Abstract
The effect of a single dose of ceftazidime on circulating concentrations of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) in a rat model of sepsis was studied. IL-6 concentrations were significantly elevated (100 to 200 times the baseline) 6 h after ceftazidime administration in both septic and nonseptic (control) rats. TNF-α concentrations increased significantly in nonseptic (∼40 times the baseline) rats but not septic (∼2 to 3 times the baseline) rats. Ceftazidime administration was not associated with an increase in endotoxin concentrations. These findings suggest that ceftazidime modulation of proinflammatory cytokine concentrations may be independent of its antimicrobial properties.
Sepsis is a diffuse inflammatory disorder that is mediated by the activity of multiple mediators, including cytokines. Infections with gram-negative organisms remain the major source of sepsis because of their virulent nature. Although antimicrobials are the primary treatment options for sepsis, they also have the potential to worsen the inflammatory state in some patients (5, 7). This has been attributed to the release of large amounts of endotoxin (i.e., lipopolysaccharide) as the result of bacterial killing by bactericidal and cell wall-active antimicrobials (4, 6, 9). Endotoxin is known to enhance the expression of proinflammatory cytokines (e.g., tumor necrosis factor alpha [TNF-α], interleukin-1 [IL-1], and IL-6) that have been implicated in the pathogenesis of sepsis and multiple-organ-dysfunction syndrome (1, 3). Ceftazidime is a third-generation cephalosporin that has excellent activity against gram-negative aerobic bacteria (13). Ceftazidime, however, may increase the concentration of proinflammatory cytokines, such as TNF-α and IL-6, by liberating endotoxin from the cell wall of gram-negative bacteria (12, 14). Some antimicrobials may also modulate the function of the immune system by a direct effect on immune cells and/or the expression of key inflammatory mediators, including cytokines. Several agents, including some β-lactams, quinolones, and macrolides, have been found to alter polymorphonuclear leukocyte migration and/or phagocytosis, which may ultimately affect the outcome for infected patients (16). Additionally, some of these antimicrobials have been shown to regulate the activity of the cytokine network, independently of their effects on endotoxin release. The direct effect of ceftazidime on proinflammatory cytokine (e.g., TNF-α and IL-6) expression in vivo has not been evaluated. In this study, an animal model of sepsis was used to evaluate the effect of ceftazidime on circulating concentrations of IL-6 and TNF-α.
The effect of ceftazidime administration on cytokine concentrations was evaluated in three groups of rats. Group A was comprised of rats that had undergone cecal ligation and puncture (CLP) (n = 6) and group B included healthy rats (n = 6). Both groups A and B received ceftazidime. Group C consisted of rats that underwent CLP surgery (n = 6) but did not receive ceftazidime. The effect of ceftazidime administration on endotoxin levels was assessed in CLP rats (n = 3) (group D) and healthy rats (n = 3) (group E). All surgical procedures were performed under aseptic techniques. Healthy rats only underwent carotid artery and femoral vein cannulation for drug administration and blood sampling. Ceftazidime was administered as a single intravenous bolus (30 mg/kg of body weight) at time zero. Blood samples (0.25 ml each) were drawn prior to ceftazidime administration (i.e., at baseline) for the measurement of both cytokines and 3 and 6 h after ceftazidime administration for TNF-α and IL-6 determinations, respectively. The amount of blood drawn was replaced with an equal volume of lactated Ringer's solution. From the group C rats that did not receive ceftazidime, a blood sample was drawn 14 h after surgery for IL-6 and TNF-α determinations. Additional samples were collected 20 and 17 h after CLP for TNF-α and IL-6 determinations, respectively. Among groups D and E, blood samples for the measurement of endotoxin concentrations were drawn before and 6 h after ceftazidime administration. IL-6 and TNF-α concentrations were measured using rat enzyme-linked immunosorbent assay kits. Endotoxin levels were determined using the chromogenic Limulus amoebocyte lysate assay. The absolute change (from baseline) in TNF-α and IL-6 concentrations 3 and 6 h after ceftazidime administration, respectively, was compared using a one-way analysis of variance followed by the Student-Newman-Keuls multiple-comparison test. An unpaired t test was used to compare the endotoxin concentrations of CLP and healthy rats treated with ceftazidime. All statistical tests were performed following log transformation of the data, and statistical significance was assumed when P was ≤0.05.
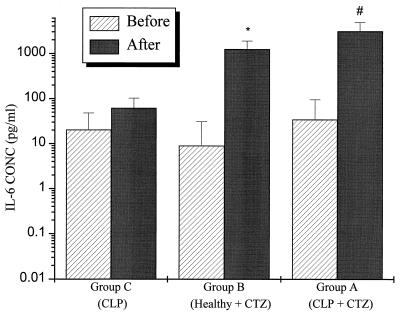
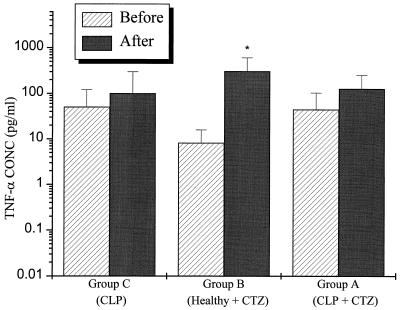
The change in systemic concentrations of IL-6 (relative to baseline) was significantly higher at 6 h after ceftazidime administration in the group A and group B rats than in the group C rats, which did not receive ceftazidime (P < 0.001) (Fig. 1). TNF-α concentrations at 3 h were also significantly higher in group B than in group C (P < 0.05) (Fig. 2). Although the TNF-α concentrations were increased in group A, the increase did not achieve statistical significance. Low but detectable levels of endotoxin were found in both CLP and healthy rats before ceftazidime administration, 2.68 ± 0.45 and 1.96 ± 0.17 endotoxin units (EU)/ml, respectively. The concentration did not change significantly after ceftazidime administration in either group: 2.38 ± 0.57 and 1.81 ± 1.07 EU/ml (P > 0.05).
FIG. 1.
Systemic IL-6 concentrations are significantly elevated after ceftazidime administration to CLP rats (group A) or healthy rats (group B) but not in the CLP rats that did not receive ceftazidime (group C) (P < 0.001). ∗, P < 0.001 versus CLP; #, P < 0.001 versus CLP; CTZ, ceftazidime.
FIG. 2.
Healthy rats that received a 30-mg/kg ceftazidime intravenous bolus (group B) had a significantly greater change in concentrations of TNF-α than did CLP rats that did not receive CTZ (group C). ∗, P < 0.05 versus CLP. An increase in TNF-α concentrations was also noted in the CLP rats that received ceftazidime (group A). This increase, however, did not achieve statistical significance. CTZ, ceftazidime.
Peak serum cytokine concentrations after CLP are generally lower than those observed after the administration of lipopolysaccharide, but they usually remain elevated longer (17). In this study, CLP was associated with small increments in IL-6 and TNF-α concentrations 14 h after the insult. In contrast, the concentrations of these cytokines were significantly increased after ceftazidime administration; IL-6 concentrations were significantly elevated 6 h after the administration of ceftazidime in group A (3,070 ± 1,709 pg/ml) and group B (1,245 ± 650 pg/ml) rats (Fig. 1). These levels exceeded the concentrations observed in the control group of rats (group C), which did not receive ceftazidime, by up to 30-fold. Serum TNF-α concentrations also appear to be affected by ceftazidime administration, although the magnitude of this effect was less than that seen for IL-6 (Fig. 2). The reason for the different effects of ceftazidime on IL-6 and TNF-α could be attributed to a difference in time profiles of the rise and fall of TNF-α and IL-6 concentrations, a more pronounced activity of ceftazidime on IL-6 biosynthesis, and/or a dysregulation of immune cells that has been previously observed during the course of sepsis or after endotoxin treatment of blood in vitro (10, 15). This may result in a less optimal response mounted against the inflammatory trigger by the host than that observed under nonseptic conditions. Since IL-6 concentrations peak at ∼20 h after CLP (unpublished observations), we measured IL-6 concentration before administering ceftazidime (i.e., 14 h after surgery), and 6 h thereafter. The time at which serum TNF-α concentrations peak after surgery or drug administration, however, can be quite variable and sometimes difficult to detect, since they decline rapidly after reaching the maximum value. Since TNF-α concentration elevations usually precede the attainment of IL-6 peak concentrations, we believe that our measured TNF-α values at 3 h after ceftazidime administration are representative of but perhaps not precisely the “peak” response. Several in vitro and in vivo studies have found that antimicrobials with high affinity for the penicillin-binding protein class 3 (PBP 3) have a marked potential to activate cytokine transcription (8, 11). Ceftazidime is known to bind to and inhibit PBP 3 and therefore may potentially affect the proinflammatory cytokine cascade. In this study, the ceftazidime modulation of cytokine concentrations appears to be endotoxin independent. This is suggested by the findings in nonseptic rats (i.e., sham and healthy). In addition, CLP rats that did not receive ceftazidime did not demonstrate a similar increase in IL-6, as compared to septic rats treated with ceftazidime. Since endotoxin concentrations did not change within 6 h after ceftazidime administration, the effect of ceftazidime on IL-6 and TNF-α in this study is not likely to be due to endotoxin release. The mechanism of these effects may involve a direct effect on transcription. Charak and coworkers have suggested that the myelosuppressive effect of ceftazidime on bone marrow progenitor cells is mediated by the release of TNF-α, since monoclonal antibodies against TNF-α completely inhibited ceftazidime-induced myelosuppression in vitro (2). This finding supports our hypothesis that ceftazidime administration modulates the concentration of proinflammatory cytokines in vivo. Follow-up studies will be needed to evaluate the mechanism(s) and the clinical significance of these observations.
REFERENCES
- 1.Bone R C. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 2.Charak B S, Brown E G, Mazumder A. Role of granulocyte colony-stimulating factor in preventing ceftazidime-induced myelosuppression in vitro. Bone Marrow Transplant. 1995;15:749–755. [PubMed] [Google Scholar]
- 3.Damas P, Canivet J L, de Groote D, Vrindts Y, Albert A, Franchimont P, Lamy M. Sepsis and serum cytokine concentrations. Crit Care Med. 1997;25:405–412. doi: 10.1097/00003246-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Eng R H, Smith S M, Fan-Havard P, Ogbara T. Effect of antibiotics on endotoxin release from gram-negative bacteria. Diagn Microbiol Infect Dis. 1993;16:185–189. doi: 10.1016/0732-8893(93)90109-k. [DOI] [PubMed] [Google Scholar]
- 5.Gelfand J A, Elin R J, Berry F W, Jr, Frank M M. Endotoxemia associated with the Jarisch-Herxheimer reaction. N Engl J Med. 1976;295:211–213. doi: 10.1056/NEJM197607222950409. [DOI] [PubMed] [Google Scholar]
- 6.Holzheimer R G. The significance of endotoxin release in experimental and clinical sepsis in surgical patients—evidence for antibiotic-induced endotoxin release? Infection. 1998;26:77–84. doi: 10.1007/BF02767765. [DOI] [PubMed] [Google Scholar]
- 7.Hopkin D A. Frapper fort ou frapper doucement: a gram-negative dilemma. Lancet. 1978;ii:1193–1194. [PubMed] [Google Scholar]
- 8.Jackson J J, Kropp H. Beta-lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J Infect Dis. 1992;165:1033–1041. doi: 10.1093/infdis/165.6.1033. [DOI] [PubMed] [Google Scholar]
- 9.Mock C N, Jurkovich G J, Dries D J, Maier R V. Clinical significance of antibiotic endotoxin-releasing properties in trauma patients. Arch Surg. 1995;130:1234–1240. doi: 10.1001/archsurg.1995.01430110092017. [DOI] [PubMed] [Google Scholar]
- 10.Munoz C, Carlet J, Fitting C, Misset B, Bleriot J P, Cavaillon J M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Investig. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prins J M, Kuijper E J, Mevissen M L C M, Speelman P, van Deventer S J H. Release of tumor necrosis factor alpha and interleukin 6 during antibiotic killing of Escherichia coli in whole blood: influence of antibiotic class, antibiotic concentration, and presence of septic serum. Infect Immun. 1995;63:2236–2242. doi: 10.1128/iai.63.6.2236-2242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prins J M, van Agtmael M A, Kuijper E J, van Deventer S J, Speelman P. Antibiotic-induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J Infect Dis. 1995;172:886–891. doi: 10.1093/infdis/172.3.886. [DOI] [PubMed] [Google Scholar]
- 13.Rains C P, Bryson H M, Peters D H. Ceftazidime. An update of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1995;49:577–617. doi: 10.2165/00003495-199549040-00008. [DOI] [PubMed] [Google Scholar]
- 14.Schneider C M, Huzly D, Vetter C, von Specht B U, Daschner F D. Tumor necrosis factor alpha and interleukin 6 release induced by antibiotic killing of Pseudomonas aeruginosa and Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1997;16:467–471. doi: 10.1007/BF02471914. [DOI] [PubMed] [Google Scholar]
- 15.Setrakian J C, Yee J, Christou N V. Reduced tumor necrosis factor alpha production in lipopolysaccharide-treated whole blood from patients in the intensive care unit. Arch Surg. 1994;129:187–192. doi: 10.1001/archsurg.1994.01420260083011. [DOI] [PubMed] [Google Scholar]
- 16.Van Vlem B, Vanholder R, De Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–291. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]
- 17.Villa P, Sartor G, Angelini M, Sironi M, Conni M, Gnocchi P, Isetta A M, Grau G, Buurman W, van Tits L J, Ghezzi P. Pattern of cytokines and pharmacomodulation in sepsis induced by cecal ligation and puncture compared with that induced by endotoxin. Clin Diagn Lab Immunol. 1995;2:549–553. doi: 10.1128/cdli.2.5.549-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]