Abstract
Ten unrelated Hafnia alvei clinical isolates were grouped according to either their low-level and inducible cephalosporinase production or their high-level and constitutive cephalosporinase production phenotype. Their AmpC sequences shared 85 to 100% amino acid identity. The immediate genetic environment of ampC genes was conserved in H. alvei isolates but was different from that found in other ampC-possessing enterobacterial species.
As with other cephalosporinase-producing enterobacterial species (3, 15), Hafnia alvei isolates may be grouped into two β-lactamase expression phenotypes, i.e., low-level and inducible cephalosporinase production and ceftazidime susceptibility on the one hand and high-level and constitutive cephalosporinase production and ceftazidime resistance on the other hand (18). We have previously shown (i) that an ampR gene is located upstream of the ampC gene and that its product acts as a repressor on the basal level of AmpC biosynthesis and as an activator upon addition of a β-lactam inducer and (ii) that transformation of an in vitro-obtained ceftazidime-resistant H. alvei mutant with ampD from Escherichia coli restores an inducible phenotype (5). The chromosome-borne AmpC of H. alvei clinical isolate 1, ACC-2, shares 94% amino acid identity with ACC-1, a plasmid-borne cephalosporinase from Klebsiella pneumoniae KUS (2, 5).
The aims of this study were to investigate the molecular heterogeneity of the ampC genes present in several unrelated H. alvei clinical isolates expressing either of the two cephalosporinase expression phenotypes and to analyze their immediate genetic environment.
Bacterial strains, plasmid, and pulsed-field gel electrophoresis (PFGE) analysis.
Ten H. alvei isolates were isolated from biliary fluids (n = 3), a tracheobronchial aspirate (n = 1), stool specimens (n = 3), blood cultures (n = 1), and urinary tract infection specimens (n = 2) of patients hospitalized in 1997 and 1998 at the Hôpital de Bicêtre (Le Kremlin-Bicêtre, France). These isolates, identified as described previously (5), were chosen in order to exclude those (i) isolated from patients hospitalized in the same department during the same 2-month period and (ii) showing a β-lactam resistance phenotype consistent with that of a penicillinase as deduced from a routine antibiogram. Plasmid DNA extractions (14) revealed that none of the isolates harbored the plasmid.
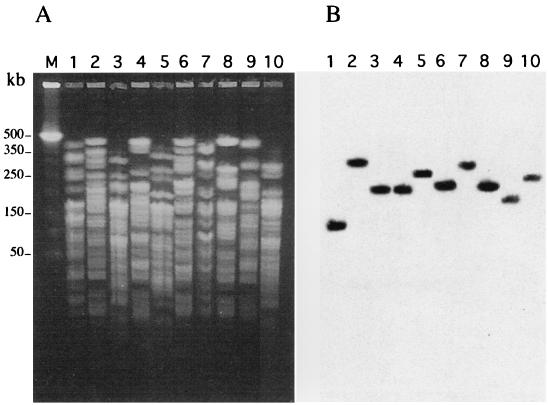
Comparison of H. alvei genomic DNAs was performed by a PFGE technique as reported previously (14). It showed that H. alvei isolates were not clonally related (Fig. 1A). A PFGE gel of SfiI-restricted DNAs of H. alvei isolates 1 to 10 followed by Southern hybridization using an intragenic probe of blaACC-2 made of a PCR-amplified fragment (primer A, 5′-GCGTAAAAAAATGCAGAACACC-3′; primer B, 5′-CACTTCCAACGAGCTCAGGATT-3′) (5, 16) revealed blaACC-2-like genes in each isolate and on separate macrorestriction fragments (Fig. 1B).
FIG. 1.
PFGE patterns of SfiI-restricted DNAs of 10 H. alvei isolates (A) and their Southern transfer and hybridization with an internal probe of blaACC-2 from H. alvei 1 (B). Lanes 1 through 10, H. alvei 1 to 10, respectively; lane M, molecular size markers are in kilobase pairs.
Susceptibility testing and β-lactamase assays.
The MICs of selected β-lactams were determined and β-lactamase assays were performed as described previously (5). Cephalosporinase basal-level and induction experiments allowed the division of H. alvei isolates into two groups: those with low-level and inducible expression of cephalosporinase and those with high-level and constitutive expression of cephalosporinase (Table 1). The first phenotype (H. alvei isolates 1 to 6) conferred susceptibility to extended-spectrum cephalosporins and cefoxitin (Table 1) except in H. alvei 6, for which the MICs of ceftazidime, cefotaxime, and cefpirome were increased, which might be due to an additional decrease in the permeability of the outer membrane (Table 1). The second phenotype (H. alvei isolates 7 to 10) conferred resistance or intermediate susceptibility to extended-spectrum cephalosporins, including cefpirome, and susceptibility to cefoxitin and cefepime. For H. alvei isolates that expressed a constitutive phenotype, full susceptibility to cefoxitin and the uncommon decreased susceptibility to cefpirome for an AmpC cephalosporinase corresponded to the atypical biochemical properties described for ACC-2 of H. alvei 1 (5). Isoelectric focusing of cultures of H. alvei clinical isolates (5) identified pIs ranging from 7.7 to 8.1 (Table 1), which lies close to the pI of 8 determined for ACC-2 (5).
TABLE 1.
β-Lactamase expression phenotypes, MICs of selected β-lactams, pIs, and sequences of β-lactamases for 10 H. alvei clinical isolates
| Phenotype | Isolate | β-Lactamase activity (mU/mg of protein)a
|
MICb (μg/ml) of:
|
pI | AAC-1-like sequence | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Basal | Induced | CAZ | CTX | CPO | FOX | FEP | ||||
| Low-level inducible | 1 | 46 | 4,500 | 2 | 0.25 | 0.5 | 8 | 0.03 | 8.0 | ACC-2 |
| 2 | 60 | 8,200 | 1 | 0.25 | 0.12 | 8 | 0.03 | 8.0 | AAC-1a | |
| 3 | 135 | 14,800 | 0.25 | 0.12 | 0.06 | 8 | 0.03 | 8.0 | ACC-3 | |
| 4 | 97 | 4,500 | 4 | 0.25 | 0.25 | 8 | 0.03 | 8.1 | ACC-1b | |
| 5 | 103 | 4,700 | 2 | 0.25 | 0.06 | 8 | 0.03 | 7.7 | ACC-3a | |
| 6 | 150 | 5,700 | 64 | 4 | 2 | 8 | 0.06 | 8.0 | ACC-1a | |
| High-level constitutive | 7 | 9,100 | 8,200 | 512 | 16 | 4 | 8 | 0.50 | 8.1 | ACC-1c |
| 8 | 10,600 | 9,000 | 64 | 32 | 4 | 8 | 0.50 | 8.0 | ACC-3 | |
| 9 | 8,200 | 12,600 | 512 | 32 | 4 | 8 | 0.50 | 8.1 | ACC-1d | |
| 10 | 12,100 | 16,400 | 256 | 16 | 4 | 8 | 0.50 | 8.0 | ACC-1a | |
One unit of β-lactamase was defined in this case as 1 μmol of cephalothin hydrolyzed per min. The standard deviations were within 10%.
Abbreviations are as follows: CAZ, ceftazidime; CTX, cefotaxime; CPO, cefpirome; FOX, cefoxitin; and FEP, cefepime.
H. alvei clinical isolates 7 and 8, which express a constitutive phenotype, were transformed with plasmid pNH5, which contains the ampD gene of E. coli (5). Both recombinant strains exhibited a low-level and inducible cephalosporinase expression phenotype (data not shown) associated with a decrease in β-lactam MICs such as those of ceftazidime, cefpirome, and cefepime, being 1, 0.5, and 0.03 μg/ml, respectively. Thus, the role of an AmpD-like protein in the regulation of the expression of H. alvei cephalosporinase was confirmed with clinical isolates as shown with an in vitro-obtained ceftazidime-resistant H. alvei 1 mutant (5).
Sequence analysis of ampCs and their genetic environment.
Using a set of internal and external primers to the blaACC-2 sequence (5) (primers A, B, C [5′-TCTTTTGCATGCTGATTGGC-3′], D [5′-CCGAGAAATCGGTGACTC-3′], E [5′-AATCAGGCGGCGATAGCGGATAT-3′], and F [5′-GCTTCAAGGTGTTCTGCATTT-3′]), PCR amplification products were obtained by using genomic DNAs of H. alvei 2 to 10 as templates. The products were sequenced and analyzed as described previously (5). AmpC amino acid identities among H. alvei isolates ranged from 85 to 100%, allowing us to divide them into three subgroups (Fig. 2): point mutant derivatives of ACC-1, namely, ACC-1a (H. alvei 2, 6, and 10), ACC-1b (H. alvei 4), ACC-1c (H. alvei 7), and ACC-1d (H. alvei 9); ACC-2, previously identified from H. alvei 1, which possesses 94% amino acid identity with ACC-1 (5); and ACC-3 (H. alvei 3 and 8), which possesses 87% amino acid identity with ACC-1, and a point mutant derivative, ACC-3a (H. alvei 5). The amino acid identity between ACC-2 and ACC-3 was 85%. The ACC-1-like enzymes clustered in the same subgroup as that of enterobacterial cephalosporinase (data not shown). As exemplified by ACC-1a found in H. alvei 2, 6, and 10, no correlation was established between the AmpC sequence and its cephalosporinase phenotype. Moreover, relative activities of restricted and extended-spectrum cephalosporins were similar for each H. alvei cephalosporinase, whatever the expression phenotype was (data not shown).
FIG. 2.
Amino acid sequence comparison of the chromosome-borne AmpCs from H. alvei isolates with the plasmid-mediated cephalosporinase ACC-1 from K. pneumoniae KUS (2) and the previously identified chromosome-borne ACC-2 from H. alvei 1 (5). Dashes indicate identical amino acids. ACC-1a was from H. alvei 2, 6, and 10; ACC-1b was from H. alvei 4, ACC-1c was from H. alvei 7, ACC-1d was from H. alvei 9, ACC-3 was from H. alvei 3 and 8, and ACC-3a was from H. alvei 5. Numbering is according to that of the ACC-1 sequence (2), to which 4 amino acids have been added at its N terminus in order to match the consensus sequence derived from the analysis of the cephalosporinase sequences.
Amino acid changes in H. alvei cephalosporinases occurred throughout the entire cephalosporinase sequence, as has been described for cephalosporinases of Citrobacter freundii, Enterobacter cloacae, and Pseudomonas aeruginosa (4, 8, 17) (Fig. 2). However, none of these amino acid changes were located in the putative active site of the H. alvei cephalosporinase, unlike those identified in the active sites of some cephalosporinases of E. cloacae, C. freundii, and Serratia marcescens that confer an extended hydrolytic profile (7, 9, 10–13, 19).
The genetic variability of AmpC sequences in H. alvei corresponded to that found in C. freundii; the amino acid identity between AmpCs of C. freundii I113 and 0S60 was only 82% (8). On the other hand, the sequence identity of AmpCs of three E. cloacae isolates was higher, since they differed only by point mutations at eight positions (4). In P. aeruginosa, the sequence identity of AmpCs is much higher than in H. alvei (99.6 versus 85%) (17).
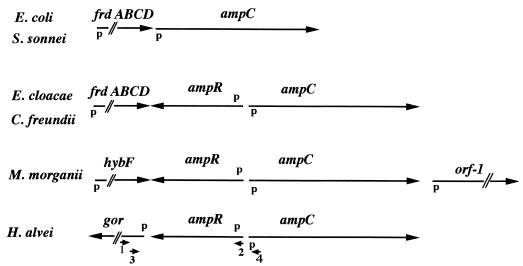
An ampR gene had been identified upstream of the ampC gene in H. alvei 1 (5). A further 540 bp upstream of this ampR gene, part of an open reading frame, the deduced protein of which shared 74% identity with the glutathione reductase of E. coli, was found in H. alvei 1 (5, 6). Using several sets of primers (primer 1 [5′-GTACGCACAGTAGCAGGATC-3′], primer 2 [5′-GCTCTTCGCGCATTTGAAGC-3′], primer 3 [5′-CCGCCAATTGCGAGATAGTC-3′], and primer 4 [5′-GGTGTTCTGCATTTTTTTACGC-3′]) (Fig. 3), PCR amplifications were attempted using H. alvei 2 to 10 DNAs as templates. Similar-sized PCR fragments indicated that ampR-like and glutathione reductase-like genes were present in each H. alvei isolate in the same positions relative to that of the ampC gene (Fig. 3). This result indicated that the genetic environment of H. alvei ampC genes was conserved, as with other enterobacterial ampC genes (Fig. 3). However, the genetic environments of the ampC genes differ from one enterobacterial species to the next (Fig. 3).
FIG. 3.
Comparison of the sequences surrounding ampC in several enterobacterial species. The positions and directions of the fumarate operon (frdABCD), hybF (hydrogenase), orf-1 (unknown function), gro (glutathione reductase), ampC, and ampR genes are indicated with arrows. The locations of the putative promoters (p) and the primers used in this study (small arrows and numbers) are also indicated.
Conclusion.
This work further underlines the relationship between the plasmid-borne cephalosporinase ACC-1 and the chromosome-borne point mutant ACC-1 derivatives of several unrelated H. alvei isolates. Similarly, 100% amino acid identity is known for the plasmid-mediated cephalosporinase DHA-1 of Salmonella enteritidis and the chromosome-borne cephalosporinase of Morganella morganii (1, 15). The heterogeneity of AmpC sequences of H. alvei may explain why several plasmid-mediated cephalosporinases are not just point mutant derivatives of known chromosome-borne cephalosporinases and why they appear to be distantly related or even unrelated to chromosome-borne cephalosporinases.
Nucleotide sequence accession number.
The nucleotide sequences data reported in this paper will appear in the GenBank and EMBL nucleotide databases under the accession no. AF180953 to AF180961.
Acknowledgments
This work was funded by the Ministère de l'Education Nationale et de la Recherche, Université Paris XI, Faculté de Médecine Paris Sud (UPRES, grant JE-2227), and the network Les β-lactamases: de l'observation clinique à la structure, Paris, France.
REFERENCES
- 1.Barnaud G, Arlet G, Verdet C, Gaillot O, Lagrange P H, Philippon A. Salmonella enteritidis: AmpC plasmid-mediated inducible β-lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob Agents Chemother. 1998;42:2352–2358. doi: 10.1128/aac.42.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauernfeind A, Schneider I, Jungwirth R, Sahly H, Ullmann U. A novel type of AmpC β-lactamase, ACC-1, produced by a Klebsiella pneumoniae strain causing nosocomial pneumonia. Antimicrob Agents Chemother. 1999;43:1924–1931. doi: 10.1128/aac.43.8.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett P M, Chopra I. Molecular basis of beta-lactamase induction in bacteria. Antimicrob Agents Chemother. 1993;37:153–158. doi: 10.1128/aac.37.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galleni M, Lindberg F, Normark S, Cole S, Honoré N, Joris B, Frère J-M. Sequence and comparative analysis of three Enterobacter cloacae ampC β-lactamase genes and their products. Biochem J. 1988;250:753–760. doi: 10.1042/bj2500753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girlich D, Naas T, Bellais S, Poirel L, Karim A, Nordmann P. Biochemical-genetic characterization, and regulation of expression of ACC-1-like chromosome-borne cephalosporinase from Hafnia alvei. Antimicrob Agents Chemother. 2000;44:1470–1478. doi: 10.1128/aac.44.6.1470-1478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greer S, Perham R N. Glutathione reductase from Escherichia coli: cloning and sequence analysis of the gene and relationship to other flavoprotein disulfide oxidoreductases. Biochemistry. 1986;25:2736–2742. doi: 10.1021/bi00357a069. [DOI] [PubMed] [Google Scholar]
- 7.Haruta S, Nukaga M, Taniguchi K, Sawai T. Resistance to oxyimino β-lactams due to a mutation of chromosomal β-lactamase in Citrobacter freundii. Microbiol Immunol. 1998;42:165–169. doi: 10.1111/j.1348-0421.1998.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones M E, Avison M B, Damdinsuren E, MacGowan A P, Bennett P M. Heterogeneity at the β-lactamase structural gene ampC amongst Citrobacter spp. assessed by polymerase chain reaction analysis: potential for typing at a molecular level. J Med Microbiol. 1994;41:209–214. doi: 10.1099/00222615-41-3-209. [DOI] [PubMed] [Google Scholar]
- 9.Lobkovsky E, Moews P C, Liu H, Zhao H, Frère J M, Knox J R. Evolution of an enzyme activity: crystallographic structure at 2-Å resolution of cephalosporinase from the ampC gene of Enterobacter cloacae P99 and comparison with a class A penicillinase. Proc Natl Acad Sci USA. 1993;90:11257–11261. doi: 10.1073/pnas.90.23.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura N, Minami S, Mitsuhashi S. Sequences of homologous β-lactamases from clinical isolates of Serratia marcescens with different substrate specificities. Antimicrob Agents Chemother. 1998;42:176–179. doi: 10.1128/aac.42.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morosini M I, Negri M C, Shoichet B, Baquero M R, Baquero F, Blazquez J. An extended-spectrum AmpC-type β-lactamase obtained by in-vitro antibiotic selection. FEMS Microbiol Lett. 1998;165:85–90. doi: 10.1111/j.1574-6968.1998.tb13131.x. [DOI] [PubMed] [Google Scholar]
- 12.Nukaga M, Haruta S, Tanimoto K, Kogure K, Taniguchi K, Tamaki M, Sawai T. Molecular evolution of a class C β-lactamase extending its substrate specificity. J Biol Chem. 1995;270:5729–5735. doi: 10.1074/jbc.270.11.5729. [DOI] [PubMed] [Google Scholar]
- 13.Oefner C, D'Arcy A, Daly J J, Gubernator K, Charnas R L, Henize I, Hubschwerlen C, Winkler F K. Refined crystal structure of β-lactamase from Citrobacter freundii indicates a mechanism for β-lactam hydrolysis. Nature. 1990;343:284–288. doi: 10.1038/343284a0. [DOI] [PubMed] [Google Scholar]
- 14.Poirel L, Guibert M, Girlich D, Bellais S, Naas T, Nordmann P. Integron- and carbenicillinase-mediated reduced susceptibility to amoxicillin-clavulanic acid in isolates of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 from French patients. Antimicrob Agents Chemother. 1999;43:1098–1104. doi: 10.1128/aac.43.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poirel L, Guibert M, Girlich D, Naas T, Nordmann P. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob Agents Chemother. 1999;43:769–776. doi: 10.1128/aac.43.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 17.Spangenberg C, Montie T C, Tümmler B. Structural and functional implications of sequence diversity of Pseudomonas aeruginosa genes oriC, ampC, and fliC. Electrophoresis. 1998;19:545–550. doi: 10.1002/elps.1150190414. [DOI] [PubMed] [Google Scholar]
- 18.Thomson K S, Sanders C C, Washington J A., II Ceftazidime resistance in Hafnia alvei. Antimicrob Agents Chemother. 1993;37:1375–1376. doi: 10.1128/aac.37.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukamoto K, Ohno R, Sawai T. Extension of the substrate spectrum by an amino acid substitution at residue 219 in the Citrobacter freundii cephalosporinase. J Bacteriol. 1990;172:4348–4351. doi: 10.1128/jb.172.8.4348-4351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]