Abstract
Bacteriophages (phages) use specialized tail machinery to deliver proteins and genetic material into a bacterial cell during infection. Attached at the distal ends of their tails are receptor binding proteins (RBPs) that recognize specific molecules exposed on host bacteria surfaces. Since the therapeutic capacity of naturally occurring phages is often limited by narrow host ranges, there is significant interest in expanding their host range via directed evolution or structure-guided engineering of their RBPs. Here, we describe the design principles of different RBP engineering platforms and draw attention to the mechanisms linking RBP binding and the correct spatial and temporal attachment of the phage to the bacterial surface. A deeper understanding of these mechanisms will directly benefit future engineering of more effective phage-based therapeutics.
Graphical Abstract

Introduction
As the most abundant biological entities within Earth’s biosphere [1], bacteriophages (phages) present a morphologically diverse repertoire of infection machinery to ensure effective recognition and attachment to their bacterial hosts. The inherent ability of phages to kill specific species or individual strains has led to a resurgence of interest in the development of phages as therapeutics to tackle the growing antibiotic resistance crisis [2]. Most phages currently being explored for therapeutic and diagnostic applications are tailed dsDNA viruses belonging to the order Caudovirales that utilize a tail organelle (cauda is tail in Latin) for translocation of their genomic DNA and proteins into the host cytoplasm during infection. These phages interact with specific ligands displayed on their bacterial host cells using receptor binding proteins (RBPs) that emanate from the tail. Physical proximity to the tail allows RBPs to spatially and temporally coordinate host recognition, irreversible attachment, and genome release.
RBPs can be categorized into two classes – tail fibers (TFs) and tailspike proteins (TSPs) – depending on their morphology. TFs are long and slender fibrous proteins lacking enzymatic activity. TSPs are shorter and stockier and usually have enzymatic activity towards a particular surface structure (commonly, a sugar moiety). RBPs interact with a variety of structures displayed on the bacterial surface, such as outer membrane proteins, lipopolysaccharides, teichoic acids, capsular polysaccharides and even organelles (e.g., flagella or pili) [3]. As the first point of contact with a bacterial host, the binding range of a phage’s RBP is the primary determinant of its host range. Thus, RBPs serve as the first and most important checkpoint in the infection process.
A bacterium can quickly become resistant to phage infection by altering its surface molecules through spontaneous mutation or phenotypic variation [4,5]. Akin to the spread of antibiotic resistance, the emergence of phage-resistant subpopulations of bacteria can be a major bottleneck when using phages as therapeutics or antibacterial agents [6,7]. Despite the impressive array of counterstrategies employed by phages for altering their host range [8], such adaptation does not occur within a suitable time frame to prolong their antibacterial activity. As a result, phages are typically applied as a cocktail of multiple unrelated phages that have different host ranges and are known (or sometimes assumed) to target different surface structures, which draws selective mutation pressure away from a single receptor. Unfortunately, while phage cocktails have proven successful in various clinical cases [9,10], their formulation and production can be time and labor intensive, especially if isolating and characterizing new phages is required to target a given pathogen or if phage resistance develops. For this reason, there is significant interest in engineering the genomes of individual phages with adaptable host ranges to bypass the need for continuous modulation of phage cocktail compositions and isolation of new phages.
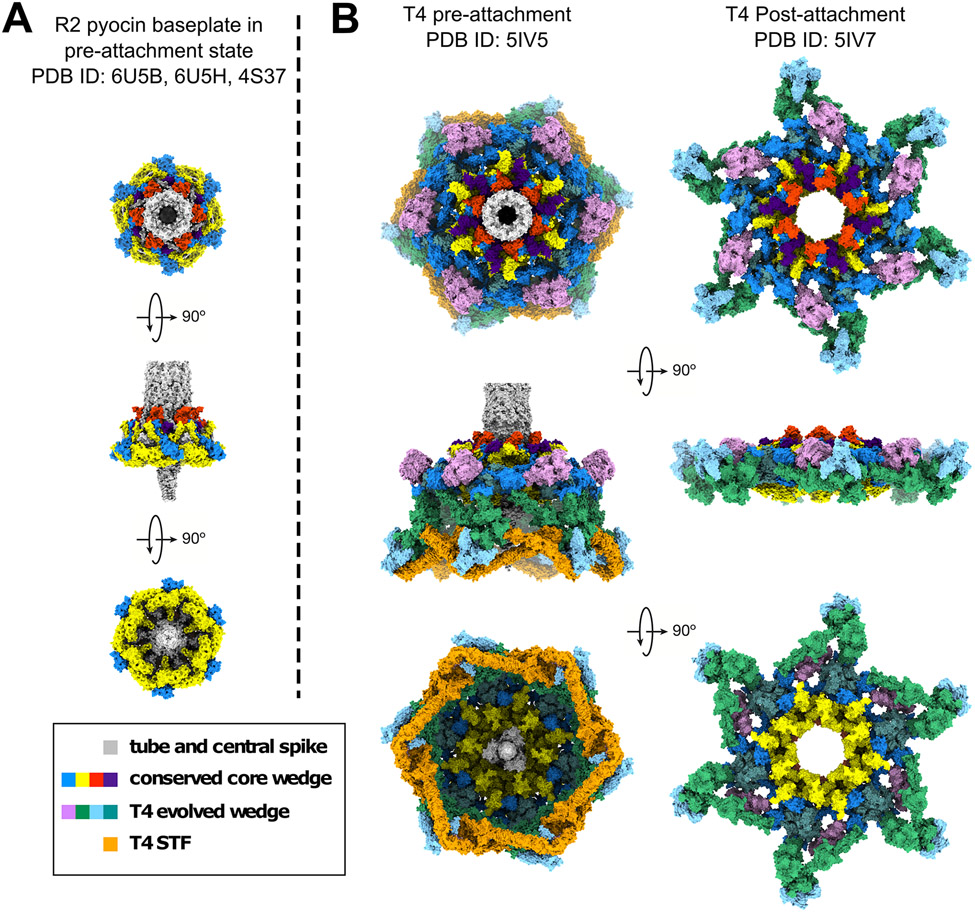
Over the last decade, a combination of X-ray crystallography [11-15], cryo-electron microscopy (cryo-EM) [16-25] and biochemical studies [26-31] have provided high-resolution models of various tail architectures (e.g., myo-, sipho-, or podo-viral) and revealed the atomic structures of different RBPs [32] (Figure 1). These “blueprints” are being used to guide structure-based engineering of RBPs to modulate the host range, with different strategies generally falling into two categories:
Figure 1. Minimal and evolved baseplates of two contractile tail nanomachines.
Shown are the structure of the P. aeruginosa R2 pyocin baseplate in the pre-attachment state (A) and the E. coli phage T4 baseplate in pre- and post-attachment states (B). Orthologues are shown in the same colors. A mutant used for the determination of the T4 baseplate structure contained no sheath. In the contracted state of the baseplate, the tube does not interact with it and dissociates away from it. The short tail fibers are disordered, and their electron density averages out in the cryoEM reconstruction. Figures generated using UCSF Chimera [84].
Domain swapping. The modular architecture of RBPs can be exploited to produce chimeric RBPs with alternative receptor binding domains to recognize different hosts [33-38]. Given the increasing number of sequenced phage genomes and high-resolution structures of diverse RBPs, it is becoming relatively easy to identify boundaries of receptor binding domains within RBPs in order to design chimeric RBPs. This modular strategy has also been applied to engineer pyocins into strain-specific antibacterial agents.
Structure-guided mutagenesis. Analogous to antibody engineering [39] and reverse transcriptase-mediated tropism switching [40], sequence variability can be introduced into the receptor binding sites of RBPs via targeted [41] or random [33,35] mutagenesis. This creates a library of phage mutants that feature highly diverse RBPs with distinct ligand specificities. Subsequent screening of these libraries can identify phages capable of infecting a broader range of bacteria, including strains refractory to the parental phage.
Both approaches have their own advantages and disadvantages, for instance, swapping domains (or even whole tail components) can be done with minimal understanding of the receptor binding or potential enzymatic capabilities of an RBP; however, a major bottleneck lies in identifying an alternative RBP featuring the desired binding range. On the other hand, targeted randomization does require knowledge of the binding site. However, the payoff with this approach is great, as it is possible to produce a synthetic assortment of binding residues with novel binding properties. Random mutagenesis, e.g., by using mutagenizing agents such as ethyl methanesulfonate [35], can also achieve the same; however, there can be a lack of control over the locations and frequency of mutation.
Structure and function of receptor binding proteins
A significant amount of information regarding the organization and function of RBPs can be derived solely from the analysis of their amino acid sequences. For example, the modular architecture of RBPs and the size of the modules that are exchanged between different phages are apparent [36,42]. Furthermore, it is possible to predict the host range of the phage by finding all bacterial hosts carrying prophages with sufficiently similar RBPs [26]. However, the location of the ligands on the surface of the RBP – the information required for precise engineering of the RBP’s ligand specificity – cannot be derived from the sequence and a detailed knowledge of the RBP structure (desirably, with ligand bound) is needed.
Tail fibers
The “spines” of many TFs feature a variation of a homotrimeric β-helix. Their distal tips that confer specificity, however, come in different shapes and sizes [11-15] (Figure 2). For example, the tip of the T4 long tail fiber (LTF) is a thin rod (Figure 2A) [11], whereas the T7 fiber carries a large globular head domain (Figure 2D) [12]. The T7 TF binds LPS on the E. coli surface, while the T4 LTF can interact with LPS or the OmpC porin [11,31] Some TFs (and TSPs) feature C-terminal intramolecular chaperone domains that assist with the folding of the trimer prior to specific cleavage and dissociation from the mature protein [12,14,43]. Many other TFs require a chaperone for assembly that is often encoded by a small gene immediately downstream from it [15,44].
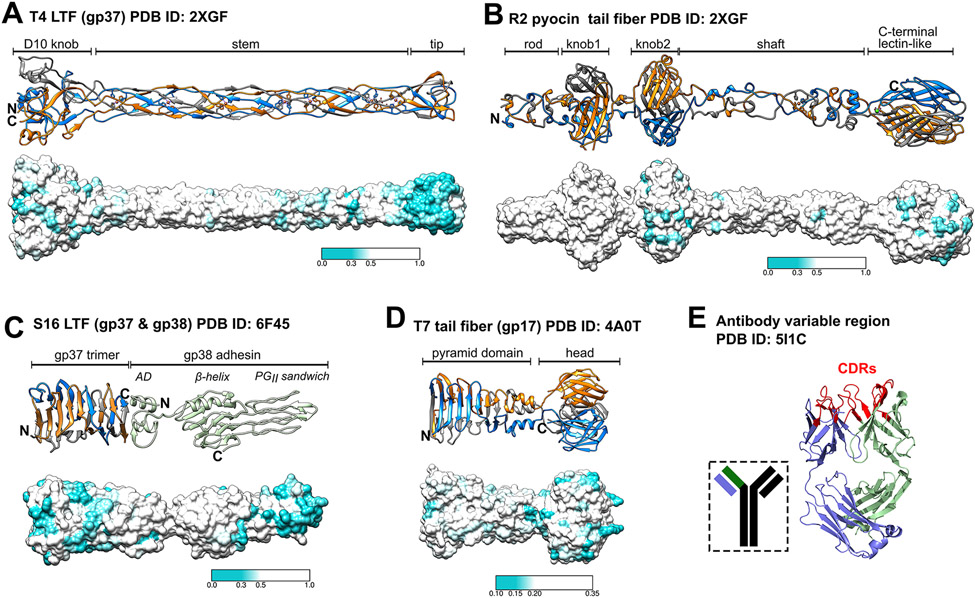
Figure 2. A selection of structurally distinct tail fiber binding tips.
The distal ends of tail fibers are shown as ribbon representations (top) and with sequence diversity mapped onto surface representations (bottom) for E. coli phage T4 gp37 (A) [11], P. aeruginosa pyocin R2 (B) [13], Salmonella phage S16 (C) [14] and E. coli phage T7 (D) [12]. Sequence variable regions are colored cyan as indicated in the color bars provided for each panel. In panel (C), AD stands for attachment domain. E) The variable region of a human antibody (light chain, purple; heavy chain, green) is shown. Highlighted are the sequence-variable complementarity determining regions (CDRs; red) that closely resemble the distal binding loops of phage tail fibers. For panels (A)-(D), the first 250 most similar sequences found by a BLAST [85] search were aligned by COBALT [86] and mapped on the molecular surface of the protein using UCSF Chimera [84]. Panel E was generated using PyMOL Molecular Graphics System, Version 1.4 Schrödinger, LLC.
Only a handful of TF structures are known, with the majority of structural information limited to their most distal fragments. The largest segment of a TF structure is known for R-type pyocins [13] (Figure 2B).
Interestingly, not only the C-terminal lectin-like domain of the pyocin fiber, but also its middle domains display patches of high sequence diversity. This pattern is characteristic to other TFs as well. Senseless mutations accumulate in proteins over time, but such mutations should be randomly scattered on the surface of the protein. However, as seen in Fig. 2, diverse residues in LPS-binding TFs appear to form patches and the size of these surface patches is similar to that of a typical sugar binding site. For this reason, this sequence diversity is unlikely to be senseless but instead is driven by natural selection. We suspect that these mutations are selected because they are beneficial for phage function and hence can participate in LPS binding. Thus, a fiber is likely to bind the sugar moiety of the LPS in several places along its length, which both orients the fiber with respect to the cell surface and fixes the fiber to it (i.e., results in ‘irreversible’ attachment). This line of thought is supported by images of phages T4 and P1 attached to the cell surface in which the distal part of the TF is oriented perpendicular to the cell surface [23,45]. We further speculate that such a restriction of the TF’s conformational space decreases the entropy of the fiber-baseplate system, which supplies the energy needed for triggering tail contraction.
The same activation principle could likely be at work in systems featuring TSPs and can explain how a TSP could first actively digest a surface sugar and then, upon reaching the core part of the LPS, trigger a conformational change in the tail that accompanies irreversible attachment. In this case, the restriction of conformational space must be even more severe as TSPs are less mobile and less rigid than TFs, meaning their initial entropy is lower.
In some TFs, distal tips are formed by separate proteins – adhesins and tail assembly proteins – that can play critical roles in or assist with receptor binding [14,15,46,47]. Among known T4-like phages most are, in fact, T2-like and their LTFs are equipped with an adhesin. The structure of one such LTF tip – that of Salmonella phage S16 – was recently solved [14] (Figure 2C). The C-terminus of the S16 adhesin is composed of ten polyglycine type II (PGII) helices that form a PGII sandwich with exposed residues and distal loops that confer host binding [48]. Organizational similarities can be drawn between the terminal receptor binding domains of tail fibers and the antigen-binding sites formed by the heavy and light variable chains of antibodies (Figure 2E). The conserved framework of the variable chains of antibodies is interspersed by three complementarity-determining regions (CDRs) that closely resemble the distal loops formed by the S16 LTF adhesin and the loops present at the tips of all known TFs (Figure 2A-D).
Tailspikes
Compared to their fibrous TF counterparts, TSPs appear more rigid in their architecture, which makes them relatively easier to crystallize. A much greater number of high-resolution structures of TSPs are currently known, including those interacting with fragments of their cell surface ligands [26,27,30,49-55] (Figure 3).
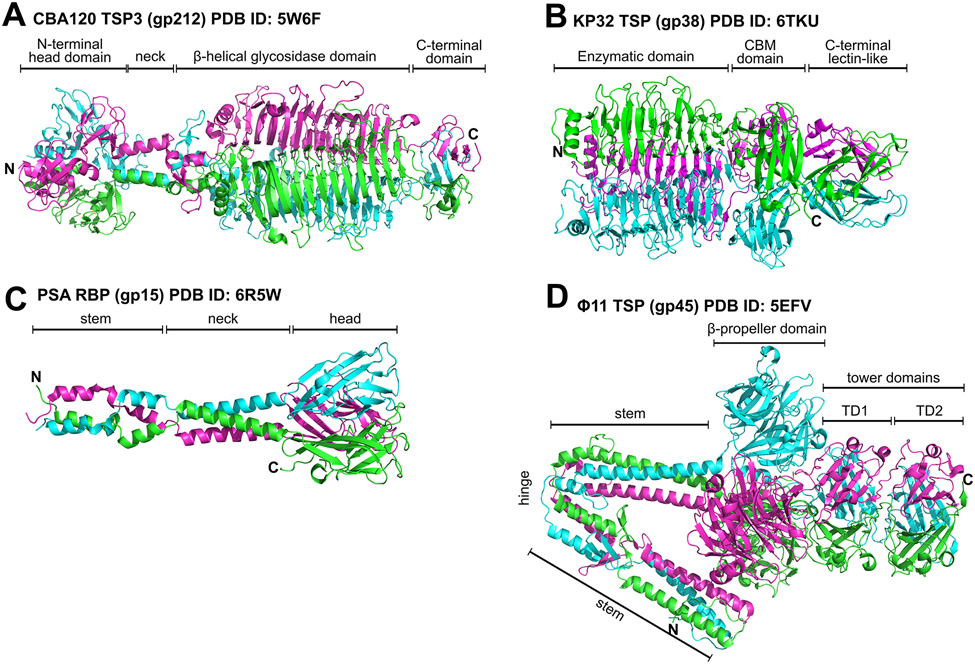
Figure 3. Representative RBPs used by phages to target different cell wall ligands.
Ribbon diagrams are shown for: (A) TSP3 (orf212/gp164) of E. coli phage CBA120 that features a middle β-helical glycosidase domain that digests the O77 O-antigen of E. coli [26,59], (B) the capsule-degrading TSP (gp38) of Klebsiella phage KP32 [52], (C) the RBP (gp15) of Listeria phage PSA that recognizes serovar 4b wall teichoic acids via the head binding domain [33], and (D) the large multidomain TSP of Staphylococcus phage Φ11 that binds α- or β-N-acetylglucosamine moieties of S. aureus wall teichoic acids via the central propeller domain [87]. CBM, carbohydrate binding module. Figures produced using PyMOL Molecular Graphics System, Version 1.4 Schrödinger, LLC.
TSPs are attached to the tail or other TSPs (as part of a multicomponent network [26,56]) with help of their N-terminal domains that could be as short as a few amino acids. Such attachment domains comprise a smaller, more conserved set than ligand-binding (sometimes called catalytic) modules that form the rest of the TSP structure. These attachment domains are “reused” across diverse families of phages alongside their interacting partners, e.g., T4 gp10-like docking domains are found in different phage families that contain multiple TSPs [26,27,57]. The rest of the TSP structure (the ligand-binding “module”) is also reused as it is linked to recognition and enzymatic processing of a sugar displayed on the surface of a particular bacterial host.
The ligand-binding module usually consists of at least two domains, one of which typically exhibits either lyase, hydrolase, or esterase activity (e.g., G7C [27]) towards cell surface sugar moieties such as lipo- and capsular polysaccharides (O- and K-antigens, respectively) or teichoic acids (Gram-positive hosts) (Figure 3A&B). Most TSPs disassemble their polymeric sugar substrate into short fragments thus creating a path for the phage particle to reach the cell membrane [26,49,54,55,58,59]. However, TSPs with esterase activity remove the small O-acetyl group from specific sugar residues and leave the polymeric chain intact, which raises a question of how the phage particle equipped with such a TSP reaches the cell surface [27]. Unlike TFs, where their least conserved regions are likely responsible for ligand binding (Figure 2), the active site in catalytically active TSPs constitutes their most conserved part. The enzymatic domain is therefore the key determinant of TSP specificity and, as a consequence, of the phage’s host range. The other domain(s) of the ligand-binding module play(s) a role in substrate binding and, therefore, host recognition, but the function of these domains in the initial host recognition and irreversible attachment is unknown. Many TSPs feature a lectin-like domain at the C-terminus that is most proximal to the cell membrane during infection.
We can assume with sufficient confidence that TSPs represent bona fide TFs with enzymatic activity that emerged as a part of the arms race between phages and bacteria after the latter evolved protective extracellular layers that are difficult for fibers to penetrate [60,61]. Most likely, phages captured non-critical catabolic enzymes (e.g., sialidases and pectate lyases) from host bacteria and incorporated them into preexisting RBPs, which allowed phages to “drill” through different outer bacterial polysaccharidic layers [62]. Nevertheless, such acquisition places serious constrains on the host range of the phage as all known TSPs typically recognize one substrate or at best a few very closely related ones [26,27,58]. Clearly, efficient receptor recognition and processing require a precise combination of affinity and optimal receptor processing kinetics, which limits the number of possible substrates [63,64]. TSP-carrying phages with wider host ranges have complex adsorption devices that contain several types of TSPs, each responsible for recognizing a certain host [65,66].
An important concept for the function of TF-like RBPs (i.e., TSPs lacking enzymatic activity) (Figure 3 C&D) is avidity as exemplified by the composition of host cell adsorption organelles of certain phages. To overcome the weak binding of an individual RBP, Lactococcus phage TP901-1 [67], Listeria phage A511 [19], Bordetella phage BPP-1[25], and many others, feature a staggering number of identical RBPs emanating from their tails [68]. To further improve the probability of interacting with a host many phages carry carbohydrate binding modules (CBMs) on various components of the phage particle, for instance, protruding from “evolved” distal tail proteins (Dits), neck passage proteins (NPSs) or major tail proteins (MTPs) [69]. These CBMs appear to have similar ligand specificities as the phage RBPs and should be careful considered when attempting to engineer the host range of such a phage.
Current RBP engineering strategies
Non-targeted recombination of phage genomes
Random mutation and recombination between phages is a long-used but mostly unreported approach to adapt phage host ranges [70-72]. For example, the so-called “Appelmans protocol” [70] involves cycling a cocktail of phages with a group of susceptible and resistant bacteria until a recombinant phage appears that lyses the resistant strains. This approach was recently used to generate a phage capable of infecting ten P. aeruginosa strains starting with two parental phages infecting only one or two strains [70]. This procedure contained over 30 rounds of co-infection in which a minimum of 48 recombination events between the parental phages and one point mutation took place. A large fraction of recombination events occurred within structural and RBP genes.
Trading tail fibers by directed homologous recombination
A more direct approach to modify host range is to only recombine the RBP gene(s) of an infecting phage or electroporated phage genome with a cytosolic RBP template featuring the desired host range. The limitation of this approach is the two RBP genes must feature high sequence similarity to allow recombination. This approach has been used to expand the host range of T4- and T2-like phages [38], for instance by swapping T2 LTF genes 37 and 38 with counterparts from phages PP01 or IP008 to expand T2 infectivity towards different E. coli strains [37,73]. To increase the frequency of recombinant phage identification, a CRISPR/Cas counterselection step can be included to remove any wildtype phages remaining after recombination [74-78]. Such a two-step approach has allowed replacing both short (gp12) and long tail fibers of T2 with those of PP01. The resulting T2 phage had improved adsorption to E. coli O157:H7 hosts, especially when compared to wildtype T2 or when only the LTFs had been switched with PP01 [79].
Complete replacement of tail components
Ando et al. developed a yeast-based platform to produce synthetic phage genomes assembled from individual fragments [34]. This approach allows the exchange of RBP and tail components between different phage backbones. The utility of the platform was demonstrated by swapping whole TFs or bioengineering chimeric TFs of different T7/T3-like phages with modified host ranges. A synthetic T3 phage carrying Yersinia phage TFs was able to cross the genus barrier and infect Y. pseudotuberculosis and E. coli strains. The advantage of this approach is the ability to synthesize phages with structurally different host recognition machinery by exchanging multiple components of the phage tail apparatus all at once regardless of their genomic position. For example, the tail proteins gp11 and gp12 and the TF protein gp17 of T7 can be replaced with their counterparts from the Klebsiella phage K11, which is equipped with a capsule-degrading TSP instead of a TF. The resulting synthetic T7K11(gp11-12-17) phage infected Klebsiella. Similar to recombination-based engineering, the major drawback of this approach is that the replacement of phage components still requires sufficient sequence similarity at chosen domain boundaries to ensure they can be attached to the engineered phage.
An alternative platform for producing synthetic Gram-positive targeting phages was developed by Kilcher et al. for rebooting in vitro-assembled synthetic genomes in Listeria L-forms [80]. This platform was recently used to re-engineer the temperate Listeria monocytogenes phage PSA with a broader host range [33]. Error-prone PCR was used to produce a library of randomized genomic fragments encoding the phage RBP (gp15), which were assembled into synthetic phage genomes. Rebooted phages carrying different RBP mutations presented a shift in host range from serovar 4b to 4d strains, which removed the need for galactose as an essential binding element of the teichoic acid receptor. Secondly, a polyvalent phage encoding both wildtype and mutated (S334R) RBPs demonstrated infectivity against both serovars. Finally, a structure-guided approach was used to generate chimeras, whereby heterologous RBPs of low sequence similarity identified using BLAST within genomes of Listeria lysogens were fused at conserved domain boundaries (e.g., “neck chimeras” were fused after the α3 coiled coil (Figure 3C) to produce phages capable of infecting a broader selection of species and serovars (4a, 4b, 4d, 5 and 6b).
Directed mutagenesis of tail fiber binding loops
As sequence composition of the distal loops of TFs is directly responsible for binding specificity [48] it is not surprising that randomization of these loops can generate phages with new host ranges. The crystal structure of the phage T7 TF (Figure 2D) was used as a blueprint by Yehl et al. to identify four exposed distal loops for targeted randomization of related phage T3 [41]. Recombination plasmids featuring the TF gene of T3 were generated using site-directed mutagenesis to replace codons within the loops with NNK codons, such that loop sequences were completely randomized at the DNA level. Upon infection, T3 recombined with the randomized TF to produce phages with unique loop compositions. The mutant phage libraries were capable of infecting T3-resistant E. coli and provided long-term suppression of E. coli resistance development when tested in vitro and in a murine model [41]. It is important to note that not all loop mutations are functional, or provide a benefit compared to the parental phage. However, as sequence variability can be concentrated to specific regions (i.e., TF binding loops), the probability of generating a mutant phage with the desired binding is considerably greater than relying on natural phage evolution. For example, the smallest loop randomized consisted of only four amino acids, yet this provided a possible 106 unique sequences within the phage library. The current limitation of this approach is our lack of understanding of the mechanism of cell surface receptor binding by TFs because not a single ligand-bound high-resolution structure of a TF is available.
Yosef et al. [35] recently demonstrated the expansion of host range through similar randomization of tail and TF genes. In their GOTrap (general optimization of transducing particles) platform, a T7 mutant lacking tail (genes 11 and 12) and TF genes infected E. coli hosts that carried randomized tail-TF genes on a plasmid containing an antibiotic resistance marker and a T7 packaging sequence. After infection, only phage particles that carried functional tail and TF complexes and contained a plasmid encoding for these proteins could successfully transduce a novel host that could be selected using the antibiotic marker (e.g., Klebsiella, Enterobacter, Salmonella etc.). Successive rounds of plasmid purification and mutation using ethyl methanesulfonate were used to select for tail and TF mutants with improved binding ranges [35].
Pyocin engineering
Phage tail-like bacteriocins, such as the R-type pyocins produced by P. aeruginosa, employ a P2 tail-like contractile machinery to kill competing bacteria by dissipating their membrane potential [16,81]. However, unlike phages that propagate during infection, pyocins can be used only as “single-shot” precision antimicrobials. While the wildtype pyocins have very narrow killing spectra, many of the engineering strategies developed for phages can be used to expand their therapeutic potential. For example, the Pseudomonas aeruginosa pyocin pyR2 (i.e., R2-type pyocin) could be retargeted to kill uropathogenic E. coli or Y. pestis strains by replacing most of its cognate TFs with those from E. coli phage P2 or Yersinia phage L-413c, respectively [36] (Figure 2B). Using the same strategy, the pyR2 pyocin was further developed into an O157-specific antimicrobial by fusing an O157-specific TSP from podovirus φV10 to the native fiber [82]. This hybrid pyocin could prevent and ameliorate E. coli-induced diarrhea and intestinal inflammation when tested in a rabbit model [83]. It is interesting to note that in the absence of structural “blueprints” of the fiber and TSP components, libraries of chimeric fibers had to be constructed and tested, which was a time-consuming task [36,82]. In addition to the structure of R1- and R2-type pyocin fibers described above, structures of the entire R2 pyocin particle were recently reported in pre- and post-contracted states providing intricate details of its internal mechanics [16]. This high-resolution model has already been used to engineer certain interface residues within the baseplate to produce an acid-stable pyocin, demonstrating the significance of high-resolution structures for future engineering of pyocins and phages.
Conclusion
Our current understanding of how a phage recognizes its host and attaches to its surface is far from complete. For example, cell envelope components that are responsible for “uncorking” the phage particle are unknown for most podo- and myo-phages that have already been studied for decades. Nevertheless, the necessary step in the infection process – the interaction of the protein that defines the specificity of the phage, its RBP – is much better understood. The work with the R2 pyocin platform demonstrates that both TSPs (with enzymatic activity) and TFs (sans enzymatic activity), can place the pyocin particle onto the path of irreversible attachment. This shows that the functions of TSPs and TFs are fundamentally similar. Not only are they responsible for the specificity, but they are capable of triggering a conformational change in the particle that is required for irreversible attachment. Together with the enormous progress in structural studies of bacterial surface polysaccharides and genetics of their biosynthetic pathways, it will soon be possible to identify in the existing pool or generate synthetically a comprehensive set of RBPs that will target all known cell surface sugars. Such RBPs can then be used in a phage- or pyocin-like mono- or multi-valent platform to provide more effective phage-based therapeutics.
Highlights.
Phages use structurally diverse receptor binding proteins (RBPs) to target different cell wall structures.
RBPs play a critical role in spatial and temporal positioning of phages on the bacterial surface to ensure correct attachment of the tail apparatus.
High-resolution structures of RBPs are essential for understanding the mechanism of receptor recognition and provide “blueprints” to guide phage engineering.
Footnotes
CRediT authorship contribution statement
Matthew Dunne: Conceptualization, Visualization, Writing - original draft, Writing - review & editing. Nikolai S. Prokhorov: Conceptualization, Visualization, Writing - original draft, Writing – review & editing. Martin J. Loessner: Writing – review & editing. Petr G. Leiman: Conceptualization, Visualization, Writing - original draft, Writing - review & editing.
Conflict of interest statement
Nothing declared.
References
- 1.Clokie MR, Millard AD, Letarov AV, Heaphy S: Phages in nature. Bacteriophage 2011, 1:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakemeyer M, Zhao W, Mandl FA, Hammann P, Sieber SA: Thinking Outside the Box—Novel Antibacterials To Tackle the Resistance Crisis. Angew Chem Int Ed 2018, 57:14440–14475. [DOI] [PubMed] [Google Scholar]
- 3.Bertozzi Silva J, Storms Z, Sauvageau D: Host receptors for bacteriophage adsorption. FEMS Microbiol Lett 2016, 363. [DOI] [PubMed] [Google Scholar]
- 4.Wright RCT, Friman V-P, Smith MCM, Brockhurst MA: Resistance Evolution against Phage Combinations Depends on the Timing and Order of Exposure. mBio 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burmeister AR, Turner PE: Trading-off and trading-up in the world of bacteria–phage evolution. Curr Biol 2020, 30:R1120–R1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labrie SJ, Samson JE, Moineau S: Bacteriophage resistance mechanisms. Nat Rev Microbiol 2010, 8:317–327. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Y, Wang L, Li X, Tan D, Cong C, Xu Y: Efficacy of a phage cocktail in controlling phage resistance development in multidrug resistant Acinetobacter baumannii. Virus Res 2019, 272:197734. [DOI] [PubMed] [Google Scholar]
- 8.de Jonge PA, Nobrega FL, Brouns SJJ, Dutilh BE: Molecular and Evolutionary Determinants of Bacteriophage Host Range. Trends Microbiol 2019, 27:51–63. [DOI] [PubMed] [Google Scholar]
- 9.Furfaro LL, Payne MS, Chang BJ: Bacteriophage Therapy: Clinical Trials and Regulatory Hurdles. Front Cell Infect Microbiol 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslam S, Lampley E, Wooten D, Karris M, Benson C, Strathdee S, Schooley RT: Lessons Learned From the First 10 Consecutive Cases of Intravenous Bacteriophage Therapy to Treat Multidrug-Resistant Bacterial Infections at a Single Center in the United States. Open Forum Infect Dis 2020, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartual SG, Otero JM, Garcia-Doval C, Llamas-Saiz AL, Kahn R, Fox GC, van Raaij MJ: Structure of the bacteriophage T4 long tail fiber receptor-binding tip. Proc Natl Acad Sci 2010, 107:20287–20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Doval C, van Raaij MJ: Structure of the receptor-binding carboxy-terminal domain of bacteriophage T7 tail fibers. Proc Natl Acad Sci 2012, 109:9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buth SA, Shneider MM, Scholl D, Leiman PG: Structure and Analysis of R1 and R2 Pyocin Receptor-Binding Fibers. Viruses 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunne M, Denyes JM, Arndt H, Loessner MJ, Leiman PG, Klumpp J: Salmonella Phage S16 Tail Fiber Adhesin Features a Rare Polyglycine Rich Domain for Host Recognition. Struct Lond Engl 1993 2018, doi: 10.1016/j.str.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 15.North OI, Sakai K, Yamashita E, Nakagawa A, Iwazaki T, Büttner CR, Takeda S, Davidson AR: Phage tail fibre assembly proteins employ a modular structure to drive the correct folding of diverse fibres. Nat Microbiol 2019, 4:1645–1653. [DOI] [PubMed] [Google Scholar]
- 16.Ge P, Scholl D, Prokhorov NS, Avaylon J, Shneider MM, Browning C, Buth SA, Plattner M, Chakraborty U, Ding K, et al. : Action of a minimal contractile bactericidal nanomachine. Nature 2020, 580:658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spinelli S, Bebeacua C, Orlov I, Tremblay D, Klaholz BP, Moineau S, Cambillau C: Cryo-Electron Microscopy Structure of Lactococcal Siphophage 1358 Virion. J Virol 2014, 88:8900–8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge P, Scholl D, Leiman PG, Yu X, Miller JF, Zhou ZH: Atomic structures of a bactericidal contractile nanotube in its pre- and postcontraction states. Nat Struct Mol Biol 2015, 22:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerrero-Ferreira RC, Hupfeld M, Nazarov S, Taylor NM, Shneider MM, Obbineni JM, Loessner MJ, Ishikawa T, Klumpp J, Leiman PG: Structure and transformation of bacteriophage A511 baseplate and tail upon infection of Listeria cells. EMBO J 2019, 38:e99455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor NMI, Prokhorov NS, Guerrero-Ferreira RC, Shneider MM, Browning C, Goldie KN, Stahlberg H, Leiman PG: Structure of the T4 baseplate and its function in triggering sheath contraction. Nature 2016, 533:346–352. [DOI] [PubMed] [Google Scholar]
- 21.Hrebík D, Štveráková D, Škubník K, Füzik T, Pantůček R, Plevka P: Structure and genome ejection mechanism of Staphylococcus aureus phage P68. Sci Adv 2019, 5:eaaw7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farley MM, Tu J, Molineux I, Liu J: Structural Remodeling of Bacteriophage Φ29 during Infection of Gram-Positive Bacterium. Biophys J 2016, 110:22a. [Google Scholar]
- 23.Hu B, Margolin W, Molineux IJ, Liu J: Structural remodeling of bacteriophage T4 and host membranes during infection initiation. Proc Natl Acad Sci 2015, 112:E4919–E4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu B, Margolin W, Molineux IJ, Liu J: The Bacteriophage T7 Virion Undergoes Extensive Structural Remodeling During Infection. Science 2013, 339:576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai W, Hodes A, Hui WH, Gingery M, Miller JF, Zhou ZH: Three-dimensional structure of tropism-switching Bordetella bacteriophage. Proc Natl Acad Sci 2010, 107:4347–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Plattner M, Shneider MM, Arbatsky NP, Shashkov AS, Chizhov AO, Nazarov S, Prokhorov NS, Taylor NMI, Buth SA, Gambino M, et al. : Structure and Function of the Branched Receptor-Binding Complex of Bacteriophage CBA120. J Mol Biol 2019, 431:3718–3739. ** The assembly, structure, and function of a multivalent RBP complex in a large bacteriophage have been described for the first time. The findings have been generalized to other multivalent RBP complexes.
- 27. Prokhorov NS, Riccio C, Zdorovenko EL, Shneider MM, Browning C, Knirel YA, Leiman PG, Letarov AV: Function of bacteriophage G7C esterase tailspike in host cell adsorption. Mol Microbiol 2017, 105:385–398. ** The structure and function of the first deacetylase TSP has been described. This TSP attaches to the particle via interaction with the second TSP of G7C that contains a T4 gp10-like module. This property turned out to be common to many multivalent TSP complexes found in all tail architectures.
- 28.Collins B, Bebeacua C, Mahony J, Blangy S, Douillard FP, Veesler D, Cambillau C, van Sinderen D: Structure and Functional Analysis of the Host Recognition Device of Lactococcal Phage Tuc2009. J Virol 2013, 87:8429–8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broeker NK, Barbirz S: Not a barrier but a key: How bacteriophages exploit host’s O-antigen as an essential receptor to initiate infection. Mol Microbiol 2017, 105:353–357. [DOI] [PubMed] [Google Scholar]
- 30.Broeker NK, Roske Y, Valleriani A, Stephan MS, Andres D, Koetz J, Heinemann U, Barbirz S: Time-resolved DNA release from an O-antigen–specific Salmonella bacteriophage with a contractile tail. J Biol Chem 2019, 294:11751–11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islam MZ, Fokine A, Mahalingam M, Zhang Z, Garcia-Doval C, van Raaij MJ, Rossmann MG, Rao VB: Molecular anatomy of the receptor binding module of a bacteriophage long tail fiber. PLOS Pathog 2019, 15:e1008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobrega FL, Vlot M, de Jonge PA, Dreesens LL, Beaumont HJE, Lavigne R, Dutilh BE, Brouns SJJ: Targeting mechanisms of tailed bacteriophages. Nat Rev Microbiol 2018, 16:760–773. [DOI] [PubMed] [Google Scholar]
- 33. Dunne M, Rupf B, Tala M, Qabrati X, Ernst P, Shen Y, Sumrall E, Heeb L, Plückthun A, Loessner MJ, et al. : Reprogramming Bacteriophage Host Range through Structure-Guided Design of Chimeric Receptor Binding Proteins. Cell Rep 2019, 29:1336–1350.e4. ** Applied different genetic engineering strategies from targeted randomization to structure guided chimeric RBP generation to redirect and expand the host range of the first Gram-positive phage, Listeria phage PSA.
- 34.Ando H, Lemire S, Pires DP, Lu TK: Engineering Modular Viral Scaffolds for Targeted Bacterial Population Editing. Cell Syst 2015, 1:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yosef I, Goren MG, Globus R, Molshanski-Mor S, Qimron U: Extending the Host Range of Bacteriophage Particles for DNA Transduction. Mol Cell 2017, 66:721–728.e3. **A new method that harnesses T7 transducing capability to select and/or generate new tail and tail fiber components is proposed and experimentally validated.
- 36. Williams SR, Gebhart D, Martin DW, Scholl D: Retargeting R-Type Pyocins To Generate Novel Bactericidal Protein Complexes. Appl Environ Microbiol 2008, 74:3868–3876. * The killing spectrum of R-type pyocins was changed from Pseudomonas aeruginosa to E. coli and Yersinia pestis by replacing most of the pyocin fiber with large fragments of RBPs of phages infecting E. coli and Yersinia pestis.
- 37.Mahichi F, Synnott AJ, Yamamichi K, Osada T, Tanji Y: Site-specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol Lett 2009, 295:211–217. [DOI] [PubMed] [Google Scholar]
- 38.Tétart F, Desplats C, Krisch HM: Genome plasticity in the distal tail fiber locus of the T-even bacteriophage: recombination between conserved motifs swaps adhesin specificity. J Mol Biol 1998, 282:543–556. [DOI] [PubMed] [Google Scholar]
- 39.Saeed AFUH, Wang R, Ling S, Wang S: Antibody Engineering for Pursuing a Healthier Future. Front Microbiol 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Deora R, Doulatov SR, Gingery M, Eiserling FA, Preston A, Maskell DJ, Simons RW, Cotter PA, Parkhill J, et al. : Reverse transcriptase-mediated tropism switching in Bordetella bacteriophage. Science 2002, 295:2091–2094. [DOI] [PubMed] [Google Scholar]
- 41. Yehl K, Lemire S, Yang AC, Ando H, Mimee M, Torres MDT, de la Fuente-Nunez C, Lu TK: Engineering Phage Host-Range and Suppressing Bacterial Resistance through Phage Tail Fiber Mutagenesis. Cell 2019, 179:459–469.e9. ** The emergence of bacterial resistance to phage infection resulting from spontaneous mutations and heterogeneity of cell surface structures can be suppressed by synthetic tail fibers and tail components.
- 42.Casjens SR, Thuman-Commike PA: Evolution of mosaically related tailed bacteriophage genomes seen through the lens of phage P22 virion assembly. Virology 2011, 411:393–415. [DOI] [PubMed] [Google Scholar]
- 43.Schwarzer D, Stummeyer K, Gerardy-Schahn R, Mühlenhoff M: Characterization of a novel intramolecular chaperone domain conserved in endosialidases and other bacteriophage tail spike and fiber proteins. J Biol Chem 2007, 282:2821–2831. [DOI] [PubMed] [Google Scholar]
- 44.Hashemolhosseini S, Stierhof YD, Hindennach I, Henning U: Characterization of the helper proteins for the assembly of tail fibers of coliphages T4 and lambda. J Bacteriol 1996, 178:6258–6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Chen C-Y, Shiomi D, Niki H, Margolin W: Visualization of bacteriophage P1 infection by cryo-electron tomography of tiny Escherichia coli. Virology 2011, 417:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.North OI, Davidson AR: Phage proteins required for tail fiber assembly also bind specifically to the surface of host bacterial strains. J Bacteriol 2020, doi: 10.1128/JB.00406-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez-Serrano R, Dunne M, Rosselli R, Martin-Cuadrado A-B, Grosboillot V, Zinsli LV, Roda-Garcia JJ, Loessner MJ, Rodriguez-Valera F: Alteromonas Myovirus V22 Represents a New Genus of Marine Bacteriophages Requiring a Tail Fiber Chaperone for Host Recognition. mSystems 2020, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trojet SN, Caumont-Sarcos A, Perrody E, Comeau AM, Krisch HM: The gp38 Adhesins of the T4 Superfamily: A Complex Modular Determinant of the Phage’s Host Specificity. Genome Biol Evol 2011, 3:674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbirz S, Müller JJ, Uetrecht C, Clark AJ, Heinemann U, Seckler R: Crystal structure of Escherichia coli phage HK620 tailspike: podoviral tailspike endoglycosidase modules are evolutionarily related. Mol Microbiol 2008, 69:303–316. [DOI] [PubMed] [Google Scholar]
- 50.Thompson JE, Pourhossein M, Waterhouse A, Hudson T, Goldrick M, Derrick JP, Roberts IS: The K5 Lyase KflA Combines a Viral Tail Spike Structure with a Bacterial Polysaccharide Lyase Mechanism. J Biol Chem 2010, 285:23963–23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stummeyer K, Dickmanns A, Mühlenhoff M, Gerardy-Schahn R, Ficner R: Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat Struct Mol Biol 2005, 12:90–96. [DOI] [PubMed] [Google Scholar]
- 52.Squeglia F, Maciejewska B, Łątka A, Ruggiero A, Briers Y, Drulis-Kawa Z, Berisio R: Structural and Functional Studies of a Klebsiella Phage Capsule Depolymerase Tailspike: Mechanistic Insights into Capsular Degradation. Struct Lond Engl 1993 2020, 28:613–624.e4. [DOI] [PubMed] [Google Scholar]
- 53.Chen C, Bales P, Greenfield J, Heselpoth RD, Nelson DC, Herzberg O: Crystal structure of ORF210 from E. coli O157:H1 phage CBA120 (TSP1), a putative tailspike protein. PloS One 2014, 9:e93156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olszak T, Shneider MM, Latka A, Maciejewska B, Browning C, Sycheva LV, Cornelissen A, Danis-Wlodarczyk K, Senchenkova SN, Shashkov AS, et al. : The O-specific polysaccharide lyase from the phage LKA1 tailspike reduces Pseudomonas virulence. Sci Rep 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee I-M, Tu I-F, Yang F-L, Ko T-P, Liao J-H, Lin N-T, Wu C-Y, Ren C-T, Wang AH-J, Chang C-M, et al. : Structural basis for fragmenting the exopolysaccharide of Acinetobacter baumannii by bacteriophage ΦAB6 tailspike protein. Sci Rep 2017, 7:42711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golomidova AK, Kulikov EE, Prokhorov NS, Guerrero-Ferreira RС, Knirel YA, Kostryukova ES, Tarasyan KK, Letarov AV: Branched Lateral Tail Fiber Organization in T5-Like Bacteriophages DT57C and DT571/2 is Revealed by Genetic and Functional Analysis. Viruses 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latka A, Leiman PG, Drulis-Kawa Z, Briers Y: Modeling the Architecture of Depolymerase-Containing Receptor Binding Proteins in Klebsiella Phages. Front Microbiol 2019, 10:2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarzer D, Browning C, Stummeyer K, Oberbeck A, Mühlenhoff M, Gerardy-Schahn R, Leiman PG: Structure and biochemical characterization of bacteriophage phi92 endosialidase. Virology 2015, 477:133–143. [DOI] [PubMed] [Google Scholar]
- 59.Greenfield J, Shang X, Luo H, Zhou Y, Heselpoth RD, Nelson DC, Herzberg O: Structure and tailspike glycosidase machinery of ORF212 from E. coli O157:H7 phage CBA120 (TSP3). Sci Rep 2019, 9:7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warwick-Dugdale J, Buchholz HH, Allen MJ, Temperton B: Host-hijacking and planktonic piracy: how phages command the microbial high seas. Virol J 2019, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bryan MJ, Burroughs NJ, Spence EM, Clokie MRJ, Mann NH, Bryan SJ: Evidence for the intense exchange of MazG in marine cyanophages by horizontal gene transfer. PloS One 2008, 3:e2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stummeyer K, Schwarzer D, Claus H, Vogel U, Gerardy-Schahn R, Mühlenhoff M: Evolution of bacteriophages infecting encapsulated bacteria: lessons from Escherichia coli K1-specific phages. Mol Microbiol 2006, 60:1123–1135. [DOI] [PubMed] [Google Scholar]
- 63.O’Leary TR, Xu Y, Liu J: Investigation of the substrate specificity of K5 lyase A from K5A bacteriophage. Glycobiology 2013, 23:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwarzer D, Stummeyer K, Haselhorst T, Freiberger F, Rode B, Grove M, Scheper T, von Itzstein M, Mühlenhoff M, Gerardy-Schahn R: Proteolytic release of the intramolecular chaperone domain confers processivity to endosialidase F. J Biol Chem 2009, 284:9465–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gebhart D, Williams SR, Scholl D: Bacteriophage SP6 encodes a second tailspike protein that recognizes Salmonella enterica serogroups C2 and C3. Virology 2017, 507:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scholl D, Rogers S, Adhya S, Merril CR: Bacteriophage K1-5 Encodes Two Different Tail Fiber Proteins, Allowing It To Infect and Replicate on both K1 and K5 Strains of Escherichia coli. J Virol 2001, 75:2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veesler D, Spinelli S, Mahony J, Lichière J, Blangy S, Bricogne G, Legrand P, Ortiz-Lombardia M, Campanacci V, van Sinderen D, et al. : Structure of the phage TP901-1 1.8 MDa baseplate suggests an alternative host adhesion mechanism. Proc Natl Acad Sci U S A 2012, 109:8954–8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dunne M, Hupfeld M, Klumpp J, Loessner MJ: Molecular Basis of Bacterial Host Interactions by Gram-Positive Targeting Bacteriophages. Viruses 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayes S, Mahony J, Vincentelli R, Ramond L, Nauta A, van Sinderen D, Cambillau C: Ubiquitous Carbohydrate Binding Modules Decorate 936 Lactococcal Siphophage Virions. Viruses 2019, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burrowes BH, Molineux IJ, Fralick JA: Directed in Vitro Evolution of Therapeutic Bacteriophages: The Appelmans Protocol. Viruses 2019, 11:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sant DG, Woods LC, Barr JJ, McDonald MJ: Host diversity slows bacteriophage adaptation by selecting generalists over specialists. Nat Ecol Evol 2021, doi: 10.1038/s41559-020-01364-1. [DOI] [PubMed] [Google Scholar]
- 72.Habusha M, Tzipilevich E, Fiyaksel O, Ben-Yehuda S: A mutant bacteriophage evolved to infect resistant bacteria gained a broader host range. Mol Microbiol 2019, 111:1463–1475. [DOI] [PubMed] [Google Scholar]
- 73.Yoichi M, Abe M, Miyanaga K, Unno H, Tanji Y: Alteration of tail fiber protein gp38 enables T2 phage to infect Escherichia coli O157:H7. J Biotechnol 2005, 115:101–107. [DOI] [PubMed] [Google Scholar]
- 74.Hupfeld M, Trasanidou D, Ramazzini L, Klumpp J, Loessner MJ, Kilcher S: A functional type II-A CRISPR-Cas system from Listeria enables efficient genome editing of large non-integrating bacteriophage. Nucleic Acids Res 2018, 46:6920–6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tao P, Wu X, Tang W-C, Zhu J, Rao V: Engineering of Bacteriophage T4 Genome Using CRISPR-Cas9. ACS Synth Biol 2017, 6:1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martel B, Moineau S: CRISPR-Cas: an efficient tool for genome engineering of virulent bacteriophages. Nucleic Acids Res 2014, 42:9504–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemay M-L, Tremblay DM, Moineau S: Genome Engineering of Virulent Lactococcal Phages Using CRISPR-Cas9. ACS Synth Biol 2017, 6:1351–1358. [DOI] [PubMed] [Google Scholar]
- 78.Kiro R, Shitrit D, Qimron U: Efficient engineering of a bacteriophage genome using the type I-E CRISPR-Cas system. RNA Biol 2014, 11:42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoshiga F, Yoshizaki K, Takao N, Miyanaga K, Tanji Y: Modification of T2 phage infectivity toward Escherichia coli O157:H7 via using CRISPR/Cas9. FEMS Microbiol Lett 2019, 366. [DOI] [PubMed] [Google Scholar]
- 80.Kilcher S, Studer P, Muessner C, Klumpp J, Loessner MJ: Cross-genus rebooting of custom-made, synthetic bacteriophage genomes in L-form bacteria. Proc Natl Acad Sci 2018, doi: 10.1073/pnas.1714658115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scholl D: Phage Tail-Like Bacteriocins. Annu Rev Virol 2017, 4:453–467. [DOI] [PubMed] [Google Scholar]
- 82.Scholl D, Cooley M, Williams SR, Gebhart D, Martin D, Bates A, Mandrell R: An engineered R-type pyocin is a highly specific and sensitive bactericidal agent for the food-borne pathogen Escherichia coli O157:H7. Antimicrob Agents Chemother 2009, 53:3074–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ritchie JM, Greenwich JL, Davis BM, Bronson RT, Gebhart D, Williams SR, Martin D, Scholl D, Waldor MK: An Escherichia coli O157-specific engineered pyocin prevents and ameliorates infection by E. coli O157:H7 in an animal model of diarrheal disease. Antimicrob Agents Chemother 2011, 55:5469–5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE: UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 2004, 25:1605–1612. [DOI] [PubMed] [Google Scholar]
- 85.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ: Basic local alignment search tool. J Mol Biol 1990, 215:403–410. [DOI] [PubMed] [Google Scholar]
- 86.Papadopoulos JS, Agarwala R: COBALT: constraint-based alignment tool for multiple protein sequences. Bioinforma Oxf Engl 2007, 23:1073–1079. [DOI] [PubMed] [Google Scholar]
- 87.Koç C, Xia G, Kühner P, Spinelli S, Roussel A, Cambillau C, Stehle T: Structure of the host-recognition device of Staphylococcus aureus phage ϕ11. Sci Rep 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]