Abstract
Background
The COVID-19 pandemic and related restriction measures have affected our daily life, sleep, and circadian rhythms worldwide. Their effects on hypersomnolence and fatigue remain unclear.
Methods
The International COVID-19 Sleep Study questionnaire which included items on hypersomnolence such as excessive daytime sleepiness (EDS), and excessive quantity of sleep (EQS), as well as sociodemographic factors, sleep patterns, psychological symptoms, and quality of life was distributed in 15 countries across the world from May to September in 2020.
Results
Altogether responses from 18,785 survey participants (65% women, median age 39 years) were available for analysis. Only 2.8% reported having had COVID-19. Compared to before the pandemic, the prevalence of EDS, EQS, and fatigue increased from 17.9% to 25.5%, 1.6%–4.9%, and 19.4%–28.3% amid the pandemic, respectively. In univariate logistic regression models, reports of having a COVID-19 were associated with EQS (OR 5.3; 95%-CI 3.6–8.0), EDS (2.6; 2.0–3.4), and fatigue (2.8; 2.1–3.6). In adjusted multivariate logistic regression, sleep duration shorter than desired (3.9; 3.2–4.7), depressive symptoms (3.1; 2.7–3.5), use of hypnotics (2.3; 1.9–2.8), and having reported COVID-19 (1.9; 1.3–2.6) remained strong predictors of EDS. Similar associations emerged for fatigue. In the multivariate model, depressive symptoms (4.1; 3.6–4.6) and reports of having COVID-19 (2.0; 1.4–2.8) remained associated with EQS.
Conclusions
A large increase in EDS, EQS, and fatigue occurred due to the COVID-19 pandemic, and especially in self-reported cases of COVID-19. These findings warrant a thorough understanding of their pathophysiology to target prevention and treatment strategies for long COVID condition.
Keywords: ICOSS, COVID-19, Sleepiness, Hypersomnia, Fatigue, Pandemic
Abbreviations
- EDS
excessive daytime sleepiness
- EQS
excessive quantity of sleep
- ETFS
excessive tendency to fall asleep
- ICOSS
The International COVID-19 Sleep Study
- OSA
obstructive sleep apnea
- PHQ-4
Patient Health Questionnaire-4
- WHO-5
World Health Organization Well-Being Index
1. Introduction
The COVID-19 pandemic caused by the SARS-CoV-19 virus has enormously impacted us. As of October 2022, there have been more than 623 million confirmed COVID-19 cases and 6.5 million deaths documented globally [1]. Measures aiming to prevent the spread of the disease, especially in the beginning of the pandemic in 2020, such as social distancing, confinements, lockdowns, and remote working, have caused major changes in our daily lives, including alterations in sleep and circadian rhythms [[2], [3], [4], [5], [6], [7]].
Several studies have documented the impact of the COVID-19 pandemic on insomnia, social jetlag, dream content, and symptoms of anxiety, and depression, with a meta-analysis confirming its large impact on sleep alterations and impaired sleep quality [[6], [7], [8], [9]]. In contrast, only a few studies have focused on the direct and indirect consequences of the COVID-19 pandemic on daytime functioning related to sleep problems [4,9]. This is surprising since sleep disturbance, fatigue, and hypersomnolence are increasingly recognized as public health concerns [[10], [11], [12]].
Hypersomnolence refers to a difficulty staying awake during the daytime, i.e. excessive daytime sleepiness (EDS) and/or increased need for sleep associated with daytime impairment [11,13]. Sleep duration exceeding 9 h per night in adults is generally considered as long sleep. Excessive quantity of sleep (EQS) as ≥ 9 h night sleep is associated with problems with impaired vigilance or daytime functioning [14]. Excessive need for sleep indicates as a total sleep duration of at least 10 h per 24 h period and at least 9 h nocturnal sleep with impaired vigilance [13].
The clinical presentation of hypersomnolence may vary greatly from one subject to another as well as for the same subject with time, in terms of frequency, severity, cause, and consequence [11]. Separating the frequency from the intensity of EDS can be challenging; however several questionnaires have assessed these different markers of the severity of hypersomnolence in the general population [15,16]. The reported prevalence of EDS is wide-ranging, from 2.5% to 33% in the general population [11], with a worsening in 16% of cases in the context of the COVID-19 pandemic [4]. Risk factors and causes of such an increase remain unclear. EDS may be the consequence of behavioural issues leading to insufficient or disrupted sleep, sleep and circadian disorders, other medical or psychiatric disorders/diseases, or side effects of medications. Additionally, EDS can be comorbid with long sleep and also with taking several long naps during daytime [11]. EQS is also frequent in the general population, with a reported prevalence of 8.4% and 1.6% when associated with distress or impairment in daytime functioning [14]. EQS is frequently associated with poor health status, including depression, diabetes, hypertension, obstructive sleep apnea, obesity, inflammation, and sometime also direct damage to wake-promoting neural networks [17]. Hypersomnolence may also be associated with daytime fatigue defined as an overwhelming sense of tiredness, lack of energy, and a feeling of exhaustion, associated with impaired physical and/or cognitive functioning [14]. The different components of hypersomnolence and their relationships with fatigue remain underexplored in the context of the COVID-19 pandemic.
The International COVID-19 Sleep Study (ICOSS) was designed to evaluate direct and indirect effects of the first wave of the COVID-19 pandemic on different aspects of sleep and circadian rhythms in adults in several countries across four continents. The main results showed increased sleep problems, fatigue, and EDS during the first phase of the COVID-19 pandemic [4,16]. The aim of this study was to assess the prevalence, the changes, and the factors associated with the frequency and intensity of EDS, EQS, and fatigue before and during the COVID-19 pandemic across different countries.
2. Methods
2.1. Study design
Altogether 15 countries/areas took part in the first ICOSS study with a cross-sectional survey collecting data online in each country in their native language between May and August 2020. The methods of the first ICOSS and the development of the questionnaire have been described elsewhere [4,16,18]. Briefly, the first ICOSS questionnaire comprised 106 items (50 questions) on sociodemographic variables, sleep patterns, sleep disorders, psychological symptoms and quality of life [16]. The survey was completed anonymously on a web survey platform on a University website or in the public domain. Information relating to the questionnaire was distributed via local and social media, departmental newsletters, and websites of different sleep societies. The study protocol was in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The anonymous online survey made re-identification of the respondents impossible. Subjects gave their implied consent by agreeing to participate in the survey.
After gathering data on important sociodemographic variables (i.e. age, gender, marital status, ethnicity, education, profession, working status, confinement, and economic situation), the survey included questions on EDS, EQS, and fatigue. EDS and fatigue were defined by answering “at least three days per week” to the following questions originally from the Basic Nordic Sleep Questionnaire, respectively: “Did/do you feel excessively sleepy during daytime?” and “Did/do you feel fatigued/exhausted at daytime?“, respectively [19]. The survey also asked about the intensity of EDS using a single question derived from the UK Biobank questionnaire (i.e., “How likely was/is it, that you would fall asleep during the daytime without intending to, or that you would struggle to stay awake while you were doing things?”). Having a moderate or high chance of falling asleep indicated an extensive tendency to fall asleep (ETFS) [15]. The perception of total night sleep duration was assessed using the questions “How many hours per night did/do you sleep on average?” with a cut point for long sleep set to 9 h per night [11]. In addition, EQS was defined as long sleep with either EDS or fatigue (or both). The presence and severity of these four symptoms were asked retrospectively at one time point “before” and “during” the pandemic period. The increase, decrease, and stability of these symptoms during the pandemic was computed by subtracting the response during the pandemic by the prepandemic value. Only those respondents with complete answers to these four questions were included to the analytic sample and no imputations for missing data were used.
The following information was also collected with estimates for the periods before and during the pandemic: The use of sleeping pills (hypnotics by prescription)”, the quality of life and health using the 5-item World Health Organization Well-Being Index (WHO-5) [20], and depressive and anxiety symptoms by the Patient Health Questionnaire-4 (PHQ-4) [16,21,22]. Sleep deprivation was assessed by subtracting reported sleep need per 24 h from the time slept per 24 h. The lower limit of ‘sleep deprivation’ was defined as there being a greater than 1 h difference between reported need and actual sleep durations, and likewise the threshold for ‘excessive sleeping’ as being more than 1 h of sleep duration greater than needed. The wordings of asking for confinement and financial suffering have been described in details previously [16]. Another question focused on whether the participant had been infected with coronavirus and been tested positive for the virus. A few questions were asked about the sense of smell and the risk of obstructive sleep apnea (OSA) using the four-item STOP questionnaire [23]. Finally, the metabolic equivalent task values were calculated according to the Compendium of Physical Activities. Metabolic equivalent task were reported rounded in metabolic equivalent task -classes of <100, 100 to 399, 400 to 1,199, and 1200 or more metabolic equivalent task -minutes per week.
2.2. Statistical analysis
The normality of the distributions were tested using the Shapiro-Wilk test. Means and standard deviations are given for the normally distributed variables and medians and interquartile ranges are reported for non-parametric variables. Meta-analyses were used to compute occurrences of EDS, ETFS, EQS, and fatigue in different countries. To strengthen the results, we used random-effects models in all tests. For occurrences and rates, the 95% confidence limits are given. Logistic regression analyses were conducted by weighting of data by the number of inhabitants in the country/area of interest and by the number of responders in that country. Different countries were used as strata. Observations were used as sampling units and non-parametric statistics were applied with proportions, and 95% confidence intervals were calculated. For the differences by groups, the Kruskal–Wallis equality-of-populations rank test was used if the distributions were not normal. For the paired differences in occurrence before and during the pandemic, the Wilcoxon signed-rank test was used. Kendall's Tau-b rank correlation coefficients were computed to search for associations between the changes during the pandemic of the outcome variables (i.e. EDS, ETFS, EQS, and fatigue). Logistic regression analyses were used to assess the influence of the explanatory variables on EDS, ETFS, EQS, and fatigue (see the variables on Table 2 ). The linear assumption for the logistic regression was analysed using Box-Tidwell test. The different logistic models were compared using the Bayesian information criteria for unweighted data. Fitness of the logistic models was estimated by McFadden's pseudo-R2 [24]. A McFadden's pseudo-R2 value of 0.2–0.4 was considered to be a good fit. Values between 0.1 and 0.2 were considered to be mediocre. To allow comparability between different rates and models, the same matrix of variables was used in all regression models. All statistical computations were conducted using STATA version 17.0 (StataCorp, College Station, USA). P-values <.05 were considered statistically significant (two-sided tests). Fig. 2 was produced using R and ggplot2 package.
Table 2.
Predictors of excessive daytime sleepiness during COVID-19 pandemic (significant predictors are highlighted in red).
| Univariate logistic regression |
Multivariate logistic regression using backward selection |
|||||||
|---|---|---|---|---|---|---|---|---|
| OR | P | Lower 95% CI | Upper 95% CI | OR | P | Lower 95% CI | Upper 95% CI | |
| Fatigue | 18.01 | < .001 | 15.69 | 20.71 | – | – | – | – |
| ETFS | 8.66 | < .001 | 7.53 | 9.96 | – | – | – | – |
| Woman gender | 1.17 | .007 | 1.04 | 1.31 | – | – | – | – |
| Age group, years | ||||||||
| < 25 | 1.39 | < .001 | 1.180 | 1.65 | 1.10 | .315 | 0.91 | 1.33 |
| 25-34 | 1.22 | .013 | 1.04 | 1.44 | 0.89 | .223 | 0.74 | 1.07 |
| 35-44 | 1.24 | .011 | 1.05 | 1.48 | 0.88 | .205 | 0.73 | 1.07 |
| 45-54 | 1.04 | .687 | 0.87 | 1.23 | 0.77 | .010 | 0.64 | 0.94 |
| 55-64 | 0.96 | .753 | 0.76 | 1.22 | 0.76 | .044 | 0.58 | 0.99 |
| ≥ 65 | 1 | 1 | . | . | . | |||
| BMI groups, kg/m2 | ||||||||
| < 25 | 1 | 1 | . | . | . | |||
| 25–29.9 | 1.10 | .119 | 0.98 | 1.25 | 0.99 | .870 | 0.86 | 1.13 |
| 30–34.9 | 1.71 | < .001 | 1.43 | 2.05 | 1.33 | .007 | 1.08 | 1.64 |
| ≥ 35 | 2.22 | < .001 | 1.78 | 2.77 | 1.40 | .010 | 1.09 | 1.81 |
| Ethnicity | – | – | – | – | ||||
| Caucasian/White | 1.45 | < .001 | 1.31 | 1.60 | – | – | – | – |
| Asian | 1 | – | – | – | – | |||
| Other | 1.58 | < .001 | 1.28 | 1.91 | – | – | – | – |
| COVID-19 infection | 2.63 | < .001 | 2.00 | 3.45 | 1.85 | < .001 | 1.31 | 2.61 |
| Smell loss | 1.80 | < .001 | 1.39 | 2.33 | – | – | – | – |
| Confinement | 1.41 | < .001 | 1.25 | 1.57 | – | – | – | – |
| Financial suffering | 1.74 | < .001 | 1.42 | 2.12 | – | – | – | – |
| Snoring | 1.64 | < .001 | 1.46 | 1.83 | 1.410 | < .001 | 1.24 | 1.61 |
| Apneas | 1.70 | < .001 | 1.45 | 1.98 | – | – | – | – |
| High blood pressure | 1.23 | .004 | 1.07 | 1.42 | – | – | – | – |
| TST per night | – | – | – | – | ||||
| < 6 h | 3.06 | < .001 | 2.67 | 3.51 | – | – | – | – |
| 6–8.9 h | 1 | – | – | – | – | |||
| > 9 h | 1.74 | < .001 | 1.49 | 2.02 | – | – | – | – |
| Sleep duration vs. sleep need | ||||||||
| TST > sleep need | 1.62 | < .001 | 1.40 | 1.89 | 1.41 | < .001 | 1.21 | 1.64 |
| 0–0.9 h sleep deprivation | 1 | 1 | ||||||
| 1–1.9 sleep deprivation | 2.07 | < .001 | 1.81 | 2.37 | 1.88 | < .001 | 1.64 | 2.16 |
| > 2 h sleep deprivation | 5.31 | < .001 | 4.55 | 6.19 | 3.88 | < .001 | 3.21 | 4.70 |
| METs | – | – | – | – | ||||
| 0-99 | 1.13 | .062 | 0.99 | 1.29 | – | – | – | – |
| 100-400 | 1.16 | .085 | 0.98 | 1.37 | – | – | – | – |
| 400-1200 | 1.10 | .191 | 0.95 | 1.27 | – | – | – | – |
| > 1200 | 1 | – | – | – | – | |||
| Anxiety | 3.98 | < .001 | 3.56 | 4.45 | – | – | – | – |
| Depression | 4.46 | < .001 | 3.99 | 4.99 | 2.99 | < .001 | 2.64 | 3.39 |
| WHO-5 | 0.97 | < .001 | 0.96 | 0.97 | – | – | – | – |
| Hypnotic use (≥1 night per wk) | 3.541 | < .001 | 3.00 | 4.18 | 2.25 | < .001 | 1.87 | 2.71 |
ETFS, Excessive tendency to fall asleep; TST, total sleep time; MET, metabolic equivalent of task; World Health Organization Well-Being Index; -, not included in the model.
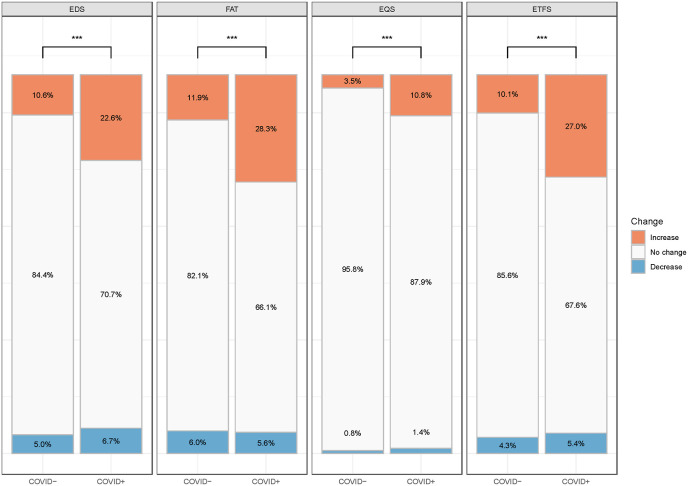
Fig. 2.
Relative change in the occurrence of excessive daytime sleepiness, fatigue, excessive quantity of sleep (≥9-h night sleep associated with EDS or FAT), and extensive tendency to fall asleep in COVID-19 positive (n = 519) and negative (n = 18,266) subjects amid the pandemic compared to before the pandemic. ∗∗∗, p < .001, as derived from Chi-Square test.
EDS, excessive daytime sleepiness; FAT, fatigue; EQS, excessive quantity of sleep (≥9 h night sleep associated with EDS or FAT); ETFS, excessive tendency to fall asleep; COVID -, reported not having COVID-19; COVID +, reported having COVID-19. ∗∗∗, Chi-Square test P < .001.
3. Results
A total of 20,598 subjects from 15 different countries/areas participated in the survey (see Table 1). Altogether 18,785 subjects (91.3%) answered all demographic questions and items on fatigue, EDS, EQS, ETFS, long sleep, and life habits. Most participants were women (65%), and the median age was 39 years. Of note, 519 respondents (2.8%) reported having had a COVID-19 infection. COVID-19 was associated with being older (median = 39, interquartile ranges = 28–54 vs 36; 28–48 years, p = .0012), and with higher BMI than those without infection (24.6; 21.6–28.6 vs 22.9; 20.5–26.1 kgm−2, p < .001). Most subjects lived in urban areas, had postsecondary education, and were working or studying. Many responders (7666; 40.8%) had been in confinement during the pandemic because of COVID-19 (n = 420, 5.5%) or for some other reason for restriction (n = 7,246, 94.5%). At least moderate financial suffering was experienced by 2415 (12.9%) participants, being more common in those with COVID-19 (n = 110, 4.6%) compared to subjects without (n = 409, 2.5%, p < .001).
Table 1.
Demographic distributions of the study population (N = 18,785).
| Reported a COVID-19 infection |
P-value |
||||
|---|---|---|---|---|---|
| No |
Yes |
||||
| n | % | n | % | ||
| Number of respondents | 18,266 | 97.2 | 519 | 2.8 | |
| Gender | .446 | ||||
| Women | 11,805 | 64.6 | 327 | 2.7 | |
| Men | 6461 | 35.4 | 192 | 2.9 | |
| Age-groups | <.001 | ||||
| < 25 y | 2887 | 15.7 | 65 | 2.2 | |
| 25–34 y | 4695 | 25.9 | 167 | 3.4 | |
| 35–44 y | 3355 | 18.5 | 121 | 3.5 | |
| 45–54 y | 3009 | 16.4 | 74 | 2.4 | |
| 55–64 y | 2326 | 12.7 | 62 | 2.6 | |
| > 65 y | 1994 | 10.8 | 30 | 1.5 | |
| BMI, kg/m2 | <.001 | ||||
| < 25 | 12,422 | 67.6 | 273 | 2.2 | |
| 25–29.9 | 3944 | 21. | 152 | 3.7 | |
| 30–34.9 | 1302 | 7.2 | 60 | 4.4 | |
| ≥ 35 | 598 | 3.4 | 34 | 5.4 | |
| Ethnicity | <.001 | ||||
| Caucasian | 7248 | 40.3 | 321 | 4.2 | |
| Asian | 8463 | 45.4 | 71 | 0.8 | |
| African | 284 | 1.7 | 41 | 12.6 | |
| Hispanic | 672 | 3.9 | 53 | 7.3 | |
| Other/indigenous | 1599 | 8.7 | 33 | 2.0 | |
| Living area | .003 | ||||
| Urban | 16,160 | 88.4 | 437 | 2.6 | |
| Rural | 2106 | 11.6 | 82 | 3.7 | |
| Education | <.001 | ||||
| Primary/secondary, high school | 4275 | 23.2 | 80 | 1.8 | |
| Vocational training | 2007 | 10.9 | 47 | 2.3 | |
| University Bachelor's or equivalent | 7570 | 41.4 | 196 | 2.5 | |
| Master or Doctoral level | 4148 | 23.1 | 195 | 4.5 | |
| Other/not given | 266 | 1.4 | 1 | 0.4 | |
| Working during pandemic | <.001 | ||||
| Student | 2509 | 13.6 | 49 | 1.9 | |
| Regular day work | 607 | 46.9 | 274 | 3.1 | |
| Irregular day work/freelancer/artist | 1525 | 8.4 | 49 | 3.1 | |
| Shift/night work | 896 | 5.0 | 47 | 5.0 | |
| Unemployed | 751 | 4.1 | 27 | 3.5 | |
| Retired | 1474 | 8.0 | 24 | 1.6 | |
| At home (no salary) | 1654 | 8.9 | 20 | 1.2 | |
| Temporary laid off | 319 | 1.8 | 13 | 3.9 | |
| Lost job due to pandemic | 201 | 1.1 | 10 | 4.7 | |
| Other | 401 | 2.2 | 6 | 1.5 | |
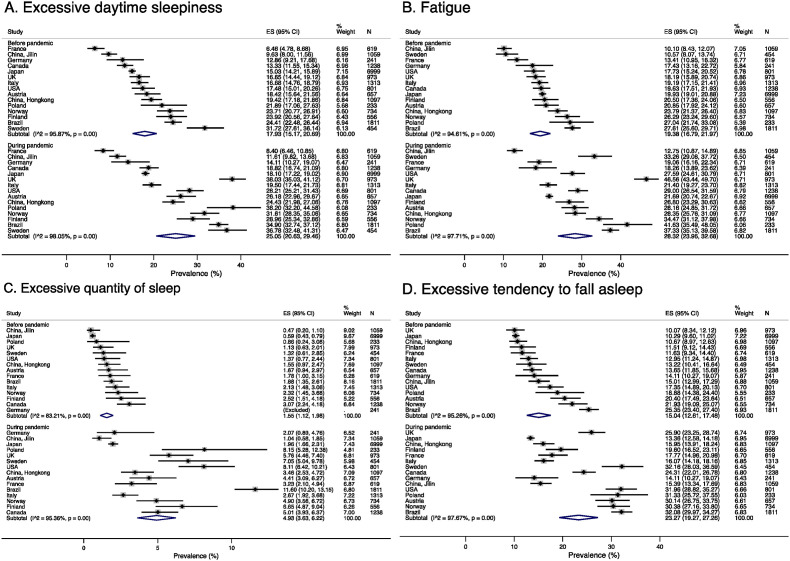
In random effects meta-analysis, before the pandemic, the pooled estimate of prevalence of EDS was 17.9% (95% CI 15.2%–20.7%) and during the pandemic 25.1% (95% CI 20.7–29.5%) (Fig. 1) The prevalence of ETFS before the pandemic was also high 15.4% overall (95% CI 12.6%–17.5%), especially in Brazil (25.4%), followed by Norway (21.9%), and Austria (20.4%) that increased greatly during the pandemic in most countries with an overall prevalence of 20.1%. The prevalence of fatigue before the pandemic was similarily high, ranging from 10.1% in the Jilin region in China to 27.6% in Brazil, and 19.4% overall (95% CI 16.8%–22.0%). The prevalence of fatigue also increased to 28.3% (95% CI 24.0%–32.7%) during the pandemic, especially in the UK and Poland being above 40% in these countries. The increase in the EQS was proportionally largest, from pooled estimate of 1.55% (95% CI 1.1–2.0%) before the pandemic to 4.9% (95% CI 3.6%–6.2%) during the pandemic with an increase in every country, particularly in Brazil, the USA, and Canada.
Fig. 1.
Forest plot for prevalence of A. Excessive daytime sleepiness (EDS). B. Fatigue, C. Excessive quantity of sleep (EQS, ≥9 h sleep per night, associated with EDS or fatigue) and D. Excessive tendency to fall asleep (ETFS) across participating countries before and during the COVID-19 pandemic.
The frequency of EDS, ETFS, EQS, and fatigue is higher in participants who had a COVID-19 twice as much, on average, as non-infected participants (Fig. 2). For instance, EDS increased in 22.6% of subjects with COVID-19 compared to 10.6% in those without (p < .001). The differences in increase in EDS, ETFS, EQS, and fatigue between subjects with and without COVID-19 were 12.0%, 16.9%, 7.3%, and 16.4%, respectively (p < .001). Decreases in any of the four symptoms were rare, and did not differ by the presence or absence of COVID-19 infection.
At the baseline, before the pandemic, we found moderate association between EDS and fatigue (τb = 0.520, p < .001), and with EDS and ETFS (τb = 0.393, p < .001). Fatigue had moderate association with ETFS (τb = 0.342, p < .001). EQS had only a weak association with EDS (τb = 0.158), fatigue (τb = 0.163), and ETFS (τb = 0.086, p < .001 in all comparisons). We found a strong association between change in EDS and fatigue during the pandemic (τb = 0.501), and a moderate association between change in EDS and ETFS (τb = 0.375), and a moderate association between change in ETFS and fatigue (τb = 0.346, p < .001 in all comparisons). Change in EQS had weak correlation with EDS (τb = 0.178), fatigue (τb = 0.173), and ETFS (τb = 0.0.914, p < .001 in all comparisons).
Logistic regression analyses were conducted to assess the factors associated with EDS, EQS, ETFS, and fatigue during the pandemic. Some variables (e.g. depressive symptoms, anxiety symptoms and WHO5 score) were associated in the models but with high multicollinearity between them. Based on changes in the Bayesian information criteria, we decided to keep only the PHQ depression item in the models. After a backward stepwise selection of variables in the final model for EDS during the pandemic, sleep deprivation, and excessive sleeping, depressive symptoms, use of hypnotics, COVID-19 infection, higher BMI, age, and snoring were the strongest associated factors. Confinement was in the final logistic regression model not associated with EDS (Table 2). McFadden's pseudo-R2 for fitness of the model was 0.13 for EDS during the pandemic. Similar results with McFadden's pseudo-R2 of 0.12 were found in the final model for ETFS, with excessive sleeping, sleep deprivation, depressive symptoms, use of hypnotics, COVID-19 infection, higher BMI, age, confinement and the risk of having OSA being the significant factors (Table S3). Woman gender, age, high BMI, ethnicity, excessive sleeping, sleep deprivation, confinement, depressive symptoms, use of hypnotics, COVID-19 infection and snoring were associated with fatigue (McFadden's pseudo-R2 = 0.16) (Table S1). We also found that age, BMI, ethnicity, COVID-19 infection, depressive symptoms, and use of hypnotics were associated with EQS (Table S2). McFadden's pseudo-R2 for EQS was 0.112.
4. Discussion
Based on our study assessing the effects of the COVID-19 pandemic on sleep in 18,785 adults, we found an increase in EDS, EQS, ETFS, and fatigue across the course of the COVID-19 pandemic in all 15 countries studied. Also, reports of a COVID-19 infection were associated with a 2- to 3-fold increase in all symptoms of hypersomnolence and fatigue.
EDS was assessed in the present study via both its frequency and intensity by responding to two validated items. We were also interested in the evaluation of EQS and fatigue, symptoms often associated with EDS [25]. The transition from wakefulness to sleep can result from physiological regulation of wakefulness, but sometimes also from an impaired arousal mechanism as reported in narcolepsy with orexin deficiency [26]. EDS can also be related to conditions causing fragmented sleep and unrefreshing sleep such as OSA, and other medical or psychiatric conditions, or the use of medications acting on the central nervous system (CNS) [27]. In the first wave of ICOSS study underlying the present analysis, 4.1% reported having diagnosed OSA, and the prevalence of having a high risk of OSA was 9.5% [7,28].
Due to large variabilities in the definition proposed, the pre-pandemic prevalence of fatigue may range from 10% to 30% [10]. In the context of the COVID-19 pandemic, we found that the prevalence of EDS, ETFS, EQS and fatigue were high (i.e. 25.1%, 20.1%, 4.9%, and 28.3%, respectively), more than doubling compared to the pre-pandemic period across all the studied countries. Some differences in the prevalence of hypersomnolence symptoms were found between countries both before and during the pandemic, which may be due to epidemiological and stressful conditions at the time of survey, but also to confinement, ethnicity, financial hardship, and cultural differences.
We reported several associated factors for EDS, ETFS, EQS and fatigue during the pandemic, being similar in most of the models, including sleep deprivation, depressive symptoms, confinement, use of hypnotics, COVID-19 infection, higher BMI, age, and proxy for OSA. Such associations have been found in previous epidemiological studies, which are often reported along with altered sleep architecture and homeostatic sleep regulation [29,30]. In contrast, we found no association between hypersomnolence symptoms, physical activities and smell loss. The absence of the latter association is interesting. In addition to COVID-19, smell loss is often found with dream-enacting behavior and the so-called REM sleep behavior disorder, a well-known risk factor of synucleinopathies [31]. EDS is highly prevalent in synucleionopathies suggesting that similar neural networks regulate sleepiness, dream-enacting, and olfactory function. On the other hand, in our previous study we found an association between COVID-19 infection and dream enactment behavior that may suggest the involvement of mental factors rather than central effects of COVID-19 infection [32].
Early reports suggested that neurotropic effects of SARS-CoV-2 by either direct CNS invasion or an indirect activation of immune system (i.e. pro-inflammatory cytokines storm, blood biomarkers, immune cells and autoantibodies) could explain many CNS symptoms including hypersomnolence that are related to COVID-19 [[33], [34], [35], [36]]. Disrupted glymphatic flow caused by e.g. glial cell inflammation and amyloid accumulation have also been proposed as a mechanism of fatigue and brain dysfunction caused by COVID-19 infection [[37], [38], [39]]. However, sleep deprivation and lack of recuperative sleep may also decrease glymphatic flow [40]. Nevertheless, a German prospective study, albeit with limited sample size, has shown that the nervous system is rarely directly affected in patients with post-COVID-19 syndrome [41]. In our study, we found an association between COVID-19 infection and symptoms of hypersomnolence and fatigue, but this result needs to be interpreted with caution as the associations with sleep deprivation, depressive and anxiety symptoms, hypnotic use, and OSA were stronger. It could be that the effect of COVID-19 on hypersomnolence symptoms is partially explained by social and psychological effects, which is in line with the German study [41]. In previous pandemics, short and long-term neurological consequences have often been reported, but inconsistently with severe sleep symptoms except with the Spanish flu in 1918 with encephalitis lethargica and narcolepsy, and the 2009–2010 H1N1 pandemic, especially the Pandemrix® vaccination against it, with narcolepsy [42,43]. In contrast, no increased risk of narcolepsy from COVID-19 or its vaccination has been reported to date; however during the pandemic patients with narcolepsy presented more daytime sleepiness with a lower quality of life [44].
Symptoms of hypersomnolence, often associated with each other, may have other health implications, acting as potential risk factors for cardiovascular, metabolic, inflammatory, neurological and psychiatric disorders [45]. Furthermore, fatigue is prevalent in post-COVID-19 recovery and is one of the most pervasive symptoms in long COVID [[46], [47], [48]]. In conclusion, EDS is one of the key symptoms in long COVID [48]; however, whether EQS constitutes a common symptom in long COVID remains to be further studied. In patients with severe COVID-19 necessitating hospital treatment, prolonged symptoms may be readily understood. They resemble symptoms of Post Intensive Care Syndrome [49]. Guedj and coworkers found alterations in brain metabolism by FDG-PET in long COVID, but the subjects in that study had in fact a severe disease [50]. For mild acute COVID-19, treated at home, the cause of the long COVID remains unclear. It may be important to differentiate these two phenotypes (i.e., severe COVID-19 with hospital treatment versus mild COVID-19 treated at home) from each other; the latter phenotype may be self-sustaining long-lasting symptoms by psychosocial factors confirming our earlier results [4]. However, the cross-sectional nature of the present analsyis preclude further speculation on potential mechanisms linking EDS, EQS and fatigue in the context of COVID-19.
4.1. Limitations
There are limitations to our study. Firstly, measuring EDS and fatigue are challenging and we could not use objective sleepiness measures such as the Multiple Sleep Latency Test. In addition, there are no adequately validated objective tests to quantify fatigue, so we had to rely on questionnaires. Existing comprehensive sleepiness and fatigue measurement tools, e.g. Epworth Sleepiness Scale and Fatigue Severity Scale, were not included in the survey. Therefore, we used only single questions from the Basic Nordic Sleep Questionnaire and UK Biobank. Secondly, due to the anonymous survey design and the size of our study, we could not collect the respondents' clinical data. Therefore, we could not confirm the infection status from health records. However, the subjects were asked if they were tested positive for COVID-19 in addition to just reporting the presumed infection. Thirdly, there is a risk for recall bias when asking about information before the pandemic. The survey was conducted relatively early in the pandemic resulting in a limited number of subjects with COVID-19, thus underpowering to compare risk factors associated with COVID-19 pandemic vs. the infection per se. Finally, the study was introduced as a sleep investigation which may have introduced a bias by recruiting a population with increased such problems, as compared to the general public.
4.2. Conclusion
In conclusion, we found a large increase in EDS, ETFS, EQS, and fatigue during the COVID-19 pandemic in all the studied countries, and especially in participants with previous COVID-19. These findings warrant a thorough understanding of their pathophysiology to target prevention and treatment strategies for long COVID condition.
Funding
Markku Partinen and Ilona Merikanto have received grants (nr 5961 and 5815, respectively) from Signe & Ane Gyllenberg Foundation for the funding of this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank all the people who have responded to the survey in different countries. We also thank all other in the ICOSS-1 collaboration. They are in alphabetical order: Fang Han (Beijing, China) and Jonathan Cedernaes (Uppsala, Sweden).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sleep.2023.04.024.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.WHO WHO coronavirus (COVID-19) dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data. https://covid19.who.int/ n.d.
- 2.Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatr. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Zhu L.-Y., Ma Y.-F., Bo H.-X., Deng H.-B., Cao J., et al. Association of insomnia disorder with sociodemographic factors and poor mental health in COVID-19 inpatients in China. Sleep Med. 2020;75:282–286. doi: 10.1016/j.sleep.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Partinen M., Holzinger B., Morin C.M., Espie C., Chung F., Penzel T., et al. Sleep and daytime problems during the COVID-19 pandemic and effects of coronavirus infection, confinement and financial suffering: a multinational survey using a harmonised questionnaire. BMJ Open. 2021;11 doi: 10.1136/BMJOPEN-2021-050672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu T., Jia X., Shi H., Niu J., Yin X., Xie J., et al. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord. 2021;281:91–98. doi: 10.1016/j.jad.2020.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merikanto I., Kortesoja L., Benedict C., Chung F., Cedernaes J., Espie C.A., et al. Evening-types show highest increase of sleep and mental health problems during the COVID-19 pandemic - multinational study on 19 267 adults. Sleep. 2021;45 doi: 10.1093/sleep/zsab216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holzinger B., Nierwetberg F., Chung F., Bolstad C.J., Bjorvatn B., Chan N.Y., et al. Has the COVID-19 pandemic traumatized us collectively? The impact of the COVID-19 pandemic on mental health and sleep factors via traumatization: a multinational survey. Nat Sci Sleep. 2022;14:1469–1483. doi: 10.2147/NSS·S368147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarpelli S., De Santis A., Alfonsi V., Gorgoni M., Morin C.M., Espie C., et al. The role of sleep and dreams in long-COVID. J Sleep Res. 2022 doi: 10.1111/JSR.13789. [DOI] [PubMed] [Google Scholar]
- 9.Chung F., Waseem R., Pham C., Penzel T., Han F., Bjorvatn B., et al. The association between high risk of sleep apnea, comorbidities, and risk of COVID-19: a population-based international harmonized study. Sleep Breath. 2021;25:849–860. doi: 10.1007/S11325-021-02373-5/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galland-Decker C., Marques-Vidal P., Vollenweider P. Prevalence and factors associated with fatigue in the Lausanne middle-aged population: a population-based, cross-sectional survey. BMJ Open. 2019;9 doi: 10.1136/BMJOPEN-2018-027070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Carbonell L., Mignot E., Leschziner G., Dauvilliers Y. Understanding and approaching excessive daytime sleepiness. Lancet. 2022;400:1033–1046. doi: 10.1016/s0140-6736(22)01018-2. [DOI] [PubMed] [Google Scholar]
- 12.Chattu V.K., Manzar M.D., Kumary S., Burman D., Spence D.W., Pandi-Perumal S.R. vol. 7. Healthc; Basel, Switzerland: 2018. (The global problem of insufficient sleep and its serious public health implications). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lammers G.J., Bassetti C.L.A., Dolenc-Groselj L., Jennum P.J., Kallweit U., Khatami R., et al. Diagnosis of central disorders of hypersomnolence: a reappraisal by European experts. Sleep Med Rev. 2020;52 doi: 10.1016/J.SMRV.2020.101306. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon M.M., Reynolds C.F., Dauvilliers Y. Excessive sleep duration and quality of life. Ann Neurol. 2013;73:785–794. doi: 10.1002/ANA.23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UK Biobank - UK Biobank https://www.ukbiobank.ac.uk/ n.d.
- 16.Partinen M., Bjorvatn B., Holzinger B., Chung F., Penzel T., Espie C.A., et al. Sleep and circadian problems during the coronavirus disease 2019 (COVID-19) pandemic: the International COVID-19 Sleep Study (ICOSS) J Sleep Res. 2021;30 doi: 10.1111/JSR.13206. [DOI] [PubMed] [Google Scholar]
- 17.Grandner M.A., Drummond S.P.A. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11:341–360. doi: 10.1016/J.SMRV.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morin C.M., Bjorvatn B., Chung F., Holzinger B., Partinen M., Penzel T., et al. Insomnia, anxiety, and depression during the COVID-19 pandemic: an international collaborative study. Sleep Med. 2021;87:38–45. doi: 10.1016/J.SLEEP.2021.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partinen M., Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4:150–155. doi: 10.1111/J.1365-2869.1995.TB00205.X. [DOI] [PubMed] [Google Scholar]
- 20.Topp C.W., Østergaard S.D., Søndergaard S., Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom. 2015;84:167–176. doi: 10.1159/000376585. [DOI] [PubMed] [Google Scholar]
- 21.De Boer A.G.E.M., Van Lanschot Jjb, Stalmeier P.F.M., Van Sandick J.W., Hulscher J.B.F., De Haes J.C.J.M., et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13:311–320. doi: 10.1023/B:QURE.0000018499.64574.1F. 132 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K., Spitzer R.L., Williams J.B.W., Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ–4. Psychosomatics. 2009;50:613–621. doi: 10.1016/S0033-3182(09)70864-3. [DOI] [PubMed] [Google Scholar]
- 23.Chung F., Yegneswaran B., Liao P., Chung S.A., Vairavanathan S., Islam S., et al. STOP QuestionnaireA tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–821. doi: 10.1097/ALN.0B013E31816D83E4. [DOI] [PubMed] [Google Scholar]
- 24.McFadden D. Conditional logit analysis of qualitative choice behavior. Front Econom. 1974:105–142. [Google Scholar]
- 25.Shen J., Barbera J., Shapiro C.M. Distinguishing sleepiness and fatigue: focus on definition and measurement. Sleep Med Rev. 2006;10:63–76. doi: 10.1016/J.SMRV.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Scammell T.E., Arrigoni E., Lipton J. Neural circuitry of wakefulness and sleep. Neuron. 2017;93:747. doi: 10.1016/J.NEURON.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagel J.F. Excessive daytime sleepiness. Am Fam Physician. 2009;79:391–396. [PubMed] [Google Scholar]
- 28.Chung F., Waseem R., Pham C., Penzel T., Han F., Bjorvatn B., et al. The association between high risk of sleep apnea, comorbidities, and risk of COVID-19: a population-based international harmonized study. Sleep Breath. 2021;25:849–860. doi: 10.1007/s11325-021-02373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bixler E.O., Vgontzas A.N., Lin H.M., Calhoun S.L., Vela-Bueno A., Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–4515. doi: 10.1210/JC.2005-0035. [DOI] [PubMed] [Google Scholar]
- 30.Hayley A.C., Williams L.J., Berk M., Kennedy G.A., Jacka F.N., Pasco J.A. The relationship between excessive daytime sleepiness and depressive and anxiety disorders in women. Aust N Z J Psychiatr. 2013;47:772–778. doi: 10.1177/0004867413490036. [DOI] [PubMed] [Google Scholar]
- 31.Boeve B.F., Silber M.H., Ferman T.J., Lucas J.A., Parisi J.E. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16:622–630. doi: 10.1002/MDS.1120. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Partinen E., Chan N.Y., Dauvilliers Y., Inoue Y., De Gennaro L., et al. Dream-enactment behaviours during the COVID-19 pandemic: an international COVID-19 sleep study. J Sleep Res. 2022;9 doi: 10.1111/JSR.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Z., Kang H., Li S., Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267:1. doi: 10.1007/S00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J., Jolkkonen J., Zhao C. Neurotropism of SARS-CoV-2 and its neuropathological alterations: similarities with other coronaviruses. Neurosci Biobehav Rev. 2020;119:184. doi: 10.1016/J.NEUBIOREV.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C.-H., Giuliani F. The role of inflammation in depression and fatigue. Front Immunol. 2019;0:1696. doi: 10.3389/FIMMU.2019.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charnley M., Islam S., Bindra G.K., Engwirda J., Ratcliffe J., Zhou J., et al. Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: potential implications for neurological symptoms in COVID-19. Nat Commun. 2022:1–11. doi: 10.1038/s41467-022-30932-1. 131 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews M.G., Mukhtar T., Eze U.C., Simoneau C.R., Ross J., Parikshak N., et al. Tropism of {SARS}-{CoV}-2 for human cortical astrocytes. Proc Natl Acad Sci USA. 2022;119 doi: 10.1073/pnas.2122236119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasmussen M.K., Mestre H., Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17:1016–1024. doi: 10.1016/s1474-4422(18)30318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spudich S., Nath A. Nervous system consequences of COVID-19. Science. 2022;375:267–269. doi: 10.1126/science.abm2052. 80- [DOI] [PubMed] [Google Scholar]
- 40.Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. 80- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleischer M., Szepanowski F., Tovar M., Herchert K., Dinse H., Schweda A., et al. Post-COVID-19 syndrome is rarely associated with damage of the nervous system: findings from a prospective observational cohort study in 171 patients. Neurol Ther. 2022;11:1637–1657. doi: 10.1007/s40120-022-00395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valerio F., Whitehouse D.P., Menon D.K., Newcombe V.F.J. The neurological sequelae of pandemics and epidemics. J Neurol. 2020;268:2629–2655. doi: 10.1007/S00415-020-10261-3. 2688 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarkanen T.O., Alakuijala A.P.E., Dauvilliers Y.A., Partinen M.M. Incidence of narcolepsy after H1N1 influenza and vaccinations: systematic review and meta-analysis. Sleep Med Rev. 2018;38:177–186. doi: 10.1016/j.smrv.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Barateau L., Chenini S., Rassu A.L., Denis C., Lorber Q., Dhalluin C., et al. Changes in sleep pattern during the COVID-19 lockdown in patients with narcolepsy, idiopathic hypersomnia, and restless legs syndrome. Neurology. 2022;99:E1475–E1485. doi: 10.1212/WNL.0000000000200907. [DOI] [PubMed] [Google Scholar]
- 45.Partinen M., Kronholm E. Epidemiology: principles and application in sleep medicine. Sleep disord. Med. Springer; New York: 2017. pp. 485–521. [DOI] [Google Scholar]
- 46.Bliddal S., Banasik K., Pedersen O.B., Nissen J., Cantwell L., Schwinn M., et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci Rep. 2021:1–11. doi: 10.1038/s41598-021-92045-x. 111 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Augustin M., Schommers P., Stecher M., Dewald F., Gieselmann L., Gruell H., et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Heal – Eur. 2021;6 doi: 10.1016/J.LANEPE.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merikanto I., Dauvilliers Y., Chung F., Wing Y.K., Gennaro L De, Holzinger B., et al. Sleep symptoms are essential features of long-COVID - comparing healthy controls with COVID-19 cases of different severity in the international COVID sleep study (ICOSS-II) J Sleep Res. 2022 doi: 10.1111/jsr.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodgson C.L., Higgins A.M., Bailey M.J., Mather A.M., Beach L., Bellomo R., et al. Comparison of 6-month outcomes of survivors of COVID-19 versus non-COVID-19 critical illness. Am J Respir Crit Care Med. 2022;205:1159–1168. doi: 10.1164/RCCM.202110-2335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guedj E., Campion J.Y., Dudouet P., Kaphan E., Bregeon F., Tissot-Dupont H., et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imag. 2021;489:2823–2833. doi: 10.1007/S00259-021-05215-4. 2021;48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.