Abstract
Background
Hypercholesterolaemia is a significant risk factor for cardiovascular diseases. Isoflavones may be effective in improving hypercholesterolaemia.
Objectives
To assess the effects of isoflavones for hypercholesterolaemia.
Search methods
We searched the following databases: The Cochrane Library (Issue 9, 2012), MEDLINE, EMBASE, Chinese BioMedical Database and China National Knowledge Infrastructure (all to September 2012).
Selection criteria
We considered randomized controlled clinical trials in hypercholesterolaemic participants comparing isoflavones versus placebo, or soy isolated protein added with isoflavones versus soy isolated protein alone.
Data collection and analysis
Two review authors independently abstracted relevant population and intervention characteristics. We resolved any disagreements through discussion, or if required by a third party. We assessed the risk of bias of trials against key criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias.
Main results
We included five randomized trials (208 participants, 104 in the intervention group and 104 in the control group). Interventions ranged from three to six months. Four trials reported results in non‐Asian populations published in English. One trial reported results in Chinese people published in Chinese. Overall, the risk of bias of included trials was high or unclear. There were no outcome data on death from any cause, morbidity, complications, health‐related quality of life and costs. Two trials reported adverse effects, including gastrointestinal discomfort (bloating and constipation) and an increased number of hot flushes. None of the trials found serious adverse events. There was a slight significant effect on triglycerides in favour of isoflavones when compared with placebo (mean difference (MD) ‐0.46 mmol/L (95% confidence interval (CI) ‐0.84 to ‐0.09; P = 0.02; 52 participants; 2 trials). No statistically significant effects on total cholesterol, low‐density lipoprotein cholesterol and high‐density lipoprotein cholesterol were shown in favour of isoflavones.
Authors' conclusions
We found no evidence for effects of isoflavones on patient‐important outcomes or lowering of cholesterol levels in people with hypercholesterolaemia. Our findings have to be interpreted with caution due to high or unclear risk of bias in several risk of bias domains, and low number of participants in trials.
Plain language summary
Isoflavones for hypercholesterolaemia
Hypercholesterolaemia is the presence of high levels of cholesterol in the blood. In humans, hypercholesterolaemia is often due to high low‐density‐lipoprotein (LDL)‐cholesterol levels, the so‐called 'bad' cholesterol. People with hypercholesterolaemia have a higher risk of developing cardiovascular diseases such as heart attacks or strokes. Isoflavones, which are chemicals in plants similar to phyto‐oestrogen, may be helpful in improving hypercholesterolaemia. Soy and red clover are rich sources of isoflavones. Asian people consume more isoflavones from their regular diet than Western people.
To assess the effects of isoflavones for the treatment of hypercholesterolaemia, we examined five randomized controlled trials of isoflavones or soy protein containing isoflavones. The trials lasted three to six months and involved 208 participants. There were no outcome data on death from any cause, cardiovascular events such as heart attack or stroke, morbidity, complications, health‐related quality of life and costs. Two trials reported adverse effects, including gastrointestinal discomfort (bloating and constipation) and an increased number of hot flushes. They observed no serious adverse events. In our included studies, we found no cholesterol‐lowering effect of isoflavones. However, the quality of the included trials had some considerable limitations and the number of the participants was low. Further higher‐quality and rigorously performed studies on patient‐important outcome measures such as cardiovascular diseases and health‐related quality of life are required.
Summary of findings
for the main comparison.
| Isoflavones for hypercholesterolaemia in adults | ||||
|
Patient or population: postmenopausal women with hypercholesterolaemia Settings: outpatients or not specified Intervention: isoflavones or soy protein‐containing isoflavones Comparison: placebo or soy protein | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Cardiovascular events | See comment | See comment | See comment | Not investigated |
| Death from any cause | See comment | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | See comment | Not investigated |
|
Adverse events [follow‐up: 3 to 6 months] |
See comment | 208 (5) |
⊕⊕⊝⊝ lowa | No serious adverse events reported |
|
Low‐density lipoprotein (LDL) cholesterol [follow‐up: 3 to 6 months] |
See comment | 132 (3) |
⊕⊝⊝⊝ very lowb | No statistically significant differences between groups |
| Costs | See comment | See comment | See comment | Not investigated |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
aDue to serious risk of bias, low number of participants and studies, short duration of treatment.
bDue to serious risk of bias, serious indirectness, low number of participants and studies, short duration of treatment.
Background
Description of the condition
Hypercholesterolaemia is the presence of abnormally high levels of cholesterol in the blood, and it has reached epidemic proportions worldwide. In 2008, Australasia, North America and Western Europe reported the highest serum total cholesterol (TC) concentrations worldwide (Farzadfar 2011). The regional mean of the serum TC levels was 5.24 mmol/L (95% confidence interval (CI) 5.08 to 5.39) for men and 5.23 mmol/L (95% CI 5.03 to 5.43) for women, which means that approximately 50% of the population in these countries had hypercholesterolaemia (Farzadfar 2011). In developing countries, the number of people with hypercholesterolaemia has risen since the early 1980s, particularly in Asia (Farzadfar 2011). For example, in 2001, the prevalence of hypercholesterolaemia was 33% in middle‐aged and older‐aged Chinese people (He 2004).
Hypercholesterolaemia is a significant risk factor for cardiovascular diseases (CVDs). In humans, hypercholesterolaemia is often due to high serum low‐density‐lipoprotein (LDL)‐cholesterol levels, which are generally atherogenic. Atherosclerosis is the pathological basis for most CVDs and is often characterized as the progressive accumulation of lipids in the vessel wall. The subsequent rate of atherogenesis is positively associated with the severity of associated risk factors including serum cholesterol levels (Badimon 2011). High levels of serum cholesterol particles may increase vascular superoxide production in the blood, alter endothelium‐dependent vasodilation (widening of the cavity in a blood vessel), and then promote the formation of atherosclerosis plaques, and finally facilitate CVDs. Thus, lowering the blood TC levels may be of benefit to people with CVDs. Several trials have demonstrated that treatment of high cholesterol levels played the most important role on more than half the decline in coronary heart disease mortality in the last decades (Ford 2007; Laatikainen 2005).
Description of the intervention
Isoflavones are plant‐based chemicals related to phyto‐oestrogen, which are found in soy and red clover. Asian people consume more isoflavones from their regular diet than Western people. Daily isoflavones intake in Chinese and Japanese people was estimated to be 15 to 50 mg/day (Kang 2010; Liu 2010; Shimazu 2010), whereas it was likely to be less than 3 mg/day in European and US populations (Chun 2007; Keinan 2002).
Hypercholesterolaemia can be modified by dietary changes including lowering cholesterol by diet or supplementing the diet with certain nutrients such as isoflavones. Isoflavones have many benefits for human health, such as improving osteoporosis and menopausal syndromes. In the 2000s, several systematic reviews and meta‐analyses assessed the effects of isoflavones on lipid profiles in humans (Taku 2007; Taku 2008; Weggemans 2003; Zhan 2005; Zhuo 2004). However, the conclusions of these reviews were inconsistent, which may be due to the different isoflavones used, dose levels, styles and durations of the trials, and the various initial serum lipid concentrations of the participants. Natural food (including soy, tofu and red clover), soy protein containing isoflavones, isoflavones extracts and single compounds of isoflavones such as genistein, daidzein or glycetein were often supplemented with isoflavones. Other components such as soy protein may have contributed to and distorted the effects of isoflavones on the serum concentrations of total and LDL‐cholesterol (Taku 2007; Taku 2008).
Adverse effects of the intervention
Isoflavones have a safe side‐effect profile with moderately elevated rates of abdominal bloating (Albertazzi 2005; Garrido 2006), gastralgia (stomach pain) (Nikander 2004) and back pain (Albertazzi 2005). The rare, but serious, adverse effects include endometrial hyperplasia (Unfer 2004) and recurrence of breast cancer (Nikander 2004).
How the intervention might work
The activities of the phyto‐oestrogen component of isoflavones may play an important role in the effects of isoflavones on serum lipid profiles. Clinical intervention studies have demonstrated that blood isoflavones metabolites (such as equol) levels were associated with the clinical effects of isoflavones, including several positive outcomes for vasomotor symptoms, increasing bone mineral density, and decreasing the cardiovascular risk factors LDL‐cholesterol and C‐reactive protein (Jackson 2011). The gut bacterial biotransformation of daidzein in certain individuals produces equol. There is a higher frequency of 'equol‐producers' in Asian populations (50% to 60%) than in Western populations (25% to 30%) (Setchell 2010). Several clinical studies have concluded that isoflavones may produce better clinical effects in equol‐producers than in non‐equol‐producers (Duncan 2000; Kreijkamp 2005; Setchell 2002).
The effects of isoflavones on serum lipid profiles may also contribute to their activities, independent of phyto‐oestrogen. Cellular and animal studies demonstrated that genistein can act as an inhibitor of protein tyrosine kinase. In pancreatic beta cells, genistein acutely stimulates insulin secretion through a cyclic adenosine monophosphate (cAMP)‐dependent protein kinase pathway (Liu 2006).
Why it is important to do this review
There were some limitations of previously published meta‐analyses on this topic. First, only one meta‐analysis searched databases such as MEDLINE, EMBASE, The Cochrane Library, Chinese BioMedical Database and China National Knowledge Infrastructure (CNKI) (Taku 2008). Other meta‐analyses only reviewed studies published in PubMed and only those published in English (Taku 2007; Weggemans 2003; Zhan 2005; Zhuo 2004). Second, all of these meta‐analyses focused on the short‐term effects of isoflavones on lipid profiles (Taku 2007; Taku 2008; Weggemans 2003; Zhan 2005; Zhuo 2004). Some clinical trials that focused on the long‐term effects of isoflavones on postmenopausal osteoporosis also evaluated blood lipid changes. However, the meta‐analyses seldom analyzed these clinical trials. Third, only one meta‐analysis mentioned adverse effects of isoflavones interventions (Taku 2008). We undertook this review to resolve these limitations and to provide a better understanding of the effects of isoflavones for hypercholesterolaemia. In the present meta‐analysis, we screened the studies through PubMed, EMBASE, The Cochrane Library, CNKI and CBM, and primarily attempted to evaluate the long‐term effects of isoflavones for hypercholesterolaemia.
Objectives
To assess the effects of isoflavones for hypercholesterolaemia.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled clinical trials.
Types of participants
Adults (aged 18 years or older) with hypercholesterolaemia.
Diagnostic criteria
A fasting blood TC greater than 5.20 mmol/L (200 mg/dL) is indicative of hypercholesterolaemia. We also accepted other definitions. We excluded people with familial hypercholesterolaemia and secondary hypercholesterolaemia.
We planned to subject diagnostic criteria to a sensitivity analysis.
Types of interventions
We investigated the following comparisons of intervention verus control/comparator where the same letters indicate direct comparisons.
Interventions
(a) Natural food, isoflavones extracts, single compound of isoflavones with total isoflavones amount of 15 mg/day or greater.
(b) Soy protein containing isoflavones with total isoflavone amount of 15 mg/day or greater.
Comparators
(a1) Placebo or other food with total isoflavones amount less than 5 mg/day.
(b1) Soy protein containing no or low isoflavones with total isoflavones amount less than 5 mg/day.
Types of outcome measures
Primary outcomes
Death from any cause.
Cardiovascular events (both fatal and non‐fatal events, including myocardial infarction, angina pectoris, stroke, peripheral arterial disease, sudden death).
LDL‐cholesterol levels.
Secondary outcomes
Adverse events.
Health‐related quality of life.
TC levels.
Other lipid levels (including high‐density‐lipoprotein (HDL) cholesterol, triglycerides, apolipoprotein A and B).
Costs.
Timing of outcome measurement
The minimum treatment duration was three months. If one study had several results over time, we used the longest time point for overall effect analysis and used other time points for subgroup analysis (i.e. six months or less and longer than six months). For cardiovascular events, intervention duration had to be at least six months.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception to the specified date.
The Cochrane Library (Issue 9, 2012).
MEDLINE (to September 2012).
EMBASE (to September 2012).
Chinese BioMedical Database (to September 2012).
CNKI (to September 2012).
We also searched databases of ongoing trials (ClinicalTrials.gov (www.clinicaltrials.gov/), Current Controlled Trials metaRegister (www.controlled‐trials.com/), the EU Clinical Trials register (www.clinicaltrialsregister.eu/).
For detailed search strategies see Appendix 1 (searches were not older than six months at the moment the final review draft was checked into the Cochrane Information and Management System for editorial approval).
If we had detected additional key words of relevance during any of the electronic or other searches, we would have modified the electronic search strategies to incorporate these terms. We included studies published in any language.
Searching other resources
We tried to identify additional studies by searching the reference lists of included trials, systematic reviews, meta‐analyses and health technology assessment reports found as a result of the searches.
Data collection and analysis
Selection of studies
Two review authors (YQ, KN) independently scanned the abstract, title or both sections of every record retrieved to determine the studies to be assessed further. We investigated all potentially relevant articles as full text. A third party resolved any differences in opinion. If resolving disagreement was not possible, we added the article to those 'awaiting assessment' and we contacted authors for clarification. We attach a PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐chart of study selection (Figure 1) (Liberati 2009).
1.
Study flow diagram.
Data extraction and management
For studies that fulfilled inclusion criteria, two review authors (YQ, KN) independently abstracted relevant population and intervention characteristics using standard data extraction templates (for details see Characteristics of included studies; Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9) with any disagreements resolved by discussion, or, if required, by a third party.
1. Overview of study populations.
|
Characteristic Study ID |
Intervention and comparator | [N] Screened / eligible | [N] Randomised | [N] Safety | [N] ITT | [N] Finishing study | [%] Randomised finishing study |
| Aubertin‐Leheudre 2008 | Isoflavones (70 mg) | ‐ | ‐ | ‐ | ‐ | 8 | N/A |
| Placebo | ‐ | ‐ | ‐ | 11 | N/A | ||
| total: | ‐ | ‐ | ‐ | 19 | N/A | ||
| Dewell 2002 | Isoflavones (150 mg) | ‐ | 20 | ‐ | 20 | 20 | 100 |
| Maltodextrin with 10% caramel | 16 | ‐ | 16 | 16 | 100 | ||
| total: | 36 | ‐ | 36 | 36 | 100 | ||
| Gardner 2001 | Isoflavones (80 mg) | ‐ | 34 | ‐ | ‐ | 31 | 91.2 |
| Trace amounts of isoflavones | 34 | ‐ | ‐ | 33 | 97.1 | ||
| total: | 68 | ‐ | ‐ | 64 | 94.1 | ||
| Mackey 2000 | Isoflavones (65 mg) | ‐ | ‐ | ‐ | ‐ | 25 | N/A |
| Less than 4 mg isoflavones | ‐ | ‐ | ‐ | 24 | N/A | ||
| total: | 54 | ‐ | ‐ | 49 | 90.7 | ||
| Wang 2005 | Isoflavones (158 mg) | ‐ | 20 | ‐ | 20 | 20 | 100 |
| Placebo | 20 | ‐ | 20 | 20 | 100 | ||
| total: | 40 | ‐ | 40 | 40 | 100 | ||
| Total | All interventions | ‐ | 104 | ||||
| All controls | ‐ | 104 | |||||
| All interventions and comparators | ‐ | 208 | |||||
"‐" denotes not reported.
ITT: intention‐to‐treat; N/A: not applicable.
We sent an email request to authors of published studies to enquire whether they would answer questions regarding their trials. Appendix 10 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the study authors of the article, if required.
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, we tried to maximize yield of information by simultaneous evaluation of all available data.
Assessment of risk of bias in included studies
Two review authors (YQ, KN) assessed each trial independently. Possible disagreements were resolved by consensus, or by consultation with a third party. In cases of disagreement, we consulted the rest of the group and made a judgement based on consensus.
We assessed risk of bias using The Cochrane Collaboration's tool (Higgins 2011). We used the following bias criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment.
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We judged risk of bias criteria as 'low risk', 'high risk' or 'unclear risk' and use individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We present a 'Risk of bias' figure and a 'Risk of bias' summary figure.
We assessed the impact of individual bias domains on study results at endpoint and study levels.
Measures of treatment effect
We expressed dichotomous data as odds ratios (OR) or risk ratios (RR) with 95% CIs. We expressed continuous data as mean differences (MD) with 95% CI.
Unit of analysis issues
We took into account the level at which randomization occurred, such as cross‐over trials, cluster‐randomized trials and multiple observations for the same outcome.
Dealing with missing data
We obtained relevant missing data from authors, if feasible and carefully performed evaluation of important numerical data such as screened, randomized participants as well as intention‐to‐treat (ITT), as‐treated and per‐protocol (PP) populations. We investigated attrition rates, for example, drop‐outs, losses to follow up and withdrawals and critically appraised issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward (LOCF)).
Assessment of heterogeneity
In the event of substantial clinical or methodological or statistical heterogeneity, we did not report study results as meta‐analytically pooled effect estimates.
We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We specifically examined heterogeneity employing the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011).
Had we found heterogeneity, we planned to determine potential reasons for it by examining individual study and subgroup characteristics.
We expected the following characteristics to introduce clinical heterogeneity.
Asian and non‐Asian populations (due to their different genotype, diet style, and equol metabolism phenotype).
Degree of hypercholesterolaemia.
Previous cardiovascular events.
Other cardiovascular risk factors.
Assessment of reporting biases
We planned to use funnel plots in case we included more than 10 studies for a given outcome to assess small study bias. There are a number of explanations for the asymmetry of a funnel plot (Sterne 2001) and we planned to interpret results carefully (Lau 2006).
Data synthesis
We primarily summarized low risk of bias data by means of a random‐effects model. We performed statistical analyses according to the statistical guidelines referenced in the most recent version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses of the primary outcome parameter(s) (see above) and investigate interaction.
Asian and non‐Asian population.
Males and females.
Hypercholesterolaemia with or without hypertriglyceridaemia.
Equol and non‐equol producers.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size.
Restricting the analysis to published studies.
Restricting the analysis taking into account risk of bias, as specified above.
Restricting the analysis to very long or large studies to establish how much they dominate the results.
Restricting the analysis to studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
We also tested the robustness of the results by repeating the analysis using different measures of effect size (RR, OR, etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
Results of the search
The initial search using the electronic search strategies listed in Appendix 1 yielded 9859 records. We did not identify any unpublished studies. After we removed duplicates from different databases, we kept potentially relevant articles for further assessment. We identified 45 articles that could not be excluded by screening of the title, the abstract or both. Further investigation of the full‐text articles revealed five included studies (six publications). We found no ongoing studies. We prepared a PRISMA flow diagram to describe the articles found from our searches (Figure 1). The review authors found no differences in their independent assessment of the eligibility of the studies.
Missing data
We sent emails to study authors to obtain relevant missing data, only one author replied and provided data. On the basis of this information, the study could be included (Aubertin‐Leheudre 2008).
Dealing with duplicate publications/companion papers
One included study (Aubertin‐Leheudre 2008) had one companion paper (Aubertin‐Leheudre 2007a), and the publication presenting cholesterol data was used. Other included studies had no duplicate publications or companion papers.
Included studies
We included five randomized controlled trials (five publications) in this review (see Characteristics of included studies for further details). Four of the included trials (five articles) were published in English. One trial (one publication) was published in Chinese (Wang 2005). Of the five trials, two were conducted in the US (Dewell 2002;Gardner 2001), and the other three trials were conducted in Canada (Aubertin‐Leheudre 2008), Australia (Mackey 2000) and China (Wang 2005). All trials employed a parallel design. Three studies compared isoflavones versus placebo (Aubertin‐Leheudre 2008;Dewell 2002;Wang 2005), and the other two studies compared soy isolated protein plus isoflavones versus soy isolated protein alone or plus trace amounts of isoflavones.
Participants
The five trials included 208 participants with hypercholesterolaemia, 100 in US populations (Dewell 2002; Gardner 2001) and 108 in other countries. Sample size of the trials ranged from 19 to 64 participants per trial. The age of the participants ranged from 40 to 83 years. All participants were postmenopausal women. Two trials included outpatients (Gardner 2001;Wang 2005); others did not specify trial settings. Table 2, Appendix 2 and Appendix 3 provide detailed information.
Interventions
There were small variations in the formulations, dosages, duration of treatments and control interventions in the included trials among the isoflavones tested (see Appendix 2 and Characteristics of included studies). Three trials used isoflavones capsules (Aubertin‐Leheudre 2008;Wang 2005) or tablets (Dewell 2002), and compared these with placebo. The other two trials used soy isolated protein plus isoflavones powers, and compared them to soy isolated protein plus less than 4 mg isoflavones/day. Treatment duration of isoflavones therapy was six months in two trials (Aubertin‐Leheudre 2008;Dewell 2002) and three months in one trial (Wang 2005). The duration of soy protein containing isoflavones treatment was 12 weeks in two trials (Gardner 2001; Mackey 2000).
Outcomes
One trial reported serum TC, HDL‐cholesterol, triglycerides and non‐HDL‐cholesterol levels, but did not report serum LDL‐cholesterol levels (Dewell 2002). The other four trials reported plasma (Aubertin‐Leheudre 2008), serum (Gardner 2001; Mackey 2000 ) or blood (Wang 2005) lipids and lipoprotein levels, including TC, triglycerides, LDL‐ and HDL‐cholesterol. One trial assessed LDL‐cholesterol by calculation according to the method of Friedewald (Gardner 2001), and another trial may have used the same method (Mackey 2000). No trial reported outcomes on cardiovascular events, such as myocardial infarction, angina pectoris or stroke, peripheral arterial disease or sudden death. One trial reported details of adverse events in the soy isolated protein added with isoflavones group (see Appendix 8), including gastrointestinal discomfort (bloating and constipation) and an increased number of hot flushes (Gardner 2001). One trial found no adverse effects (Aubertin‐Leheudre 2008). Three trials did not report adverse events in the intervention groups (Dewell 2002;Mackey 2000;Wang 2005). The other outcomes that were reported included body weight, body mass index (BMI), androstenedione, estrone, oestradiol, follicle‐stimulating hormone, luteinizing hormone, thyroid‐stimulating hormone, sex hormone binding globulin (SHBG), osteocalcin and bone‐specific alkaline phosphatase. All trials measured outcomes at the end of treatment. No trial reported health‐related quality of life or socioeconomic costs.
Study details
Two trials had a run‐in period (Gardner 2001;Mackey 2000). No studies stopped before the scheduled end.
Publication details
Four studies were published in English and one study in Chinese (Wang 2005). None of the studies reported on commercial funding. Three studies reported non‐commercial funding (Aubertin‐Leheudre 2008;Dewell 2002;Wang 2005). All studies were published in peer‐reviewed journals (see Characteristics of included studies).
Excluded studies
We excluded 39 (reasons for exclusion are listed under Characteristics of excluded studies). The major exclusion reason was that not all participants were hypercholesterolaemic. Other exclusion reasons included less than three months' intervention time, improper control or improper intervention.
Risk of bias in included studies
Two trials provided limited information about design and methodology (Mackey 2000;Wang 2005). All trials were conducted in one study centre. Two trials reported a sample size calculation (Aubertin‐Leheudre 2008;Gardner 2001). All the trials had prespecified inclusion criteria and four trials had prespecified exclusion criteria. One trial prespecified diagnostic criteria (Mackey 2000). No trial stated that they performed an ITT analysis to evaluate the data. Two studies may have performed ITT analyses (Dewell 2002;Wang 2005). Three trials did not report missing data. The Characteristics of included studies table shows the assessment of risk of bias. Review authors' judgements about each risk of bias item is presented as percentages across all included studies in Figure 2, and review authors' judgements about each risk of bias item for each included study is shown in Figure 3. The review authors had the same judgements on their risk of bias assessments of the included studies.
2.
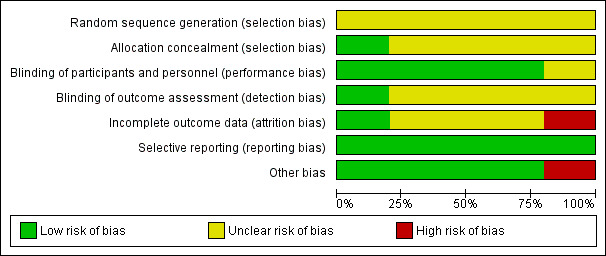
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
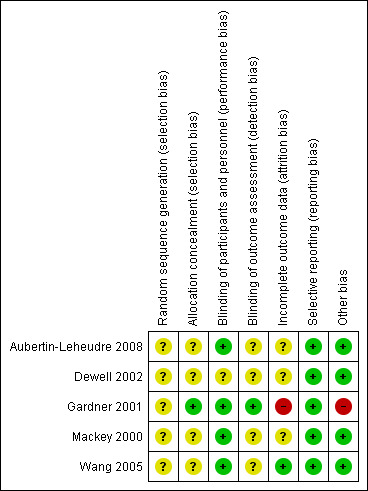
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One study reported an adequate allocation concealment (Gardner 2001).
Blinding
Four studies reported on blinding of participants and personnel (Aubertin‐Leheudre 2008; Gardner 2001; Mackey 2000; Wang 2005) and one study on outcome assessment (Gardner 2001).
Incomplete outcome data
Only one study addressed incomplete outcome data adequately (Wang 2005).
Selective reporting
None of the trials reviewed had published protocols of their study. No reporting bias was detected according to the stated outcomes in the methods section and the reported outcomes in the results section of the publication.
Other potential sources of bias
We considered four trials to be free of other potential sources of bias with one trial showing high risk of bias (Gardner 2001).
Effects of interventions
See: Table 1
We were only able to perform meta‐analyses on four outcomes in this review. One trial performed in an Asian population demonstrated a major difference to the other studies with participants being postmenopausal women with abnormal endocrine function and showing very low baseline fasting serum HDL‐cholesterol concentrations (0.28 to 0.42 mmol/L). Thus, we excluded this trial from meta‐analysis.
Primary outcomes
There were no data on the primary outcomes death from any cause and cardiovascular events. One trial compared isoflavones with placebo in 19 participants including eight in the intervention and 11 in the control group (Aubertin‐Leheudre 2008). Two trials compared soy protein containing isoflavones versus soy protein alone in 113 participants including 56 in the intervention and 57 in the control groups (Gardner 2001;Mackey 2000). For LDL‐cholesterol, there were no statistically significant differences between isoflavones and placebo (MD ‐0.23 mmol/L, 95% CI ‐0.5 to 0.04; P value = 0.10; 113 participants; 2 trials; Analysis 1.1 subgroup 1.1.2; Figure 4).
1.1. Analysis.
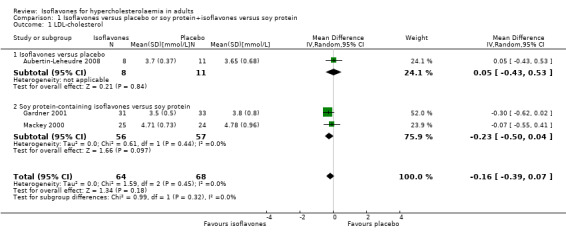
Comparison 1 Isoflavones versus placebo or soy protein+isoflavones versus soy protein, Outcome 1 LDL‐cholesterol.
4.
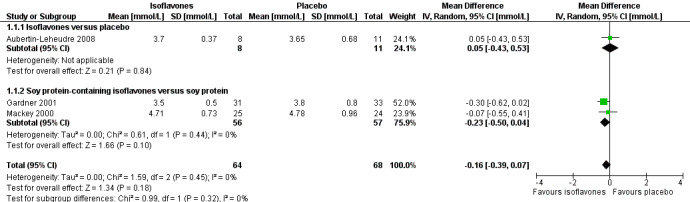
Forest plot of comparison: 1 Isoflavones versus placebo, outcome: 1.1 LDL‐cholesterol [mmol/L].
Secondary outcomes
There were no data on the secondary outcomes health‐related quality of life and costs in any of the trials. The trials reported no serious adverse events (Appendix 8; Appendix 9).
A meta‐analysis of four trials (Aubertin‐Leheudre 2008;Dewell 2002;Gardner 2001;Mackey 2000) showed no statistically significant effects of isoflavones on TC (Analysis 1.2; Figure 5).
1.2. Analysis.
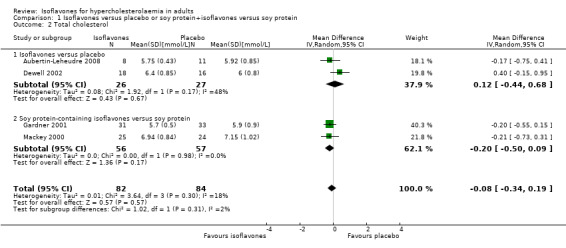
Comparison 1 Isoflavones versus placebo or soy protein+isoflavones versus soy protein, Outcome 2 Total cholesterol.
5.
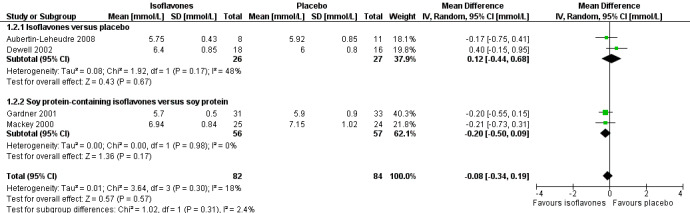
Forest plot of comparison: 1 Isoflavones versus placebo, outcome: 1.2 Total cholesterol [mmol/L].
A meta‐analysis of three trials (Aubertin‐Leheudre 2008;Gardner 2001;Mackey 2000) showed no statistically significant effects of isoflavones on HDL‐cholesterol (Analysis 1.3).
1.3. Analysis.
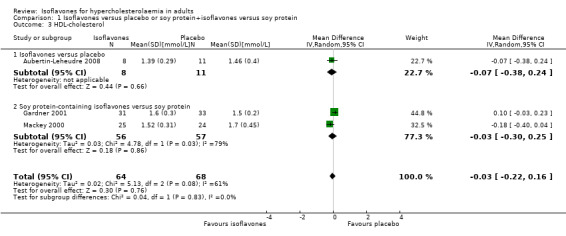
Comparison 1 Isoflavones versus placebo or soy protein+isoflavones versus soy protein, Outcome 3 HDL‐cholesterol.
Two trials compared isoflavones with placebo in 52 participants including 25 in the intervention and 27 in the control groups (Aubertin‐Leheudre 2008; Dewell 2002) and showed a statistically significant reduction of triglycerides (MD ‐0.46 mmol/L, 95% CI ‐0.84 to ‐0.09; P value = 0.02; Analysis 1.4) in favour of isoflavones (Figure 6).
1.4. Analysis.
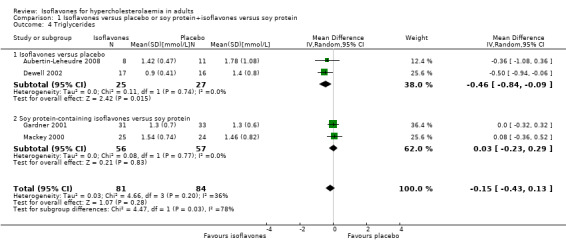
Comparison 1 Isoflavones versus placebo or soy protein+isoflavones versus soy protein, Outcome 4 Triglycerides.
6.
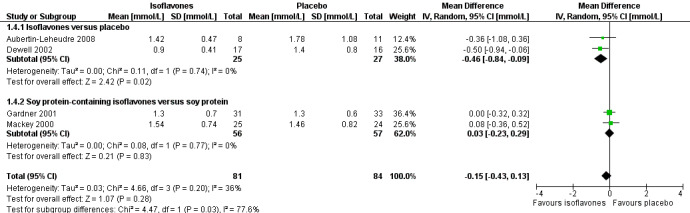
Forest plot of comparison: 1 Isoflavones versus placebo, outcome: 1.4 Triglycerides [mmol/L].
Reporting biases
We did not draw funnel plots due to the low number of included studies.
Subgroup analyses
All participants of the five included trials were postmenopausal women. We could not identify equol or non‐equol producers, or participants with hypercholesterolaemia with or without hypertriglyceridaemia. We did not perform subgroup analyses due to lack of data.
Sensitivity analyses
We were not able to do sensitivity analyses on language of publication, source of funding and country due to the limited number of trials.
Discussion
Summary of main results
Five randomized controlled clinical trials with 208 participants were included in this review, but we excluded one study (Wang 2005) from meta‐analysis due to substantial differences in clinical characteristics of participants. Duration of treatment ranged from three to six months. There were no outcome data on death from any cause, morbidity, complications, health‐related quality of life and costs.
Compared with placebo, there was a statistically significant effect of isoflavones alone on triglycerides and no statistically significant effect on TC, LDL‐cholesterol or HDL‐cholesterol in hypercholesterolaemic women. There was no statistically significant effect of soy protein containing isoflavones on hypercholesterolaemia. Compared with placebo, isoflavones also showed no statistically significant effects on body weight, BMI, waist circumference, total fat mass (FM), abdominal FM, visceral FM, resting energy expenditure, daily energy expenditure, diastolic and systolic blood pressure, HDL/TC, fasting plasma glucose, insulin and HOMA2‐IR (no data were provided in these studies). Compared with soy protein, soy protein containing isoflavones showed no statistically significant effects on body weight, BMI, androstenedione, estrone, oestradiol, follicle‐stimulating hormone, luteinizing hormone, SHBG, thyroid‐stimulating hormone, osteocalcin, bone‐specific alkaline phosphatase (with the exception of SHBG, no data of other variables were provided in these studies).
Overall completeness and applicability of evidence
The participants in the included trials were postmenopausal women. These populations may have been chosen due to the theory that isoflavones have phyto‐oestrogen effects. However, these participants may not be representative of all people with hypercholesterolaemia. Most of the participants were recruited from non‐Asian populations. The major source of isoflavones is soy, which is consumed largely by Asian populations. In addition, the included trials reported no long‐term data on cardiovascular events and other patient‐important outcomes. Therefore, the evaluation of the effects of isoflavones on hypercholesterolaemia especially in Asian populations and on patient‐relevant end points should be addressed in future studies.
Quality of the evidence
All of the randomized trials included in this review were of rather poor quality in terms of their design, reporting and methodology. They provided only limited descriptions of study design, randomization, allocation concealment and baseline data. We identified no multicentre, large‐scale randomized controlled clinical trials. Moreover, the included trials were heterogeneous in the populations (adults or elderly people, or hypercholesterolaemia with or without hypertriglyceridaemia), interventions and the reported outcomes.
Diagnostic criteria
Among the five included trials, only one trial diagnosed participants with hypercholesterolaemia (Mackey 2000). The other four trials did not describe exact diagnostic criteria.
Interventions
Three trials used isoflavones interventions and placebo controls (Aubertin‐Leheudre 2008;Dewell 2002; Wang 2005). Two trials used soy protein‐containing isoflavones and compared this with soy isolated protein plus less than 4 mg isoflavones/day (Gardner 2001;Mackey 2000). No trial lasted longer than six months
Surrogate outcomes
The primary target of hypercholesterolaemia treatment is to prevent cardiovascular events. No trial reported cardiovascular events in people with hypercholesterolaemia. Other outcomes from the included trials were also surrogate outcomes, that is TC, LDL‐ and HDL‐cholesterol, and triglycerides. We excluded 40 randomized trials from this review. The main reasons were participants not being hypercholesterolaemic, less than three‐month interventions and improper control or intervention.
Adverse outcomes
There was inadequate reporting on adverse events in the included trials (Appendix 8; Appendix 9). Two trials reported results about adverse effects. Three trials did not report adverse events. In general, isoflavones were safe for postmenopausal women although there were gastrointestinal side effects.
Potential biases in the review process
Although we conducted comprehensive searches, we only identified and included five trials. Among the five trials, four trials were performed in non‐Asian populations and one trial was performed in an Asian population. All studies were of small sample sizes. We tried to avoid location bias, but we could not exclude potential dissemination bias. We have undertaken extensive searches for unpublished material and identified no trials that qualified for inclusion.
Agreements and disagreements with other studies or reviews
Several systematic reviews and meta‐analyses on isoflavones for dyslipidaemia have been published (Taku 2007; Taku 2008; Weggemans 2003; Zhan 2005; Zhuo 2004). Only one review assessed the effects of extracted soy isoflavones alone on lipids (Taku 2008). Two reviews assessed the effects of soy protein‐containing isoflavones on lipids (Weggemans 2003;Zhan 2005). Two reviews assessed the effects of soy isoflavones alone and soy protein‐containing isoflavones on lipids, and performed subgroup analysis by normal cholesterolaemia and primary hypercholesterolaemia (Taku 2007; Zhuo 2004). All reviews included participants with normal cholesterolaemia and primary hypercholesterolaemia. These reviews included 36 trials. Of these trials, only three were included in our review (Dewell 2002; Gardner 2001;Mackey 2000). The other included trials in these reviews were excluded from this review, mostly because the intervention durations were less than three months or the participants were not hypercholesterolaemic. We included one trial in this review because of information provided by contacting the study author and another was published in Chinese. The conclusions of the above‐mentioned reviews were inconsistent due to various factors. Two reviews performed similar meta‐analyses to our review and concluded that supplementation of soy protein‐containing isoflavones for one to three months only slightly decreased TC (Taku 2007) and LDL‐cholesterol (Zhuo 2004), but had no statistically significant effects on HDL‐cholesterol or triglycerides compared to soy protein‐containing no or traces of isoflavones in hypercholesterolaemia. In contrast to these latter two reviews, our systematic review found that three to six months' consumption of isoflavones compared with placebo, or soy protein‐containing isoflavones compared with soy protein had no statistically significant effects on TC, LDL‐cholesterol or HDL‐cholesterol. Our review only found that isoflavones alone significantly lowered triglycerides compared with placebo in hypercholesterolaemia.
Authors' conclusions
Implications for practice.
We found no evidence for effects of isoflavones on patient‐important outcomes or lowering of cholesterol levels in people with hypercholesterolaemia.
Implications for research.
From the results of the present review, it would be interesting to test isoflavones compared with placebo in Asian hypercholesterolaemic populations. International, multicentre, rigorously designed, adequately powered, long‐term, high‐quality studies are required to provide better evidence. Long‐term trials should consider the definition of cardiovascular events measures, the incidence of adverse events and other patient‐important endpoints in long‐term trials.
Acknowledgements
None.
Appendices
Appendix 1. Search strategies
| Search terms and databases |
| Unless otherwise stated, search terms are free text terms. Abbreviations: '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| #1 MeSH descriptor Hypercholesterolemia explode all trees #2 MeSH descriptor Hyperlipoproteinemias explode all trees #3 MeSH descriptor Cholesterol, HDL explode all trees #4 MeSH descriptor Cholesterol, LDL explode all trees #5 MeSH descriptor Lipoproteins, HDL explode all trees #6 MeSH descriptor Lipoproteins, LDL explode all trees #7 (hypercholesterolaemi* in All Text or hypercholesterolemi* in All Text or hyperlipoproteinaemi* in All Text or hyperlipoproteinemi* in All Text or hyperlipaemi* in All Text or hyperlipemi* in All Text) #8 (HDL in All Text or LDL in All Text or cholesterol in All Text) #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Isoflavones explode all trees #11 MeSH descriptor Soybeans explode all trees #12 (biochanin* in All Text or formononetin* in All Text or prunetin* in All Text) #13 ((red in All Text and glover* in All Text) or (alfalfa in All Text and sprout* in All Text)) #14 soybean* in All Text #15 (coumestrol in All Text or pterocarpans in All Text or rotenone in All Text) #16 (daidzin* in All Text or daidzein* in All Text or genistin* in All Text or genistein* in All Text or glycetin* in All Text or glycetein* in All Text) #17 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18 (#9 AND #17) |
| MEDLINE |
| 1 exp hypercholesterolemia/ or exp hyperlipoproteinemias/ 2 (hypercholesterol?emi* or hyperlipoprotein?emi* or hyperlip?emi*).tw,ot. 3 (HDL or LDL or cholesterol).tw,ot. 4 exp Lipoproteins, HDL/ or exp Cholesterol, HDL/ 5 exp Cholesterol, LDL/ or exp Lipoproteins, LDL/ 6 or/1‐5 7 exp Isoflavones/ 8 exp Genistein/ or exp Soybeans/ 9 (daidzin* or daidzein* or genistin* or genistein* or glycetin* or glycetein*).tw,ot. 10 (biochanin* or formononetin* or prunetin*).tw,ot. 11 (coumestrol or pterocarpans or rotenone).tw,ot. 12 (red glover* or alfalfa sprout* or soybean*).tw,ot. 13 or/7‐12 14 randomized controlled trial.pt. 15 controlled clinical trial.pt. 16 randomi?ed.ab. 17 placebo.ab. 18 drug therapy.fs. 19 randomly.ab. 20 trial.ab. 21 groups.ab. 22 or/14‐21 23 Meta‐analysis.pt. 24 exp Technology Assessment, Biomedical/ 25 exp Meta‐analysis/ 26 exp Meta‐analysis as topic/ 27 hta.tw,ot. 28 (health technology adj6 assessment$).tw,ot. 29 (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 30 ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systemat$)).tw,ot. 31 or/23‐30 32 22 or 31 33 (comment or editorial or historical‐article).pt. 34 32 not 33 35 (animals not (humans and animals)).sh. 36 34 not 35 37 6 and 13 and 36 |
| EMBASE |
| 1 exp hyperlipidemia/ 2 exp high density lipoprotein/ 3 exp low density lipoprotein/ 4 exp high density lipoprotein cholesterol/ 5 exp low density lipoprotein cholesterol/ 6 (hypercholesterol?emi* or hyperlipoprotein?emi* or hyperlipid?emi*).tw,ot. 7 exp cholesterol/ 8 ((HDL or LDL) adj6 (lipoprotein* or cholesterol*)).tw,ot. 9 or/1‐8 10 exp isoflavone derivative/ 11 exp isoflavone/ 12 exp genistein/ 13 exp soybean/ 14 exp daidzin/ 15 exp daidzein/ 16 exp genistin/ 17 exp glycitein/ 18 exp biochanin A/ 19 exp formononetin/ 20 exp prunetin/ 21 isoflavone*.tw,ot. 22 (coumestrol or pterocarpans or rotenone).tw,ot. 23 (genistein* or genistin* or soybean* or daidzein* or glycitein* or biochanin* or formononetin* or prunetin*).tw,ot. 24 (red glover* or alfalfa sprout*).tw,ot. 25 or/10‐24 26 exp Randomized Controlled Trial/ 27 exp Controlled Clinical Trial/ 28 exp Clinical Trial/ 29 exp Comparative Study/ 30 exp Drug comparison/ 31 exp Randomization/ 32 exp Crossover procedure/ 33 exp Double blind procedure/ 34 exp Single blind procedure/ 35 exp Placebo/ 36 exp Prospective Study/ 37 ((clinical or control$ or comparativ$ or placebo$ or prospectiv$ or randomi?ed) adj3 (trial$ or stud$)).ab,ti. 38 (random$ adj6 (allocat$ or assign$ or basis or order$)).ab,ti. 39 ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ab,ti. 40 (cross over or crossover).ab,ti. 41 or/26‐40 42 exp meta analysis/ 43 (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 44 ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 45 exp Literature/ 46 exp Biomedical Technology Assessment/ 47 hta.tw,ot. 48 (health technology adj6 assessment$).tw,ot. 49 or/42‐48 50 41 or 49 51 (comment or editorial or historical‐article).pt. 52 50 not 51 53 9 and 25 and 52 54 limit 53 to human |
| China National Knowledge Infrastructure |
| #1 (soy or soya):ti,ab,kw #2 (tofu):ti,ab,kw #3 (red clover):ti,ab,kw #4 (isoflavone or isoflavones):ti,ab,kw #5 (daidzin or daidzein):ti,ab,kw #6 (genistin or genistein):ti,ab,kw #7 (glycetin or glycetein):ti,ab,kw #8 #1 or #2 or #3 or #4 or #5 or $6 or #7 #9 (cholesterol):ti,ab,kw #10 (triglyceride):ti,ab,kw #11 (lipid or lipids):ti,ab,kw #12 (LDL‐C or HDL‐C):ti,ab,kw #13 #9 or #10 or #11 or #12 #14 #8 and #13 |
| Chinese BioMedical Database |
| #1 (soy or soya):ti,ab,kw #2 (tofu):ti,ab,kw #3 (red clover):ti,ab,kw #4 (isoflavone or isoflavones):ti,ab,kw #5 (daidzin or daidzein):ti,ab,kw #6 (genistin or genistein):ti,ab,kw #7 (glycetin or glycetein):ti,ab,kw #8 #1 or #2 or #3 or #4 or #5 or $6 or #7 #9 (cholesterol):ti,ab,kw #10 (triglyceride):ti,ab,kw #11 (lipid or lipids):ti,ab,kw #12 (LDL‐C or HDL‐C):ti,ab,kw #13 #9 or #10 or #11 or #12 #14 #8 and #13 |
Appendix 2. Description of interventions
|
Characteristic Study ID |
Intervention [route, frequency, total dose/day] |
Comparator [route, frequency, total dose/day] |
| Aubertin‐Leheudre 2008 | Isoflavones capsules (orally, 4 times daily, 70 mg/day (44 mg daidzein, 16 mg glycetein, 10 mg genistein)) |
Placebo capsules (orally, 4 times daily) |
| Dewell 2002 | Soy‐derived isoflavones tablets (orally, 3 times daily, 150 mg/day) |
Maltodextrin with 10% caramel colour tablets, (orally, 3 times daily, 150 mg/day) |
| Gardner 2001 | 42 g soy proteins containing 80 mg aglycone isoflavones (orally, twice daily, 80 mg/day) |
42 g soy proteins containing trace amounts of isoflavones (orally, twice daily, 3 mg/day) |
| Mackey 2000 | 28 g soy protein powder with 65 mg isoflavones (orally, once daily, 65 mg/day) |
28 g soy protein powder with less than 4 mg isoflavones (orally, once daily, < 4 mg/day) |
| Wang 2005 | Isoflavones capsules (orally, 3 times daily, 157.5 mg/day) |
Placebo capsules (orally, 3 times daily) |
Appendix 3. Baseline characteristics (I)
|
Characteristic Study ID |
Intervention and comparator | Duration of intervention | Duration of follow‐up | Participating population | Country | Setting | Ethnic groups (%) | Duration of disease [mean years (SD)/range] |
| Aubertin‐Leheudre 2008 | Isoflavones (70 mg) | 6 months | 6 months | Hypercholesterolaemic postmenopausal women | Canada | ‐ | ‐ | ‐ |
| Placebo | ||||||||
| Dewell 2002 | Isoflavones (150 mg) | 6 months | 6 months | Moderately hypercholesterolaemic postmenopausal women | US | ‐ | ‐ | ‐ |
| Maltodextrin with 10% caramel | ||||||||
| Gardner 2001 | Isoflavones (80 mg) | 12 weeks | 16 weeks | Moderately hypercholesterolaemic postmenopausal women | US | Outpatients | White, non‐Hispanic (69) | ‐ |
| Trace amounts of isoflavones | White, non‐Hispanic (87) | |||||||
| Mackey 2000 | Isoflavones (65 mg) | 12 weeks | 22 weeks | Hypercholesterolaemic postmenopausal women | Australia | ‐ | ‐ | ‐ |
| Less than 4 mg isoflavones | ||||||||
| Wang 2005 | Isoflavones (158 mg) | 3 months | 3 months | Hypercholesterolaemic postmenopausal women | China | Outpatients | ‐ | ‐ |
| Placebo | ||||||||
|
Footnotes "‐" denotes not reported. SD: standard deviation. | ||||||||
Appendix 4. Baseline characteristics (II)
|
Characteristic Study ID |
Intervention and comparator | Sex [female %] | Age [mean years (SD)/range] | TC [mean mmol/L (SD)] | BMI [mean kg/m2 (SD)] | Co‐medications / Co‐interventions | Co‐morbidities |
| Aubertin‐Leheudre 2008 | Isoflavones (70 mg) | 100 | 50‐70 | 5.90 (0.59) | 31.2 (4.5) | ‐ | ‐ |
| Placebo | 100 | 50‐70 | 6.02 (0.90) | 32.8 (4.8) | |||
| Dewell 2002 | Isoflavones (150 mg) | 100 | 69 (4) 64‐83 |
6.8 (0.9) | 25 (4) | 1 participant took simvastatin and 1 took fluvastatin | ‐ |
| Maltodextrin with 10% caramel | 100 | 70 (4) 65‐77 |
6.3 (2.0) | 25 (4) | |||
| Gardner 2001 | Isoflavones (80 mg) | 100 | 62.6 (7.3) | 6.1 (0.6) | 25.6 (4.4) | ‐ | ‐ |
| Trace amounts of isoflavones | 100 | 58.4 (7.2) | 6.2 (0.9) | 25.4 (3.6) | |||
| Mackey 2000 | Isoflavones (65 mg) | 100 | 56.4 (4.9) | 7.29 (0.90) | ‐ | ‐ | ‐ |
| Less than 4 mg isoflavones | 100 | 56.8 (4.2) | 7.47 (1.04) | ||||
| Wang 2005 | Isoflavones (158 mg) | 100 | 45‐55 | 7.63 (0.18) | ‐ | ‐ | ‐ |
| Placebo | 100 | 45‐55 | 7.74 (0.23) | ||||
|
Footnotes "‐" denotes not reported. BMI: body mass index; SD: standard deviation; TC: total cholesterol. | |||||||
Appendix 5. Matrix of study endpoints (publications)
|
Characteristic Study ID |
Primarya endpoint(s) | Secondaryb endpoint(s) | Otherc endpoint(s) | Time points for outcome measurement |
| Aubertin‐Leheudre 2008 | Fasting plasma LDL‐C | Adverse events, fasting plasma TC, HDL‐C, triglycerides | BMI, body weight, waist circumference, total FM, abdominal FM, visceral FM, REE, DEE, diastolic blood pressure, systolic blood pressure, HDL‐C/TC, fasting plasma glucose and insulin, HOMA2‐IR | 6 months |
| Dewell 2002 | ‐ | Fasting serum TC, triglycerides | ‐ | 6 months |
| Gardner 2001 | Fasting plasma LDL‐C | Adverse events, fasting plasma TC, HDL‐C, triglycerides | Androstenedione, BMI, oestradiol, estrone, FSH | 12 weeks |
| Mackey 2000 | Fasting serum LDL‐C | Fasting serum TC,HDL‐C, triglycerides | Body weight, FSH, LH, TSH, SHBG, osteocalcin, bone‐specific alkaline phosphatase | 12 weeks |
| Wang 2005 | Fasting blood LDL‐C | Fasting blood TC, HDL‐C, triglycerides | ‐ | 3 months |
|
Footnotes a,b verbatim statement in the publication; c not explicitly stated as primary or secondary endpoint(s) in the publication. "‐" denotes not reported. BMI: body mass index; DEE: daily energy expenditure; FFM: fat‐free mass; FM: fat mass; FSH: follicle‐stimulating hormone; HDL‐C: high‐density lipoprotein cholesterol; HOMA2‐IR: homeostasis model assessment; LDL‐C: low‐density lipoprotein cholesterol; LH: luteinizing hormone; REE: resting energy expenditure; SHBG: sex hormone binding globulin; TC: total cholesterol; TSH: thyroid‐stimulating hormone. | ||||
Appendix 6. Matrix of study endpoints (protocol/trial documents)
|
Characteristic Study ID |
Trial identifier |
| Aubertin‐Leheudre 2008 | ‐ |
| Dewell 2002 | ‐ |
| Gardner 2001 | ‐ |
| Mackey 2000 | ‐ |
| Wang 2005 | ‐ |
|
Footnotes "‐" denotes no protocol documents available | |
Appendix 7. Definition of endpoint measurement
|
Characteristic Study ID |
Cardiovascular events | Health‐related quality of life | Costs | Severe/serious adverse events |
| Aubertin‐Leheudre 2008 | ‐ | ‐ | ‐ | ‐ |
| Dewell 2002 | ‐ | ‐ | ‐ | ‐ |
| Gardner 2001 | ‐ | ‐ | ‐ | ‐ |
| Mackey 2000 | ‐ | ‐ | ‐ | ‐ |
| Wang 2005 | ‐ | ‐ | ‐ | ‐ |
|
Footnotes "‐" denotes not reported. | ||||
Appendix 8. Adverse events (I)
|
Characteristic Study ID |
Intervention and comparator | Deaths [n/N] | All adverse events [n/N] |
Severe/serious adverse events [n/N] |
Left study due to adverse events [n/N] |
| Aubertin‐Leheudre 2008 | Isoflavones (70 mg) | 0 | 0 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 | |
| Dewell 2002 | Isoflavones (150 mg) | 0 | ‐ | ‐ | ‐ |
| Maltodextrin with 10% caramel | 0 | ‐ | ‐ | ‐ | |
| Gardner 2001 | Isoflavones (80 mg) | 0 | 2/34 gastrointestinal discomfort (bloating and constipation); an increased number of hot flushes |
0 | 2/34 |
| Trace amounts of isoflavones | 0 | 1/34 gastrointestinal discomfort (bloating and constipation) |
0 | 1/34 | |
| Mackey 2000 | Isoflavones (65 mg) | 0 | ‐ | ‐ | ‐ |
| Less than 4 mg isoflavones | 0 | ‐ | ‐ | ‐ | |
| Wang 2005 | Isoflavones (158 mg) | 0 | ‐ | ‐ | ‐ |
| Placebo | 0 | ‐ | ‐ | ‐ | |
|
Footnotes "‐" denotes not reported. | |||||
Appendix 9. Adverse events (II)
|
Characteristic Study ID |
Intervention and comparator | Hospitalization [n/N] | Outpatient treatment [n/N] | Symptoms [n/N] |
| Aubertin‐Leheudre 2008 | Isoflavones (70 mg) | 0 | 0 | 0 |
| Placebo | 0 | 0 | 0 | |
| Dewell 2002 | Isoflavones (150 mg) | ‐ | ‐ | ‐ |
| Maltodextrin with 10% caramel | ‐ | ‐ | ‐ | |
| Gardner 2001 | Isoflavones (80 mg) | ‐ | ‐ | ‐ |
| Trace amounts of isoflavones | ‐ | ‐ | ‐ | |
| Mackey 2000 | Isoflavones (65 mg) | ‐ | ‐ | ‐ |
| Less than 4 mg isoflavones | ‐ | ‐ | ‐ | |
| Wang 2005 | Isoflavones (158 mg) | ‐ | ‐ | ‐ |
| Placebo | ‐ | ‐ | ‐ | |
|
Footnotes "‐" denotes not reported. | ||||
Appendix 10. Survey of authors providing information on included trials
|
Characteristic Study ID |
Study author contacted | Study author replied | Study author provided data | Comments |
| Aubertin‐Leheudre 2008 | Y | Y | Y | Authors provided data of participants with baseline cholesterol levels higher than 5.2 mmol/L; therefore, this trial was included |
| Dewell 2002 | Y | N | N | The study did not provide the email address of the authors. We tried to find the email address by searching other publications by the same authors in PubMed, but the email address was invalid |
| Gardner 2001 | Y | Y | N | We asked the corresponding author for the results of estrone, oestradiol, androstendione and follicle‐stimulating hormone measurements of participants at weeks 0 and 12. He replied but provided no data (due to no access to 12‐year‐old data) |
| Mackey 2000 | Y | N | N | ‐ |
| Wang 2005 | Y | N | N | ‐ |
|
Footnotes N: no; Y: yes. | ||||
Data and analyses
Comparison 1. Isoflavones versus placebo or soy protein+isoflavones versus soy protein.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LDL‐cholesterol | 3 | 132 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.39, 0.07] |
| 1.1 Isoflavones versus placebo | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.43, 0.53] |
| 1.2 Soy protein‐containing isoflavones versus soy protein | 2 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.50, 0.04] |
| 2 Total cholesterol | 4 | 166 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.34, 0.19] |
| 2.1 Isoflavones versus placebo | 2 | 53 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.44, 0.68] |
| 2.2 Soy protein‐containing isoflavones versus soy protein | 2 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.50, 0.09] |
| 3 HDL‐cholesterol | 3 | 132 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.22, 0.16] |
| 3.1 Isoflavones versus placebo | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.38, 0.24] |
| 3.2 Soy protein‐containing isoflavones versus soy protein | 2 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.30, 0.25] |
| 4 Triglycerides | 4 | 165 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.43, 0.13] |
| 4.1 Isoflavones versus placebo | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.84, ‐0.09] |
| 4.2 Soy protein‐containing isoflavones versus soy protein | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.23, 0.29] |
| 5 Sex hormone binding globulin | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐4.22 [‐15.62, 7.18] |
1.5. Analysis.
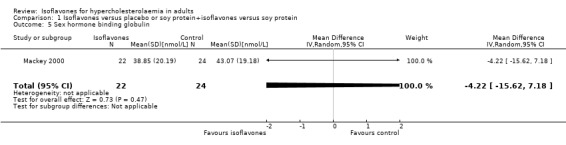
Comparison 1 Isoflavones versus placebo or soy protein+isoflavones versus soy protein, Outcome 5 Sex hormone binding globulin.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aubertin‐Leheudre 2008.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1 : 1 | |
| Participants |
Inclusion criteria: postmenopausal women aged 50‐70 years, obese (fat mass > 40%), without major physical incapacity, without hormone therapy (at the time of the study, women had never been on hormone therapy or were off hormone therapy for at least 1 year), sedentary (practiced 3 hours/wk of physical activities), weight stable (2 kg) for the last 6 months, non‐smoker, moderate drinking (maximum 15 g of alcohol/d, the equivalent of 1 alcoholic beverage/d), no medication that could influence body composition and metabolism, and absence of menses for the past 12 months Exclusion criteria: no Diagnostic criteria: not specified |
|
| Interventions |
Number of study centres: 1 Treatment before study: no Titration period: 6 months |
|
| Outcomes | Outcomes reported in abstract of publication: body composition, medical and social characteristics, daily energy expenditure, dietary intake and blood biochemical analyses (lipid profile, insulin, glucose) | |
| Study details |
Run‐in period: no Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Commercial funding: not reported Non‐commercial/other funding: supported by the Canadian Institutes of Health Research (CIHR) and the Research Centre on Aging Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To investigate whether 6 months of isoflavone supplementation, which has been shown to be sufficient to improve menopausal symptoms, could also improve clinical cardiovascular disease (CVD) risk factors in obese postmenopausal women, compared with a placebo" | |
| Notes | Not all participants were hypercholesterolaemic. The authors provided the data of participants with the baseline cholesterol higher than 5.2 mmol/L. 8 and 11 women with hypercholesterolaemia in the isoflavones and placebo groups, respectively, completed the trial. Thus, this trial was included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Women were randomly assigned to one of two groups, isoflavones (ISO) or placebo (PLA)" Comment: no information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from publication: "Identical active and placebo capsules were supplied and encapsulated by Arkopharma Ltd. (Carros, France)" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information about blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "Among these 50 participants, 39 completed the study (21 in ISO vs. 18 in PLA)" Comment: number of participants analyzed was less than number of participants randomized |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: no other sources of bias were found in this study |
Dewell 2002.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio intervention: control = 5 : 4 | |
| Participants |
Inclusion criteria: healthy moderately hypercholesterolaemic (mean TC 6.6 ± 1.3 mmol/L) postmenopausal women (mean age 69 ± 4 years) Exclusion criteria: receiving hormone replacement therapy, clinical or biochemical evidence of diabetes or renal, hepatic or cardiovascular disease Diagnostic criteria: not specified |
|
| Interventions |
Number of study centres: 1 Treatment before study: two individuals were taking medication known to affect carbohydrate or lipid metabolism. 1 woman was taking simvastatin and 1 woman fluvastatin for hypercholesterolaemia. Both women had been on a stable dose for at least 1 year before the study, and medications were not altered during the study period Titration period: 6 months |
|
| Outcomes | Outcomes reported in abstract of publication: triacylglycerol, TC and HDL‐C | |
| Study details |
Run‐in period: no Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Commercial funding: not reported Non‐commercial/other funding: yes; a small research grant from the College of Applied Sciences and Arts at San Jose State University and a research award from the Circle of Friends/Department of Nutrition and Food Science of San Jose State University Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "to investigate the effects of PE supplementation (150 mg) on serum lipids and lipoproteins in moderately hypercholesterolemic, elderly, postmenopausal women" | |
| Notes | The trial reported the outcomes of HDL‐C and non‐HDL‐C at baseline and at 2 months, but no data at 6 months Abbreviations: HDL: high‐density‐lipoprotein |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Thirty‐six subjects were randomized into two groups" Comment: no information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from publication: "Subjects were recruited initially as part of a larger randomized, double blind, placebo‐controlled trial with a parallel design to assess the role of PE supplementation on bone mineral health" Comment: no information about blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information about blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "Because of the unavailability of serum, determinations of triacylglycerol and cholesterol concentrations at 6 months were performed only on 17 and 18 of the 20 subjects in the PE‐treated group, respectively" Comment: number of participants analyzed was less than number of participants randomized |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: no other sources of bias were found in this study |
Gardner 2001.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1 : 1 | |
| Participants |
Inclusion criteria: the fasting plasma LDL‐cholesterol concentration of 3.37‐4.92 mmol/L (130‐190 mg/dL) and a triacylglycerol concentration < 2.82 mmol/L (< 250 mg/dL); postmenopausal (≥ 1 year since their last menstrual cycle), age < 80 years, and a BMI of 20–31 kg/m2 Exclusion criteria: smokers, had been taking hormone replacements or lipid‐lowering medication during the previous 3 months, had a history of cardiovascular disease or diabetes, or had breast, endometrial or ovarian cancer in the previous 10 years Diagnostic criteria: not specified |
|
| Interventions |
Number of study centres: 1 Treatment before study: no Titration period: 12 wks |
|
| Outcomes | Outcomes reported in abstract of publication: TC, LDL‐C, HDL‐C and triacylglycerol | |
| Study details |
Run‐in period: yes Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Commercial/non‐commercial/other funding: not reported Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "to determine the effect of soy protein and isoflavones on plasma lipid concentrations in postmenopausal, moderately hypercholesterolemic women" | |
| Notes | "LDL‐cholesterol was calculated according to the method of Friedewald unless the triacylglycerols were > 4.52 mmol/L (> 400 mg/dL), in which case the LDL‐C value was considered missing data (3 LDL‐cholesterol data points were excluded: 1 in the Milk group, 0 in the Soy– group, and 2 in the Soy+ group)" Abbreviations: HDL: high‐density‐lipoprotein; LDL: low‐density‐lipoprotein |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The subjects were randomly assigned to 12 wk of dietary protein supplementation (42 g/d) with either a milk protein (Milk group) or 1 of 2 soy proteins containing either trace amounts of isoflavones (Soy– group) or 80 mg aglycone isoflavones (Soy+ group)", "Randomization was performed in blocks of 30 participants" Comment: no details provided |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Dietary supplements containing a mixture of protein, carbohydrate, and calcium in powder form (Shaklee Corporation, Hayward, CA) were provided in sealed packets, each containing one‐half of the daily dose (21 g protein/packet 2 packets/d)", "All supplements were formulated to be identical in taste, color, and odor" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from publication: "Participants, investigators, study staff, and laboratory technicians were blinded to treatment assignments until the conclusion of the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from publication: "Participants, investigators, study staff, and laboratory technicians were blinded to treatment assignments until the conclusion of the trial" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from publication: "These analyses showed a stable composition of isoflavone concentrations throughout the study (data not shown)", "Of the 115 women who entered the study, 21 withdrew before study completion", "Statistical analyses were performed on data" Comment: number of participants analyzed was less than number of participants randomized |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | High risk | Quote "At the onset of the study, the participants were instructed to take the full dose of the protein supplements. However, we had a higher than anticipated dropout rate early in the study because of adverse gastrointestinal responses to the acute dietary change. Therefore, gradual adaptation to the protein supplements was recommended for the latter four‐fifths of participants. These participants began the 4‐wk run‐in phase by consuming one‐half of the goal dose rather than the full dose. These participants were then encouraged to increase their intake to the full dose by week 3 of the run‐in phase". "Exceptions to group comparability at randomization included a higher average age and a higher number of years since menopause in the Soy+ group and a lower percentage of married women in the Soy– group than in the Soy+ group" Comment: not all participants performed the run‐in phase; several baseline characteristics of participants were not equally distributed between the intervention and placebo groups |
Mackey 2000.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio not reported | |
| Participants |
Inclusion criteria: postmenopausal women aged 45‐65 years Exclusion criteria: a history of allergy to soy or if any of them were taking cholesterol‐lowing agents Diagnostic criteria: a fasting TC > 5.5 mmol/L |
|
| Interventions |
Number of study centres: 1 Treatment before study: no Titration period: 12 wks |
|
| Outcomes | Outcomes reported in abstract of publication: TC, LDL‐C, HDL‐C, SHBG and LH | |
| Study details |
Run‐in period: yes Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Commercial funding: yes Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "We performed a series of studies in men and women using soy protein with or without isoflavones to study the effect on the lipoprotein profile as well as other biochemical indices such as sex hormones, pituitary hormones, markers of bone turnover and glucose tolerance" | |
| Notes | The female study was a prospective, double‐blind, randomised controlled study. The male study was an open prospective observational pilot study. Thus, only female study was included. The concentrations of LDL‐C maybe calculated Abbreviations: HDL: high‐density‐lipoprotein; LDL: low‐density‐lipoprotein |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Fifty four female subjects were randomised to receive 28 g of protein powder; either a) a soy protein with an isoflavone content of 65 mg isoflavones daily (ISP+) or b) a soy protein isolate with less than 4 mg isoflavones per daily (ISP‐)" Comment: no information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from publication: "The female study was a prospective, double‐blind, randomised controlled study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information about blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Of the 54 women who were randomised into the study, 49 women completed the study" Comment: number of participants analyzed was less than number of participants randomized |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: no other sources of bias were found in this study |
Wang 2005.
| Methods | Parallel randomised controlled clinical trial, randomisation ratio 1 : 1 | |
| Participants |
Inclusion criteria: postmenopausal women (≥ 6 months since their last menstrual cycle) with perimenopausal symptoms, dyslipidaemia and abnormal endocrine function, aged 45‐55 years Exclusion criteria: use of steroid drugs Diagnostic criteria: not specified |
|
| Interventions |
Number of study centres: 1 Treatment before study: no Titration period: 3 month |
|
| Outcomes | Outcomes reported in abstract of publication: serum TC, HDL‐C, LDL‐C and triacylglycerol | |
| Study details |
Run‐in period: no Study terminated before regular end (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: Chinese Commercial/non‐commercial/other funding: not reported Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To study the effects of soybean isoflavones on lipids metabolism in the perimenopausal female" | |
| Notes | Baseline serum HDL‐C concentrations were very low | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Subjects were randomised to 3 groups" Comment: no information about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from publication:"The placebo group consumed the placebo capsules that looked like the isoflavones capsules" Comment: participants and personnel were potentially masked |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information about blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: number of participants analyzed was equal to the number of participants randomized |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: no other sources of bias were found in this study |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atkinson 2004 | Randomized clinical trial comparing 43.5 mg red clover‐derived isoflavones versus placebo in 177 women. It was excluded because not all participants were hypercholesterolaemic |
| Badeau 2007 | 56 postmenopausal women were treated with either isoflavone or placebo tablets for 3 months in a cross‐over design, separated by a 2‐month washout period. It was excluded because not all participants were hypercholesterolaemic |
| Blakesmith 2003 | Randomized clinical trial comparing isoflavone versus placebo in 25 healthy premenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Cancellieri 2007 | Multicentre, randomized, double‐blind, placebo‐controlled clinical investigation on 125 menopausal women randomly assigned to 2 groups treated for 6 months with placebo or 1 tablet daily of an herbal product containing 72 mg/dose of isoflavones of different plants origin and other plant extracts. It was excluded because not all participants were hypercholesterolaemic |
| Chedraui 2008 | Randomized controlled cross‐over clinical trial conducted in 60 postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Cheng 2007 | Randomized clinical trial comparing isoflavone versus placebo in 60 healthy postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Cianci 2012 | 120 women with a mean age of 54.8 ± 0.6 years were enrolled and randomized to treatment with isoflavones and berberine or calcium and vitamin D(3). It was excluded because 1) not all participants were hypercholesterolaemic, and 2) intervention was isoflavones and berberine |
| Colacurci 2005 | Randomized clinical trial comparing isoflavone versus placebo in 60 healthy postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Dent 2001 | Randomized clinical trial comparing isoflavone‐rich soy versus isoflavone‐poor soy in perimenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Fornaro 2006 | 54 healthy postmenopausal women were randomly allocated to 60 mg/d of both genistein and daidzein for 6 months (active group, n = 27) or no therapy (control group, n = 27). It was excluded because not all participants were hypercholesterolaemic |
| Gallagher 2004 | A total of 65 women, with a mean age of 55 years and 7.5 years since menopause, were randomized to 1 of 3 groups; soy protein with 96 mg isoflavones/d, soy with 52 mg isoflavones/d, or soy without isoflavones (< 4 mg isoflavones/d). It was excluded because not all participants were hypercholesterolaemic |
| Garrido 2006 | 29 healthy postmenopausal women were invited to take part in a randomized study to receive either 100 mg/d isoflavone supplement (n = 15) or identical placebo capsules (n = 14). It was excluded because not all participants were hypercholesterolaemic |
| Gonzalez 2007 | A randomized, double‐blind, placebo‐controlled, cross‐over study was conducted in 32 Caucasian, postmenopausal women with diet‐controlled type 2 diabetes. It was excluded because not all participants were hypercholesterolaemic |
| Han 2002 | Randomized clinical trial comparing isoflavone versus placebo in postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Hidalgo 2005 | 60 postmenopausal women aged > 40 years, non‐users of hormone therapy, with Kupperman index score 15, were randomized in a double‐blind method to receive either a commercially available red clover isoflavone supplement (80 mg/d) or placebo for 90 d. It was excluded because not all participants were hypercholesterolaemic |
| Ho 2007 | A double‐blind, randomized, placebo‐controlled trial was conducted in 203 postmenopausal Chinese women aged 48‐62 years. They were randomly assigned to receive daily doses of 500 mg calcium, and 0 mg isoflavones (placebo, n = 67), 40 mg isoflavones (n = 68) and 80 mg isoflavones (n = 68). It was excluded because not all participants were hypercholesterolaemic |
| Jiang 2008 | Randomized clinical trial comparing isoflavone versus placebo in 189 normal cholesterolaemic and hypercholesterolaemic perimenopausal women. It was excluded because lack of cholesterol data in hypercholesterolaemic women in the placebo group |
| Lee 2012 | In this randomized, double‐blind, placebo‐controlled trial, 51 subjects with a body mass index of 23 kg/m2 or greater and a waist‐to‐hip ratio of 0.90 or greater for men or 0.85 or greater for women were randomly assigned to take 9.9 g/d of either a placebo or doenjang for 12 weeks. It was excluded because 1) not all participants were hypercholesterolaemic, and 2) doenjang may contain isoflavones and soy protein |
| Liang 2007 | It was excluded because the intervention was soy lecithin |
| Llaneza 2010 | 116 postmenopausal women with insulin resistance were randomly assigned to a group of Mediterranean diet and physical exercise (control group) or a group of Mediterranean diet, physical exercise and daily oral ingestion of 40 mg of soy isoflavones (soy isoflavones group). It was excluded because not all participants were hypercholesterolaemic |
| Marini 2010 | Randomized clinical trial comparing genistein versus placebo in postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Nikander 2004 | Randomized clinical trial comparing isoflavone versus placebo in 56 non‐diabetic postmenopausal women with a history of breast cancer. It was excluded because not all participants were hypercholesterolaemic |
| Oliveira 2012 | In a prospective, randomized and single‐blinded clinical trial, we compared people with chronic hepatitis C who had casein as a supplement (n = 80) (control group), with people who consumed a soy supplement diet (n = 80) (intervention group). It was excluded because 1) not all participants were hypercholesterolaemic, and 2) the intervention was soy protein and isoflavones, but the control was placebo |
| Ozturk 2009 | Randomized clinical trial comparing isoflavone versus placebo in 22 postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Petri Nahas 2004 | Randomized clinical trial comparing isoflavone versus placebo in 50 postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Rios 2008 | In this double‐blind, placebo‐controlled study, 47 postmenopausal women 47‐66 years of age received 40 mg of isoflavone (n = 25) or 40 mg of casein placebo (n = 22). It was excluded because not all participants were hypercholesterolaemic |
| Schult 2004 | Randomized clinical trial comparing isoflavones versus placebo in 252 menopausal women aged 45‐60 years. It was excluded because not all participants were hypercholesterolaemic |
| Swain 2002 | Perimenopausal women (n = 69) were randomly assigned (double blind) to treatment: isoflavone‐rich soy‐protein isolate (n = 24), isoflavone‐poor soy‐protein isolate (n = 24) or whey protein (control; n = 21). Each subject consumed 40 g soy or whey protein daily for 24 weeks. It was excluded because not all participants were hypercholesterolaemic |
| Terzic 2009 | Randomized clinical trial comparing a red clover‐derived isoflavone versus placebo in 40 healthy postmenopausal women with a mean age of 56 years. It was excluded because not all participants were hypercholesterolaemic |
| Törmälä 2006 | 30 postmenopausal women were treated in a randomized, placebo‐controlled, cross‐over trial with isoflavones or placebo for 3 months interrupted by a 2‐month washout period. It was excluded because not all participants were hypercholesterolaemic |
| Uesugi 2003 | Randomized clinical trial comparing isoflavone versus placebo in postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Villa 2009 | A randomized placebo controlled study was conducted in 50 postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Wong 2012 | Randomized controlled cross‐over clinical trial conducted in 41 participants (23 men, 18 women). It was excluded because the intervention duration was 4 weeks |
| Woo 2003 | Randomized clinical trial comparing isoflavone versus placebo in postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
| Wu 2006a | Randomized clinical trial comparing isoflavone versus placebo, and isoflavone plus walking versus walking in 136 postmenopausal women at < 5 years after the onset of menopause. It was excluded because the mean daily dietary isoflavones intakes of participants in all groups at baseline and during the trial were greater than 37 mg/d, and not all participants were hypercholesterolaemic. This paper was also republished |
| Wu 2006b | Randomized clinical trial comparing isoflavone versus placebo, and isoflavone plus walking versus walking in 128 postmenopausal women at < 5 years after the onset of menopause. It was excluded because the mean daily dietary isoflavones intakes of participants in all groups at baseline and during the trial were greater than 37 mg/d, and not all participants were hypercholesterolaemic. This paper was also republished |
| Xiao 2003 | It was excluded because the intervention duration was 8 weeks |
| Ye 2012 | A randomized placebo‐controlled trial. 90 early postmenopausal Chinese women, aged 45‐60 years, were randomly assigned to 3 treatment groups (30 each) receiving daily doses of 0 (placebo), 84 and 126 mg of soy germ isoflavones. It was excluded because most of participants were not hypercholesterolaemic |
| Yildiz 2005 | Randomized clinical trial comparing 40 mg of genistein versus placebo in postmenopausal women. It was excluded because not all participants were hypercholesterolaemic |
d: day.
Differences between protocol and review
Review authors have changed since the published protocol and we added six more review authors (Yuan Zeng, Peng Liu, Long Yi, Ting Zhang, Qian Yong Zhang, Jun Dong Zhu). We changed the contact person to Man Tian Mi.
We added the Chinese BioMedical Database (to September 2012) to the search.
Contributions of authors
Yu Qin (YQ): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and update draft.
Kai Niu (KN): search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft and update draft.
Yuan Zeng (YZ): acquiring trial reports, trial selection and data extraction.
Peng Liu (PL): acquiring trial reports and data extraction.
Long Yi (LY): acquiring trial reports and data extraction.
Ting Zhang (TZ): data analysis and data interpretation.
Qian Yong Zhang (QYZ): data analysis and data interpretation.
Jun Dong Zhu (JDZ): data analysis and data interpretation.
Man Tian MI (MTM): search strategy development, data analysis, data interpretation and review draft.
Sources of support
Internal sources
No sources of support supplied
External sources
National Natural Science Foundation of China (No. 81072288), China.
-
Chongqing Science and Technology Commission, China.
(No. CSTC, 2011AB5040)
Declarations of interest
None known.
New
References
References to studies included in this review
Aubertin‐Leheudre 2008 {published data only}
- Aubertin‐Leheudre M, Lord C, Khalil A, Dionne IJ. Effect of 6 months of exercise and isoflavone supplementation on clinical cardiovascular risk factors in obese postmenopausal women: a randomized, double‐blind study. Menopause 2007;14(4):624‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Aubertin‐Leheudre M, Lord C, Khalil A, Dionne IJ. Isoflavones and clinical cardiovascular risk factors in obese postmenopausal women: a randomized double‐blind placebo‐controlled trial. Journal of Womens Health 2008;17:1363‐9. [DOI] [PubMed] [Google Scholar]
Dewell 2002 {published data only}
- Dewell A, Hollenbeck CB, Bruce B. The effects of soy‐derived phytoestrogens on serum lipids and lipoproteins in moderately hypercholesterolemic postmenopausal women. Journal of Clinical Endocrinology and Metabolism 2002;87(1):118‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gardner 2001 {published data only}
- Gardner CD, Newell KA, Cherin R, Haskell WL. The effect of soy protein with or without isoflavones relative to milk protein on plasma lipids in hypercholesterolemic postmenopausal women. American Journal of Clinical Nutrition 2001;73(4):728‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mackey 2000 {published data only}
- Mackey R, Ekangaki A, Eden JA. The effects of soy protein in women and men with elevated plasma lipids. Biofactors 2000;12(1‐4):251‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang YS, Zhang GR, Liu YJ, Zhuo HX, Xia FL. Effects of isoflavones on blood lipid profiles in perimenopausal women [大豆异黄酮对女性围绝经期血脂代谢的影响]. Zhong Hua Xian Dai Zhong Xi Yi Za Zhi 2005;3(19):1738‐9. [Google Scholar]
References to studies excluded from this review
Atkinson 2004 {published data only}
- Atkinson C, Oosthuizen W, Scollen S, Loktionov A, Day NE, Bingham SA. Modest protective effects of isoflavones from a red clover‐derived dietary supplement on cardiovascular disease risk factors in perimenopausal women, and evidence of an interaction with ApoE genotype in 49‐65 year‐old women. Journal of Nutrition 2004;134(7):1759‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Badeau 2007 {published data only}
- Badeau R, Jauhiainen M, Metso J, Nikander E, Tikkanen MJ, Ylikorkala O, et al. Effect of isolated isoflavone supplementation on ABCA1‐dependent cholesterol efflux potential in postmenopausal women. Menopause 2007;14(2):293‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blakesmith 2003 {published data only}
- Blakesmith SJ, Lyons‐Wall PM, George C, Joannou GE, Petocz P, Samman S. Effects of supplementation with purified red clover (Trifolium pratense) isoflavones on plasma lipids and insulin resistance in healthy premenopausal women. British Journal of Nutrition 2003;89(4):467‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cancellieri 2007 {published data only}
- Cancellieri F, Leo V, Genazzani AD, Nappi C, Parenti GL, Polatti F, et al. Efficacy on menopausal neurovegetative symptoms and some plasma lipids blood levels of an herbal product containing isoflavones and other plant extracts. Maturitas 2007;56(3):249‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chedraui 2008 {published data only}
- Chedraui P, San Miguel G, Hidalgo L, Morocho N, Ross S. Effect of Trifolium pratense‐derived isoflavones on the lipid profile of postmenopausal women with increased body mass index. Gynecological Endocrinology 2008;24(11):620‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cheng 2007 {published data only}
- Cheng G, Wilczek B, Warner M, Gustafsson JA, Landgren BM. Isoflavone treatment for acute menopausal symptoms. Menopause 2007;14:468‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cianci 2012 {published data only}
- Cianci A, Cicero AF, Colacurci N, Matarazzo MG, Leo V. Activity of isoflavones and berberine on vasomotor symptoms and lipid profile in menopausal women. Gynecological Endocrinology 2012;28:699‐702. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Colacurci 2005 {published data only}
- Colacurci N, Chiàntera A, Fornaro F, Novellis V, Manzella D, Arciello A. Effects of soy isoflavones on endothelial function in healthy postmenopausal women. Menopause 2005;12(3):299‐307. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dent 2001 {published data only}
- Dent SB, Peterson CT, Brace LD, Swain JH, Reddy MB, Hanson KB, et al. Soy protein intake by perimenopausal women does not affect circulating lipids and lipoproteins or coagulation and fibrinolytic factors. Journal of Nutrition 2001;131(9):2280‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fornaro 2006 {published data only}
- Fornaro F, Carotenuto V, Bisogni C, Trabucco E, Russo C, Manzella D, et al. Brachial reactivity in postmenopausal women: Impact of soy isoflavone administration. Italian Journal of Gynaecology and Obstetrics 2006;18:106‐10. [EMBASE: 44822700] [Google Scholar]
Gallagher 2004 {published data only}
- Gallagher JC, Satpathy R, Rafferty K, Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause 2004;11(3):290‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garrido 2006 {published data only}
- Garrido A, Maza MP, Hirsch S, Valladares L. Soy isoflavones affect platelet thromboxane A2 receptor density but not plasma lipids in menopausal women. Maturitas 2006;54(3):270‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gonzalez 2007 {published data only}
- Gonzalez S, Jayagopal V, Kilpatrick ES, Chapman T, Atkin SL. Effects of isoflavone dietary supplementation on cardiovascular risk factors in type 2 diabetes. Diabetes Care 2007;30:1871‐3. [EMBASE: 47036769; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Han 2002 {published data only}
- Han KK, Soares JM Jr, Haidar MA, Lima GR, Baracat EC. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstetrics and Gynecology 2002;99(3):389‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hidalgo 2005 {published data only}
- Hidalgo LA, Chedraui PA, Morocho N, Ross S, San Miguel G. The effect of red clover isoflavones on menopausal symptoms, lipids and vaginal cytology in menopausal women: a randomized, double‐blind, placebo‐controlled study. Gynecological Endocrinology 2005;21(5):257‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ho 2007 {published data only}
- Ho SC, Chen YM, Ho SS, Woo JL. Soy isoflavone supplementation and fasting serum glucose and lipid profile among postmenopausal Chinese women: a double‐blind, randomized, placebo‐controlled trial. Menopause 2007;14(5):905‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jiang 2008 {published data only}
- Jiang M, Xu YF, Miao ZL, Zhong XM, Zhang M, Liu N. Effects of isoflavones on blood lipid profiles in perimenopausal women [大豆异黄酮对围绝经期妇女血脂水平的影响]. ZhongGuo Shi Yong Yi Yao 2008;3(17):49‐50. [Google Scholar]
Lee 2012 {published data only}
- Lee M, Chae S, Cha Y, Park Y. Supplementation of Korean fermented soy paste doenjang reduces visceral fat in overweight subjects with mutant uncoupling protein‐1 allele. Nutrition Research 2012;32:8‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liang 2007 {published data only}
- Liang SP, Zhang DP. Observation and analysis of the lipid‐lowering effects of oat soy flour [燕麦大豆粉降脂作用的观察与分析]. ZhongGuo Wu Zhen Xue Za Zhi 2007;7(3):466. [Google Scholar]
Llaneza 2010 {published data only}
- Llaneza P, Gonzalez C, Fernandez‐Iñarrea J, Alonso A, Diaz‐Fernandez MJ, Arnott I, et al. Soy isoflavones, Mediterranean diet, and physical exercise in postmenopausal women with insulin resistance. Menopause 2010;17(2):372‐8. [EMBASE: 2010168638; MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marini 2010 {published data only}
- Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Stefano V, et al. Efficacy of genistein aglycone on some cardiovascular risk factors and homocysteine levels: a follow‐up study. Nutrition Metabolism and Cardiovascular Diseases 2010;20(5):332‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nikander 2004 {published data only}
- Nikander E, Tiitinen A, Laitinen K, Tikkanen M, Ylikorkala O. Effects of isolated isoflavonoids on lipids, lipoproteins, insulin sensitivity, and ghrelin in postmenopausal women. Journal of Clinical Endocrinology and Metabolism 2004;89(7):3567‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oliveira 2012 {published data only}
- Oliveira LP, Jesus RP, Boulhosa RS, Mendes CM, Gnoatto MC, Lemaire DC, et al. Effect of soy protein supplementation in patients with chronic hepatitis C: a randomized clinical trial. World Journal of Gastroenterology 2012;18(18):2203‐11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozturk 2009 {published data only}
- OzturkTurhan N, Iltemir Duvan C, Bolkan F, Onaran Y. Effect of isoflavone on plasma nitrite/nitrate, homocysteine, and lipid levels in Turkish women in the early postmenopausal period: a randomized controlled trial. Turkish Journal of Medical Sciences 2009;39:367‐75. [EMBASE: 354824750] [Google Scholar]
Petri Nahas 2004 {published data only}
- Petri Nahas E, Nahás Neto J, Luca L, Traiman P, Pontes A, Dalben I. Benefits of soy germ isoflavones in postmenopausal women with contraindication for conventional hormone replacement therapy. Maturitas 2004;48(4):372‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rios 2008 {published data only}
- Rios DR, Rodrigues ET, Cardoso AP, Montes MB, Franceschini SA, Toloi MR. Lack of effects of isoflavones on the lipid profile of Brazilian postmenopausal women. Nutrition 2008;24(11‐12):1153‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schult 2004 {published data only}
- Schult TM, Ensrud KE, Blackwell T, Ettinger B, Wallace R, Tice JA. Effect of isoflavones on lipids and bone turnover markers in menopausal women. Maturitas 2004;48(3):209‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Swain 2002 {published data only}
- Swain JH, Alekel DL, Dent SB, Peterson CT, Ready MB. Iron indexes and total antioxidant status in response to soy protein intake in perimenopausal women. American Journal of Clinical Nutrition 2002;76:165‐71. [EMBASE: 34686562] [DOI] [PubMed] [Google Scholar]
Terzic 2009 {published data only}
- Terzic MM, Dotlic J, Maricic S, Mihailovic T, Tosic‐Race B. Influence of red clover‐derived isoflavones on serum lipid profile in postmenopausal women. Journal of Obstetrics and Gynaecology Research 2009;35(6):1091‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Törmälä 2006 {published data only}
- Törmälä RM, Nikander E, Tiitinen A, Väisänen‐Tommiska M, Ylikorkala O, Mikkola TS. Serum cholesterol efflux potential in postmenopausal women treated with isolated isoflavones. Menopause 2006;13(1):96‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Uesugi 2003 {published data only}
- Uesugi T, Toda T, Okuhira T, Chen JT. Evidence of estrogenic effect by the three‐month‐intervention of isoflavone on vaginal maturation and bone metabolism in early postmenopausal women. Endocrine Journal 2003;50:613‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Villa 2009 {published data only}
- Villa P, Costantini B, Suriano R, Perri C, Macrì F, Ricciardi L, et al. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: relationship with the metabolic status. Journal of Clinical Endocrinology and Metabolism 2009;94(2):552‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wong 2012 {published data only}
- Wong JM, Kendall CW, Marchie A, Liu Z, Vidgen E, Holmes C, et al. Equol status and blood lipid profile in hyperlipidemia after consumption of diets containing soy foods. American Journal of Clinical Nutrition 2012;95(3):564‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woo 2003 {published data only}
- Woo J, Lau E, Ho SC, Cheng F, Chan C, Chan AS, et al. Comparison of Pueraria lobata with hormone replacement therapy in treating the adverse health consequences of menopause. Menopause 2003;10(4):352‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 2006a {published data only}
- Wu J, Oka J, Tabata I, Higuchi M, Toda T, Fuku N, et al. Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: a 1‐year randomized placebo‐controlled trial. Journal of Bone and Mineral Research 2006;21(5):780‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 2006b {published data only}
- Wu J, Oka J, Higuchi M, Tabata I, Toda T, Fujioka M, et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo‐controlled trial. Metabolism: Clinical and Experimental 2006;55(4):423‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Xiao 2003 {published data only}
- Xiao LZ, Jiang ZP, Xu X, Huang Z, Lai SY, Hu BH. Effects of isoflavones on blood lipid profiles and endothelial function in postmenopausal women [大豆异黄酮对绝经后X综合征患者血脂及内皮功能的影响]. Lin Chung Xin Xue Guan Bing Za Zhi 2003;19(5):302‐3. [Google Scholar]
Ye 2012 {published data only}
- Ye YB, Wang ZL, Zhuo SY, Lu W, Liao HF, Verbruggen M, et al. Soy germ isoflavones improve menopausal symptoms but have no effect on blood lipids in early postmenopausal Chinese women: a randomized placebo‐controlled trial. Menopause 2012;19:791‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yildiz 2005 {published data only}
- Yildiz MF, Kumru S, Godekmerdan A, Kutlu S. Effects of raloxifene, hormone therapy, and soy isoflavone on serum high‐sensitive C‐reactive protein in postmenopausal women. International Journal of Gynaecology and Obstetrics 2005;90(2):128‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Albertazzi 2005
- Albertazzi P, Steel SA, Bottazzi M. Effect of pure genistein on bone markers and hot flushes. Climacteric 2005;8(4):371‐9. [PUBMED: 16390772] [DOI] [PubMed] [Google Scholar]
Badimon 2011
- Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thrombosis and Haemostasis 2011;105 Suppl 1:S34‐42. [PUBMED: 21479344] [DOI] [PubMed] [Google Scholar]
Chun 2007
- Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. Journal of Nutrition 2007;137(5):1244‐52. [PUBMED: 17449588] [DOI] [PubMed] [Google Scholar]
Duncan 2000
- Duncan AM, Merz‐Demlow BE, Xu X, Phipps WR, Kurzer MS. Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiology, Biomarkers and Prevention 2000;9(6):581‐6. [PUBMED: 10868692] [PubMed] [Google Scholar]
Farzadfar 2011
- Farzadfar F, Finucane MM, Danaei G, Pelizzari PM, Cowan MJ, Paciorek, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country‐years and 3.0 million participants. Lancet 2011;377(9765):578‐86. [PUBMED: 21295847] [DOI] [PubMed] [Google Scholar]
Ford 2007
- Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980‐2000. New England Journal of Medicine 2007;356(23):2388‐98. [PUBMED: 17554120] [DOI] [PubMed] [Google Scholar]
He 2004
- He J, Gu D, Reynolds K, Wu X, Muntner P, Zhao J, et al. Serum total and lipoprotein cholesterol levels and awareness, treatment, and control of hypercholesterolemia in China. Circulation 2004;110(4):405‐11. [PUBMED: 15238453] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jackson 2011
- Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of S‐equol, an estrogen receptor β agonist. Nutrition Reviews 2011;69(8):432‐48. [PUBMED: 21790611] [DOI] [PubMed] [Google Scholar]
Kang 2010
- Kang X, Zhang Q, Wang S, Huang X, Jin S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. Canadian Medical Association Journal 2010;182(17):1857‐62. [PUBMED: 20956506] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keinan 2002
- Keinan‐Boker L, Peeters PH, Mulligan AA, Navarro C, Slimani N, Mattisson I, et al. Soy product consumption in 10 European countries: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutrition 2002;5(6B):1217‐26. [PUBMED: 12639228] [DOI] [PubMed] [Google Scholar]
Kreijkamp 2005
- Kreijkamp‐Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. American Journal of Clinical Nutrition 2005;81(1):189‐95. [PUBMED: 15640479] [DOI] [PubMed] [Google Scholar]
Laatikainen 2005
- Laatikainen T, Critchley J, Vartiainen E, Salomaa V, Ketonen M, Capewell S. Explaining the decline in coronary heart disease mortality in Finland between 1982 and 1997. American Journal of Epidemiology 2005;162(8):764‐73. [PUBMED: 16150890] [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333(7568):597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2006
- Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. Genistein acutely stimulates insulin secretion in pancreatic beta‐cells through a cAMP‐dependent protein kinase pathway. Diabetes 2006;55(4):1043‐50. [PUBMED: 16567527] [DOI] [PubMed] [Google Scholar]
Liu 2010
- Liu B, Qin L, Liu A, Uchiyama S, Ueno T, Li X, et al. Prevalence of the equol‐producer phenotype and its relationship with dietary isoflavone and serum lipids in healthy Chinese adults. Journal of Epidemiology 2010;20(5):377‐84. [PUBMED: 20671375] [DOI] [PMC free article] [PubMed] [Google Scholar]
Setchell 2002
- Setchell KD, Brown NM, Lydeking‐Olsen E. The clinical importance of the metabolite equol‐a clue to the effectiveness of soy and its isoflavones. Journal of Nutrition 2002;132(12):3577‐84. [PUBMED: 12468591] [DOI] [PubMed] [Google Scholar]
Setchell 2010
- Setchell KD, Clerici C. Equol: history, chemistry, and formation. Journal of Nutrition 2010;140(7):1355S‐62S. [PUBMED: 20519412] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shimazu 2010
- Shimazu T, Inoue M, Sasazuki S, Iwasaki M, Sawada N, Yamaji T, et al. Isoflavone intake and risk of lung cancer: a prospective cohort study in Japan. American Journal of Clinical Nutrition 2010;91(3):722‐8. [PUBMED: 20071645] [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
Taku 2007
- Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta‐analysis of 11 randomized controlled trials. American Journal of Clinical Nutrition 2007;85(4):1148‐56. [PUBMED: 17413118] [DOI] [PubMed] [Google Scholar]
Taku 2008
- Taku K, Umegaki K, Ishimi Y, Watanabe S. Effects of extracted soy isoflavones alone on blood total and LDL cholesterol: meta‐analysis of randomized controlled trials. Therapeutics and Clinical Risk Management 2008;4(5):1097‐103. [PUBMED: 19209289] [DOI] [PMC free article] [PubMed] [Google Scholar]
Unfer 2004
- Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Renzo GC. Endometrial effects of long‐term treatment with phytoestrogens: a randomized, double‐blind, placebo‐controlled study. Fertility and Sterility 2004;82(1):145‐8. [PUBMED: 15237003] [DOI] [PubMed] [Google Scholar]
Weggemans 2003
- Weggemans RM, Trautwein EA. Relation between soy‐associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta‐analysis. European Journal of Clinical Nutrition 2003;57(8):940‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
Zhan 2005
- Zhan S, Ho SC. Meta‐analysis of the effects of soy protein containing isoflavones on the lipid profile. American Journal of Clinical Nutrition 2005;81(2):397‐408. [PUBMED: 15699227] [DOI] [PubMed] [Google Scholar]
Zhuo 2004
- Zhuo XG, Melby MK, Watanabe S. Soy isoflavone intake lowers serum LDL cholesterol: a meta‐analysis of 8 randomized controlled trials in humans. Journal of Nutrition 2004;134(9):2395‐400. [PUBMED: 15333734] [DOI] [PubMed] [Google Scholar]


