Abstract
Patient registries serve to overcome the research limitations inherent in the study of rare diseases, where patient numbers are typically small. Despite the value of real-world data collected through registries, adequate design and maintenance are integral to data quality. We aimed to describe an overview of the challenges in design, quality management, and maintenance of rare disease registries.
A systematic search of English articles was conducted in PubMed, Ovid Medline/Embase, and Cochrane Library. Search terms included “rare diseases, patient registries, common data elements, quality, hospital information systems, and datasets”. Inclusion criteria were any manuscript type focused upon rare disease patient registries describing design, quality monitoring or maintenance. Biobanks and drug surveillances were excluded.
A total of 37 articles, published between 2001 and 2021, met the inclusion criteria. Patient registries covered a wide range of disease areas and covered multiple geographical locations, with a predisposition for Europe. Most articles were methodological reports and described the design and setup of a registry. Most registries recruited clinical patients (92%) with informed consent (81%) and protected the collected data (76%). Whilst the majority (57%) collected patient-reported outcome measures, only few (38%) consulted PAGs during the registry design process. Few reports described details regarding quality management (51%) and maintenance (46%).
Rare disease patient registries are valuable for research and evaluation of clinical care, and an increasing number have emerged. However, registries need to be continuously evaluated for data quality and long-term sustainability to remain relevant for future use.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-023-02719-0.
Keywords: Rare disease, Patient registry, Data quality, Design, Maintenance
Background
Patient registries, organized systems that use observational study methods to collect uniform data to evaluate specified outcomes for a population defined by a particular disease or condition, are powerful tools to evaluate outcomes when randomized controlled trials are difficult to conduct [1]. Therefore, patient registries have the potential to solve one of the main challenges of research in rare diseases, where small sample sizes often lead to limited possibilities. With the low prevalence consequential to rare diseases, patient data are scarce and scattered. However, the rise of large online databases and data protection policies allow different centers and different countries to collaborate and share data to enhance research possibilities. Rare disease registries have become increasingly popular: more than 800 rare disease registries were listed in a December 2021 report of registries in or affiliated with Europe [2].
In line with the increasing number of patient registries for rare diseases, the European Union Committee of Experts on Rare Diseases (EUCERD) published recommendations in 2013 on patient registration and data collection. They emphasize interoperability with other registries through use of ontological coding language and minimum common data sets, involvement of patients in registry governance, and adaptability and sustainability for registry continuation [3]. However, with the exception that quality should be assured, no constructive descriptions on measures for quality were outlined, even though experts agree that registries should always be created using well-established quality criteria, and quality should be one of the most important elements in design and maintenance of a registry [4, 5]. Fortunately, many European registries do dedicate attention to data quality, but comprehensive quality assurance plans are not yet common practice [6].
In 2015, the Cross-border Patient Registries Initiative (PARENT) published specific methodological guidelines for governance of patient registries, delving deeper into the quality dimensions of a patient registry [7]. PARENT categorized the quality dimensions into governance, data quality, information quality, and ethical and legal issues regarding data privacy and protection. However, with the increasing number and widely varying types of (online) registries, guidelines on management and infrastructure on (re)use of data were necessary, and the FAIR principles were born in 2016 [8]. The four principles of findability, accessibility, interoperability and reusability (FAIR) aimed to navigate the expanding terrain of big data and electronic data capturing in research and have also been successfully applied and implemented in rare disease registries [8, 9]. The Italian National Center of Rare Diseases recognized the need for guidelines specifically for data quality management in rare disease patient registries. Together with other European countries, they published recommendations aligned with the FAIR principles in 2018, focusing not only on establishment of registries, but also on maintenance and sustainability [10].
The design, development, and establishment of a registry comprises a multitude of aspects: technicalities of coding language and data capturing programs; ethical and legal issues to ensure data privacy and protection whilst simultaneously enabling data sharing and reuse; governance and managerial aspects attending to the different interests of patients, clinicians, researchers, policy makers, pharmaceutical companies, and other stakeholders. Initiatives worldwide provide support to the development of rare disease registries. The “Building Consensus and Synergies for the European Union Registration of Rare Disease Patients” (EPIRARE) project aims to address regulatory, ethical and technical issues associated with the registration of rare disease patients in Europe, and the American Patient Registry Item Specifications and Metadata (PRISM) Library for rare diseases centralizes important questions and answers when creating a new registry [11, 12].
However, the establishment of a registry is just a first step, and although several guidelines have been published, the quality of patient registries remains a challenge, and data quality and bias are amongst the limitations of using patient registry data [13]. Utility, relevance, and sustainability are also amongst the issues that continuously need to be addressed. In this review, we aimed to describe the literature that pertains to the design, quality management, and maintenance of rare disease patient registries to learn from and improve existing registries, and to act as a basis for the setup of new registries.
Methods
A systematic search for English language publications in Medline (Ovid), Embase (Ovid), Pubmed, and Cochrane Library was conducted. Search items included “rare diseases”, “patient registries”, “common data elements”, “quality”, “hospital information systems” and “datasets”, in free text and keyword (MeSH) versions (See Additional File 1 for full search methods). There was no time frame limit on publication date of the literature search. After removing duplicates, studies were screened across two stages. In the first stage, all titles and abstracts of all studies were screened against the inclusion criteria. In the second stage, the potentially relevant studies underwent full text screening. Using Covidence systematic review software, one person (ICH) completed all screening [14].
Inclusion criteria:
No restriction on types of studies.
Subjects must be human and have a rare disease.
Study must involve a patient registry, defined as an organized system that uses observational study methods to collect uniform data to evaluate specified outcomes for a population defined by a particular disease or condition [1].
Study must include a description of a registry component such as setup/design, maintenance/sustainability, and/or quality monitoring/assurance.
Aim of the registry must at least include either surveillance or, gaining knowledge on the understanding of natural history, evolution, risk and/or outcomes of a specific disease.
Exclusion criteria:
Study only describing results with patient data extracted from a registry.
Study involves a registry that does not collect clinical data (e.g., biobanks).
Study involves a registry that is designed for the sole purpose to develop or evaluate (pharmacological) products.
The primary data points for extraction of this literature review were at least one description of:
-
(i)
Design or setup of a registry:
- use of informed consent (yes/no).
- use of a set of common data elements (yes/no).
- the (electronic) data capturing system/interface (e.g., REDCap).
- use of ontology/diagnostic codes (yes/no).
- collection of patient-reported outcomes (yes/no).
- involvement of patient advocacy groups (PAGs) in the design (yes/no).
- description of governance or structure of management (e.g., coordinating centers, dedicated working group, electoral selection, stakeholders).
- description of data protection and sharing, (e.g., data access policies, anonymization processes)
- method of patient recruitment (through clinic, PAGs, insurance records, pharmacy bills, voluntarily through social media/websites, other).
-
(ii)
Quality management or assurance of a registry (yes/no), such as quality assessment measures, audits, data entry training programs, site monitoring.
-
(iii)
Maintenance or sustainability of a registry (yes/no), such as long-term or specific end goals, funding, partnerships, or collaborations.
Secondary data points included general characteristics, including article type and aim, characteristics of the patient registry, year launched, country of coordinating entity, population description, inclusion criteria, number of registered patients at time of publications, aim of the registry, and type of data collected.
A data extraction template was created in Covidence systematic review software to collect relevant information according to the aforementioned datapoints [14]. The data were exported to Microsoft Excel 2016 for analysis [15]. Only data published in the articles were collected, with no approaches made to the registry developers and/or websites.
Results
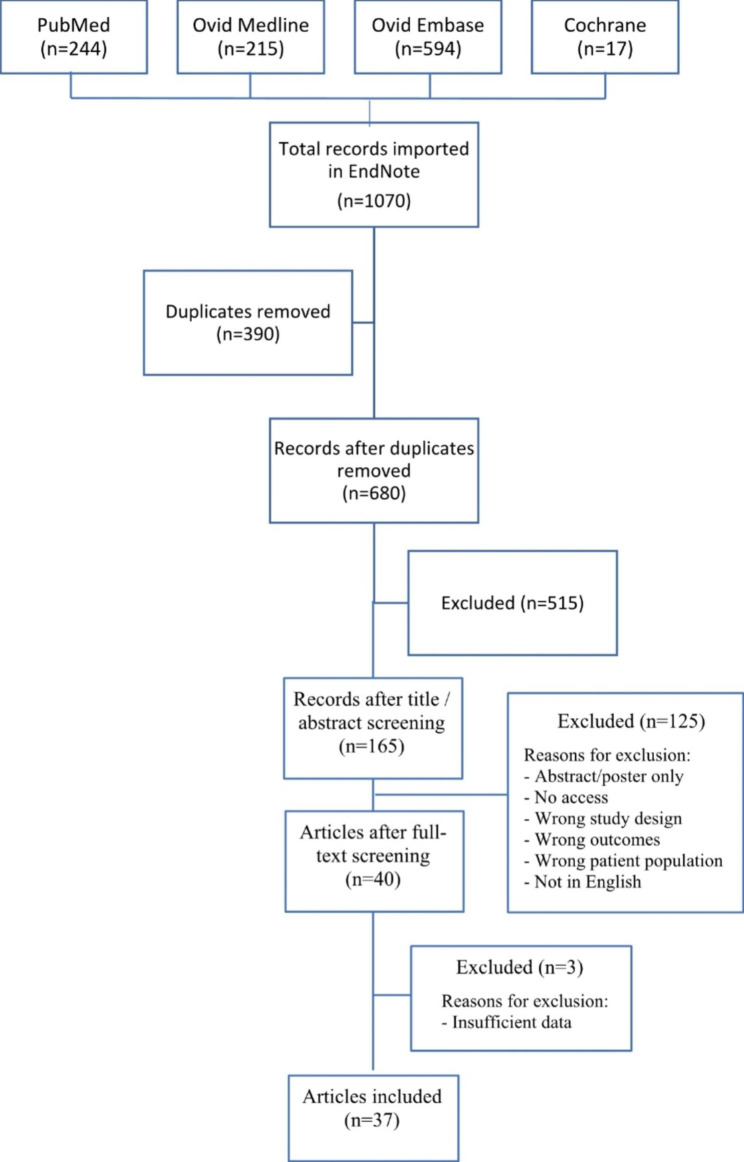
A literature search in the four databases resulted in a total of 1070 records. With the removal of 390 duplicates, 680 records were eligible for title and abstract screening. After title and abstract screening, 165 records were selected for full text screening. Forty articles were selected for inclusion, with subsequent exclusion of 3 articles due to insufficient data, resulting in a total of 37 articles [16–52] (Fig. 1).
Fig. 1.
PRISMA flow chart
The characteristics of the selected studies and respective registries are displayed in Table 1. Registries were launched between 2001 and 2021 with a geographical coverage of national (10/37, 27%), continental (limited to one continent; 8/37, 22%), or global (across multiple continents; 19%), and with most of their coordinating entities in the United States (8/37, 22%), United Kingdom (8/37, 22%), or Germany (7/37, 19%) (Figs. 2 and 3). Number of cases included at time of publication ranged from 0 to more than 30,000 cases. The time between the launch of the registry and the year of publication of the article was median 3 years (range 1–12 years). Most of the registries (23/37; 62%) covered a multitude of related diseases, and 14/37 (38%) registries focused on a single specific disease only. All registries included multiple participating centers, except one single center-based registry [21].
Table 1.
Characteristics of included articles and respective registries
| First author | Publication Year | Registry name | Disease area(s) | Country* | Coverage† | Launch | n‡ |
|---|---|---|---|---|---|---|---|
| Ali [16] | 2020 | European Registries for Rare Endocrine Conditions (EuRRECa) | Rare endocrine conditions | United Kingdom | Continental | 2018 | 5500 |
| Alvis [17] | 2020 | Colombian registry of haemophilia and other coagulopathies | Hemophilia and other coagulopathies | Colombia | National | 2015 | 4395 |
| Bassanese [18] | 2021 | European Rare Kidney Disease Registry (ERKReg) | Rare kidney diseases | Germany | Continental | 2019 | 7607 |
| Bellgard [19] | 2012 | Australian National Duchenne Muscular Dystrophy Registry | Duchenne and Becker’s muscular dystrophy | Australia | National | 2010 | / |
| Beswick [20] | 2016 | Cole-Reagins Registry for Sinonasal Cancer (CORSICA) | Malignancy of the paranasal sinuses | United States | National | / | / |
| Blankshain [21] | 2016 | The University of Illinois at Chicago (UIC) Neuro-Ophthalmology Registry | Neuro-ophthalmic diseases | United States | National | / | / |
| Chalmers [22] | 2017 | European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) registry | Bronchiectasis | United Kingdom | Continental | 2015 | > 8000 |
| Clarke [23] | 2011 | Fabry Outcome Survey (FOS) | Fabry disease | Sweden | Global | 2001 | 1616 |
| De Antonio [24] | 2019 | French myotonic dystrophy registry (DM-Scope) | MD | France | National | 2008 | 2970 |
| Eades-Perner [25] | 2007 | European registry of primary immunodeficiencies (ESID) | Primary immunodeficiencies | Germany | Continental | 2004 | 2386 |
| Evangelista [26] | 2016 | UK FSHD registry | FSHD | United Kingdom | Regional | 2012 | 518 |
| Feenstra [27] | 2006 | European Cytogeneticists Association Register of Unbalanced Chromosome Aberrations (ECARUCA) | Rare chromosome aberrations | United Kingdom | Continental | 2003 | ~ 4000 |
| Finkel [28] | 2020 | Registry of Patients with a Diagnosis of Spinal Muscular Atrophy (RESTORE Registry) | SMA | United States | Global | 2018 | 64 |
| Fischer [29] | 2014 | PedNet Haemophilia registry | Hemophilia | Netherlands | Global | 2004 | 1094 |
| Guien [30] | 2018 | French National FSHD Registry | FSHD | France | National | 2013 | 638 |
| Hilber(31) | 2012 | National Registry of MD and FSHD | MD and FSHD | United States | National | 2002 | 1611 |
| Jaussaud [32] | 2006 | The French ‘observatoire’ on Gaucher’s disease (FROG) | Gaucher’s disease | France | National | 2005 | 0 |
| Javaid [33] | 2016 | Rare UK Diseases Study (RUDY) platform | Rare disorders of the musculoskeletal system or blood vessels | United Kingdom | Regional | 2014 | 380 |
| Khatami [34] | 2016 | The European Narcolepsy Network (EU-NN) database | Narcolepsy and other hypersomnias | Switzerland | Continental | 2008 | 1079 |
| Kingswood [35] | 2014 | TuberOus SClerosis registry to increase disease Awareness (TOSCA) | Tuberous sclerosis complex | United Kingdom | Global | 2011 | 2216 |
| Mallbris [36] | 2007 | Swedish Hereditary Angioedema Registry (Sweha-Reg) | Hereditary angioedema | Sweden | National | 2007 | / |
| Marques [37] | 2020 | Portuguese inherited retinal dystrophies registry (IRD-PT) | Inherited retinal dystrophies | Portugal | National | 2017 | 1800 |
| Mercier [38] | 2019 | Desmoid Tumor Research Foundation (DTRF) Patient Registry | Desmoid tumors | United States | Global | 2017 | 329 |
| Ng [39] | 2011 | UK Primary Sjogren’s Syndrome Registry (UKPSSR) | Primary Sjogren’s Syndrome | United Kingdom | Regional | 2009 | 500 |
| Nurok [40] | 2010 | International lymphangioleiomyomatosis (LAM) Registry | Lymphangioleiomyomatosis | United States | Global | 2010 | / |
| Opladen [41] | 2016 | International Working Group on Neurotransmitter Related Disorders (iNTD) | Primary and secondary neurotransmitter-related disorders | Germany | Global | 2014 | 95 |
| Opladen [42] | 2021 | Unified European Registry for Inherited Metabolic Disease (U-IMD registry) | inherited metabolic diseases | Germany | Continental | 2019 | 1193 |
| Orbach [43] | 2021 | Paediatric Rare Tumours Network -European Registry (PARTNER) | Very rare pediatric tumors | Italy | Global | 2016 | / |
| Osara [44] | 2017 | Newborn Screening (NBS) Connect | Inherited metabolic disorders | United States | National | 2012 | 442 |
| Patel [45] | 2010 | North American Skull Base Society (NASBS) database | Skull base tumors treated with craniofacial surgery | United States | Continental | 2004 | / |
| Pechmann [46] | 2019 | SMA patient registry (SMArtCARE) | SMA | Germany | Regional | 2017 | / |
| Reincke [47] | 2006 | German Acromegaly Registry | Acromegaly | Germany | National | 2003 | 1543 |
| Roy [48] | 2015 | Belgian Neuromuscular Disease Registry | Neuromuscular diseases | Belgium | National | 2008 | 3424 |
| Seidel [49] | 2017 | Global Rare Fungal Infection Registry (FungiScope™) | Rare invasive fungal diseases | Germany | Global | 2003 | 794 |
| Spahr [50] | 2021 | MyeliNeuroGene Database | Rare diseases | Canada | National | 2011 | 1000 |
| Tingley [51] | 2020 | Canadian Inherited Metabolic Diseases Research Network (CNMDRN) database | Inherited metabolic diseases | Canada | National | 2012 | 798 |
| Viviani [52] | 2015 | European Cystic Fibrosis Society Patient Registry (ECFSPR) | Cystic fibrosis | United Kingdom | Continental | 2003 | > 30,000 |
Abbreviations: MD, myotonic dystrophy; FSHD, facioscapulohumeral muscular dystrophy; SMA, spinal muscular atrophy
*Country of coordinating entity
†Geographical coverage
‡Number of participants included in registry at time of publication
Fig. 2.
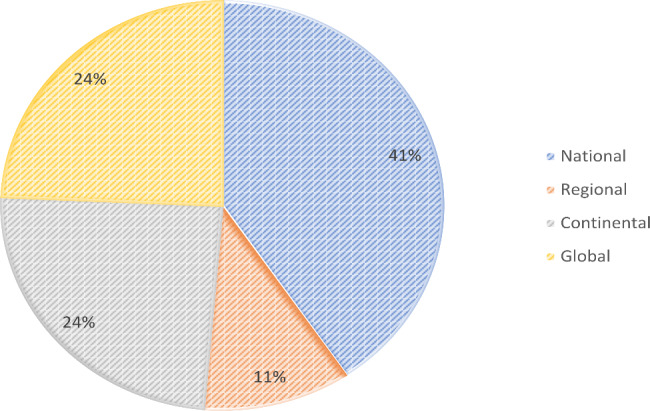
Geographical coverage of included registries
Fig. 3.
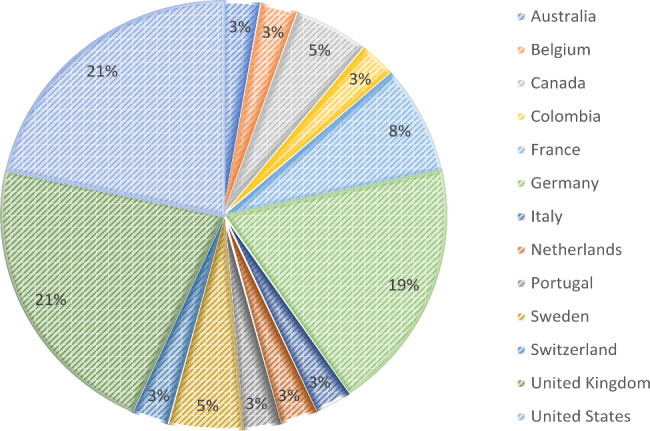
Countries of coordinating entities of included registries
The majority (36/37, 97%) of the articles described elements of the design, 19/37 (51%) described some form of quality management, and 17/37 (46%) had a description of registry maintenance. A summary of these main findings can be found in Table 2, and a detailed overview per registry in Additional File 2.
Table 2.
Summary of main findings on design, quality management, and maintenance of included registries
| Registry attribute | Frequency | ||||||
|---|---|---|---|---|---|---|---|
| Aims | N/37 | % | |||||
| Providing subjects for clinical studies | 12 | 32 | |||||
| Evaluating/improving clinical care | 9 | 24 | |||||
| Describing epidemiology | 8 | 22 | |||||
| Improving the understanding of natural history | 7 | 19 | |||||
| Evaluating/improving health-related outcomes | 6 | 16 | |||||
| Creating collaborations or clinical networks | 6 | 16 | |||||
| Describing clinical characteristics of disease | 5 | 14 | |||||
| Evaluating therapies or interventions | 3 | 8 | |||||
| Providing evidence for management decisions | 1 | 3 | |||||
| Unclear | 5 | 14 | |||||
| Recruitment method | |||||||
| Clinic | 34 | 92 | |||||
| PAGs | 6 | 16 | |||||
| Voluntarily* | 6 | 16 | |||||
| Other† | 1 | 3 | |||||
| Yes | No | Unclear | |||||
| N/37 | % | N/37 | % | N/37 | % | ||
| Informed consent | 30 | 81 | 2 | 5 | 5 | 14 | |
| Core data set | 8 | 22 | 27 | 73 | 2 | 5 | |
| Coding language | 9 | 24 | 24 | 65 | 4 | 11 | |
| PROMS collection | 21 | 57 | 3 | 8 | 13 | 35 | |
| PAG involvement | 14 | 38 | 19 | 51 | 4 | 11 | |
| Governance description | 21 | 57 | 11 | 30 | 5 | 14 | |
| Data security description | 28 | 76 | 6 | 16 | 3 | 8 | |
| Quality monitoring | 19 | 51 | 15 | 41 | 3 | 8 | |
| Maintenance description | 17 | 46 | 18 | 49 | 2 | 5 | |
| Funding description | 30 | 81 | 5 | 14 | 2 | 5 | |
Abbreviations: PROMS, patient-reported outcome measures; PAG, patient advocacy groups
* e.g., through social media, websites
† e.g., mandatory by law
Registry design
The aims of the registries, as reported, were providing subjects for clinical studies (32%), evaluating or improving clinical care (24%), describing epidemiology (22%) improving the understanding of natural history (19%), evaluating or improving health-related outcomes (16%), creating collaborations or clinical networks (16%), describing clinical characteristics of a disease (14%), evaluating therapies or interventions (8%), and providing evidence for management decisions (3%). Five registries had no clear description of their aim.
The type of data collected was mostly sociodemographic data (e.g., sex, date of birth or age, country of birth), diagnosis, medical history (e.g., signs and symptoms, date of onset, diagnostic tests, physical examination), care pathway (e.g., treatment center, number of visits, date of contact, physician), and treatment history (e.g., interventions, drugs). Other data collected were health-related outcomes (e.g., quality of life, disability, adverse events), research information (e.g., participation in trials), genetics, and biobank specimens.
Participants were recruited mostly through clinical care (34/37, 92%). For one national registry, all participants were registered by law through health care providers and health payers (e.g., insurance companies [17]. The majority of the registries collected informed consent (30/37, 81%) and described some form of data access, data sharing, or data protection strategies (28/37, 76%). The main findings on design description of the included registries are described in Table 2. In terms of development, 8/37 (22%) used a common or core data set and 9/37 (24%) used an ontological coding language such as the International Statistical Classification of Diseases (ICD) [53], Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) [54], Online Mendelian Inheritance in Man (OMIM) [55], Human Phenotype Ontology (HPO) [56], Human Genome Variation Society (HGVS) [57], or Orphanet Rare Disease Ontology (ORDO) [58]. Electronic data capture software programs were poorly reported, but most of the registries had an online web portal programmed using HTML and Javascript technologies, such as Research Electronic Data Capture (REDCap). In terms of governance, nearly half (16/37, 43%) of the registries had no or unclear descriptions on the included stakeholders or members of the governing body or structure of management. Whilst many (21/37, 57%) of the registries collected patient-reported outcome measures (PROM), only few (15/37, 38%) consulted PAGs of their respective disease areas during the design of the registry. PROMs collected in the registries included general quality of life (e.g., Pediatric Quality of Life Inventory [59], Short Form 36 [60], World Health Organisation Quality of Life questionnaire [61]), health-related quality of life (e.g., European Quality of Life-5 Dimension 5 Levels [62]), disease-specific quality of life (e.g., Acromegaly Quality of Life Questionnaire [63], Sinonasal Outcome Test-22 [64], Individualised Neuromuscular Quality of Life Questionnaire [65]), pain (e.g., McGill Pain Questionnaire [66], PainDetect [67]), patient experience (Hospital Anxiety and Depression Scale [68]), burden of disease (e.g., Zarit Burden Interview [69], Work Productivity and Activity Impairment Questionnaire [70], Nottingham Activities of Daily Living score activity [71]), sleep quality (e.g., Pittsburgh Sleep Quality Index [72], Epworth Sleepiness Scale [73]), and symptom assessment (e.g., Composite Autonomic Symptom Scale [74], Profile of Fatigue and Discomfort and Sicca Symptoms Inventory [75, 76]).
Registry quality
About half (19/37, 51%) of all registries mentioned some description of quality maintenance, but measures varied widely. The described quality measures could generally be divided into assessment at the system input level, during data collection, and assessment at the user level, before or after data collection. Measures of assessment at the system input level included automated quality assurance checks (e.g., error alerts for duplicate records, predefined ranges for numeric data, calculation checks for dates), closed-ended items, validating data types (string vs. numeric), and mandatory data elements or items. At the user level, before data collection, measures described were data input training and support, prerequisite credentials of capability or knowledge, and selection of patients through predefined inclusion and exclusion criteria. After data collection, measures such as periodical quality monitoring (or auditing or peer-reviewing), performed by specific members of the governing body, a dedicated data management team, or independent professionals were described. Of the 19 registries that described some form of quality maintenance, 14 registries mentioned quality monitoring at least once during the lifetime of the registry.
Registry maintenance
Similar to quality management, approximately half (17/37, 46%) of the included registries had a clear description of maintenance of the registry (Table 2). Descriptions of funding, long-term goals, or sustainability were considered descriptions of maintenance. Sources of funding were frequently described (30/37; 81%) and varied from federal or European Union authoritative bodies (18/30; 60%), private pharmaceutical or technical companies (12/30; 40%), research institutes, societies, or foundations (10/30; 33%), PAGs (3/30; 10%), and private philanthropy (1/30; 3%). Clear long-term or end-goals included descriptions such as predefined follow-up or recruitment periods and aims in gaining of understanding or developments of treatments. Only two registries mentioned the malleability of a registry, recognizing how it may evolve over time through feedback, new knowledge and technologies, and capacity to expand [38, 48]. Another interesting measure for maintenance and sustainability described was a financial compensation per registered patient, to encourage regular and continuous updating of data [25].
Discussion
The majority of registries included in the review registered clinical patients from all over the world, with the United States, United Kingdom, and Germany in the lead as coordinating entities. A wide variety of rare diseases were covered, with an apparent representation of (neuro)muscular diseases. Most registries were developed for the provision of participants for scientific research. Most patient registration used informed consent, and often data security policies were in place as per the General Data Protection Regulation (GDPR) of the European Union [77]. Only a minority of registries used ontological coding systems. Although patient-reported outcome measures were frequently collected by the registries in this review, PAGs had not equally been consulted during the developmental process. Elements on registry design were most frequently described, but less attention was paid to descriptions on quality management and maintenance.
The findings in this review highlight the imbalance between designing and sustaining a registry, challenged by difficulties in collecting quality data and the continued relevance of a registry. These results are in line with the findings of other similar studies [1, 6, 11, 12, 78, 79). With an average of only three years between launch of the registry and its publication, long-term functionality of the registries is questionable. Funding is frequently described in the included registries, with a large portion of the registries maintained by private pharmaceutical or technological companies. This may also influence maintenance, as this type of funding could contribute to greater registry visibility as part of regulated industry requirements [1]. Furthermore, registries with industry funding also frequently have policies in place to ensure long-term sustainability and are more likely to be of high quality (78). Although sustainability of a registry may be supported by adequate funding, it does not necessarily constitute longevity, as funding may not be renewed after a certain period of time.
There are several limitations to this study. Firstly, the inclusion criteria and definitions of specific datapoints might not always have been an accurate representation of the included registries. Certain datapoints, for example regarding a description on data access policies, might have been regarded as absent despite the respective registry still having these policies. Secondly, the selected search terms required studies describing the design, quality management, or maintenance of a rare disease patient registry. Some articles, including those describing a registry and its collected data, which focused primarily on their results rather than on the framework of the registry, might have been missed due to absence of important key words. Therefore, the strict inclusion criteria limited the results to articles with sufficient detail regarding methodology. On the other hand, this highlights the importance of complete and detailed descriptions of methodological aspects when publishing the introduction of a registry. Lastly, as this is a qualitative study in nature, no meta-analysis of the collected data could be conducted.
The rise of many new rare disease registries and a lacking focus on improving and sustaining existing ones leads to the production of data that is not always usable nor shareable. One of the reasons to increase data quality in existing rare disease patient registries is to reduce duplicate efforts and production of excessive data. Several measures have been developed to improve these issues, such as promoting interoperability between registries with the sets of common and domain-specific data elements of the European Commission Joint Research Center (JRC) (80, 81). Another measure to tackle the different forms of data collection is through the use of standardized coding languages, such as ICD, SNOMED CT, and ORDO [53, 54, 58]. The use of ontologies is not only important to promote interoperability, but also to facilitate the technological developments to link registries and facilitate overarching research access (82). Importantly, of the registries included in this review, only a minority have implemented these measures. Furthermore, although these measures are a refinement of quality data collection and in accordance with the FAIR principles, which do facilitate maintenance and sustainability, these measures are nevertheless also part of registry design. Although the JRC common and domain-specific data sets are good suggestions to promote interoperability, registries generally want to collect additional disease-specific or patient-reported data and, ideally, collect data through several points of follow-up over a long period of time.
Concerningly, a survey on the main activities and methodological, technical and regulatory issues of European rare disease registries conducted more than a decade ago presented findings not dissimilar to the findings in this review [83]. Quality assurance and sustainability are amongst the key issues addressed, and despite the guidelines and recommendations published in the past 10 years, are still issues that newly established registries face. Therefore, the important question is how to improve existing registries. Possibilities include periodical quality monitoring, recurrent evaluation of user feedback, implementation of coding languages, monetary incentives and mandatory items to promote complete data entry, assessments of data capturing, revision of research aims, and long-term sources of funding. However, application of multiple adequate maintenance strategies remains an important issue, with several registries describing the challenges of maintaining a registry, such as ensuring continuous data entry, assuring quality, and securing further funding [35, 37, 39, 48]. It is important to recognize that once a registry has been developed and collecting data, its design is not set in stone, and continuous evaluations and efforts to improve are necessary. Nevertheless, the limited number of registries describing any strategies on sustainability and maintenance over a longer term, and the few that recognize the challenges demonstrate how this area is still largely undermined. Therefore, strategies and protocols on maintenance and management should play an equally large role as structure design when developing a registry.
The present review illustrates that the current registries are still largely behind in complying with the 2013 guidelines on patient registration and data collection, and the field of rare disease registries has made limited improvements in the past decade. Only a minority of the registries promoted interoperability through the use of coding language and minimum common data sets, there was little involvement of patients in registry governance, and few considered sustainability strategies for registry continuation [3].
Conclusions
With this review we described that rare disease patient registries commonly describe the elements of registry design but pay less attention to quality management and maintenance. These important finding highlight the challenges of developing and maintaining a high quality and sustainable registry. Considerations during design should be made as to what is ideal and what is feasible. Lastly, recommendations on measures to improve existing databases to remain relevant and valuable for rare disease research are warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- EUCERD
European Union Committee of Experts on Rare Diseases
- EPIRARE
“Building Consensus and Synergies for the European Union Registration of Rare Disease Patients”
- FAIR
Findability, Accessibility, Interoperability and Reusability
- GDPR
General Data Protection Regulation
- HPO
Human Phenotype Ontology
- ICD
International Statistical Classification of Diseases
- JRC
Joint Research Center
- OMIM
Online Mendelian Inheritance in Man
- ORDO
Orphanet Rare Disease Ontology
- REDCap
Research Electronic Data Capture
- PARENT
Cross-border Patient Registries Initiative
- SNOMED
CT Systematized Nomenclature of Medicine Clinical Terms
Authors’ contributions
ICH, IALMvR, IB, MT, and SKK contributed to study conception and design. ICH collected the data. ICH and IALMvR analyzed and interpreted the data. ICH was the main contributor to the writing of the manuscript. ICH, IALMvR, IB, MT, and SKK contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
Professor King is generously supported in his role as an Academic Paediatric Surgeon by The Royal Children’s Hospital Foundation and the Federal Government. Dr Isabel Hageman is supported by the Academy Ter Meulen grant of the Academy Medical Sciences Fund of the Royal Netherlands Academy of Arts & Sciences (KNAW).
Data availability
The data generated, used and/or analyzed during the current study are available from the corresponding author on request.
Declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Isabel C. Hageman, Email: isabel.hageman@radboudumc.nl
Iris A.L.M. van Rooij, Email: iris.vanrooij@radboudumc.nl
Ivo de Blaauw, Email: ivo.deblaauw@radboudumc.nl.
Misel Trajanovska, Email: misel.trajanovska@mcri.edu.au.
Sebastian K. King, Email: sebastian.king@rch.org.au
References
- 1.Gliklich RE, Leavy MB, Dreyer NA. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville, MD, United States: Agency for Healthcare Research and Quality (US) September 2020. Report No.: 19(20)-EHC020. [PubMed]
- 2.Orphanet. Rare Disease Registries in Europe. 2021. Available from: http://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf.
- 3.European Union Committee of Experts on Rare Diseases: Recommendations on European Reference Networks for rare diseases, European Union Committee of Experts on Rare Diseases; 2013. Available from: https://health.ec.europa.eu/publications/eucerd-core-recommendations-rare-disease-patient-registration-and-data-collection_en.
- 4.Kodra Y, Posada de la Paz M, Coi A, Santoro M, Bianchi F, Ahmed F, et al. Data Quality in Rare Diseases Registries. Adv Exp Med Biol. 2017;1031:149–64. doi: 10.1007/978-3-319-67144-4_8. [DOI] [PubMed] [Google Scholar]
- 5.Cavero-Carbonell C, Gras-Colomer E, Guaita-Calatrava R, López-Briones C, Amorós R, Abaitua I, et al. Consensus on the criteria needed for creating a rare-disease patient registry. A Delphi study. J Public Health. 2015;38(2):e178–e86. doi: 10.1093/pubmed/fdv099. [DOI] [PubMed] [Google Scholar]
- 6.Taruscio D, Vittozzi L, Choquet R, Heimdal K, Iskrov G, Kodra Y, et al. National registries of rare diseases in Europe: an overview of the current situation and experiences. Public Health Genomics. 2015;18(1):20–5. doi: 10.1159/000365897. [DOI] [PubMed] [Google Scholar]
- 7.Zalatel M, Krolj M. Methodological guidelines and recommendations for efficient and rational governance of patient registries; 2015. Available from: https://health.ec.europa.eu/system/files/2016-11/patient_registries_guidelines_en_0.pdf.
- 8.Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3:160018. doi: 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groenen KHJ, Jacobsen A, Kersloot MG, Dos Santos Vieira B, van Enckevort E, Kaliyaperumal R, et al. The de novo FAIRification process of a registry for vascular anomalies. Orphanet J Rare Dis. 2021;16(1):376. doi: 10.1186/s13023-021-02004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodra Y, Weinbach J, Posada-de-la-Paz M, Coi A, Lemonnier SL, van Enckevort D et al. Recommendations for Improving the Quality of Rare Disease Registries. Int J Environ Res Public Health. 2018;15(8). [DOI] [PMC free article] [PubMed]
- 11.European Commission. Building Consensus and Synergies for the EU Registration of Rare Disease Patients [EPIRARE] Project. 2014. Available from: https://webgate.ec.europa.eu/chafea_pdb/health/projects/20101202/summary.
- 12.Richesson R, Shereff D, Andrews J. PRISM Library: Patient Registry item specifications and Metadata for Rare Diseases. J Libr Metadata. 2010;10(2–3):119–35. doi: 10.1080/19386389.2010.506385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richesson R, Vehik K. Patient registries: Utility, Validity and Inference. In: de la Posada M, Groft SC, editors. Rare Diseases Epidemiology. Dordrecht: Springer Netherlands; 2010. pp. 87–104. [DOI] [PubMed] [Google Scholar]
- 14.Covidence . Covidence. v2903 b2820a43 ed. Melbourne, Australia: Veritas Health Innovation; 2022. [Google Scholar]
- 15.Microsoft Excel . v.16.62 ed. Redmond. Washington, United States: Microsoft Corporation; 2022. [Google Scholar]
- 16.Ali SR, Bryce J, Tan LE, Hiort O, Pereira AM, van den Akker ELT, et al. The EuRRECa Project as a model for Data Access and Governance Policies for Rare Disease Registries that collect clinical outcomes. Int J Environ Res Public Health. 2020;17(23):1–12. doi: 10.3390/ijerph17238743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvis LF, Sanchez P, Acuna L, Escobar G, Linares A, Solano MH, et al. National registry of haemophilia and other coagulopathies: a multisector initiative in the colombian Health System. Haemophilia. 2020;26(6):e254–e61. doi: 10.1111/hae.14138. [DOI] [PubMed] [Google Scholar]
- 18.Bassanese G, Wlodkowski T, Servais A, Heidet L, Roccatello D, Emma F, et al. The european rare kidney Disease Registry (ERKReg): objectives, design and initial results. Orphanet J Rare Dis. 2021;16(1):251. doi: 10.1186/s13023-021-01872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellgard MI, Macgregor A, Janon F, Harvey A, O’Leary P, Hunter A, et al. A modular approach to disease registry design: successful adoption of an internet-based rare disease registry. Hum Mutat. 2012;33(10):E2356–66. doi: 10.1002/humu.22154. [DOI] [PubMed] [Google Scholar]
- 20.Beswick DM, Holsinger FC, Kaplan MJ, Fischbein NJ, Hara W, Colevas AD, et al. Design and rationale of a prospective, multi-institutional registry for patients with sinonasal malignancy. Laryngoscope. 2016;126(9):1977–80. doi: 10.1002/lary.25996. [DOI] [PubMed] [Google Scholar]
- 21.Blankshain KD, Moss HE. Research Registries: a Tool to Advance understanding of Rare Neuro-Ophthalmic Diseases. J Neuroophthalmol. 2016;36(3):317–23. doi: 10.1097/WNO.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalmers JD, Crichton M, Goeminne PC, Loebinger MR, Haworth C, Almagro M, et al. The european multicentre bronchiectasis audit and research collaboration (EMBARC): experiences from a successful ERS clinical research collaboration. Breathe. 2017;13(3):180–92. doi: 10.1183/20734735.005117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke JT, Giugliani R, Sunder-Plassmann G, Elliott PM, Pintos-Morell G, Hernberg-Ståhl E, et al. Impact of measures to enhance the value of observational surveys in rare diseases: the Fabry Outcome Survey (FOS) Value Health. 2011;14(6):862–6. doi: 10.1016/j.jval.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 24.De Antonio M, Dogan C, Daidj F, Eymard B, Puymirat J, Mathieu J, et al. The DM-scope registry: a rare disease innovative framework bridging the gap between research and medical care. Orphanet J Rare Dis. 2019;14(1):122. doi: 10.1186/s13023-019-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eades-Perner AM, Gathmann B, Knerr V, Guzman D, Veit D, Kindle G, et al. The european internet-based patient and research database for primary immunodeficiencies: results 2004-06. Clin Exp Immunol. 2007;147(2):306–12. doi: 10.1111/j.1365-2249.2006.03292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evangelista T, Wood L, Fernandez-Torron R, Williams M, Smith D, Lunt P, et al. Design, set-up and utility of the UK facioscapulohumeral muscular dystrophy patient registry. J Neurol. 2016;263(7):1401–8. doi: 10.1007/s00415-016-8132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feenstra I, Fang J, Koolen DA, Siezen A, Evans C, Winter RM, et al. European Cytogeneticists Association Register of Unbalanced chromosome aberrations (ECARUCA); an online database for rare chromosome abnormalities. Eur J Med Genet. 2006;49(4):279–91. doi: 10.1016/j.ejmg.2005.10.131. [DOI] [PubMed] [Google Scholar]
- 28.Finkel RS, Day JW, De Vivo DC, Kirschner J, Mercuri E, Muntoni F, et al. RESTORE: a prospective multinational Registry of patients with genetically confirmed spinal muscular atrophy - rationale and Study Design. J Neuromuscul Dis. 2020;7(2):145–52. doi: 10.3233/JND-190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer K, Ljung R, Platokouki H, Liesner R, Claeyssens S, Smink E, et al. Prospective observational cohort studies for studying rare diseases: the european PedNet Haemophilia Registry. Haemophilia. 2014;20(4):e280–6. doi: 10.1111/hae.12448. [DOI] [PubMed] [Google Scholar]
- 30.Guien C, Blandin G, Lahaut P, Sanson B, Nehal K, Rabarimeriarijaona S, et al. The French National Registry of patients with facioscapulohumeral muscular dystrophy. Orphanet J Rare Dis. 2018;13(1):218. doi: 10.1186/s13023-018-0960-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilbert JE, Kissel JT, Luebbe EA, Martens WB, McDermott MP, Sanders DB, et al. If you build a rare disease registry, will they enroll and will they use it? Methods and data from the National Registry of Myotonic dystrophy (DM) and Facioscapulohumeral muscular dystrophy (FSHD) Contemp Clin Trials. 2012;33(2):302–11. doi: 10.1016/j.cct.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaussaud R. The French ‘observatoire’ on Gaucher’s disease. Eur J Intern Med. 2006;17 Suppl:S6-8. [DOI] [PubMed]
- 33.Javaid MK, Forestier-Zhang L, Watts L, Turner A, Ponte C, Teare H, et al. The RUDY study platform - a novel approach to patient driven research in rare musculoskeletal diseases. Orphanet J Rare Dis. 2016;11(1):1–9. doi: 10.1186/s13023-016-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khatami R, Luca G, Baumann CR, Bassetti CL, Bruni O, Canellas F, et al. The european Narcolepsy Network (EU-NN) database. J Sleep Res. 2016;25(3):356–64. doi: 10.1111/jsr.12374. [DOI] [PubMed] [Google Scholar]
- 35.Kingswood JC, Bruzzi P, Curatolo P, de Vries PJ, Fladrowski C, Hertzberg C, et al. TOSCA - first international registry to address knowledge gaps in the natural history and management of tuberous sclerosis complex. Orphanet J Rare Dis. 2014;9:182. doi: 10.1186/s13023-014-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mallbris L, Nordenfelt P, Björkander J, Lindfors A, Werner S, Wahlgren CF. The establishment and utility of Sweha-Reg: a swedish population-based registry to understand hereditary angioedema. BMC Dermatol. 2007;7:6. doi: 10.1186/1471-5945-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marques JP, Carvalho AL, Henriques J, Murta JN, Saraiva J, Silva R. Design, development and deployment of a web-based interoperable registry for inherited retinal dystrophies in Portugal: the IRD-PT. Orphanet J Rare Dis. 2020;15(1):304. doi: 10.1186/s13023-020-01591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercier KA, Walsh DM. The initiation, design, and establishment of the desmoid Tumor Research Foundation Patient Registry and Natural History Study. Rare Tumors. 2019;11:2036361319880978. doi: 10.1177/2036361319880978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng WF, Bowman SJ, Griffiths B. United Kingdom Primary Sjogren’s Syndrome Registry–a united effort to tackle an orphan rheumatic disease. Rheumatology (Oxford) 2011;50(1):32–9. doi: 10.1093/rheumatology/keq240. [DOI] [PubMed] [Google Scholar]
- 40.Nurok M, Eslick I, Moss F, Carvalho CRR, Costabel U, D’Armiento J, et al. The International LAM Registry: a component of an innovative web-based clinician, researcher, and patient-driven rare disease research platform. Lymphatic Res Biology. 2010;8(1):81–7. doi: 10.1089/lrb.2009.0028. [DOI] [PubMed] [Google Scholar]
- 41.Opladen T, Cortès-Saladelafont E, Mastrangelo M, Horvath G, Pons R, Lopez-Laso E, et al. The International Working Group on Neurotransmitter related Disorders (iNTD): a worldwide research project focused on primary and secondary neurotransmitter disorders. Mol Genet Metab Rep. 2016;9:61–6. doi: 10.1016/j.ymgmr.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opladen T, Gleich F, Kozich V, Scarpa M, Martinelli D, Schaefer F, et al. U-IMD: the first unified european registry for inherited metabolic diseases. Orphanet J Rare Dis. 2021;16(1):95. doi: 10.1186/s13023-021-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orbach D, Ferrari A, Schneider DT, Reguerre Y, Godzinski J, Bien E, et al. The european Paediatric Rare Tumours Network - European Registry (PARTNER) project for very rare tumors in children. Pediatr Blood Cancer. 2021;68(S4):e29072. doi: 10.1002/pbc.29072. [DOI] [PubMed] [Google Scholar]
- 44.Osara Y, Coakley K, Devarajan A, Singh RH. Development of newborn screening connect (NBS connect): a self-reported patient registry and its role in improvement of care for patients with inherited metabolic disorders. Orphanet J Rare Dis. 2017;12(1):132. doi: 10.1186/s13023-017-0684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel SG. Internet-based multi-institutional clinical research: a new method to conduct and manage quality of life studies. Skull Base. 2010;20(1):23–6. doi: 10.1055/s-0029-1242981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pechmann A, König K, Bernert G, Schachtrup K, Schara U, Schorling D, et al. SMArtCARE - A platform to collect real-life outcome data of patients with spinal muscular atrophy. Orphanet J Rare Dis. 2019;14(1):18. doi: 10.1186/s13023-019-0998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reincke M, Petersenn S, Buchfelder M, Gerbert B, Skrobek-Engel G, Franz H, et al. The german Acromegaly Registry: description of the database and initial results. Exp Clin Endocrinol Diabetes. 2006;114(9):498–505. doi: 10.1055/s-2006-948313. [DOI] [PubMed] [Google Scholar]
- 48.Roy AJ, Van den Bergh P, Van Damme P, Doggen K, Van Casteren V. Early stages of building a rare disease registry, methods and 2010 data from the belgian neuromuscular Disease Registry (BNMDR) Acta Neurol Belg. 2015;115(2):97–104. doi: 10.1007/s13760-014-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seidel D, Duran Graeff LA, Vehreschild MJGT, Vehreschild JJ, Ziegler M, Hamprecht A, et al. FungiScopeTM-Global Emerg Fungal Infect Registry Mycoses. 2017;60(8):508–16. doi: 10.1111/myc.12631. [DOI] [PubMed] [Google Scholar]
- 50.Spahr A, Rosli Z, Legault M, Tran LT, Fournier S, Toutounchi H, et al. The LORIS MyeliNeuroGene rare disease database for natural history studies and clinical trial readiness. Orphanet J Rare Dis. 2021;16(1):328. doi: 10.1186/s13023-021-01953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tingley K, Lamoureux M, Pugliese M, Geraghty MT, Kronick JB, Potter BK, et al. Evaluation of the quality of clinical data collection for a pan-canadian cohort of children affected by inherited metabolic diseases: lessons learned from the canadian inherited metabolic Diseases Research Network. Orphanet J Rare Dis. 2020;15(1):89. doi: 10.1186/s13023-020-01358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viviani L, Zolin A, Mehta A, Olesen HV. The european cystic Fibrosis Society Patient Registry: Valuable lessons learned on how to sustain a disease registry. Orphanet J Rare Dis. 2015;9(1):81. doi: 10.1186/1750-1172-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization. International Classification of Diseases, Eleventh Revision (ICD-11). Geneva:World Health Organization; 2019/2021.
- 54.De Silva TS, MacDonald D, Paterson G, Sikdar KC, Cochrane B. Systematized nomenclature of medicine clinical terms (SNOMED CT) to represent computed tomography procedures. Comput Methods Programs Biomed. 2011;101(3):324–9. doi: 10.1016/j.cmpb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online mendelian inheritance in man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33(Database issue):D514–7. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson PN, Köhler S, Bauer S, Seelow D, Horn D, Mundlos S. The human phenotype ontology: a tool for annotating and analyzing human hereditary disease. Am J Hum Genet. 2008;83(5):610–5. doi: 10.1016/j.ajhg.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Callenberg KM, Santana-Santos L, Chen L, Ernst WL, De Moura MB, Nikiforov YE, et al. Clinical implementation and validation of Automated Human Genome Variation Society (HGVS) nomenclature system for Next-Generation sequencing-based assays for Cancer. J Mol Diagn. 2018;20(5):628–34. doi: 10.1016/j.jmoldx.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Vasant D, Chanas L, Malone J, Hanauer M, Olry A, Jupp S, et al. editors. ORDO: an ontology connecting rare disease, epidemiology and genetic data. Proceedings of ISMB; 2014: researchgate. net.
- 59.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306(6890):1437–40. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The World Health Organization Quality of Life Assessment (WHOQOL) Development and general psychometric properties. Soc Sci Med. 1998;46(12):1569–85. doi: 10.1016/S0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 62.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 63.Webb SM, Prieto L, Badia X, Albareda M, Catalá M, Gaztambide S, et al. Acromegaly Quality of Life Questionnaire (ACROQOL) a new health-related quality of life questionnaire for patients with acromegaly: development and psychometric properties. Clin Endocrinol (Oxf) 2002;57(2):251–8. doi: 10.1046/j.1365-2265.2002.01597.x. [DOI] [PubMed] [Google Scholar]
- 64.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–54. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 65.Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL) Neurology. 2007;68(13):1051–7. doi: 10.1212/01.wnl.0000257819.47628.41. [DOI] [PubMed] [Google Scholar]
- 66.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 67.Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–20. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 68.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 69.Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden interview: a New Short Version and Screening Version. Gerontologist. 2001;41(5):652–7. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 70.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4(5):353–65. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 71.Lincoln NB, Gladman JR. The extended activities of Daily Living scale: a further validation. Disabil Rehabil. 1992;14(1):41–3. doi: 10.3109/09638289209166426. [DOI] [PubMed] [Google Scholar]
- 72.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 73.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 74.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012;87(12):1196 – 201. [DOI] [PMC free article] [PubMed]
- 75.Bowman S, Booth D, Platts R. Measurement of fatigue and discomfort in primary Sjogren’s syndrome using a new questionnaire tool. Rheumatology. 2004;43(6):758–64. doi: 10.1093/rheumatology/keh170. [DOI] [PubMed] [Google Scholar]
- 76.Bowman SJ, Booth DA, Platts RG, Field A, Rostron J. Validation of the Sicca symptoms Inventory for clinical studies of Sjögren’s syndrome. J Rhuematol. 2003;30(6):1259–66. [PubMed] [Google Scholar]
- 77.Hoofnagle CJ, van der Sloot B, Borgesius FZ. The European Union general data protection regulation: what it is and what it means. Inform Commun Technol Law. 2019;28(1):65–98. doi: 10.1080/13600834.2019.1573501. [DOI] [Google Scholar]
- 78.Coi A, Santoro M, Villaverde-Hueso A, Lipucci Di Paola M, Gainotti S, Taruscio D, et al. The Quality of Rare Disease Registries: Evaluation and Characterization. Public Health Genomics. 2016;19(2):108 − 15. [DOI] [PubMed]
- 79.Santoro M, Coi A, Lipucci Di Paola M, Bianucci AM, Gainotti S, Mollo E, et al. Rare disease registries classification and characterization: a data mining approach. Public Health Genomics. 2015;18(2):113 − 22. [DOI] [PubMed]
- 80.European Commission. Set of common data elements for rare disease registration. European Commission Joint Research Center; 2019. Available from: https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/CDS/EU_RD_Platform_CDS_Final.pdf.
- 81.Abaza H, Kadioglu D, Martin S, Papadopoulou A, dos Santos Vieira B, Schaefer F, et al. Domain-Specific Common Data Elements for Rare Disease Registration: Conceptual Approach of a European Joint Initiative Toward Semantic Interoperability in Rare Disease Research. JMIR Med Inform. 2022;10(5):e32158. [DOI] [PMC free article] [PubMed]
- 82.Sernadela P, González-Castro L, Carta C, van der Horst E, Lopes P, Kaliyaperumal R, et al. Linked Registries: Connecting Rare Diseases Patient Registries through a Semantic Web Layer. Biomed Res Int. 2017;2017:8327980. [DOI] [PMC free article] [PubMed]
- 83.Taruscio D, Gainotti S, Mollo E, Vittozzi L, Bianchi F, Ensini M, et al. The current situation and needs of rare disease registries in Europe. Public Health Genomics. 2013;16(6):288 − 98. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated, used and/or analyzed during the current study are available from the corresponding author on request.