Abstract
Purpose:
Little is known regarding the prognostic implications of variant histology in upper tract urothelial carcinoma (UTUC). We sought to evaluate the impact of variant histology UTUC on patient survival outcomes at our institution.
Materials and Methods:
We identified 705 patients who underwent nephroureterectomy for UTUC at our institution between January 1995 and December 2018. We tested the association between variant histology and cancer-specific (CSS) and overall survival (OS) using separate multivariable Cox models after adjusting for pathological stage.
Results:
Forty-seven patients (6.7%) had variant histology, with prevalence increasing over time (p=0.003). Other demographic and surgical characteristics were similar between variant histology and pure UC groups. While patients with variant histology were more likely to receive neoadjuvant chemotherapy (38% vs 15%; p<0.001), they were also more likely to have a higher pathologic T stage (p<0.001). Variant histology was associated with significantly worse CSS (HR: 2.14; 95% CI 1.33, 3.44; p=0.002) and OS (HR: 1.74; 95% CI 1.15, 2.63; p=0.008). After adjusting for pathologic T stage, variant histology was not significantly associated with CSS (HR: 1.17; 95% CI 0.72, 1.89; p=0.5) or OS (HR: 1.20; 95% CI 0.79, 1.84; p=0.4).
Conclusions:
Variant histology UTUC is associated with advanced stage and poor survival and could serve as a useful biomarker for high-risk disease when pathological stage is unknown. However, the inferior CSS and OS with variant histology can be explained by the higher tumor stage on nephroureterectomy. Thus, finding variant histology on surgical pathology does not provide additional prognostic information beyond stage.
Keywords: upper tract urothelial carcinoma, nephroureterectomy, variant histology
INTRODUCTION
Upper tract urothelial carcinoma (UTUC) is a rare malignancy, accounting for 5–10% of all urothelial carcinomas diagnosed in the United States [1, 2]. However, improvements in surveillance of bladder cancer patients, coupled with advancements in imaging and endoscopic technology, have contributed to increasing incidence of UTUC [3]. Although morphologically indistinct from urothelial carcinoma of the bladder, UTUC has a notably divergent molecular profile with a higher prevalence of mutations in FGFR3 and HRAS, and less frequent mutations in TP53 and RB1 [4]. Additionally, up to 10% of UTUC patients harbor germline deficiencies in mismatch repair genes associated with Lynch Syndrome [5]. Regardless of the genomic profile, tumor stage and grade remain the most important prognostic factors at diagnosis [6]. Due to the aggressive nature of this disease, the “gold standard” for treatment of UTUC is radical nephroureterectomy (RNU) with excision of a bladder cuff. Perioperative platinum-based chemotherapy plays an evolving but important role in management of this aggressive disease [7].
Variant histology is present in up to a third of bladder urothelial carcinoma cases, most commonly in the form of divergent differentiation (eg, squamous, glandular, micropapillary, etc.) that arises in association with the urothelial component. Occasionally, purely nonurothelial tumors can develop, primarily as squamous cell carcinoma or adenocarcinoma. Variant histology findings are associated with adverse pathologic features and worse oncologic outcomes in patients with bladder cancer [8].
The impact of variant histology in UTUC is still not well defined, and most disease management is extrapolated from the bladder cancer data. Unique challenges exist in the management of UTUC, including the frequent lack of adequate tissue prior to RNU. While ureteroscopic or percutaneous biopsy can predict tumor grade with reasonable accuracy [9], diagnosis of variant histology typically requires additional tissue afforded by extirpative surgery. As such, unlike with bladder cancer, most variant histology diagnoses are made after RNU. As diagnosis of variant histologies are increasing in UTUC, it is paramount to better define their impact on patient outcomes, with consequent tailoring of disease management, adjuvant therapies, and follow-up strategy [6].
In this study we sought to evaluate the impact of variant histology pathologic diagnosis on cancer-specific and overall survival at our institution.
MATERIALS AND METHODS
After institutional review board approval was obtained, data were collected from a prospectively updated clinical research database at our institution. The search identified 809 consecutive nephroureterectomy procedures performed at Memorial Sloan Kettering Cancer Center (MSK) from January 6, 1995, to December 17, 2018. Six patients who underwent serial nephroureterectomies had their second procedure excluded. Patients were excluded if they had renal cell carcinoma only (56), metastasis (4), or no cancer (38) on pathology. Therefore, there were 705 unique patients and procedures for analysis.
Surgery was performed by genitourinary surgeons according to the previously described standard criteria for RNU [10]. Surgical approach (open, laparoscopic, or robot-assisted) was determined according to each patient’s and surgeon’s preference. Whether to also perform lymph node dissection was at the discretion of each surgeon. Standard lymph node dissection encompassed hilar and regional lymph nodes adjacent to the ipsilateral great vessel, which included paracaval for the right side and paraaortic for the left side, above the bifurcation of the aorta. For patients with distal ureteral tumors, the lymph node dissection encompassed the common, external, and internal iliac and obturator nodes. Extended lymph node dissection, including retrocaval and interaortocaval lymphadenectomy, was not routinely performed unless locally advanced disease (pT3/4 or N+ disease) was suspected.
Pathologic Evaluation
All surgical specimens were processed according to our institution’s standard pathologic procedures and analyzed by certified pathologists. Of note, in 2005, subspecialty divisions were created, after which all cases were reviewed by specialized genitourinary pathologists. All specimens were histologically confirmed to be urothelial tumors. UTUC was defined as urothelial carcinoma in the renal pelvis or calyces as well as tumors located within the ureter. Tumors were staged according to the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) TNM classification [11].Tumor grading and variant histology identification and characterization were assessed according to the 2016 World Health Organization’s classification [12].
Follow-up Protocol
Patients undergoing RNU were followed every three months for the first year, every four months for the second year, every six months from the third through the fifth year, and at least annually thereafter. All evaluations consisted of a history and physical examination, laboratory evaluation including urine cytology, cystoscopy, cross-sectional imaging of the abdomen and pelvis, and imaging of the chest. Additional image evaluations such as bone scans, chest CT, and MRIs were performed when clinically indicated.
Disease recurrence was defined as any disease relapse—documented by radiograph, endoscope, or pathology—in the bladder, contralateral kidney, operative site, or regional lymph nodes, or distant metastases. Cause of death was determined by chart review corroborated by death certificate. Patients were censored for disease recurrence on their date of last imaging or pathologic evaluation, for cancer-specific survival their date of death due to other causes or date of last contact if still alive, and for overall survival the date of last contact.
Statistical Methods
We tested the association between variant histology (if present on final pathology) and recurrence-free, cancer-specific, and overall survival using separate multivariable Cox models, after adjusting for the pathologic stage. We repeated the primary analyses using competing risks regression for the outcomes of recurrence-free survival with death from any cause as a competing event and cancer-specific survival with death from other causes as a competing event. We also used multivariable Cox regression to evaluate the association between variant histology and cancer-specific and overall survival among those patients who experienced a recurrence, where survival time is measured from the date of recurrence. P-values <0.05 were defined as statistically significant. All analyses were performed using R version 3.6.0.
RESULTS
Variant histology was present in the nephroureterectomy specimens of 47 patients (6.7%). There were 28 with squamous differentiation, 8 with sarcomatoid, 3 with glandular differentiation, 2 with pure squamous cell carcinoma, and 1 each of lymphoepithelioma-like, large and small cell carcinoma, plasmacytoid, nested, trophoblastic differentiation, and pure small cell carcinoma. Patients’ and disease characteristics, grouped by the presence or absence of variant histology, are presented in Table 1. Prevalence increased over time (p = 0.003). Five of 47 variant histology cases were diagnosed prior to the 2005 subspecialization of genitourinary pathologists, while 42 were diagnosed afterwards. While patients with variant histology were more likely to receive neoadjuvant chemotherapy (38% vs 15%; p <0.001), they were also more likely to have a higher T stage disease on final pathology (p <0.001). The median follow-up time for those who did not experience a recurrence was 2.2 years (Q1 0.8, Q3 5.7) and was similar among those with and without variant histology 2.2 years (Q1 1.2, Q3 6.2) and 2.9 years (Q1 0.9, Q3 6.8). After the initial surgical treatment, 434 patients experienced disease progression, 28 of which had variant histology, and 326 died, 25 of which had variant histology. UTUC was responsible for 188 deaths, 19 of which had variant histology. The cause of death was correctly identified in all patients, and none were excluded from the cancer-specific survival analysis.
Table 1.
Patient and disease characteristics at time of nephroureterectomy, separately for those patients with variant histology on pathology and those without any variant histology. Results are presented as median (quartiles) and frequency (%). Groups are compared by Wilcoxon, χ2 or Fisher’s exact test.
| Characteristic | No Variant Histology, n = 658 | Variant Histology, n = 47 | p-value |
|---|---|---|---|
|
| |||
| Age at surgery (yr) | 71 (64, 77) | 70 (63, 78) | 0.9 |
| Surgical approach | 0.2 | ||
| Laparoscopic | 71 (11) | 1 (2) | |
| Open | 446 (68) | 35 (74) | |
| Robotic | 141 (21) | 11 (23) | |
| Year of surgery | 2009 (2003, 2015) | 2013 (2010, 2016) | 0.003 |
| Bilateral nephroureterectomy | 1 (0) | 0 (0) | >0.9 |
| Neoadjuvant chemotherapy | 97 (15) | 18 (38) | <0.001 |
| Pathologic T stage | <0.001 | ||
| pTA/IS/1 | 365 (55) | 3 (6) | |
| pT2 | 112 (17) | 11 (23) | |
| pT3 | 168 (26) | 28 (60) | |
| pT4 | 13 (2) | 5 (11) | |
| Lymph node dissection | 475 (72) | 38 (81) | 0.2 |
| Pathologic N stage among those who underwent a lymph node dissection | 0.2 | ||
| pN0 | 391 (82) | 28 (74) | |
| pN1–3 | 84 (18) | 10 (26) | |
| Number of nodes removed among those who underwent a lymph node dissection | 9 (4, 17) | 13 (6, 18) | 0.2 |
| Unknown | 1 | 0 | |
The presence of variant histology was associated with an increased risk for the outcome of both cancer-specific (Fig. 1; hazard ratio [HR]: 2.14; 95% confidence interval [CI]: 1.33, 3.44; p = 0.002) and overall survival (Fig. 2; HR: 1.74; 95% CI: 1.15, 2.63; p = 0.008). The association between variant histology and the outcome of recurrence-free survival was not statistically significanct (Fig. 3; HR: 1.01; 95% CI: 0.69, 1.49; p > 0.9). After adjusting for the pathologic T stage, variant histology was not significantly associated with any outcome (all p-values ≥ 0.4; Table 2). As we noted the significant difference in year of surgery and receipt of neoadjuvant chemotherapy by variant histology status (Table 1) we performed sensitivity analyses for all outcomes after adjusting for year of surgery and neoadjuvant chemotherapy receipt in addition to pathologic T stage and the results did not meaningfully change. When using competing risks regression, the association between variant histology and recurrence-free and cancer-specific survival did not appreciably change (HR=0.94; 95% CI 0.63, 1.41; p=0.8 and HR=1.13; 95% CI 0.70, 1.83; p=0.6, respectively).
Figure 1.
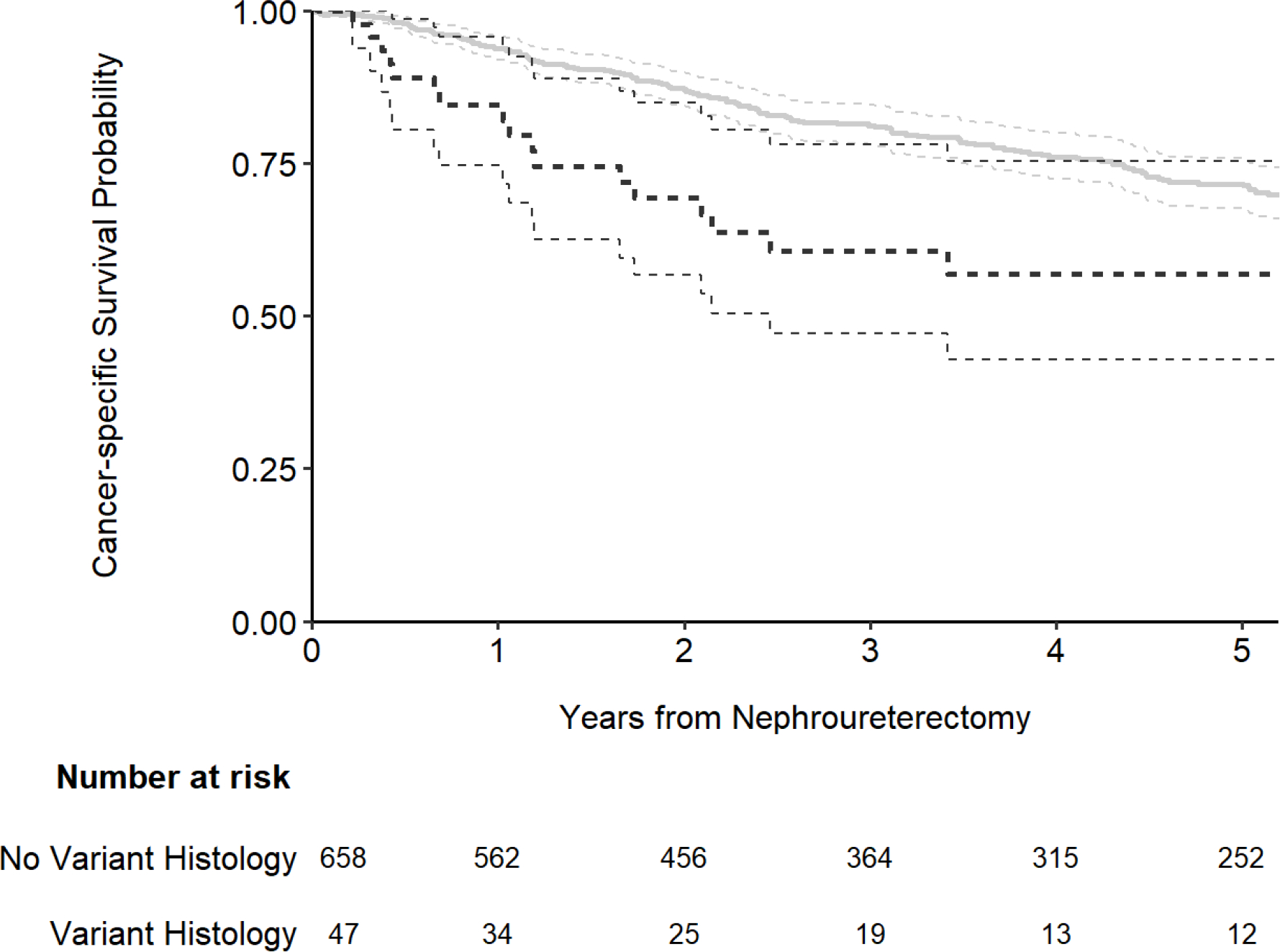
Kaplan-Meier plot for cancer-specific survival after nephroureterectomy, separately for patients with no variant histology (gray line) and those with any variant histology on pathology (black line).
Figure 2.
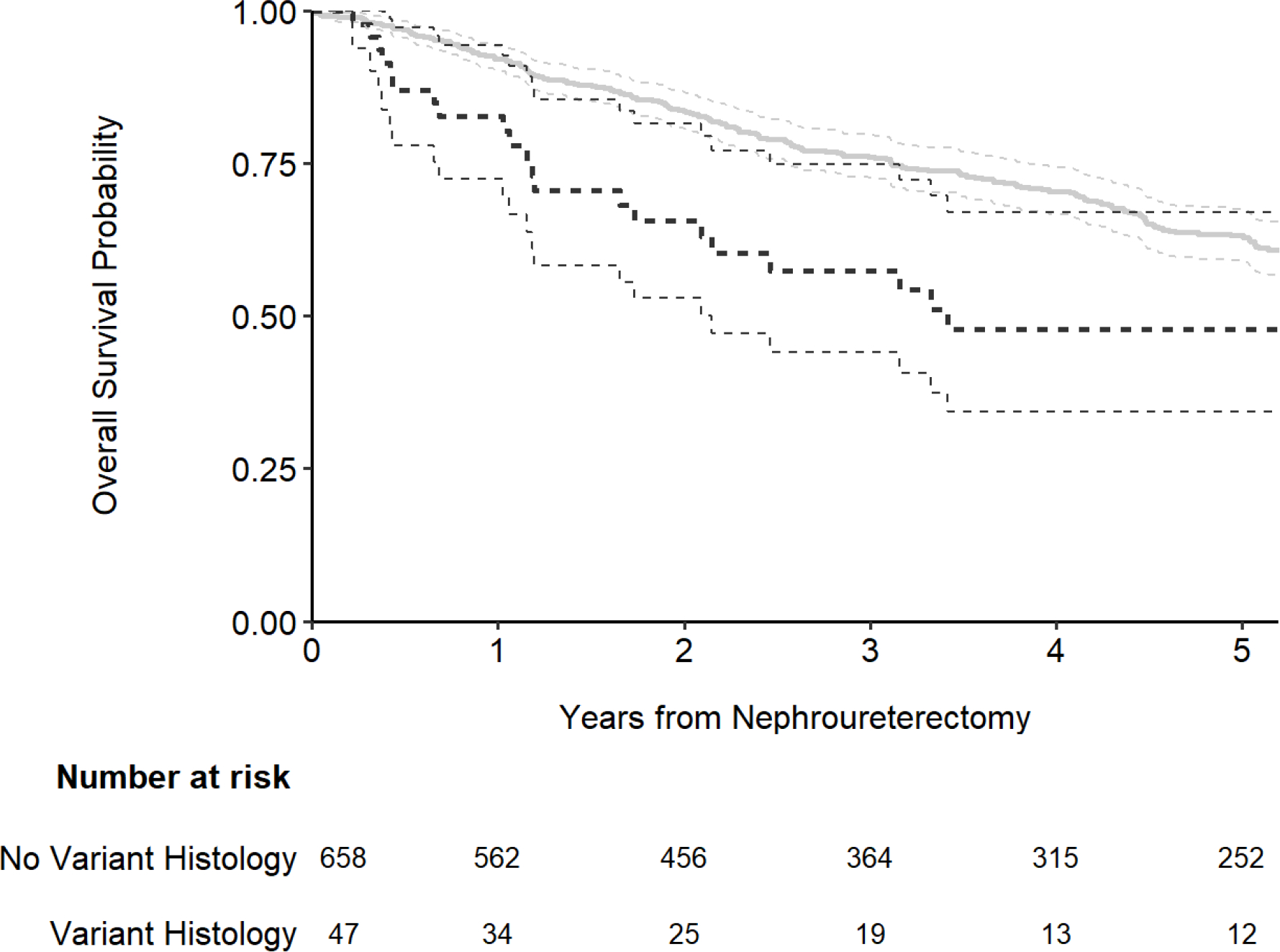
Kaplan-Meier plot for overall survival after nephroureterectomy, separately for patients with no variant histology (gray line) and those with any variant histology on pathology (black line).
Figure 3.
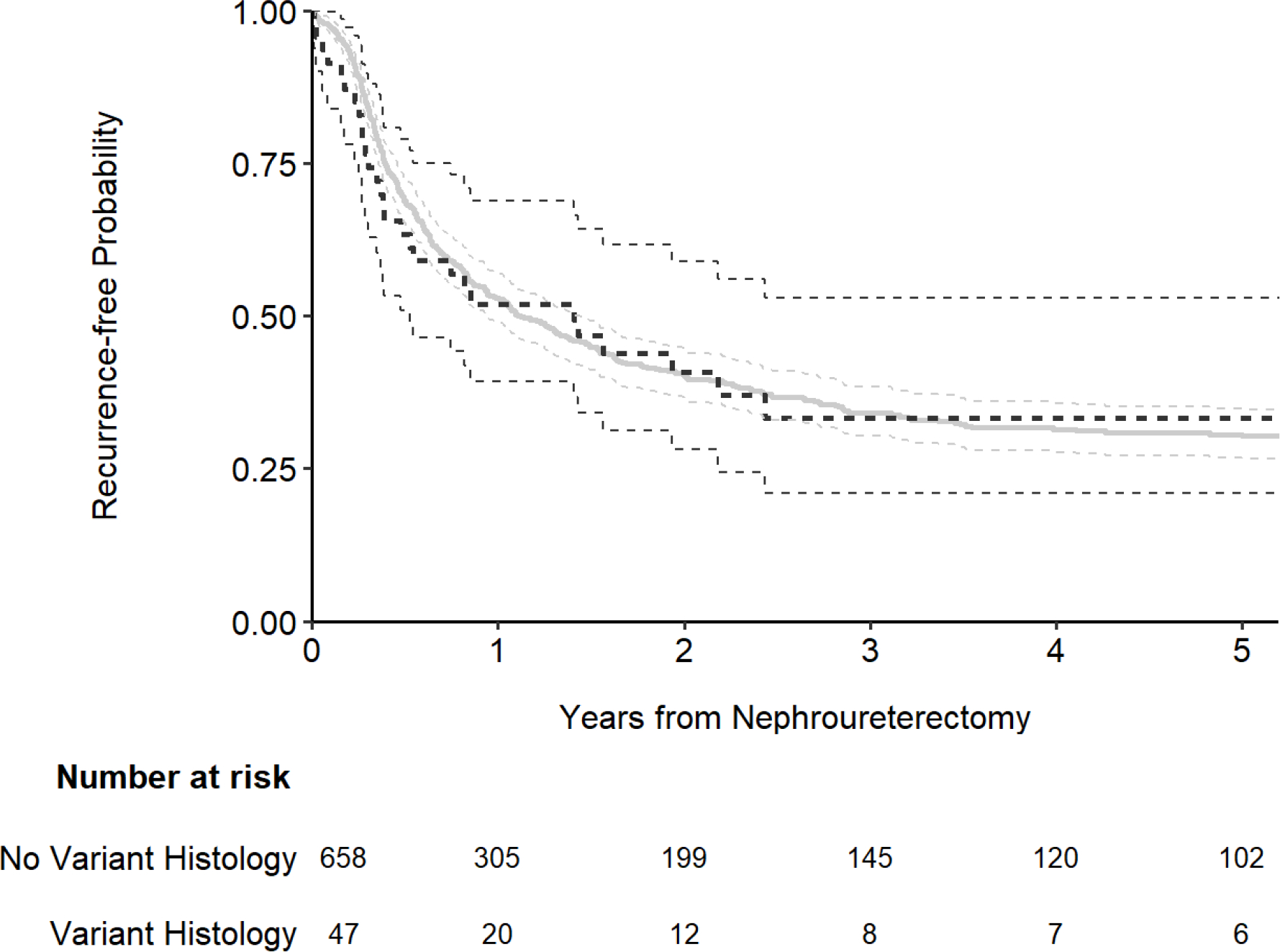
Kaplan-Meier plot for recurrence-free survival after nephroureterectomy, separately for patients with no variant histology (gray line) and those with any variant histology on pathology (black line).
Table 2.
Multivariable Cox proportional hazards regression models testing the association between variant histology on pathology and recurrence-free survival, cancer-specific survival, and overall survival
| Recurrence-free survival | Cancer-specific survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Characteristic | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
|
| |||||||||
| Pathologic variant histology | 0.88 | 0.59, 1.30 | 0.5 | 1.17 | 0.72, 1.89 | 0.5 | 1.20 | 0.79, 1.84 | 0.4 |
| Pathologic T stage | |||||||||
| pTA/IS/1 | — | — | — | — | — | — | |||
| pT2 | 1.23 | 0.95, 1.60 | 0.12 | 2.32 | 1.51, 3.57 | <0.001 | 1.36 | 1.01, 1.84 | 0.043 |
| pT3 | 1.35 | 1.08, 1.69 | 0.008 | 5.00 | 3.50, 7.16 | <0.001 | 2.28 | 1.77, 2.95 | <0.001 |
| pT4 | 1.49 | 0.81, 2.75 | 0.2 | 9.76 | 5.02, 19.0 | <0.001 | 3.97 | 2.19, 7.21 | <0.001 |
HR = hazard ratio; CI = confidence interval
Similar to the primary analysis, on univariate analysis there was evidence that variant histology was associated with worse cancer-specific and overall survival after recurrence (p < 0.001 and p < 0.001, respectively). However, the association with cancer-specific survival appears to be explained by higher pathologic T stage, while the association with overall survival remains significant (multivariable analysis in Table 3).
Table 3.
Multivariable Cox proportional hazards regression models testing the association between variant histology on pathology and cancer-specific survival and overall survival after recurrence
| Cancer-specific survival | Overall survival | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | HR | 95% CI | p-value | HR | 95% CI | p-value |
|
| ||||||
| Pathologic variant histology | 1.48 | 0.88, 2.50 | 0.14 | 1.86 | 1.15, 3.00 | 0.011 |
| Pathologic T stage | ||||||
| pTA/IS/1 | Reference | |||||
| pT2 | 1.85 | 1.17, 2.91 | 0.008 | 1.20 | 0.83, 1.73 | 0.3 |
| pT4 | 4.78 | 3.29, 6.95 | <0.001 | 3.12 | 2.29, 4.26 | <0.001 |
| pT4 | 9.91 | 4.66, 21.1 | <0.001 | 6.19 | 2.98, 12.8 | <0.001 |
HR = hazard ratio; CI = confidence interval
DISCUSSION
The lack of definitive evidence characterizing the role of a variant histology diagnosis in the management of UTUC presents a known challenge for clinicians. Our analysis of this large single-center RNU cohort showed that variant histology was present in 6.7% of the final pathologic specimens. Variant histology was associated with a higher pathologic stage and a higher chance of receiving neoadjuvant chemotherapy. Variant histology was also associated with lower cancer-specific and overall survival but not recurrence-free survival after RNU. After adjustment for pathologic T stage, variant histology was no longer associated with survival. As those with variant histology were also more likely to have a higher pathologic T stage, the perceived worse survival associated with variant histology is explained by the higher pathologic T stage. In other words, variant histology does not add predictive information to pathologic findings after nephroureterectomy. On presurgical biopsy, however, this finding may be a useful predictor of high-risk features. We did not evaluate this hypothesis in our study, as this data set relied on surgical pathology findings from nephroureterectomy, and matching biopsy material was not available in all cases.
The rates of variant histology diagnosis in patients undergoing RNU is quite variable in the literature, ranging from 7.5% to 40% in previous studies [13–16]. A recent meta-analysis showed a variant histology incidence of 13% in 2,153 RNU specimens [17]. In our study, variant histology was identified in only 6.7% of patients, a lower rate compared to other cohorts; this is likely due to our inclusion of patients from an earlier era, when there was less emphasis on identifying variant histology in the genitourinary pathology community. This is clearly demonstrated by the temporal increase in variant histology identified in our series (p = 0.003, Table 1), reflecting changes in pathology evaluation over time and increased recognition of the potential prognostic implications. Moreover, an increased proportion of cases were diagnosed after the subspecialization of genitourinary pathologists in 2005. We did not rereview all the pathology in this series, although we acknowledge that doing so could result in reclassification, as demonstrated in a recent study by Rolim et al, which described the presence of a histologic variant in 34% of patients after reviewing the pathology specimens from 115 cases of UTUC performed between 2002 and 2017 [16]. Our contemporary experience, however, does not suggest such a high prevalence.
The presence of variant histology is associated with worse outcomes after RNU. One meta-analysis from Mori et al of 26 studies with 12,865 patients reported a 2.0-fold increase in cancer-specific mortality, a 1.8-fold increase in overall mortality, and a 1.6-fold increase in disease recurrence with variant histology on RNU [17]. Our study demonstrated that although the cancer-specific and overall survival rates are univariately associated with the presence of a histological variant in the surgical specimen, this benefit is lost after controlling for pathologic stage. This is consistent with previous studies, where variant histology was associated with worse outcomes only in univariate analysis [13, 18, 19]. Moreover, these findings are in agreement with those found in bladder cancer, which demonstrated that although variant histology is frequently diagnosed at a higher stage and with higher disease burden, stage-matched survival is similar to pure urothelial carcinoma outcomes [20, 21].
Challenges with diagnosing variant histology UTUC prior to initiation of neoadjuvant chemotherapy limit the application of such information to adjuvant treatment. Xylinas et al found that the presence of variant histology on RNU did not affect the response to adjuvant systemic chemotherapy [22]. Retrospective neoadjuvant data [23, 24] and a recently published phase 3, open-label, randomized controlled multicenter adjuvant trial have demonstrated a role for neoadjuvant chemotherapy in downstaging the primary tumor and for adjuvant chemotherapy in improving long-term oncologic control [7]. At our center, we have been offering neoadjuvant gemcitabine and cisplatin to patients with high-risk UTUC in the context of a phase 2 clinical trial [25]. Thus, as the frequency of diagnosis of histological variants at our institution is related to more recent surgeries, those patients also received neoadjuvant chemotherapy at higher rates, potentially influencing the oncologic outcomes. It is possible that without neoadjuvant chemotherapy, the patients with variant histology could have fared worse even after matching for stage.
Limitations
Several limitations in the current study must be noted. Despite the homogeneity of data afforded by a single-institution study, surgical selection may represent a bias in this retrospective series. Patients selected for RNU usually present with more advanced and aggressive disease. Small and low-grade tumors may be treated with an endoscopic approach and thus would not be included in this analysis. Pre-RNU biopsy specimens were only available for a minority of patients. Findings such as node positivity, stage, and oncologic outcomes may be impacted by factors such as surgeon, adjunctive chemotherapy, and pathologic sampling. As previously noted, we relied on pathologic findings at the time of initial surgery and re-review was not performed, which might impact the observed prevalence of histologic subtypes. Due to the relatively small number of patients identified with variant histology, we did not analyze variant histology subgroups, instead grouping all variant histologic subtypes together. There is some suggestion in the literature that certain variant histologic subtypes, namely micropapillary and sarcomatoid, behave more aggressively than others [26]. Another large Surveillance, Epidemiology, and End Results (SEER) study of UTUC patients found that patients with squamous cell carcinoma had worse cancer-specific mortality, except for those with pT1–2 disease [27].
CONCLUSIONS
We found that variant histology was associated with worse cancer-specific survival and overall survival, which was explained by an association with higher pathological stage. In patients who experienced recurrence, variant histology was associated with worse cancer-specific survival, also attributed to higher stage. Data from this series suggest that variant histology in UTUC is a useful biomarker associated with higher stage and poor oncologic outcomes when pathological stage is unknown, such as in the pretreatment biopsy setting. However, finding variant histology on surgical pathology does not provide additional prognostic information beyond TNM staging risk after nephroureterectomy.
Source of Funding:
This research was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and Ruth L. Kirschstein National Research Service Award T32 CA082088 and funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations:
- RNU
radical nephroureterectomy
- UTUC
upper tract urothelial carcinoma
REFERENCES:
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2020. CA Cancer J Clin, 2020. 70(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Munoz JJ and Ellison LM, Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol, 2000. 164(5): p. 1523–5. [PubMed] [Google Scholar]
- 3.Raman JD, et al. , Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int, 2011. 107(7): p. 1059–64. [DOI] [PubMed] [Google Scholar]
- 4.Sfakianos JP, et al. , Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol, 2015. 68(6): p. 970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Audenet F, et al. , Clonal Relatedness and Mutational Differences between Upper Tract and Bladder Urothelial Carcinoma. Clin Cancer Res, 2019. 25(3): p. 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbeutcha A, et al. , Prognostic factors and predictive tools for upper tract urothelial carcinoma: a systematic review. World J Urol, 2017. 35(3): p. 337–353. [DOI] [PubMed] [Google Scholar]
- 7.Birtle A, et al. , Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet, 2020. 395(10232): p. 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobo N, et al. , What Is the Significance of Variant Histology in Urothelial Carcinoma? Eur Urol Focus, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Margolin EJ, et al. , Discordance between Ureteroscopic Biopsy and Final Pathology for Upper Tract Urothelial Carcinoma. Journal of Urology, 2018. 199(6): p. 1440–1445. [DOI] [PubMed] [Google Scholar]
- 10.Favaretto RL, et al. , The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol, 2010. 58(4): p. 574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin MB, et al. , The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin, 2017. 67(2): p. 93–99. [DOI] [PubMed] [Google Scholar]
- 12.Moch H, et al. , WHO classification of tumours of the urinary system and male genital organs. Vol. 8. 2016: International Agency for Research on Cancer (IARC). [Google Scholar]
- 13.Rink M, et al. , Impact of histological variants on clinical outcomes of patients with upper urinary tract urothelial carcinoma. J Urol, 2012. 188(2): p. 398–404. [DOI] [PubMed] [Google Scholar]
- 14.Masson-Lecomte A, et al. , Impact of micropapillary histological variant on survival after radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol, 2014. 32(2): p. 531–7. [DOI] [PubMed] [Google Scholar]
- 15.Elawdy MM, et al. , Histopathologic Characteristics of Upper Tract Urothelial Carcinoma With an Emphasis on Their Effect on Cancer Survival: A Single-Institute Experience With 305 Patients With Long-Term Follow-Up. Clin Genitourin Cancer, 2016. 14(6): p. e609–e615. [DOI] [PubMed] [Google Scholar]
- 16.Rolim I, et al. , Clinicopathologic analysis of upper urinary tract carcinoma with variant histology. Virchows Arch, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Mori K, et al. , Prognostic Value of Variant Histology in Upper Tract Urothelial Carcinoma Treated with Nephroureterectomy: A Systematic Review and Meta-Analysis. J Urol, 2019: p. 101097JU0000000000000523. [DOI] [PubMed] [Google Scholar]
- 18.Tang Q, et al. , The prognostic impact of squamous and glandular differentiation for upper tract urothelial carcinoma patients after radical nephroureterectomy. World J Urol, 2016. 34(6): p. 871–7. [DOI] [PubMed] [Google Scholar]
- 19.Sakano S, et al. , Impact of variant histology on disease aggressiveness and outcome after nephroureterectomy in Japanese patients with upper tract urothelial carcinoma. Int J Clin Oncol, 2015. 20(2): p. 362–8. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, et al. , The Impact of Plasmacytoid Variant Histology on the Survival of Patients with Urothelial Carcinoma of Bladder after Radical Cystectomy. Eur Urol Focus, 2019. 5(1): p. 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mally AD, et al. , Clinical Outcomes of Patients With T1 Nested Variant of Urothelial Carcinoma Compared to Pure Urothelial Carcinoma of the Bladder. Clin Genitourin Cancer, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xylinas E, et al. , Histologic variants of upper tract urothelial carcinoma do not affect response to adjuvant chemotherapy after radical nephroureterectomy. Eur Urol, 2012. 62(1): p. e25–6. [DOI] [PubMed] [Google Scholar]
- 23.Quhal F, et al. , Efficacy of neoadjuvant and adjuvant chemotherapy for localized and locally advanced upper tract urothelial carcinoma: a systematic review and meta-analysis. Int J Clin Oncol, 2020. [DOI] [PubMed] [Google Scholar]
- 24.Almassi N, et al. , Impact of Neoadjuvant Chemotherapy on Pathologic Response in Patients With Upper Tract Urothelial Carcinoma Undergoing Extirpative Surgery. Clin Genitourin Cancer, 2018. 16(6): p. e1237–e1242. [DOI] [PubMed] [Google Scholar]
- 25.Coleman* JA, et al. , Multicenter prospective phase ii clinical trial of gemcitabine and cisplatin as neoadjuvant chemotherapy in patients with high-grade upper tract urothelial carcinoma. Journal of Urology, 2019. 201(Supplement 4): p. e999–e999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamboni S, et al. , Incidence and survival outcomes in patients with upper urinary tract urothelial carcinoma diagnosed with variant histology and treated with nephroureterectomy. BJU Int, 2019. 124(5): p. 738–745. [DOI] [PubMed] [Google Scholar]
- 27.Deuker M, et al. , Upper Urinary Tract Tumors: Variant Histology Versus Urothelial Carcinoma. Clin Genitourin Cancer, 2021. 19(2): p. 117–124. [DOI] [PubMed] [Google Scholar]


