcohol can affect estradiol | ||
| Kulkarni | 2009 | India | 37.65 ± 6.6 38.30 ± 6.3 |
Alcohol patients and controls | 360 | 200 | 160 | 160 | T, F, L, AO | Alcoholic patients displayed significantly low levels of serum testosterone, LH, FSH. | |
| Oldereid | 1992 | Norway | Men attending our laboratory | 239 | 56 | 183 | C, M, AM, | Alcohol did not appear to have any deleterious effects on sperm quality | |||
| Close | 1990 | USA | 32.9 | Infertile men | 136 | 82 | 46 | 8 | 54 | C, M | Alcohol showed no decrease in sperm count, percentage of motility of oval sperm |
| Von Der | 2002 | Finland | 20–50 | Male residents of Helsinki | 84 | 44 | 40 | T, E, | Alcohol misuse can affect testosterone levels. | ||
| Sierksma | 2004 | Finland | 45–64 | Middle-aged men, apparently healthy nonsmoking, and moderate alcohol drinkers, | 10 | 5 | 5 | Alcohol consumption can increase testosterone levels | |||
| Frias | 2000 | Spain | 13–17 | Young adolescents using alcohol | 21 | 10 | 11 | T, E, FSH, LH, | There are many serious effects of alcohol abuse on reproductive function | ||
| Iturriaga | 1995 | Chile | 37.3 ± 7.6 | Male chronic alcoholics | 45 | 15 | 30 | T, FSH, LH, SHBG, | Alcohol consumption leads to lower FSH and higher SHBG levels | ||
| Dai | 1981 | USA | 51 ± 5.4 | Men are considered to be free of clinical heart disease | 239 | 33 | 66 | 140 | 206 | T | Alcohol intake was not found to be related to testosterone concentrations. |
V: Semen volume (mL), C: Concentration (10″6 spermatozoa/mL), M: Progressive motility (%), NM: Normal morphology, AM: Abnormal morphology and sperm defects, AO: Antioxidant, DF: Sperm DNA fragmentation (%), T: Testosterone, E: Estradiol, F: FSH, L: LH, I: Inhibin B, S: SHBG2.
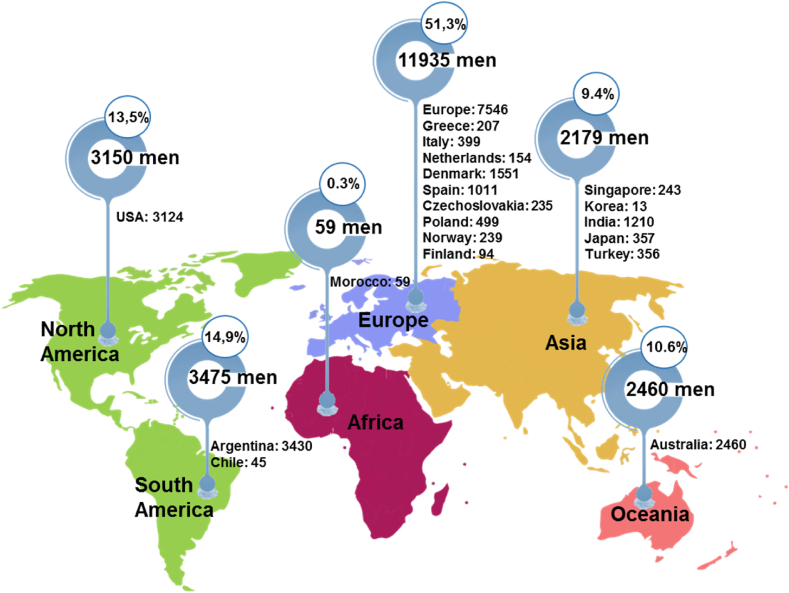
40 studies published separately over the 37 years from 1981 to 2018 examined the effects of alcohol consumption on the male reproductive health of 23,258 men from 5 continents of the world, of which 11,935 were from Europe (51.3%), 6625 people from America (South America: 14.9%, America North: 13.5%), 2460 people from Australia (10.6%), 2179 people from Asia (9.4%) and 59 people from Africa (0.3%) (Fig. 2).
Fig. 2.
Worldwide distribution of 40 studies with 23,258 men reporting in this meta-analysis.
3.2. Association between alcohol consumption and semen parameters
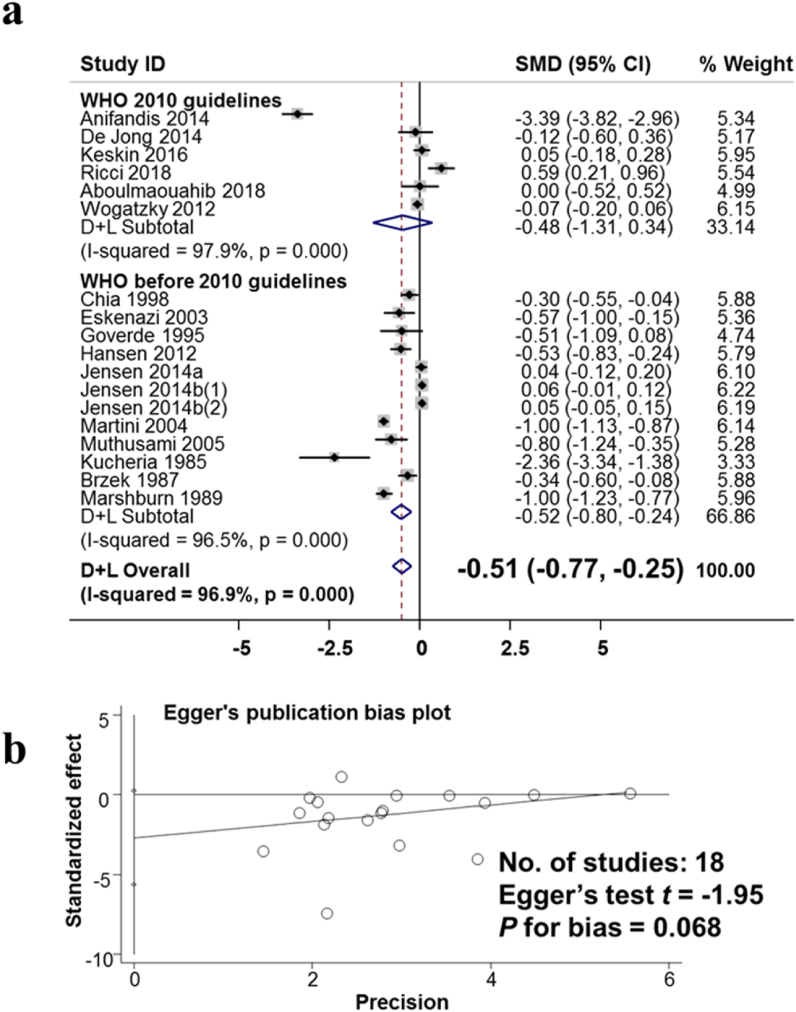
The effects of alcohol consumption on semen volume were analyzed across 18 studies involving 17,144 male adults are shown in Fig. 3a. The studies were analyzed based on the random-effects model. The difference between studies was large, with the I-squared heterogeneity index being 96.9%. Each study was weighted (%weight) from 3.33 to 6.22. The results of the meta-analysis showed that the standard mean difference (SMD) between the no-alcoholics and alcoholic groups was −0.51 (95% CI: −0.77, −0.25). This result suggests that drinking alcohol reduces the volume of semen in each ejaculation. Fig. 3b presents the results of the assessment of publication bias in publications on the effects of alcohol consumption on semen volume using the Egger test. The results showed no publication bias with p for bias being 0.068.
Fig. 3.
The observed association of alcohol consumption and the decreased semen volume. a. Forest plot for the effect of alcohol consumption and semen volume. b. Evaluating publication bias among studies by Egger publication bias plot.
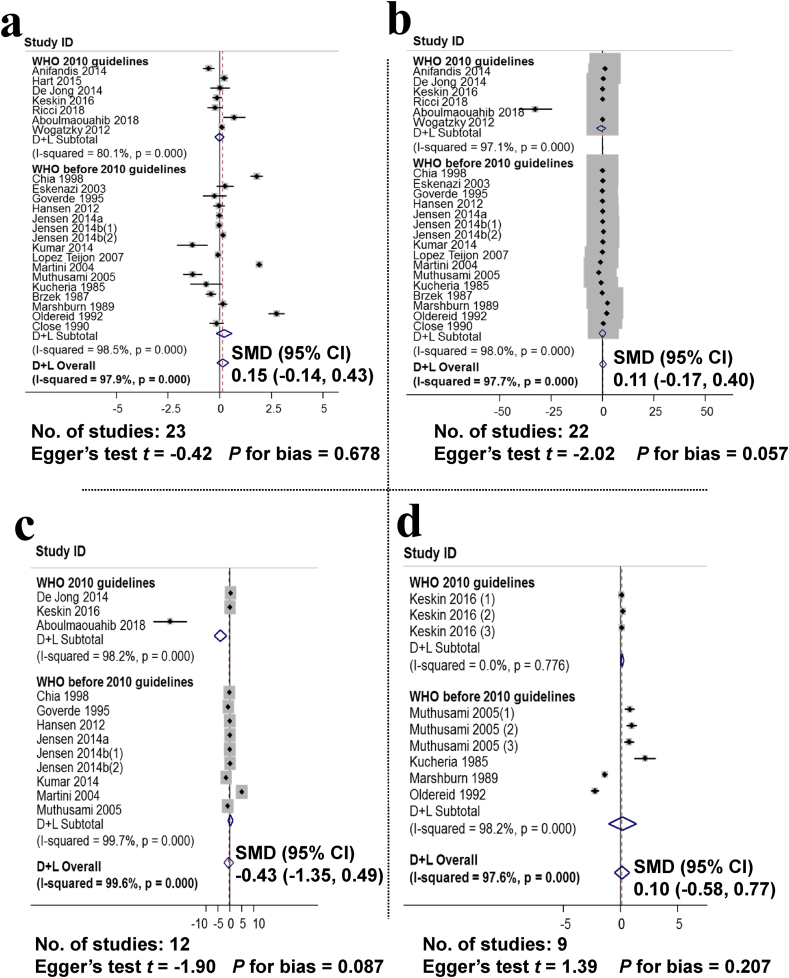
Fig. 4 presents an analysis of the effect of alcohol consumption on sperm density (23 studies, with 19,436 men), motility (22 studies, with 18,549 men), normal morphology (12 studies, with 14,296 men), and abnormal morphology (9 studies, with 2071 men). The results of the analysis showed that the SMD between the no-alcoholics and alcoholics groups was 0.15 (95% CI: −0.14, 0.43), sperm motility 0.11 (95% CI: −0.17, 0.40), SMD of normal sperm morphology is −0.43 (95% CI: −1.35, 0.49). SMD of abnormal sperm morphology was 0.10 (95% CI: −0.58, 0.77). This result shows that drinking alcohol did not significantly affect the parameters of sperm density, motility, normal morphology, and abnormal morphology.
Fig. 4.
Non-observed associations between alcohol consumption and sperm concentration, motility, normal and abnormal morphology. a. Forest plot for the effect of alcohol consumption and sperm density, assessment of publication bias using the Egger publication bias plot; b. Forest plot for the effect of alcohol consumption and sperm motility and assessment of publication bias using the Egger publication bias plot; c. Forest plot for the effect of alcohol consumption and normal morphology and assessment of publication bias using the Egger publication bias plot; d. Forest plot for the effect of alcohol consumption and abnormal morphology and assessment of publication bias using Egger publication bias plot.
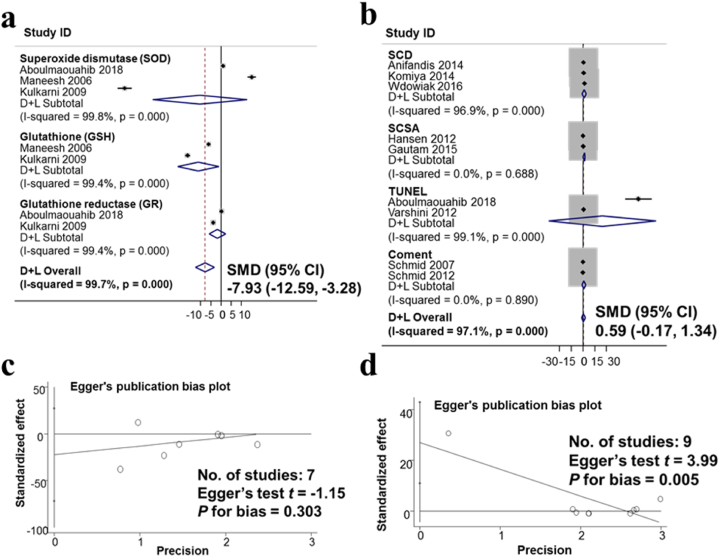
3.3. Associations between alcohol consumption and antioxidant enzymes, sperm DNA fragmentation
The results of an analysis of 1400 men from seven studies on the effects of alcohol consumption on antioxidant enzymes are shown in Fig. 5a. The studies were analyzed based on the random-effects model. The studies have a large variation in research results, the heterogeneity index I-squared heterogeneity was 96.9%. The results of the meta-analysis show that the difference in SMD between the no-alcoholics and alcoholics groups was −7.93 (95% CI: −12.59, −3.28). This result suggests that drinking alcohol reduces antioxidant enzymes in semen. Fig. 5c presents the results of the assessment of publication bias in publications on the impact of alcohol consumption on antioxidant enzymes using the Egger test. The results show no publication bias with p for bias was 0.303.
Fig. 5.
The observed association of the decreased antioxidant enzymes and non-observed association of sperm DNA fragmentation with Alcohol consumption. a. Forest plot for the effect of alcohol consumption and antioxidant enzymes in semen; b. Forest plot for the effect of alcohol consumption and sperm DNA fragmentation; c. Evaluation of publication bias regarding antioxidant enzymes in semen using Egger publication bias plot; d. Evaluation of publication bias about sperm DNA fragmentation using Egger publication bias plot.
The effect of alcohol intake on sperm DNA fragmentation in 1883 men from 9 meta-analyzed studies (Fig. 5b). The studies were analyzed based on the random-effects model. The studies have a large variation in research results, the heterogeneity index I-squared heterogeneity was 96.9%, The results of the meta-analysis show that the standard means difference in SMD between the drinking and no-drinking groups was 0.59 (95% CI: −0.17, 1.34). This result shows that drinking alcohol had not to change the sperm DNA fragmentation index. Fig. 5d presents the results of the assessment of publication bias in publications on the impact of alcohol consumption on sperm DNA fragmentation using the Egger test. The results show publication bias with p for bias being 0.005.
3.4. Associations between alcohol consumption and sex hormones
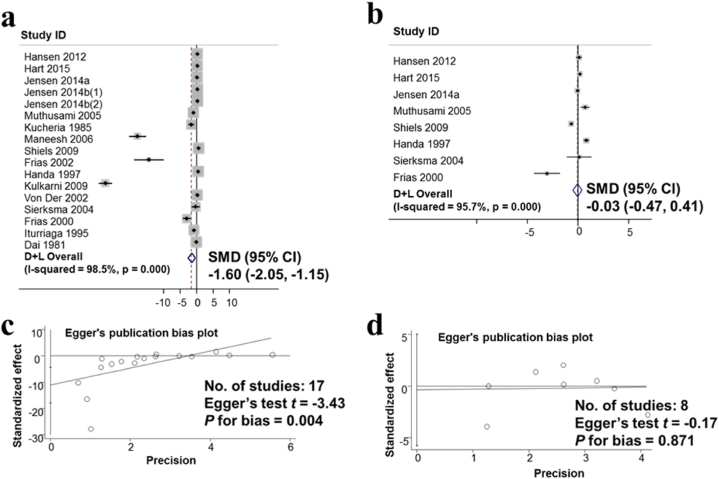
The results of the study on the effects of alcohol consumption on the hormone testosterone from 17 studies involving 13,373 men were meta-analyzed and presented in Fig. 6a. The studies were analyzed based on the random-effects model. The studies have a large variation in research results, the heterogeneity index I-squared heterogeneity was 96.9%, The results of the meta-analysis show that the standard means difference in SMD between the drinking and non-drinking groups was −1.60 (95% CI: −2.05, −1.15). These results suggest that alcohol consumption lowers the testosterone hormone. Fig. 6c presents the results of the assessment of publication bias in publications on the effects of alcohol consumption on testosterone using the Egger test. The results show publication bias with p for bias was 0.004.
Fig. 6.
The observed association of the decreased hormone testosterone and non-observed association of hormone Estradiol with Alcohol consumption. a. Forest plot for the effect of alcohol consumption and the hormone testosterone; b. Forest plot for the effect of alcohol consumption and the hormone β-Estradiol; c. Evaluation of publication bias about the hormone testosterone using Egger publication bias plot; d. Evaluation of publication bias using Egger’s publication bias plot.
The results of the estradiol hormone survey on 4143 men from 8 studies show that the alcoholic group did not change the estradiol hormone compared with the no-alcoholic group with SMD values of −0.03 (95% CI: −0.47, 0.41) in Fig. 6b. Evaluation of publication bias in publications on the impact of alcohol consumption on the hormone estradiol using the Egger test. The results show no publication bias p for bias was 0.871 in Fig. 6d.
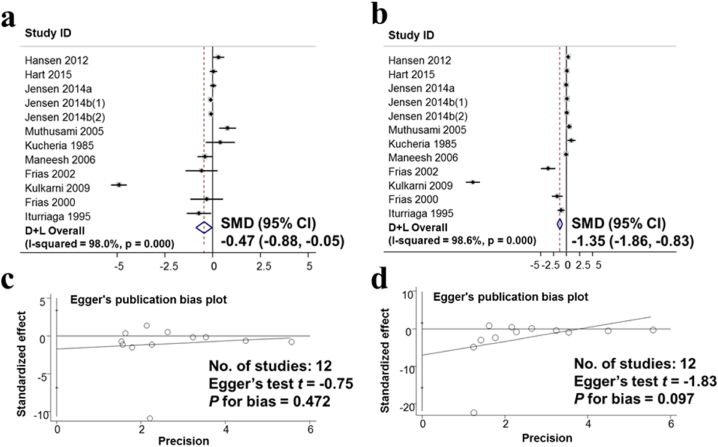
Results of the FSH hormone survey in 9375 men from 12 studies and LH hormone in 11,458 men from 12 studies show that the standard means difference in SMD were −0.47; (95% CI: −0.88, −0.05) and −1.35; (95% CI: −1.86, −0.83), respectively. This suggests that drinking alcohol lowers the hormones FSH and LH (Fig. 7a and b). Evaluation of publication bias in publications on the effects of alcohol consumption on hormones FSH and LH by the Egger test. The results showed no publication bias for both FSH or LH with p for bias being 0.472 and 0.097, respectively (Fig. 7c and d).
Fig. 7.
Observed associations between Alcohol consumption and Hormone FSH, Hormone LH. a. Forest plot for the effect of alcohol consumption and hormone FSH; b. Forest plot for the effect of alcohol consumption and hormone LH; c. Evaluation of publication bias about the hormone FSH using Egger publication bias plot; d. Evaluation of publication bias LH using Egger publication bias plot.
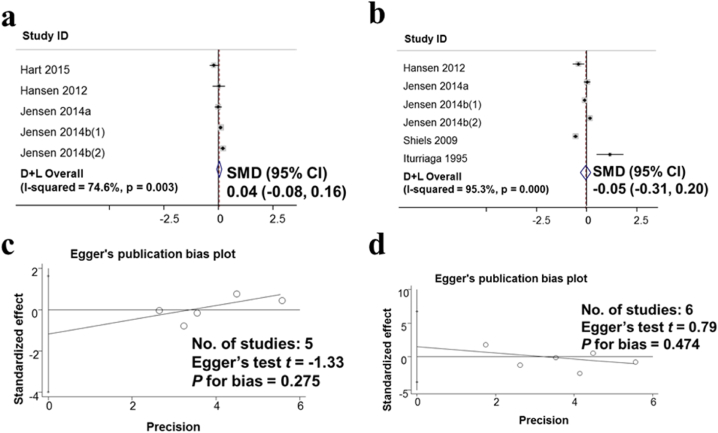
Inhibin B hormone survey from 5 studies including 10,782 men and SHBG2 hormone from 6 studies including 11,215 men are shown in Fig. 8. The standard means difference SMD for inhibin B was 0.04 (95% CI: −0.08, 0.16); SMD for SHBG2 was −0.05; (95% CI: −0.31, 0.2). Thus, using alcohol has not changed the hormone Inhibin B and SHBG2 (Fig. 8a and b). The assessment of publication bias in the publications on the impact of alcohol consumption on inhibin B and SHBG2 using the Egger test. The results show no publication bias for both inhibin B and SHBG2 with p for bias being 0.275 and 0.474, respectively (Fig. 8c and d).
Fig. 8.
Non-observed associations between Alcohol consumption Hormone Inhibin B, Hormone SHBG2. a. Forest plot for the effect of alcohol consumption and hormone Inhibin B; b. Forest plot for the effect of alcohol consumption and hormone SHBG2; c. Evaluation of publication bias Inhibin B using Egger publication bias plot; d. Evaluation of SHBG2 hormone-related publication bias using Egger publication bias plot.
3.5. Subgroup analysis of the associations between alcohol consumption at different levels on male reproductive function
Subgroup analysis was conducted to assess the impact of different levels of alcohol consumption on male fertility. The results presented in Table 2 show that the alcoholic group (drinking at least 1 unit of alcohol per week) had a decrease of semen volume with the SMD was −0.51 (95% CI: −0.77, −0.25); the antioxidant with SMD was −7.93 (95% CI: −12.59, −3.28); testosterone with SMD was −1.60 (95% CI: −2.05, −1.15); FSH with SMD was −0.47 (95% CI: −0.88, 0.05); LH with SMD was −1.35 (95% CI: −1.86, −0.83) compared to the no-alcoholic group. Meanwhile, the moderate alcoholic group (who have 1–7 units of alcohol per week) had no statistical impact on seminal parameters (volume, density, mobility, normal morphology) and sperm abnormalities as well as sex hormones (testosterone, estradiol, FSH, LH, Inhibin B, SHBG2) in comparison to the no-alcoholic group. Comparing the heavy alcoholic group (consuming more than 7 units a week) and the no-alcoholic group, there were significant associations on male reproductive function, likely to reduce the volume of semen per ejaculation with SMD was −0.48 (95% CI: −0.86, −0.11); testosterone with SMD was −1.92 (95% CI: −2.70, −1.13); FSH with SMD was −0.77 (95% CI: −1.47, −0.06), but increased estradiol with SMD was 0.22 (95% CI: 0.00, 0.44); and increased LH with SMD was 0.06= (95% CI: 0.01, 0.12). When analyzing two subgroups of the heavy alcoholic group and the moderate alcoholic group, there was no impact on seminal parameters, but the heavy alcoholic group was shown to maintain a significant association with the increase in estradiol (SMD = 0.54 (95% CI: 0.06, 1.02)) and LH (SMD = 0.22 (95% CI: 0.00, 0.43)) (Table 2).
Table 2.
Subgroup analysis of the associations between alcohol consumption at different levels on male reproductive function.
| Research Index | Comparison between subgroups of alcohol consumption at different levels |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcoholics Vs. No alcoholics |
Moderate alcoholics vs. No alcoholics |
Heavy alcoholics vs. No alcoholics |
Heavy alcoholics vs. Moderate alcoholics |
|||||||||||||
| n | men | SMD (95% CI) | n | men | SMD (95% CI) | n | men | SMD (95% CI) | n | men | SMD (95% CI) | |||||
| Sperm parameter | ||||||||||||||||
| Semen volume (ml) | 18 | 17,144 | −0.51 (−0.77, −0.25) | ↓ | 6 | 3148 | −0.71 (−1.85, 0.42) | ↔ | 9 | 6869 | −0.48 (−0.86, −0.11) | ↓ | 8 | 2820 | −0.05 (−0.15, 005) | ↔ |
| Concentration (106/mL) | 23 | 19,436 | 0.15 (−0.14, 0.43) | ↔ | 8 | 3859 | −0.02 (−0.18, 0.15) | ↔ | 11 | 7383 | −0.06 (−0.25, 0.12) | ↔ | 10 | 3641 | −0.09 (−0.29, 0.11) | ↔ |
| Progressive motility (%) | 22 | 18,549 | 0.11 (−0.17, 0.40) | ↔ | 7 | 3276 | 0.35 (−0.10, 0.79) | ↔ | 10 | 6959 | 0.19 (−0.14, 0.52) | ↔ | 9 | 2874 | −0.31 (−0.67, 0.04) | ↔ |
| Normal morphology | 12 | 14,296 | −0.43 (−1.35, 0.49) | ↔ | 3 | 1331 | −0.08 (−0.28, 0.13) | ↔ | 5 | 5700 | −0.09 (−0.15, −0.03) | ↔ | 5 | 1154 | −0.29 (−0.79, 0.21) | ↔ |
| Abnormal morphology | 9 | 2071 | 0.10 (−0.58, 0.77) | ↔ | 3 | 1011 | 0.10 (−0.05, 0.24) | ↔ | 4 | 1271 | −0.22 (−1.21, 0.77) | ↔ | 3 | 300 | 0.09 (−0.20, 0.38) | ↔ |
| Antioxidant and sperm DNA fragmentation | ||||||||||||||||
| Antioxidant | 7 | 1400 | 7.93 (−12.59, −3.28) | ↓ | N/A | N/A | N/A | |||||||||
| DNA fragmentation | 9 | 1883 | 0.59 (−0.17, 1.34) | ↔ | N/A | N/A | N/A | |||||||||
| Hormone | ||||||||||||||||
| Testosterone | 17 | 13,373 | −1.60 (−2.05, −1.15) | ↓ | 4 | 1676 | 0.05 (−0.09, 0.18) | ↔ | 7 | 6415 | −1.92 (−2.70, −1.13) | ↓ | 5 | 1981 | 0.02 (−0.26, 0.29) | ↔ |
| Estradiol | 8 | 4143 | −0.03 (−0.47, 0.41) | ↔ | 3 | 1577 | 0.01 (−0.18, 0.20) | ↔ | 3 | 855 | 0.22 (0.00, 0.44) | ↑ | 4 | 1808 | 0.54 (0.06, 1.02) | ↑ |
| FSH | 12 | 9375 | −0.47 (−0.88, 0.05) | ↓ | 3 | 1577 | 0.04 (−0.11, 0.20) | ↔ | 6 | 6208 | −0.77 (−1.47, −0.06) | ↓ | 3 | 1732 | 0.07 (−0.04, 0.18) | ↔ |
| LH | 12 | 11,458 | −1.35 (−1.86, −0.83) | ↓ | 3 | 1577 | −0.03 (−0.19, 0.13) | ↔ | 5 | 5849 | 0.06 (0.01, 0.12) | ↑ | 4 | 1808 | 0.22 (0.00, 0.43) | ↑ |
| Inhibin B | 5 | 10,782 | 0.04 (−0.08, 0.16) | ↔ | 3 | 1577 | −0.07 (−0.22, 0.08) | ↔ | 3 | 855 | −0.09 (−0.24, 0.05) | ↔ | 3 | 1732 | −0.09 (−0.24, 0.05) | ↔ |
| SHBG2 | 6 | 11,215 | −0.05 (−0.31, 0.20) | ↔ | 2 | 994 | −0.01 (−0.17, 0.15) | ↔ | 4 | 5425 | −0.11 (−0.33, 0.11) | ↔ | 2 | 965 | −0.01 (−0.17, 0.15) | ↔ |
n: publication number; men: number of men participating in the study; SMD: Standard mean difference.
↑: uptrend; ↓: downtrend; ↔: trend does not change; N/A: no data available.
4. Discussion
In developed countries in Eastern Europe and Central Asia, the burden of disease caused by alcohol accounts for 12.1%. In several developing countries in the Asia Pacific and South America, this rate is estimated at 6.2% [5,71]. This meta-analytical study, which involves 23,258 men in 40 reports on 5 continents, once again confirms the potential danger to male reproductive quality at various concentrations of alcohol in everyday life.
There is a contradiction in conclusions of the effect of alcohol on male reproductive function. Gou et al. in a report on 66 alcoholics, drinking more than 1 year, results showed that the average sperm count, volume, motility, or percentage of sperm was significantly reduced [72]. Stutz et al. concluded that alcohol use could have had detrimental effects on seminal parameters [25]. In contrast, the findings of Govern et al. demonstrated no statistically significant differences in semen volume, sperm concentration, and percentage of motile sperm of daily drinkers [73].
Oxidative stress is implicated as a cause of sperm DNA damage and it can lead to infertility [[74], [75], [76]]. In men, a high ROS ratio leads to sperm DNA damage and sperm DNA fragmentation [77]. Sperm DNA damage has been evaluated as an important attribution of semen quality [78,79], and higher ROS concentrations may also induce autophagy of spermatozoa [80]. Therefore, to be able to diagnose and treat male infertility effectively, it is necessary to evaluate the oxidative status, antioxidant defense system and DNA damage, and sperm parameters [76]. Our study results showed that drinking alcohol reduced antioxidant enzymes in semen, but did not change sperm DNA fragmentation index. In addition, we do not have enough studies to analyze how different levels of alcohol intake affect antioxidant enzymes and sperm DNA fragmentation.
The association between total testosterone levels and alcohol drinking habits was not previously found [62,[81], [82], [83], [84]]. In contrast, reports have shown that men with alcoholism will have a decrease in serum testosterone levels accompanied by a low LH and FSH, which may result from two mechanisms: alcohol destroys Leydig cells or affects the metabolism of hypothalamic-pituitary-gonadal activity [85]. Our results have also shown the effect of alcohol on reproductive hormones, which reduces testosterone, FSH, and LH.
Performing a subgroup analysis provides a clearer view and several interesting results when looking at each group separately. The report also advises that heavy drinkers should be encouraged to cut back [71]. As previous studies only focused on studying two main groups of drinkers and non-drinkers, or heavy drinkers and drinkers, our study expanded the study groups to four subgroups, including the comparison of the alcoholic vs the no-alcoholic group, the moderate alcoholic vs the non-alcoholic groups, the heavy alcoholic vs the no-alcoholic groups, the heavy alcoholic vs the moderate alcoholic groups. This helps our study to determine exactly how alcohol consumption levels affect fertility in men. The heavy alcohol consumption groups have been shown to affect sperm quality, decreasing testosterone and increasing estradiol. Drinking alcohol at all levels does not affect Inhibin B and SHBG2 which is contrary to several previous studies [19,60]. Subgroup analysis offers interesting conclusions, moderate drinking (less than 7 alcohol units per week) has no effects on male fertility. Heavy drinking (more than 7 units/week) negatively impacts male reproductive health.
Around the world, alcohol use among men should be considered an important public health issue. During the five-year period from 2000 to 2005, it was alarmed noting that the rate of alcohol use among Vietnamese people increased rapidly rate by 50% [86,87], besides that the data related to epidemiological data shows that the harmful effects of alcohol use on public health and society are increasing [88].
In conclusion, the meta-analysis of 40 studies surveying 23,258 men showed that alcohol consumption hurt ejaculate semen volume, antioxidant enzymes in semen, and sex hormone levels. However, drinking alcohol at a moderate level (less than 7 alcohol units per week) is unlikely to change the parameters of semen and sex hormones, while drinking at a heavy level (more than 7 units/per week) is likely to make negative effects on semen volume and sex hormones.
Author contribution statement
Tung Nguyen-Thanh, Ph.D.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ai-Phuong Hoang-Thi: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Dang Thi Anh Thu: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
No additional information is available for this paper.
Acknowledgments
This study was supported by The Vietnamese Ministry of Education and Training’s Research Projects in Science and Technology (Grant number B2023-DHH-11). The authors also acknowledge the partially supported by Hue University, Vietnam, under the Core Research Program, Research Group on Regenerative Medicine (Grant number NCM.DHH.2022.02).
References
- 1.Heath D.B. Routledge; 2012. Drinking Occasions: Comparative Perspectives on Alcohol and Culture. [Google Scholar]
- 2.Room R., Babor T., Rehm J. Alcohol and public health. Lancet. 2005;365(9458):519–530. doi: 10.1016/S0140-6736(05)17870-2. [DOI] [PubMed] [Google Scholar]
- 3.Rehm J., et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 4.Organization W.H. World Health Organization; 2019. Global Status Report on Alcohol and Health 2018. [Google Scholar]
- 5.Giang K.B., et al. Alcohol use and alcohol consumption–related problems in rural Vietnam: an epidemiological survey using AUDIT. Subst. Use Misuse. 2008;43(3–4):481–495. doi: 10.1080/10826080701208111. [DOI] [PubMed] [Google Scholar]
- 6.Murray C.J., Lopez A.D. Global mortality, disability, and the contribution of risk factors: global Burden of Disease Study. Lancet. 1997;349(9063):1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 7.Single E., et al. Morbidity and mortality attributable to alcohol, tobacco, and illicit drug use in Canada. Am. J. Publ. Health. 1999;89(3):385–390. doi: 10.2105/ajph.89.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutjahr E., Gmel G., Rehm J. Relation between average alcohol consumption and disease: an overview. Eur. Addiction Res. 2001;7(3):117–127. doi: 10.1159/000050729. [DOI] [PubMed] [Google Scholar]
- 9.Rehm J., et al. The global distribution of average volume of alcohol consumption and patterns of drinking. Eur. Addiction Res. 2003;9(4):147–156. doi: 10.1159/000072221. [DOI] [PubMed] [Google Scholar]
- 10.Goodlett C.R., Horn K.H. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res. Health. 2001;25(3):175. [PMC free article] [PubMed] [Google Scholar]
- 11.Ji C. Advances and new concepts in alcohol-induced organelle stress, unfolded protein responses and organ damage. Biomolecules. 2015;5(2):1099–1121. doi: 10.3390/biom5021099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obad A., et al. Alcohol-mediated organ damages: heart and brain. Front. Pharmacol. 2018;9:81. doi: 10.3389/fphar.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomes P.G., et al. Natural recovery by the liver and other organs after chronic alcohol use. Alcohol Res. Curr. Rev. 2021;41(1) doi: 10.35946/arcr.v41.1.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzo-Avalos S., Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int. J. Environ. Res. Publ. Health. 2010;7(12):4281–4304. doi: 10.3390/ijerph7124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen M.L., et al. Does last week's alcohol intake affect semen quality or reproductive hormones? A cross-sectional study among healthy young Danish men. Reprod. Toxicol. 2012;34(3):457–462. doi: 10.1016/j.reprotox.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Pajarinen J., et al. Moderate alcohol consumption and disorders of human spermatogenesis. Alcohol Clin. Exp. Res. 1996;20(2):332–337. doi: 10.1111/j.1530-0277.1996.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 17.Emanuele M.A., Emanuele N.V. Alcohol's effects on male reproduction. Alcohol Health Res. World. 1998;22:195–201. [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen T.K., et al. Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open. 2014;4(9):e005462. doi: 10.1136/bmjopen-2014-005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen T.K., et al. Alcohol and male reproductive health: a cross-sectional study of 8344 healthy men from Europe and the USA. Hum. Reprod. 2014;29(8):1801–1809. doi: 10.1093/humrep/deu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., et al. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil. Steril. 2011;95(1):116–123. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Ricci E., et al. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod. Biomed. Online. 2017;34(1):38–47. doi: 10.1016/j.rbmo.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Gaur D.S., Talekar M.S., Pathak V.P. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J. Pathol. Microbiol. 2010;53(1):35–40. doi: 10.4103/0377-4929.59180. [DOI] [PubMed] [Google Scholar]
- 23.Martini A.C., et al. Effects of alcohol and cigarette consumption on human seminal quality. Fertil. Steril. 2004;82(2):374–377. doi: 10.1016/j.fertnstert.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Muthusami K., Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil. Steril. 2005;84(4):919–924. doi: 10.1016/j.fertnstert.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Stutz G., et al. The effect of alcohol, tobacco, and aspirin consumption on seminal quality among healthy young men. Arch. Environ. Health. 2004;59(11):548–552. doi: 10.1080/00039890409603432. [DOI] [PubMed] [Google Scholar]
- 26.Aboulmaouahib S., et al. Impact of alcohol and cigarette smoking consumption in male fertility potential: looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia. 2018;50(3) doi: 10.1111/and.12926. [DOI] [PubMed] [Google Scholar]
- 27.Zini A., San Gabriel M., Baazeem A. Antioxidants and sperm DNA damage: a clinical perspective. J. Assist. Reprod. Genet. 2009;26(8):427–432. doi: 10.1007/s10815-009-9343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barratt C.L., et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance—challenges and future research opportunities. Hum. Reprod. Update. 2017;23(6):660–680. doi: 10.1093/humupd/dmx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teijon M.L., et al. Semen quality in a population of volunteers from the province of Barcelona. Reprod. Biomed. Online. 2007;15(4):434–444. doi: 10.1016/s1472-6483(10)60370-7. [DOI] [PubMed] [Google Scholar]
- 30.Luo D., et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 31.Wan X., et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan A., et al. Potential benefits of minimum unit pricing for alcohol versus a ban on below cost selling in England 2014: modelling study. BMJ. 2014;349:g5452. doi: 10.1136/bmj.g5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anifandis G., et al. The impact of cigarette smoking and alcohol consumption on sperm parameters and sperm DNA fragmentation (SDF) measured by Halosperm((R)) Arch. Gynecol. Obstet. 2014;290(4):777–782. doi: 10.1007/s00404-014-3281-x. [DOI] [PubMed] [Google Scholar]
- 35.Chia S.E., Tay S.K., Lim S.T. What constitutes a normal seminal analysis? Semen parameters of 243 fertile men. Hum. Reprod. 1998;13(12):3394–3398. doi: 10.1093/humrep/13.12.3394. [DOI] [PubMed] [Google Scholar]
- 36.Condorelli R.A., et al. Chronic consumption of alcohol and sperm parameters: our experience and the main evidences. Andrologia. 2015;47(4):368–379. doi: 10.1111/and.12284. [DOI] [PubMed] [Google Scholar]
- 37.Eskenazi B., et al. The association of age and semen quality in healthy men. Hum. Reprod. 2003;18(2):447–454. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- 38.Goverde H.J., et al. Semen quality and frequency of smoking and alcohol consumption--an explorative study. Int. J. Fertil. Menopausal Stud. 1995;40(3):135–138. [PubMed] [Google Scholar]
- 39.Hart R.J., et al. Testicular function in a birth cohort of young men. Hum. Reprod. 2015;30(12):2713–2724. doi: 10.1093/humrep/dev244. [DOI] [PubMed] [Google Scholar]
- 40.Jensen T.K., et al. Alcohol and male reproductive health: a cross-sectional study of 8344 healthy men from Europe and the USA. Hum. Reprod. 2014;29(8):1801–1809. doi: 10.1093/humrep/deu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joo K.J., et al. The effects of smoking and alcohol intake on sperm quality: light and transmission electron microscopy findings. J. Int. Med. Res. 2012;40(6):2327–2335. doi: 10.1177/030006051204000631. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S., et al. Environmental & lifestyle factors in deterioration of male reproductive health. Indian J. Med. Res. 2014;140(Suppl):S29–S35. [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez Teijon M., et al. Semen quality in a population of volunteers from the province of Barcelona. Reprod. Biomed. Online. 2007;15(4):434–444. doi: 10.1016/s1472-6483(10)60370-7. [DOI] [PubMed] [Google Scholar]
- 44.Martini A.C., et al. Effects of alcohol and cigarette consumption on human seminal quality. Fertil. Steril. 2004;82(2):374–377. doi: 10.1016/j.fertnstert.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 45.Muthusami K.R., Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil. Steril. 2005;84(4):919–924. doi: 10.1016/j.fertnstert.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Kucheria K., Saxena R., Mohan D. Semen analysis in alcohol dependence syndrome. Andrologia. 1985;17(6):558–563. doi: 10.1111/j.1439-0272.1985.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 47.Brzek A. Alcohol and male fertility (preliminary report) Andrologia. 1987;19(1):32–36. doi: 10.1111/j.1439-0272.1987.tb01853.x. [DOI] [PubMed] [Google Scholar]
- 48.de Jong A.M., et al. Effect of alcohol intake and cigarette smoking on sperm parameters and pregnancy. Andrologia. 2014;46(2):112–117. doi: 10.1111/and.12054. [DOI] [PubMed] [Google Scholar]
- 49.Komiya A., et al. Clinical factors associated with sperm DNA fragmentation in male patients with infertility. Sci. World J. 2014;2014 doi: 10.1155/2014/868303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keskin M.Z., et al. Do cigarette and alcohol affect semen analysis? Arch. Ital. Urol. Androl. 2016;88(1):56–59. doi: 10.4081/aiua.2016.1.56. [DOI] [PubMed] [Google Scholar]
- 51.Wdowiak A., et al. Relationship between alcohol consumption and sperm nuclear DNA fragmentation and pregnancy POST ĘP. Y ANDROLOGI I ONLINE. 2016;3(1):14–21. [Google Scholar]
- 52.Ricci E., et al. Alcohol intake and semen variables: cross-sectional analysis of a prospective cohort study of men referring to an Italian Fertility Clinic. Andrology. 2018;6(5):690–696. doi: 10.1111/andr.12521. [DOI] [PubMed] [Google Scholar]
- 53.Schmid T.E., et al. The effects of male age on sperm DNA damage in healthy non-smokers. Hum. Reprod. 2007;22(1):180–187. doi: 10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- 54.Varshini J., et al. Poor sperm quality and advancing age are associated with increased sperm DNA damage in infertile men. Andrologia. 2012;44(Suppl 1):642–649. doi: 10.1111/j.1439-0272.2011.01243.x. [DOI] [PubMed] [Google Scholar]
- 55.Schmid T.E., et al. Micronutrients intake is associated with improved sperm DNA quality in older men. Fertil. Steril. 2012;98(5):1130–1137 e1. doi: 10.1016/j.fertnstert.2012.07.1126. [DOI] [PubMed] [Google Scholar]
- 56.Marshburn P.B., Sloan C.S., Hammond M.G. Semen quality and association with coffee drinking, cigarette smoking, and ethanol consumption. Fertil. Steril. 1989;52(1):162–165. doi: 10.1016/s0015-0282(16)60809-9. [DOI] [PubMed] [Google Scholar]
- 57.Wogatzky J., et al. The combination matters--distinct impact of lifestyle factors on sperm quality: a study on semen analysis of 1683 patients according to MSOME criteria. Reprod. Biol. Endocrinol. 2012;10:115. doi: 10.1186/1477-7827-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gautam S., et al. Sperm DNA damage in non-familial sporadic heritable retinoblastoma (NFSHRb) Clin. Epidemiol. Global Health. 2015;3:S20–S25. [Google Scholar]
- 59.Maneesh M., et al. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J. Physiol. Pharmacol. 2006;50(3):291–296. [PubMed] [Google Scholar]
- 60.Shiels M.S., et al. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control. 2009;20(6):877–886. doi: 10.1007/s10552-009-9318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frias J., et al. Effects of acute alcohol intoxication on pituitary-gonadal axis hormones, pituitary-adrenal axis hormones, beta-endorphin and prolactin in human adults of both sexes. Alcohol Alcohol. 2002;37(2):169–173. doi: 10.1093/alcalc/37.2.169. [DOI] [PubMed] [Google Scholar]
- 62.Handa K., et al. Behavioral correlates of plasma sex hormones and their relationships with plasma lipids and lipoproteins in Japanese men. Atherosclerosis. 1997;130(1–2):37–44. doi: 10.1016/s0021-9150(96)06041-8. [DOI] [PubMed] [Google Scholar]
- 63.Kulkarni S.R., et al. Levels of plasma testosterone, antioxidants and oxidative stress in alcoholic patients attending de-addiction centre. Biol. Med. 2009;1(4):11–20. [Google Scholar]
- 64.Oldereid N.B., Rui H., Purvis K. Lifestyles of men in barren couples and their relationships to sperm quality. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992;43(1):51–57. doi: 10.1016/0028-2243(92)90243-r. [DOI] [PubMed] [Google Scholar]
- 65.Close C.E., Roberts P.L., Berger R.E. Cigarettes, alcohol and marijuana are related to pyospermia in infertile men. J. Urol. 1990;144(4):900–903. doi: 10.1016/s0022-5347(17)39618-0. [DOI] [PubMed] [Google Scholar]
- 66.von der P.B., et al. Testosterone, 5 alpha-dihydrotestosterone and cortisol in men with and without alcohol-related aggression. J. Stud. Alcohol. 2002;63(5):518–526. doi: 10.15288/jsa.2002.63.518. [DOI] [PubMed] [Google Scholar]
- 67.Sierksma A., et al. Effect of moderate alcohol consumption on plasma dehydroepiandrosterone sulfate, testosterone, and estradiol levels in middle-aged men and postmenopausal women: a diet-controlled intervention study. Alcohol Clin. Exp. Res. 2004;28(5):780–785. doi: 10.1097/01.alc.0000125356.70824.81. [DOI] [PubMed] [Google Scholar]
- 68.Frias J., et al. Effects of acute alcohol intoxication on pituitary-gonadal axis hormones, pituitary-adrenal axis hormones, beta-endorphin and prolactin in human adolescents of both sexes. Life Sci. 2000;67(9):1081–1086. doi: 10.1016/s0024-3205(00)00702-5. [DOI] [PubMed] [Google Scholar]
- 69.Iturriaga H., et al. Effects of abstinence on sex hormone profile in alcoholic patients without liver failure. J. Endocrinol. Invest. 1995;18(8):638–644. doi: 10.1007/BF03349782. [DOI] [PubMed] [Google Scholar]
- 70.Dai W.S., et al. The epidemiology of plasma testosterone levels in middle-aged men. Am. J. Epidemiol. 1981;114(6):804–816. doi: 10.1093/oxfordjournals.aje.a113251. [DOI] [PubMed] [Google Scholar]
- 71.Di Castelnuovo A., et al. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch. Intern. Med. 2006;166(22):2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 72.Guo H., et al. Effects of cigarette, alcohol consumption and sauna on sperm morphology. Zhonghua nan ke xue= Natl. J. Androl. 2006;12(3) 215-217, 221. [PubMed] [Google Scholar]
- 73.Goverde H.J., et al. 1995. Semen Quality and Frequency of Smoking and Alcohol Consumption: an Explorative Study. [PubMed] [Google Scholar]
- 74.Aitken R.J., et al. Oxidative stress and male reproductive health. Asian J. Androl. 2014;16(1):31. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bisht S., Dada R. Oxidative stress: major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci.-Scholar. 2017;9(3):420–447. doi: 10.2741/s495. [DOI] [PubMed] [Google Scholar]
- 76.Hosen M.B., et al. Oxidative stress induced sperm DNA damage, a possible reason for male infertility. Iran. J. Reproductive Med. 2015;13(9):525. [PMC free article] [PubMed] [Google Scholar]
- 77.Makker K., Agarwal A., Sharma R. Oxidative stress & male infertility. Indian J. Med. Res. 2009;129(4):357. [PubMed] [Google Scholar]
- 78.Aitken R.J., et al. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum. Reprod. 2010;25(10):2415–2426. doi: 10.1093/humrep/deq214. [DOI] [PubMed] [Google Scholar]
- 79.Lewis S.E., et al. The impact of sperm DNA damage in assisted conception and beyond: recent advances in diagnosis and treatment. Reprod. Biomed. Online. 2013;27(4):325–337. doi: 10.1016/j.rbmo.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Aitken R.J., Curry B.J. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxidants Redox Signal. 2011;14(3):367–381. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- 81.Allen N.E., et al. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002;13(4):353–363. doi: 10.1023/a:1015238102830. [DOI] [PubMed] [Google Scholar]
- 82.Medina D.L., Santisteban P. 2000. Thyrotropin-dependent Proliferation of in Vitro Rat Thyroid Cell Systems. [DOI] [PubMed] [Google Scholar]
- 83.Svartberg J., Midtby M., Bonaa K.H., Sundsfjord J., Joakimsen R.M., Jorde R. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. Eur. J. Endocrinol. 2003;149:145–152. doi: 10.1530/eje.0.1490145. [DOI] [PubMed] [Google Scholar]
- 84.Hsieh C.-c., et al. Predictors of sex hormone levels among the elderly: a study in Greece. J. Clin. Epidemiol. 1998;51(10):837–841. doi: 10.1016/s0895-4356(98)00069-9. [DOI] [PubMed] [Google Scholar]
- 85.Maneesh M., et al. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J. Physiol. Pharmacol. 2006;50(3):291. [PubMed] [Google Scholar]
- 86.Bao Giang K., Van Minh H., Allebeck P. Alcohol consumption and household expenditure on alcohol in a rural district in Vietnam. Glob. Health Action. 2013;6(1) doi: 10.3402/gha.v6i0.18937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poznyak V., et al. The world health organization's global monitoring system on alcohol and health. Alcohol Res. Curr. Rev. 2013;35(2):244–249. [PMC free article] [PubMed] [Google Scholar]
- 88.Lincoln M. Alcohol and drinking cultures in Vietnam: a review. Drug Alcohol Depend. 2016;159:1–8. doi: 10.1016/j.drugalcdep.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.