Abstract
Distal arthrogryposis (DA) is a collection of rare disorders characterized by congenital joint contractures. Most DA mutations are in muscle- and joint-related genes, and the anatomical defects originate cell-autonomously within the musculoskeletal system. However, gain-of-function (GOF) mutations in PIEZO2, a principal mechanosensor in somatosensation, cause DA subtype 5 via unknown mechanisms. We show that expression of a GOF PIEZO2 mutation in proprioceptive sensory neurons mainly innervating muscle spindles and tendons is sufficient to induce DA5-like phenotypes in mice. Overactive PIEZO2 causes anatomical defects via increased activity within the peripheral nervous system during postnatal development. Further, Botox and a dietary fatty acids that modulate PIEZO2 activity reduce DA5-like deficits. This reveals a role for somatosensory neurons: excessive mechanosensation within these neurons disrupts musculoskeletal development.
One-sentence summary:
Overactive PIEZO2 in proprioceptive neurons disrupts musculoskeletal development.
Distal Arthrogryposis (DA) is a rare disorder with congenital contractures primarily affecting joints. It is estimated to afflict ~1 in 3,000 individuals worldwide and usually requires invasive surgeries to alleviate the symptoms (1, 2). Various mutations have been identified in DA patients in genes important for musculoskeletal function (3). DA5 is an autosomal dominant disorder with distinct clinical features including ophthalmoplegia and restrictive lung disease, in addition to contractures in distal joints (4). Gain-of-function (GOF) mutations in PIEZO2, a mechanically activated ion channel, have been found in DA5 patients (5). PIEZO2 is the principal mechanosensor in somatosensory neurons and underlies touch sensation (6) and proprioception (the sense of where one’s limbs are in space) (7), as well as mechanosensory processes in internal organs such as lung, aorta, and bladder (8–10). However, direct role for this ion channel in muscles or tendons has not been described.
Overactive PIEZO2 increases mechanosensitivity of sensory neurons.
We engineered mice that conditionally express a GOF Piezo2 mutant to study the disease mechanism for DA5. One such human GOF mutation E2727del is in the C-terminus of PIEZO2 and it causes slower channel inactivation (5). We designed a knock-in strategy that allows Cre recombinase-dependent replacement of a wild type C-terminal exon of PIEZO2 with one that harbors the human-equivalent mouse GOF variant E2799del (Fig. 1A). We first generated constitutive GOF Piezo2 mice (GOFconst.) by breeding mice homozygous for the GOF allele into CmvCre driver that expresses Cre recombinase ubiquitously (11). We used whole-cell patch clamp to record PIEZO2-dependent mechanically activated currents from dorsal root ganglion (DRG) neurons of homozygous GOF Piezo2 mice and wild type littermates (Fig. 1B and Fig. S1A). We initially tested homozygous instead of heterozygous mice to avoid diluting the effects of the GOF allele by a wildtype allele. Although the majority of homozygotes died perinatally for unknown reasons, we were able to obtain DRGs from viable mice for recording. DRG neurons from homozygous GOFconst. mice showed a slower inactivation and a remaining current at the end of the stimulus (as a percentage of the peak current at the strongest stimulus applied) compared to wild type (Fig. 1B and Fig. S1B). As expected, there was no detectable difference in Imax and apparent threshold between wild type and GOF neurons (Fig. S1C). We also compared mechanically activated currents in DRG neurons of heterozygous mice to wild type to reflect the human condition. To enrich the recorded population for PIEZO2-dependent rapidly inactivating neurons, we crossed mice carrying the GOF Piezo2 allele into PvalbCre/Ai9, which express Cre recombinase and the Ai9 tdTomato reporter protein in proprioceptive neurons (7, 12). We observed robust differences in the kinetics of mechanically activated currents between heterozygous GOF and wild type DRGs (Fig. 1C and D). These results validate that the engineered mice express a functional GOF PIEZO2 channel with similar kinetics of inactivation as human GOF mutations analyzed in heterologous system (5). They also demonstrate that heterozygosity of GOF PIEZO2 is sufficient to increase mechanosensitivity in sensory neurons.
Figure 1. Gain-of-function (GOF) PIEZO2 increases mechanosensitivity of sensory neurons.
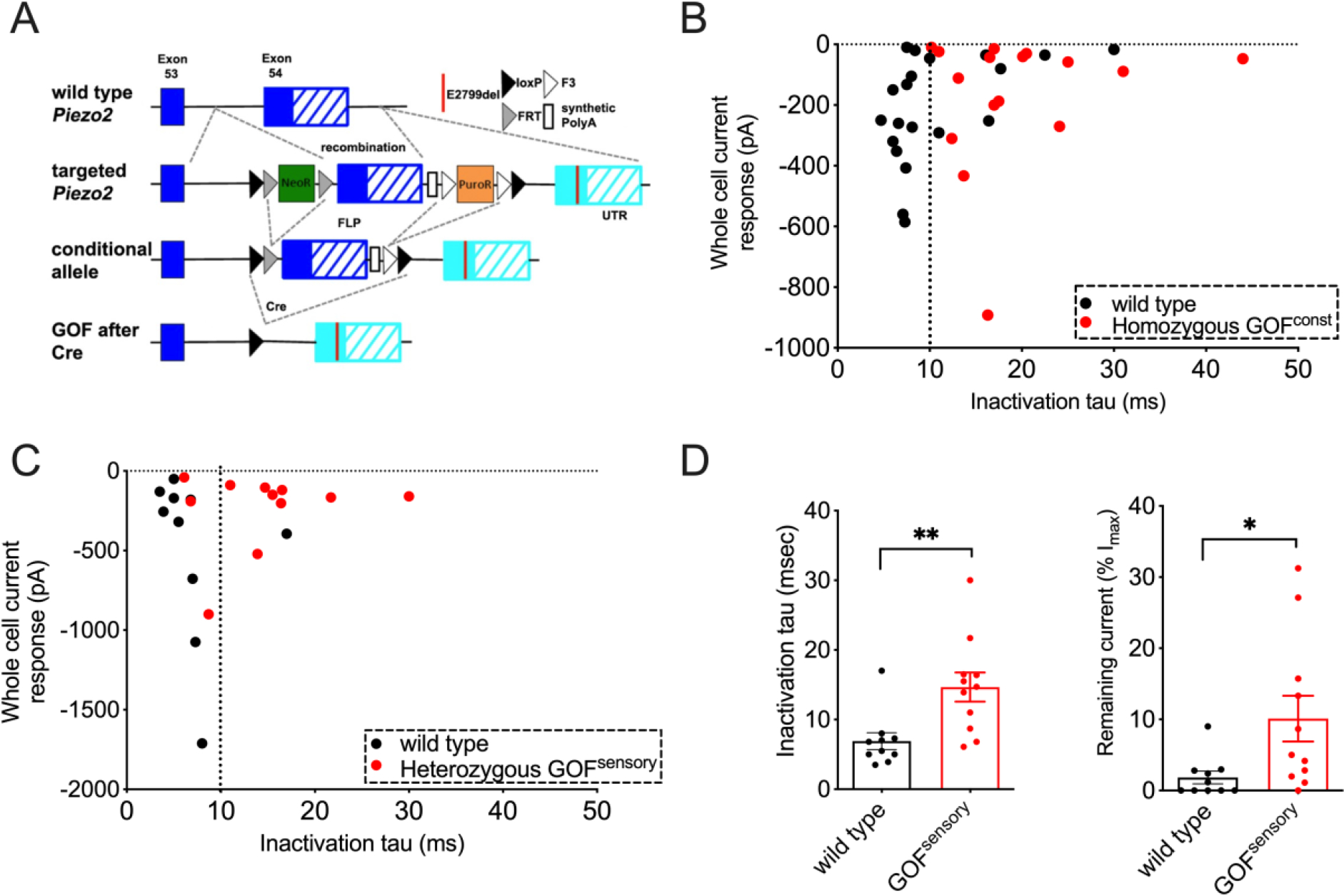
(A) Conditional GOF Piezo2 mouse model. Cre-dependent replacement of wild type exon (blue) by GOF-carrying exon (cyan). (B) Mechanically activated currents and inactivation time constant (tau) in DRG sensory neurons from wild type (black) and constitutively homozygous GOF Piezo2 mice (red). 87% WT and 70% GOF neurons were responsive to the stimulus. (C) Mechanically activated currents and inactivation time constant in Tdtomato+ neurons from PvalbCre/Ai9 (wild type, black) and GOF Piezo2; PvalbCre/Ai9 mice (red). (D) Bar graphs representing inactivation time constant (ms) and remaining current at the end of the stimulus (% of Imax value) in DRG sensory neurons. *p < 0.05, **p < 0.01, and ***p < 0.001 (Student’s t-test). Each data point represents a single DRG neuron.
Overactive PIEZO2 causes limb defects in mice.
The constitutive heterozygous GOF mice had normal body weight at different ages (Fig S2A), suggesting that overactive PIEZO2 is unlikely to impact overall growth. In addition, food intake and metabolic rate of these transgenic mice were comparable to those of wild type littermates over a period of eight days (Fig S2B and C), suggesting normal eating behavior and metabolism.
We observed that hindlimbs of the transgenic mice had contractures compared to wild type littermates (Fig. 2A). Direct visualization as well as Micro-computed tomography (CT) images showed that the angles between major hindlimb joints were smaller in GOFconst. mice than in wild type (Fig. 2A, upper panel). We measured the angle between the phalange and metacarpal, the two major bones of hindlimbs (it is challenging to quantitatively study forelimbs due to their small size) and found that phalange-metacarpal angles in hindlimbs of GOFconst. mice were significantly decreased compared to wild type mice (Fig. 2A, bar graph). DA5 patients have weak or absent tendon reflexes suggesting that defective tendon development might be associated with joint contractures (5). To evaluate tendon phenotypes, we dissected the intact tendons from hindlimbs (Fig. 2B), and we observed that the average length of tendons was significantly reduced in GOFconst. mice compared to wild type (Fig. 2B). It is therefore possible that the DA5-like contractures are caused by shortened tendons.
Figure 2. GOF Piezo2 mice develop limb defects.
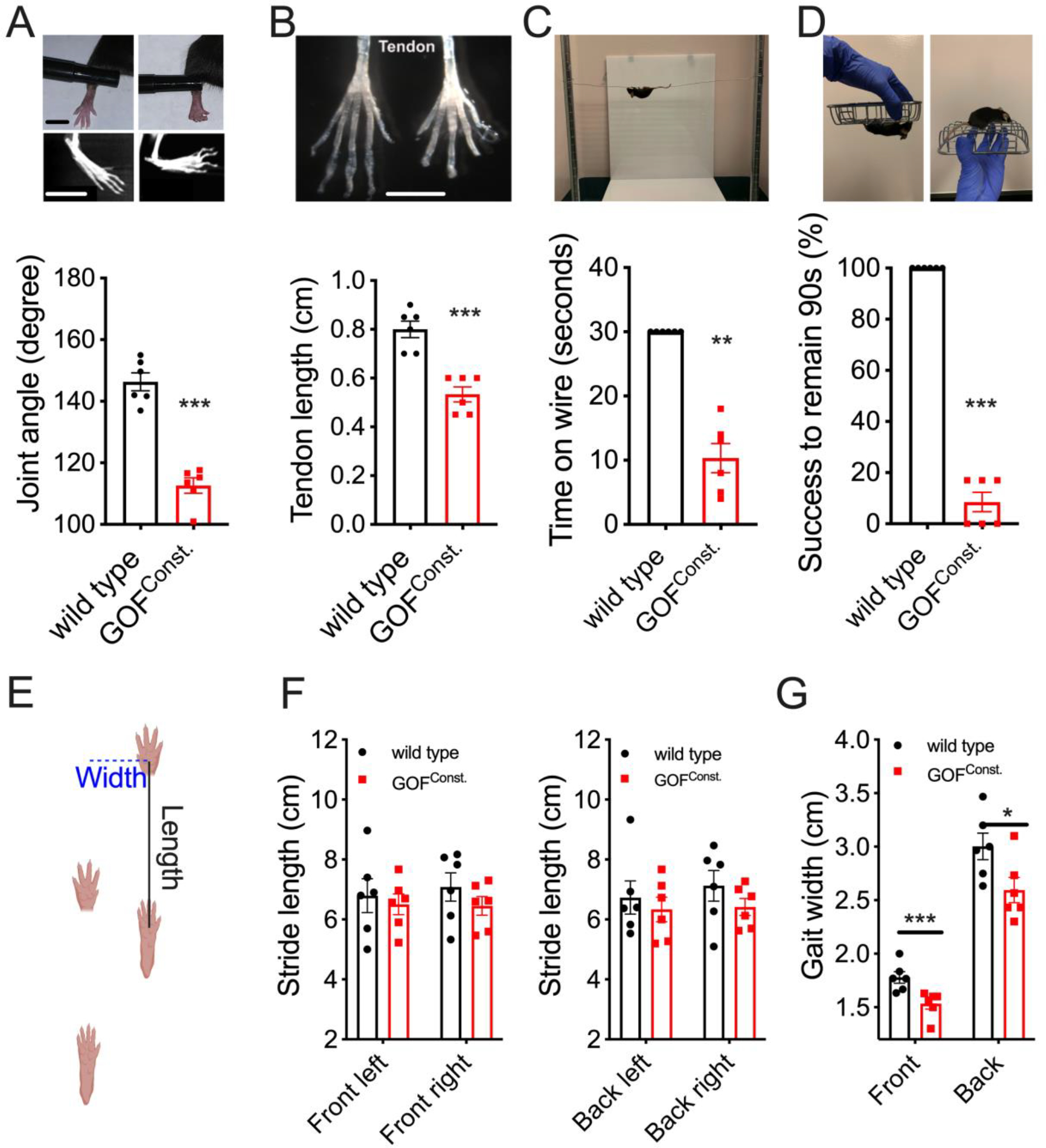
(A) Joint morphology of live animals and CT scan (upper images) and phalange-metacarpal joint angles (lower graph) in the hindlimb. Left: wild type; Right: GOF mice. Scale bars: 0.5cm. (B) Intact tendon (upper) and tendon length (lower) from hindlimbs. Scale bar: 0.5cm. (C) Hanging wire test (upper) and Time (sec) that animals remained on the metal wire, with 30 sec as cutoff (lower). (D) Inverted screen test (upper) and quantification as percentage of animals successfully remaining on the rotating screen for 90 seconds (lower). (E) Gait assay: the stride length measures forelimb-hindlimb distance in the first (front) and second (back) stride. Gait width is the distance between forelimbs (front) or between hindlimbs (back) (F-G) Quantifications for E. *p < 0.05, **p < 0.01, and ***p < 0.001 (Student’s t-test). Each data point represents a single animal.
Anatomical deformities cause limited joint movements and affect motor functions in distal arthrogryposis patients. To test whether GOF PIEZO2 impacts limb functionality in mice, we performed two behavioral tests. First, we used a hanging-wire assay (13). The test is based on the latency of a mouse to fall off from a metal wire (Fig. 2C). We found that wild type mice were able to remain on the wire for at least 30 seconds, whereas GOFconst. mice fell off significantly earlier (Fig. 2C). Second, we performed the inverted screen test (14), which measures the time until a mouse falls off a square screen after it is turned upside down (Fig. 2D and method). All wild type mice were able to hold on for at least 90 seconds. In contrast, the average success rate for GOFconst. mice was less than 10% (Fig. 2D). Thus, these results suggest that limb functionality was compromised in GOFconst. mice, consistent with the anatomical defects.
We also measured the gait of GOF Piezo2 mice (Fig 2E). The length of the stride during locomotion was similar to that of wild type (Fig 2F). In contrast, the width of their stride was decreased compared to wild type (Fig 2G). This is expected given the anatomical deficits, and is reminiscent of limited range of limb motion observed in DA5 human patients (5). Despite these defects, GOF Piezo2 mice had normal daily activity patterns in an open field arena over eight days (Fig S2D), suggesting that overactive PIEZO2 is not completely debilitating.
Somatosensory PIEZO2 overactivity causes limb defects.
To investigate which cell types are involved in the DA5 etiology, we expressed GOF Piezo2 allele in various tissues. We induced GOF PIEZO2 expression in all mesenchymal cells including skeletal muscles, cartilages and tendons using Prrx1Cre (15, 16). This Cre line induces robust recombinase activity in mouse limb mesenchyme starting from early embryogenesis. These GOF Piezo2 mice had normal phalange-metacarpal angles and tendon lengths in their hindlimbs (Fig. 3A and B). Consistently, these mice performed comparably to wild type mice on behavioral assays (Fig. 3C and D). On the other hand, we found that expression of GOF PIEZO2 in proprioceptive sensory neurons (via PvalbCre knock-in mice) that mainly innervate muscle spindles and tendon organs (7, 17) was sufficient to cause joint contractures, shortened tendons, and poor performance on behavioral assays (Fig. 3A–D). These data suggest that overactive PIEZO2 in sensory neurons, but not in mesenchyme, is sufficient to induce DA5-like phenotypes in vivo.
Figure 3. GOF PIEZO2 in sensory neurons causes limb defects.
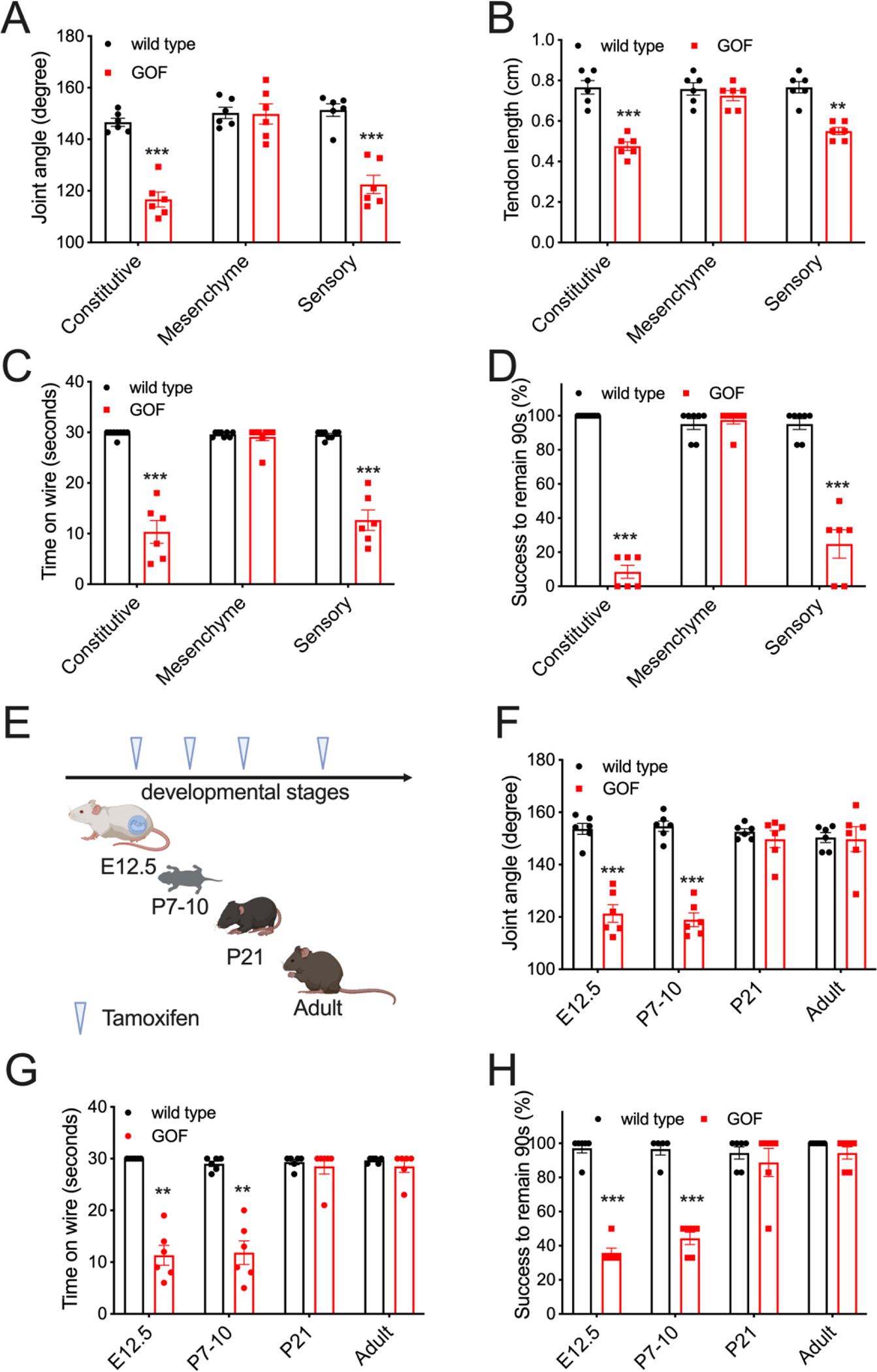
(A) Phalange-metacarpal joint angle of the hindlimbs in wild type and tissue specific GOF mice (black: wild type; red: GOF Piezo2). (B) Tendon length of hindlimbs. (C) Quantification of hanging wire test results. (D) Quantification of inverted screen test results. (E) Tamoxifen induction of GOF PIEZO2 in proprioceptive neurons by AdvillinCre-ERT2 at various developmental stages. (F) Phalange-metacarpal joint angle of the hindlimbs in E. (G) Quantification of hanging wire test results in E. (H) Quantification of inverted screen test in E. *p < 0.05, **p < 0.01, and ***p < 0.001 (One-way ANOVA followed by Tukey’s multiple comparison). Each data point represents a single animal.
Since DA is a developmental disorder presumably originating during fetal stages (2), we used our mouse model to determine the timing of PIEZO2 activity in sensory neurons that affects musculoskeletal development. We used an inducible sensory neuron-specific GOF Piezo2 mouse by crossing the GOF allele into AdvillinCre-ERT2 driver mice (18). Advillin is expressed in most DRG neurons, but not central nervous system or non-neuronal cell types, starting in early embryogenesis (19, 20). We induced GOF PIEZO2 expression in sensory neurons at various developmental stages by tamoxifen injection (Fig. 3E). When induced at embryonic day 12.5 or at postnatal day 7–10 (P7–10), these mice showed significantly decreased phalange-metacarpal joint angles (Fig. 3F), suggesting that the phenotype is mainly dependent on expression of PIEZO2 sometime after P7. However, induction of GOF PIEZO2 expression in sensory neurons after P21 or during adulthood (> 1month old) did not cause joint defects in mice (Fig. 3F). Consistent with anatomical phenotypes, mice expressing GOF PIEZO2 in sensory neurons during embryonic (E12.5) and early postnatal stage (P7–10), but not after early adulthood (>P21), performed poorly on behavioral tests (Fig. 3G and H). These results confirm our earlier findings using PvalbCre that overactive PIEZO2 in sensory neurons causes DA5-like defects (Fig. 3A–D). In addition, they suggest a critical postnatal developmental period between days 7 and 21 during which excessive mechanotransduction in somatosensory neurons causes limb malformation.
To ensure that the anatomical deficits are not an indirect consequence of compromised proprioceptive neuronal development, we examined proprioceptive nerve endings of muscle spindles and Golgi tendon organs (Fig. S3). We did not observe overt anomalies in these proprioceptive endings (Fig. S3A). In addition, we quantified the size of proprioceptors innervating muscle spindles and observed no difference between GOF Piezo2 and wild type mice (Fig. S3B). Further, we did not find significant changes in extrafusal and intrafusal muscle fiber diameters in GOF Piezo2 mice (Fig. S3C), suggesting that overactive PIEZO2 in sensory neurons does not appear to have strong effects on muscle development.
Overactive PIEZO2 causes defects via efferent pathways.
We also used a pharmacological approach to further test whether anatomical defects of GOF Piezo2 mice are attributed to hyperactivation of sensory neurons. We performed intra-muscular injection of Botulinum toxin (Botox) into the hindlimb of mice at P7–10 (Fig. 4A), at the beginning of the critical period. The contralateral hindlimb received vehicle injection as an internal control. Botox blocks neural transmission from both sensory and motor neurons by inhibiting exocytosis (21). Four weeks after a single dose of injection, we observed that Botox did not affect joint morphology and tendon length in wild-type mice (Fig. 4A). Meanwhile, it rescued joint and tendon defects in the hindlimb that received the treatment in GOF Piezo2 mice compared to hindlimb injected with vehicle control (Fig. 4A). Further, if administered on both sides of hindlimbs, Botox improved performances on the behavioral assays for GOF Piezo2 mice (Fig. 4B). However, it failed to completely restore the behavioral outcomes, probably due to deficits in forelimbs which did not receive Botox treatment. This result suggests that GOF PIEZO2-dependent neuronal activity is responsible for joint defects.
Figure 4. Pharmacological effects on limb phenotypes in GOF mice.
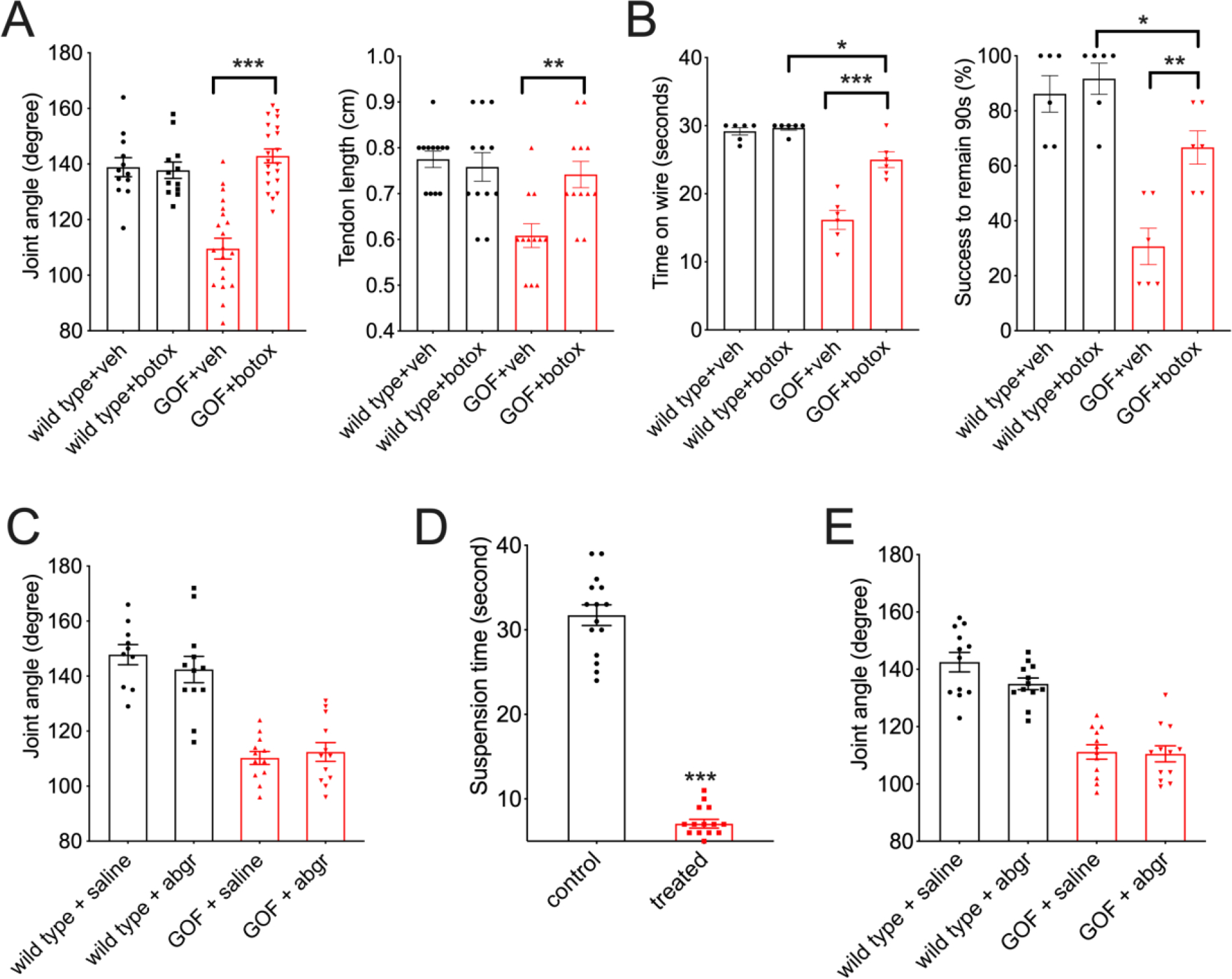
(A) Phalange-metacarpal joint angle and tendon length of the hindlimbs in young adult wild type and GOF Piezo2; PvalbCre mice that received Botulinum toxin (Botox) at P7–10. (B) Quantification of behavioral tests in mice treated with Botox. (C) Phalange-metacarpal joint angle of the hindlimbs in young adult mice receiving a single dose of alpha-Bungarotoxin (abgr) at P7–10. (D) Quantification for motor defects (in a modified hanging assay) in young adult mice receiving 5-day alpha-Bungarotoxin (abgr) treatment at P7–10. (E) Phalange-metacarpal joint angle of the hindlimbs in young adult mice receiving daily alpha-Bungarotoxin (abgr) treatment from P5–10 in D.
Mechanical activation of proprioceptive nerve endings has two consequences: 1. it causes local Ca2+-dependent signaling within the peripheral proprioceptive endings culminating in exocytosis (22), and 2. it induces an afferent signal by initiating firing of proprioceptive neurons, which activates downstream motor neurons innervating the same peripheral muscle to counteract the stretch (23). Botulinum toxin would block both these processes. To test whether the results we observed using Botox resulted from blocking motor neuron output, we injected ɑ-Bungarotoxin, which blocks neural transmission from motor neurons to muscles (24), into hindlimbs of P7–10 GOF Piezo2 mice. This treatment did not alleviate anatomical defects in these mice (Fig. 4C). It is possible that a single dose of ɑ-Bungarotoxin is insufficient to inhibit motor transmission throughout the critical developmental period because it has shorter pharmacological window than Botox (25). To assess this possibility, we injected ɑ-Bungarotoxin into the hindlimbs for five consecutive days. This treatment was sufficient to severely impact motor neuron function, as it compromised motor behavior (Fig. 4D), but it still failed to rescue anatomical defects in GOF Piezo2 mice (Fig. 4E). This implies that motor activity does not play a major role in DA5 like deficits. Together, our pharmacological experiments suggest that GOF PIEZO2 causes musculoskeletal abnormalities via ectopic efferent activity in afferent peripheral mechanosensory neuronal endings, likely involving increased levels of exocytosis.
We also tested whether GOF PIEZO2 allele caused increased proprioceptive firing (afferent function) during development. To measure muscle spindle firing, we evaluated proprioceptor mechanotransduction ex-vivo. In this assay, we mechanically stretched the extensor digitorum longus (EDL) muscle and recorded from the innervating proprioceptors (muscle spindles) to assess stretch-induced neuronal activity (Fig. S4A) (7, 26). We found that muscle spindle afferent activity in response to stretch is not significantly different between wild type and GOF Piezo2 mice at P7 postnatal stage (Fig. S4B–C). Therefore, we observe increased PIEZO2 current in cell culture assays, but no increased neuronal firing ex vivo. It is possible that somatosensory neurons have compensatory mechanisms for controlling overactive transduction responses. Thus, these recordings are consistent with the idea that local efferent action of afferent proprioceptive neurons caused by overactive PIEZO2 is responsible for the developmental deficits.
EPA diet alleviates defects in GOF mice.
Our data suggests that manipulations that would decrease overall PIEZO2 activity during a critical development time could potentially rescue the DA5-like phenotype. The ω-3 polyunsaturated fatty acid (PUFA) eicosapentaenoic acid (EPA), a membrane lipid component, significantly decreases the inactivation time of wild type and GOF PIEZO1 channels, a close relative of PIEZO2 (27, 28). Because EPA is commonly found in fish and widely used as a dietary supplement, we tested whether a diet enriched in ω-3 PUFAs has therapeutic potential.
We first showed that overnight incubation with EPA (200 μM) reduced wild type as well as GOF mutant PIEZO2 inactivation time constant in a heterologous expression system (Fig. 5A and B). This suggests that EPA has the potential to modulate disease causing mutations in PIEZO2. We then fed female mice a diet enriched in EPA during pregnancy and nursing. The sensory neurons of their offspring were later assessed for PIEZO2-dependent mechanosensitivity (Fig. S5A). We found that EPA-enriched diet intake significantly reduced inactivation time constant of mechanically stimulated currents in sensory neurons but had no effect on the current intensity and apparent threshold (Fig. S5A). Next, we determined the effect of this diet on the anatomical defects in GOF Piezo2 mice. Liquid chromatography-mass spectrometry (LC-MS) showed that the feeding protocol successfully incorporated EPA into the plasma membrane of sensory neurons that carry the GOF Piezo2 mutation (Fig. S5B). Finally, we observed that dietary EPA partially prevented joint malformations (Fig. 5C) and improved the performances on the hanging wire and inverted screen tests (Fig. 5D) in GOF Piezo2 mice.
Figure 5. EPA diet rescues joint defects in GOF mice.
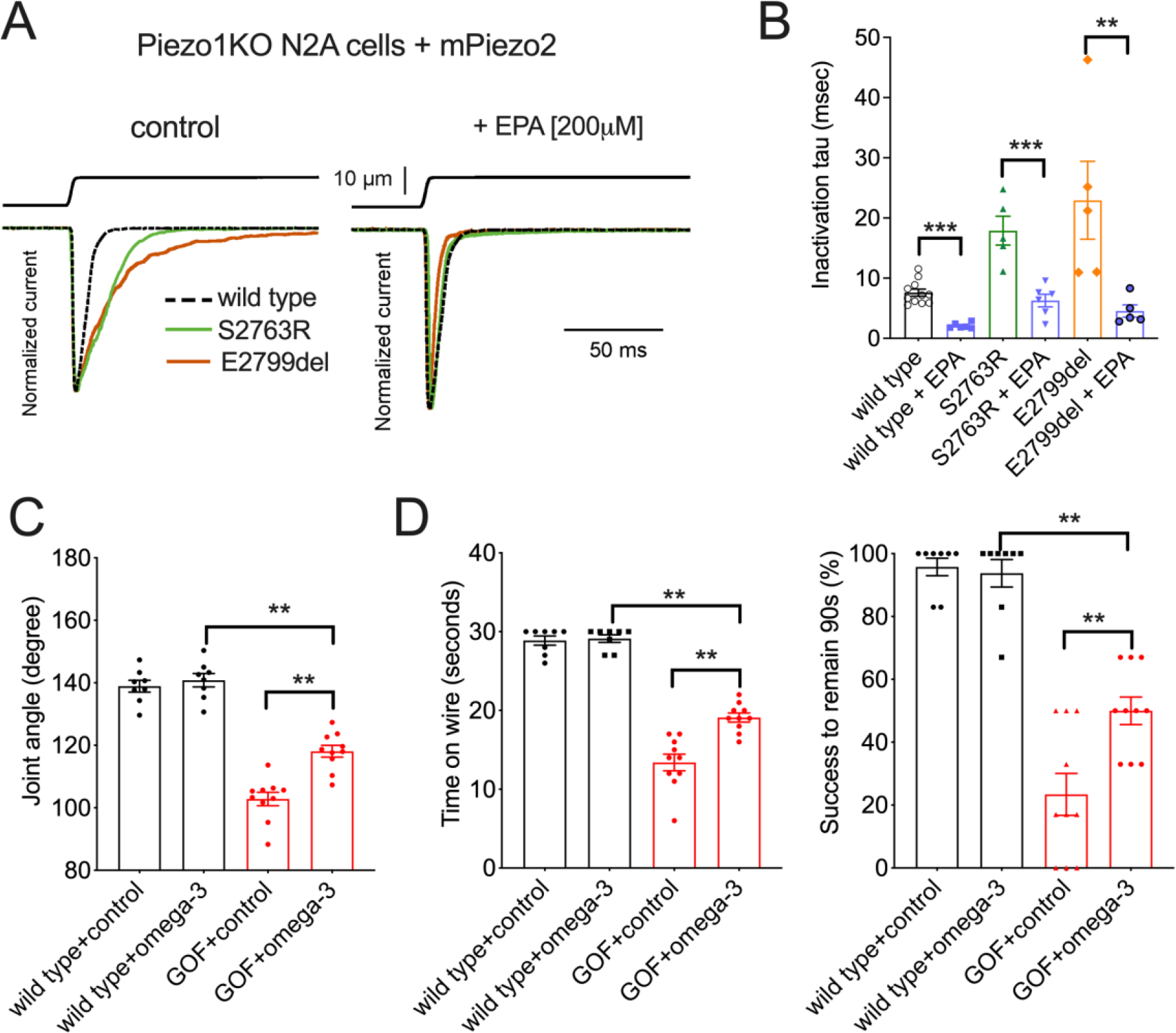
(A) Mechanically activated currents in N2APiezo1−/− cells heterologously expressing mouse PIEZO2 wild type (stippled) and GOF mutants S2763R (green) and E2799del (brown). (B) Quantification of the inactivation time constant results in A. (C) Phalange-metacarpal joint angle of the hindlimbs in young adult wild type and GOF Piezo2; PvalbCre mice that received EPA-enriched diet. Control: sunflower-oil-based food, which has a similar level of fat content and energy. (D) Quantification of hanging wire and inverted screen test results in C. *p < 0.05, **p < 0.01, and ***p < 0.001 (Two-way ANOVA followed by Tukey honestly significance test).
Our study has identified a critical postnatal period during development when relatively small excess PIEZO2 activity in somatosensory neurons can cause tendon deficits and severe joint contractures. Previous work has shown that loss of PIEZO2 in proprioceptive neurons causes skeletal abnormalities manifested as spine malalignment in mice (15), indicating a requirement of proprioceptive feedback for proper vertebral development. We hypothesize that overactive PIEZO2 causes anatomical abnormalities in joints via increased exocytosis from sensory neuron endings without involving motor circuitry. Indeed, it is known that sensory neurons secrete a variety of factors in response to neuronal stimulation, including glutamate release from proprioceptive endings (22). While the role of secreted factors such as calcitonin-gene-related peptide (CGRP) from nociceptors in mediating pain and inflammation is well documented (29), relatively little is known about the importance and identity of such efferent signaling molecules in other sensory neurons, including proprioceptors.
We also present proof-of-concept that Botox injection or dietary treatment can counteract the effect of overactive PIEZO2 function to evade DA-like phenotypes in mice when applied during a developmental critical period. These approaches might have clinical applications. Beyond this, our findings call attention to the importance of considering sensory mechanotransduction when diagnosing and treating other musculoskeletal disorders.
Supplementary Material
Acknowledgements:
We thank Anton Maximov for discussion and for critical reading of the manuscript.
Funding:
National Institutes of Health grant R35 NS105067 and R01 DE022358 (AP)
National Institutes of Health grant SC3 GM127195 (KAW)
National Institutes of Health grant R25 GM071381 (AS)
National Institutes of Health grant R01GM133845 (VV)
National Institutes of Health grant NCCIH/NINDS Intramural Program (ATC)
Howard Hughes Medical Institute (AP)
Footnotes
Competing interests: The authors declare no competing financial interests.
Data and materials availability:
GOF Piezo2 mice (C57BL/6NTac-Fam38btm3287(ED2799D)Arte are available from Ardem Patapoutian upon request or directly from Taconic Biosciences. All data are available in the main text or the supplementary materials.
References
- 1.Bamshad M, Van Heest AE, Pleasure D, Arthrogryposis: A Review and Update. Journal of Bone and Joint Surgery. 91, 40–46 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowalczyk B, Feluś J, Arthrogryposis: an update on clinical aspects, etiology, and treatment strategies. aoms. 1, 10–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajsharghi H, Kimber E, Kroksmark A-K, Jerre R, Tulinius M, Oldfors A, Embryonic Myosin Heavy-Chain Mutations Cause Distal Arthrogryposis and Developmental Myosin Myopathy That Persists Postnatally. Arch Neurol. 65 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Beals RK, Weleber RG, Distal arthrogryposis 5: A dominant syndrome of peripheral contractures and ophthalmoplegia. Am. J. Med. Genet. 131A, 67–70 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, Philippakis A, Hoischen A, Riemer G, Steen U, Steen VM, Mathur J, Cox J, Lebo M, Rehm H, Weiss ST, Wood JN, Maas RL, Sunyaev SR, Patapoutian A, Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc. Natl. Acad. Sci. U.S.A. 110, 4667–4672 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranade SS, Woo S-H, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A, Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 516, 121–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo S-H, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Jessell TM, Wilkinson KA, Patapoutian A, Piezo2 is the principal mechanotransduction channel for proprioception. Nat Neurosci. 18, 1756–1762 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nonomura K, Woo S-H, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A, Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 541, 176–181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng W-Z, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, Patapoutian A, PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science. 362, 464–467 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall KL, Saade D, Ghitani N, Coombs AM, Szczot M, Keller J, Ogata T, Daou I, Stowers LT, Bönnemann CG, Chesler AT, Patapoutian A, PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature. 588, 290–295 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwenk F, Baron U, Rajewsky K, A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23, 5080–5081 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H, A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 13, 133–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers DC, Fisher EMC, Brown SDM, Peters J, Hunter AJ, Martin JE, Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mammalian Genome. 8, 711–713 (1997). [DOI] [PubMed] [Google Scholar]
- 14.Deacon RMJ, Measuring the Strength of Mice. JoVE, 2610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assaraf E, Blecher R, Heinemann-Yerushalmi L, Krief S, Carmel Vinestock R, Biton IE, Brumfeld V, Rotkopf R, Avisar E, Agar G, Zelzer E, Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity. Nat Commun. 11, 3168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ, Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 33, 77–80 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S, A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, e159 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau J, Minett MS, Zhao J, Dennehy U, Wang F, Wood JN, Bogdanov YD, Temporal control of gene deletion in sensory ganglia using a tamoxifen-inducible Advillin-Cre-ERT2 recombinase mouse. Mol Pain. 7, 100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akopian AN, Wood JN, Peripheral Nervous System-specific Genes Identified by Subtractive cDNA Cloning. Journal of Biological Chemistry. 270, 21264–21270 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Orefice LL, Mosko JR, Morency DT, Wells MF, Tasnim A, Mozeika SM, Ye M, Chirila AM, Emanuel AJ, Rankin G, Fame RM, Lehtinen MK, Feng G, Ginty DD, Targeting Peripheral Somatosensory Neurons to Improve Tactile-Related Phenotypes in ASD Models. Cell. 178, 867–886.e24 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davletov B, Bajohrs M, Binz T, Beyond BOTOX: advantages and limitations of individual botulinum neurotoxins. Trends Neurosci. 28, 446–452 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Bewick GS, Reid B, Richardson C, Banks RW, Autogenic modulation of mechanoreceptor excitability by glutamate release from synaptic-like vesicles: evidence from the rat muscle spindle primary sensory ending. J Physiol. 562, 381–394 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H-H, Hippenmeyer S, Arber S, Frank E, Development of the monosynaptic stretch reflex circuit. Curr Opin Neurobiol. 13, 96–102 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Wilson PT, Hawrot E, Lentz TL, Distribution of alpha-bungarotoxin binding sites over residues 173–204 of the alpha subunit of the acetylcholine receptor. Mol Pharmacol. 34, 643–650 (1988). [PubMed] [Google Scholar]
- 25.Fertuck HC, Woodward W, Salpeter MM, In vivo recovery of muscle contraction after alpha-bungarotoxin binding. J Cell Biol. 66, 209–213 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson KA, Kloefkorn HE, Hochman S, Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation. PLoS One. 7, e39140 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cordero-Morales JF, Vásquez V, How lipids contribute to ion channel function, a fat perspective on direct and indirect interactions. Current Opinion in Structural Biology. 51, 92–98 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero LO, Massey AE, Mata-Daboin AD, Sierra-Valdez FJ, Chauhan SC, Cordero-Morales JF, Vásquez V, Dietary fatty acids fine-tune Piezo1 mechanical response. Nat Commun. 10, 1200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyengar S, Ossipov MH, Johnson KW, The role of calcitonin gene–related peptide in peripheral and central pain mechanisms including migraine. Pain. 158, 543–559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
GOF Piezo2 mice (C57BL/6NTac-Fam38btm3287(ED2799D)Arte are available from Ardem Patapoutian upon request or directly from Taconic Biosciences. All data are available in the main text or the supplementary materials.


