Abstract
Aims
To investigate the association between circulating lipoprotein(a) (Lp(a)) and risk of all-cause and cause-specific mortality in the general population and in patients with chronic diseases, and to elucidate the dose-response relations.
Methods and results
We searched literature to find prospective studies reporting adjusted risk estimates on the association of Lp(a) and mortality outcomes. Forty-three publications, reporting on 75 studies (957,253 participants), were included. The hazard ratios (HRs) and 95% confidence intervals (95%CI ) for the top versus bottom tertile of Lp(a) levels and risk of all-cause mortality were 1.09 (95%CI: 1.01–1.18, I2: 75.34%, n = 19) in the general population and 1.18 (95%CI: 1.04–1.34, I2: 52.5%, n = 12) in patients with cardiovascular diseases (CVD). The HRs for CVD mortality were 1.33 (95%CI: 1.11–1.58, I2: 82.8%, n = 31) in the general population, 1.25 (95%CI: 1.10–1.43, I2: 54.3%, n = 17) in patients with CVD and 2.53 (95%CI: 1.13–5.64, I2: 66%, n = 4) in patients with diabetes mellitus. Linear dose-response analyses revealed that each 50 mg/dL increase in Lp(a) levels was associated with 31% and 15% greater risk of CVD death in the general population and in patients with CVD. No non-linear dose-response association was observed between Lp(a) levels and risk of all-cause or CVD mortality in the general population or in patients with CVD (Pnonlinearity > 0.05).
Conclusion
This study provides further evidence that higher Lp(a) levels are associated with higher risk of all-cause mortality and CVD-death in the general population and in patients with CVD. These findings support the ESC/EAS Guidelines that recommend Lp(a) should be measured at least once in each adult person’s lifetime, since our study suggests those with higher Lp(a) might also have higher risk of mortality.
Supplementary information
The online version contains supplementary material available at 10.1007/s10654-022-00956-4.
Keywords: Mortality, Cause of death, Cardiovascular disease, Lipoprotein(a), Survival, Heart disease risk factors, Cohort studies, Chronic disease, Meta-analysis
Introduction
Lipoprotein(a) (Lp(a)) consists of a cholesteryl-ester rich lipid core and an apolipoprotein B-100 bound to apolipoprotein(a) (apo(a)). Lp(a) has proatherogenic and prothrombotic properties and, is considered a risk factor for the development of cardiovascular disease (CVD) [1–6]. Also, European Society of Cardiology and the European Atherosclerosis Society (ESC/EAS) Guidelines for the Management of Dyslipidemias suggest to measure Lp(a), since it helps to identify people with high levels of Lp(a) that may have a higher risk of CVD [7].
Despite well-established evidence on Lp(a) as a risk factor of CVD, findings on relation between this marker and mortality are controversial. Also, this association has been studied more extensively in the general/healthy population than in patients. The Emerging Risk Factors Collaboration in 2009 investigated the association of Lp(a) concentration and risk of coronary heart disease, stroke, and mortality in healthy and general population. The findings of this individual participant meta-analysis of cohort and nested case-control studies indicated the risk ratios (RR) of 1.14 (95% Confidence interval (95%CI): 1.07–1.22, number of studies (n)= 24) and 1.01 (95%CI: 0.98–1.05, n= 25) for aggregate of coronary and nonvascular mortality [8]. Among publications on patients with chronic diseases, the Ludwigshafen Risk and Cardiovascular Health study in patients with prevalent coronary heart disease revealed no associations between Lp(a) concentrations and genetic variants with all-cause or CVD mortality [9]. Furthermore, analyses on plasma Lp(a) concentrations and risk of CVD death in two prospective cohorts of individuals with type 2 diabetes (T2DM) found no considerable association of this marker and CVD death [10]. On the contrary, other studies have found increased risk of mortality with elevated Lp(a) in the general population [11, 12] or in patients with CVD [13, 14] or T2DM [15].
Regardless of a considerable number of publications, few studies have summarized the association of circulating Lp(a) with the risk of mortality. The latest meta-analysis on this association was published in 2011, which investigated the association with all-cause mortality as a secondary outcome in the general population [16]. Another meta-analysis [17] focused only on patients with coronary artery diseases and used the highest versus lowest approach to perform the meta-analysis which is not the best approach as the highest and lowest values of Lp(a) may vary substantially between studies. None of the above-mentioned studies have presented the dose-response associations [16, 17]. In addition, findings of an individual patient meta-analysis of clinical trials on statin treated patients revealed approximately linear association between elevated baseline and on-statin Lp(a) levels and risk of cardiovascular diseases [18]. Other studies narratively reviewed the findings [19] or focused on a few number of studies represented by consensus panel or collaborations [1]. Moreover, it remains unclear whether levels of Lp(a) might be associated with the risk of mortality in a same pattern in the general population or in patients with chronic diseases. Thus, in the current study, we systematically reviewed the association between circulating Lp(a) and all-cause and/or cause-specific mortality either in the general population or in patients with chronic diseases. Additionally, we performed dose-response analyses to clarify the strength and shape of relationship between Lp(a) and mortality outcomes.
Materials and methods
Review design
This systematic review was designed and reported in accordance with PRISMA guidelines [20]. The study protocol was registered in the PROSPERO database on Nov 16, 2020 (registration number: CRD42020213420).
Data sources and search strategy
Embase.com, Medline ALL (Ovid), Web of science Core Collection, and Cochrane Central were searched until June 8, 2021. Lipoprotein(a) and mortality-related keywords were combined in the search strategy. Furthermore, filters to exclude conference abstracts, case-report studies, non-adult populations, and animal studies were applied. The search strategy was developed by an expert research librarian (WB). The search results were imported in EndNote and de-duplicated with the method as described by Bramer et al. [21]. The details of the search strategy and keywords are presented in Supplementary Table-1. To complete our search, the references of the included studies and published reviews were manually reviewed.
Eligibility criteria
We included all original prospective observational investigations, including cohort and case-cohort studies, reporting adjusted risk estimates of the association of circulating Lp(a) with all-cause or cause-specific mortality in adults (≥ 18 years) irrespective of health and diseases status. No date restrictions were applied.
Retrospective studies, conference abstracts, ecological studies, case reports, case-series, letters to the editor, conference proceedings, narrative reviews, systematic reviews or meta-analyses as well as studies conducted in animals, children, or adolescents were excluded. We did not include non-English language articles.
It should be noted, to provide a complementary report of all available evidence of this association, the studies reporting unadjusted and/or descriptive/frequency results are also summarized in the Supplementary Tables-4 and 5.
Study selection, data extraction, and quality assessment
All titles/abstracts were screened in duplicate by two independent groups of researchers (MA and HRD together with AV, YW, AVW, and KB) according to the eligibility criteria. Afterward, all provided full-texts were reviewed similarly in duplicate as the previous step. MA and HRD extracted data from the included studies based on a predesigned form. The main extracted information from the included studies was the first author’s name, study design, publication year, location, number of participants, sex distribution of the population, participants’ health status at study entry, age, follow-up duration, Lp(a) assessment method, number of deaths, causes of mortality, adjustments, and hazard/ risk/ odds ratios.
Quality of the included studies was assessed using Newcastle-Ottawa scale [22] by MA and HRD, independently. Each study was judged based on selection, comparability, and outcome/exposure domains. Studies which achieved fewer than four points, four to six points, and seven or more points were graded as poor, fair and good quality. Discrepancies were solved through discussion and unsettled disagreements were arbitrated by the third investigator (TV).
Data analysis
We performed DerSimonian-Laird random effects model to calculate the pooled hazard ratios (HR) and corresponding 95% confidence intervals (95%CI) [23]. For studies with reported odds ratio (OR) between 0.5 and 2.5 or relative risks (RR), values were treated as the HR [24, 25] and risk estimates of the most adjusted model were extracted. To enable a consistent and standardized approach in the meta-analysis, all risk estimates for the association between Lp(a) and mortality were transformed to top versus bottom third of Lp(a) distribution in each study [26]. Further details on the risk conversion methods are provided the following section. For a study [27] that used other categories than the first one as the reference category, we changed the reference category to the lowest one using the method developed by Hamling [28]. We calculated standard errors (SE) using the method developed by Greenland [29] for studies that did not report CI or SE [30–33]. Before inclusion in the meta-analysis, the risk estimates of a study [34] providing results stratified by gender, but not overall, were pooled using a fixed-effects model.
Heterogeneity between studies was assessed using the I2 [35]. We conducted subgroup analyses by region (Asia, Europe, America, Australia, or multiple regions combined), follow-up duration (< 10 or ≥ 10 years), by whether studies adjusted for body mass index (BMI) or lipid lowering medication intake as confounders, and Lp(a) assessment methods. Additionally, among the included studies, there were some articles that were conducted on more than one cohort but did not report the association of Lp(a) and mortality in each cohort separately [8, 10, 11, 36]. Therefore, we performed a subgroup analysis stratified by whether papers included multiple studies (Yes/No). We also conducted a sensitivity analysis to explore if the results were robust using leave-one-out analysis, excluding each study at a time from analysis. Publication bias was assessed with Egger’s test and asymmetry was visually explored with funnel plots [37, 38].
To perform the linear dose-response analyses, the method by Greenland and Longnecker [39] was used. Using this method, we calculate the HRs and 95%CI from the natural logarithm of the risk estimated across the categories of Lp(a). We considered HRs for risk of mortality per 50 mg/dL Lp(a) increase, since it has been suggested that lowering of Lp(a) by 50 mg/dL may reduce the risk of CVD [40]. Also, the findings of studies on the association of extreme levels of Lp(a) with risk of CVD showed a stepwise increase in risk of CVD with no evidence of a threshold effect [41–43] and one of the biggest studies in the current systematic review performed by Langsted et al. [11] presented the HRs per 50 mg/dL increase in Lp(a) for risk of mortality (all-cause, cardiovascular and non-vascular mortality). Additionally, a nonlinear dose-response association between Lp(a) and mortality was assessed using restricted cubic splines with three knots at 10%, 50% and 90% percentiles of the distribution, which was combined using multivariate meta-analysis [44, 45]. We included studies that reported the risk of mortality for at least three categories of Lp(a) in these analyses.
We performed the statistical analyses of our meta-analysis in R (Version 4.0.5) using meta and dmetar packages and Stata (Version 16.0) was used to perform the dose-response analyses.
Risk conversions
For studies that reported the mortality in tertiles of Lp(a), the HRs or RRs were included as reported. For studies that grouped the exposure in quartile, quintile, high versus low level of the marker, or reported the results as continuous, such as per SD or per unit, we converted the HRs or RRs from the original studies to the HRs or RRs for the third versus bottom tertile of Lp(a) distribution using defined methods [26]. Based on this method, to convert log relative risks from the reported scale in the studies to top versus bottom third, we need to use conversion factors driving based on the ratio of expected differences in mean levels of the standardized exposure, for the target comparison versus reported comparison.
For example, a factor of 2.54 is the difference in the means of the upper and lower quartiles and the expected difference in means of the top versus bottom thirds of the standard normal distribution is 2.18. So, by applying a multiplication conversion factor of 2.18/2.54 to the log relative risk and its standard error, we can convert a top versus bottom quartile comparison to correspond to the top versus bottom third comparison. Similarly, a conversion factor of 2.18 and 2.18 * SD are used to obtain the risk for the third versus first tertile for the estimations reported per SD and unit increment of exposure, respectively. The multiplicative conversion factors of 2.18/2.80 and 2.18/1.59 were used to transform the RR for the top versus the bottom quintiles and high versus low levels of Lp(a). This method has been widely used in previous publications [46–50].
Results
Eligible studies and characteristics
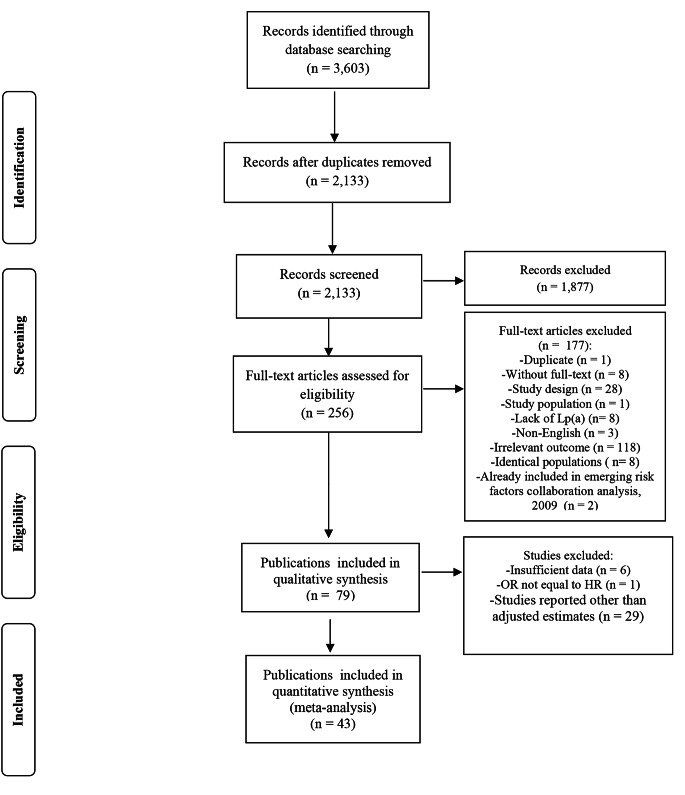
Our search returned 2,133 references. Out of 2,133 titles and abstracts, 256 articles were qualified for a full text screening. From these 256, we excluded articles with irrelevant outcomes (n = 118) or study design (n = 28) and those with non-English languages (n = 3). Additionally, 8 articles did not investigate Lp(a) as the exposure and 8 articles were excluded due to the identical populations to the other included studies. One duplicate title and one article with irrelevant population were also excluded. Furthermore, to avoid double-counting of the included studies, we excluded those studies (n = 2) that overlapped with the Risk Factors Collaboration study [8]. We could not retrieve the full-texts of 8 titles.
Hence, 79 papers were further assessed. Of these, 29 studies reported unadjusted results as either univariate risks, mortality number/rate in different levels of Lp(a), or level of Lp(a) in survivors and non-survivors, which were excluded from our analyses but their key characteristics and results are summarized in Supplementary Tables-4 and 5. 4 studies [51–54] and subgroup analysis of patients with DM from one of the included studies [36] were excluded due to insufficient data to perform the risk conversions; 2 studies used another category of Lp(a) than the first as the reference category and did not report enough information to apply Hamling method [55, 56]; and one study [57] reported OR which values could not be treated as HRs. Thus, 43 publications were included in our meta-analysis. Among these publications, 4 articles reported the pooled results of participants from 7 [36], 2 [11], 2 [10], 25 [8] studies, leading to 75 studies and 957,253 individuals. Figure 1 represents the study selection procedure.
Fig. 1.
Study selection procedure
Supplementary Table-2 presents the main characteristics of the included studies in the meta-analysis. Briefly, these studies have been published between 1998 and 2020. The association of Lp(a) concentration and all-cause or cause-specific mortality was reported in general or healthy populations [8, 11, 12, 34, 36, 58–70], individuals with CVD [13, 14, 30, 67, 69, 71–84], chronic renal failure (CRF) [27, 31–33], and DM [10, 15, 67, 85, 86]. One study was conducted in women only [78] and two in men only [64, 68]. The follow-up duration ranged from 1 to 20 years.
Additionally, 20 studies were graded as fair and the remaining as good quality, according to Newcastle – Ottawa quality assessment scale, as presented in Supplementary Table-3.
Meta-analysis and dose-response analysis
All-cause mortality
The association of Lp(a) and all-cause mortality is summarized in Table 1. Higher levels of Lp(a) was associated with a higher risk of all-cause mortality in the general population (HR: 1.09, 95%CI: 1.01–1.18, I2: 75.34%, n = 19, Supplementary Figure-1) and in patients with CVD (HR: 1.18, 95%CI: 1.04–1.34, I2: 52.5, n = 12, Supplementary Figure-2). No significant association was found in patients with CRF or DM. The results of meta-analysis were not robust in leave-one-out analysis in general population by removing several effect sizes [11, 12, 34, 62, 63, 65–67], but in patients with CVD, the association remained unchanged (Supplementary Figure-6-A and B). Funnel plots showed no asymmetry in both populations (Egger’s p-value > 0.05, Supplementary Figure-7-A and B).
Table 1.
Summary risk estimates and stratified analyses for association between circulating Lp(a) and all-cause mortality in general population, patients with CVD, CRF or DM
| No. of effect sizes | No. of studies | No. of participants | Pooled HR (95%CI) | I2% | p-value (between groups) |
|
|---|---|---|---|---|---|---|
| General population | ||||||
| Overall effect | 12 | 19 | 169,432 | 1.09 (1.01, 1.18) | 75.34 | - |
| Region | ||||||
| Asia | 3 | 3 | 15,171 | 1.24 (0.81, 1.89) | 86.8 | 0.63 |
| Europe | 7 | 14 | 149,056 | 1.05 (0.96, 1.14) | 74.9 | |
| America | 2 | 2 | 5,205 | 1.09 (0.99, 1.22) | 0 | |
| Follow-up duration | ||||||
| < 10 years | 8 | 14 | 67,054 | 1.04 (0.94, 1.16) | 54.8 | 0.36 |
| ≥ 10 years | 4 | 5 | 102,378 | 1.15 (0.96, 1.37) | 89.7 | |
| Multiple studies* | ||||||
| Yes | 2 | 9 | 121,892 | 0.99 (0.92, 1.06) | 70.8 | 0.05 |
| No | 10 | 10 | 47,540 | 1.13 (1.01, 1.27) | 67.0 | |
| Adjustment for BMI | ||||||
| Yes | 9 | 16 | 162,669 | 1.07 (0.99, 1.16) | 79.6 | 0.34 |
| No | 3 | 3 | 6,763 | 1.30 (0.87, 1.95) | 30.5 | |
| Adjustment for Lipid-lowering medication | ||||||
| Yes | 3 | 9 | 58,294 | 1.06 (0.88, 1.27) | 74.0 | 0.72 |
| No | 9 | 10 | 111,138 | 1.10 (0.98, 1.23) | 77.7 | |
| Lp(a) assessment methods | ||||||
| ELISA | 6 | 6 | 20,033 | 1.07 (0.88, 1.29) | 75.9 | 0.22 |
| ITA | 3 | 9 | 73,434 | 1.08 (0.92, 1.27) | 87.3 | |
| INA | 1 | 1 | 1,274 | 1.32 (0.82, 2.12) | - | |
| NM | 1 | 1 | 4,930 | 1.78 (1.02, 3.09) | - | |
| Multiple methods | 1 | 2 | 69,761 | 1.02 (1.005, 1.03) | - | |
| Patients with CVD | ||||||
| Overall effect | 12 | 12 | 19,762 | 1.18 (1.04, 1.34) | 52.5 | - |
| Region | ||||||
| Asia | 4 | 4 | 7,255 | 1.34 (1.04, 1.72) | 44.6 | 0.67 |
| Europe | 6 | 6 | 9,415 | 1.14 (0.87, 1.48) | 65.3 | |
| America | 1 | 1 | 1,620 | 1.14 (1.01, 1.27) | - | |
| Australia | 1 | 1 | 1,472 | 1.11 (0.82, 1.50) | - | |
| Follow-up duration | ||||||
| < 10 years | 11 | 11 | 18,798 | 1.19 (1.04, 1.37) | 56.6 | 0.50 |
| ≥ 10 years | 1 | 1 | 964 | 1.06 (0.76, 1.47) | - | |
| Adjustment for BMI | ||||||
| Yes | 5 | 5 | 12,524 | 1.12 (0.98, 1.27) | 47.3 | 0.35 |
| No | 7 | 7 | 7,238 | 1.28 (0.98, 1.68) | 58.0 | |
| Adjustment for Lipid-lowering medication | ||||||
| Yes | 4 | 4 | 11,535 | 1.08 (0.98, 1.18) | 18.8 | 0.09 |
| No | 8 | 8 | 8,227 | 1.34 (1.05, 1.72) | 54.0 | |
| Lp(a) assessment methods | ||||||
| ELISA | 4 | 4 | 5,484 | 1.27 (1.006, 1.61) | 53.1 | 0.04 |
| ITA | 3 | 3 | 5,571 | 1.24 (1.03, 1.50) | 23.1 | |
| INA | 2 | 2 | 2,956 | 0.89 (0.48, 1.64) | 62.7 | |
| IRMA | 1 | 1 | 966 | 2.59 (1.19, 5.62) | - | |
| Automated latex enhanced immunoassay | 1 | 1 | 1,472 | 1.11 (0.82, 1.50) | - | |
| Photometric assay | 1 | 1 | 3,313 | 0.95 (0.81, 1.11) | - | |
| Patients with CRF | ||||||
| Overall effect | 3 | 3 | 4,188 | 1.96 (0.89, 4.32) | 65.4 | - |
| Patients with DM | ||||||
| Overall effect | 2 | 2 | 596 | 0.97 (0.72, 1.32) | 0.0 | - |
* Some publications were conducted on more than one cohort study without reporting the separated results. CVD: Cardiovascular disease; CRF: Chronic renal failure; DM: Diabetes mellitus; HR: Hazard ratio; 95%CI: 95% confidence interval; ELISA: Enzyme-linked immunosorbent assay; ITA: Immunoturbidimetric assay; INA: Immunonephelometric assay; NM: Not mentioned
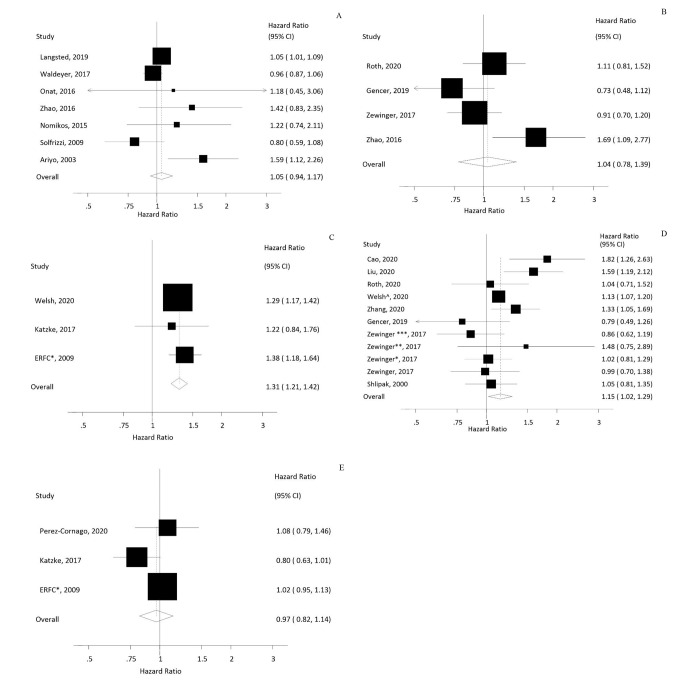
Moreover, there was no significant linear dose-response association either in general population (HR: 1.05, 95%CI: 0.94–1.17, I2: 54.6%, n = 14, Fig. 2 A) or among patients with CVD (HR: 1.04, 95%CI: 0.78–1.39, I2: 61.7%, n = 4, Fig. 2B). Also, no non-linear dose-response association was shown between Lp(a) and all-cause mortality in these populations (pnonlinearity > 0.05, Supplementary Figure-8-A and B, Supplementary Table-6).
Fig. 2.
Linear dose-response analysis of Lp(a) per 50 mg/dL and A) All-cause mortality in general population, B) All-cause mortality in patients with CVD, C) CVD- death in general population, D) CVD-death in patients with CVD, E) Non-CVD-death in general population. *ERFC: The emerging risk factor collaboration. Zwinger*: Validation study 1; Zwinger**: Validation study 2; Zwinger***: Validation study 3; Welsh^: High risk cohort
CVD and non-CVD deaths
Table 2 summarizes the risk estimates for association between circulating Lp(a) and CVD-death. Our meta-analysis indicated an association of Lp(a) with higher risk of CVD-death in general population (HR: 1.33, 95%CI: 1.11–1.58, I2: 82.8%, n = 31, Supplementary Figure-3), patients with CVD (HR: 1.25, 95%CI: 1.10–1.43, I2: 54.3%, n = 17, Supplementary Figure-4) and patients with DM (HR: 2.53, 95%CI: 1.13–5.64, I2: 66%, n = 4). These results were robust in leave-one-out analysis (Supplementary Figure-6-C and D) and no asymmetry was detected in funnel plots (Egger’s p-value > 0.05, Supplementary Figure-7-C and D).
Table 2.
Summary risk estimates and stratified analyses for association between circulating Lp(a) and CVD-death in general population, patients with CVD, CRF or DM
| No. of effect sizes | No. of studies | No. of participants | Pooled HR (95%CI) | I2% | p-value (between groups) |
|
|---|---|---|---|---|---|---|
| General population | ||||||
| Overall effect | 8 | 31 | 112,157 | 1.33 (1.11, 1.58) | 82.8 | - |
| Region | ||||||
| Asia | 1 | 1 | 10,413 | 1.45 (0.91, 2.31) | - | 0.93 |
| Europe | 6 | 6 | 29,061 | 1.33 (1.04, 1.69) | 87.4 | |
| Multiple regions combined | 1 | 24 | 72,683 | 1.33 (1.15, 1.54) | - | |
| Follow-up duration | ||||||
| < 10 years | 5 | 28 | 80,285 | 1.21 (1.03, 1.44) | 74 | 0.36 |
| ≥ 10 years | 3 | 3 | 31,872 | 1.45 (1.03, 2.05) | 74.1 | |
| Multiple studies* | ||||||
| Yes | 1 | 24 | 72,683 | 1.33 (1.15, 1.54) | - | 0.96 |
| No | 7 | 7 | 39,474 | 1.34 (1.07, 1.67) | 85 | |
| Adjustment for BMI | ||||||
| Yes | 5 | 28 | 106,328 | 1.30 (1.003, 1.69) | 88.8 | 0.47 |
| No | 3 | 3 | 5,829 | 1.70 (0.86, 3.35) | 55.7 | |
| Adjustment for Lipid-lowering medication | ||||||
| Yes | 2 | 2 | 7,669 | 1.81 (0.56, 5.84) | 79.4 | 0.61 |
| No | 6 | 29 | 104,488 | 1.32 (1.09, 1.60) | 86 | |
| Lp(a) assessment methods | ||||||
| ELISA | 2 | 2 | 10,972 | 1.47 (0.95, 2.28) | 0 | 0.01 |
| ITA | 2 | 2 | 19,060 | 1.48 (1.01, 2.16) | 94.8 | |
| IRMA | 1 | 1 | 1,773 | 0.99 (0.87, 1.13) | - | |
| NM | 2 | 2 | 7,669 | 1.80 (0.56, 5.84) | 79.4 | |
| Multiple methods | 1 | 24 | 72,683 | 1.33 (1.15, 1.54) | - | |
| Patients with CVD | ||||||
| Overall effect | 17 | 17 | 57,019 | 1.25 (1.10, 1.43) | 54.3 | |
| Region | ||||||
| Asia | 3 | 3 | 12,434 | 1.62 (1.33, 2.96) | 0.0 | 0.03 |
| Europe | 12 | 12 | 42,719 | 1.14 (0.99, 1.30) | 47.9 | |
| America | 1 | 1 | 1,383 | 1.33 (0.65, 2.72) | - | |
| Multiple regions combined | 1 | 1 | 483 | 1.39 (0.37, 5.23) | - | |
| Follow-up duration | ||||||
| < 10 years | 16 | 16 | 52,857 | 1.29 (1.12, 1.48) | 55.4 | 0.09 |
| ≥ 10 years | 1 | 1 | 4,162 | 1.02 (0.80, 1.29) | - | |
| Adjustment for BMI | ||||||
| Yes | 9 | 9 | 20,993 | 1.15 (0.97, 1.36) | 53.9 | 0.06 |
| No | 8 | 8 | 36,026 | 1.56 (1.18, 2.06) | 57.9 | |
| Adjustment for Lipid-lowering medication | ||||||
| Yes | 9 | 9 | 50,862 | 1.23 (1.03, 1.47) | 61.5 | 0.52 |
| No | 8 | 8 | 6,157 | 1.38 (1.03, 1.84) | 50.8 | |
| Lp(a) assessment methods | ||||||
| ELISA | 4 | 4 | 3,062 | 1.78 (1.24, 2.57) | 4.8 | 0.04 |
| ITA | 7 | 7 | 44,379 | 1.25 (1.06, 1.47) | 66 | |
| INA | 2 | 2 | 2,956 | 0.89 (0.55, 1.45) | 23.4 | |
| IRMA | 1 | 1 | 966 | 2.59 (1.04, 6.45) | - | |
| Immunoassay method | 1 | 1 | 483 | 1.39 (0.37, 5.23) | - | |
| Photometric assay | 1 | 1 | 3,313 | 0.99 (0.81, 1.20) | - | |
| Latex agglutination assay | 1 | 1 | 1,860 | 1.31 (0.85, 2.02) | - | |
| Patients with CRF | ||||||
| Overall effect | 2 | 2 | 658 | 1.83 (0.19, 17.96) | 89.3 | |
| Patients with DM | ||||||
| Overall effect | 3 | 4 | 4,070 | 2.53 (1.13, 5.64) | 66 |
* Some publications were conducted on more than one cohort study without reporting the separated results. CVD: Cardiovascular disease; CRF: Chronic renal failure; DM: Diabetes mellitus; HR: Hazard ratio; 95%CI: 95% confidence interval; ELISA: Enzyme-linked immunosorbent assay; ITA: Immunoturbidimetric assay; INA: Immunonephelometric assay; IRMA: Immunoradiometric assay; NM: Not mentioned
According to the findings of subgroups analyses in patients with CVD, higher levels of Lp(a) was associated with a higher risk of mortality in Asian studies (HR: 1.62, 95%CI: 1.33–2.96, I2: 0.0%) compared to the other regions (p-value between groups < 0.05).
In general population and patients with CVD, linear dose-response analysis revealed a higher risk of CVD-death with higher levels of Lp(a). A 50 mg/dL increase in Lp(a) concentration was associated with 31% and 15% greater risk of CVD-death in both general population and patients with CVD (HR: 1.31, 95%CI: 1.21–1.42, I2: 0%, n = 26 in the general population; HR: 1.15, 95%CI: 1.02–1.29, I2: 52.3%, n = 11 in patients with CVD, Figure-2-C and D). No non-linear dose response association was observed (pnonlinearity > 0.05, Supplementary Figure-8-C and D, Supplementary Table-6).
We found no significant association between Lp(a) concentration and non-CVD-death in the general population (HR: 1.05, 95%CI: 0.91–1.21, I2: 68.4%, n = 29, Supplementary Figure-5, Table 3). No asymmetry was detected and the effect estimate was robust in sensitivity analysis (Supplementary Figure-6-E and 2-E). Also, no significant linear (HR: 0.96, 95%CI: 0.79–1.18, I2: 57.1%, n = 28, Fig. 2E) or non-linear dose response associations were found (pnonlinearity > 0.05, Supplementary Figure-8-E, and Supplementary Table-6).
Table 3.
Summary risk estimates and stratified analyses for association between circulating Lp(a) and non-CVD-death in the general population
| No. of effect sizes | No. of studies | No. of participants | Pooled HR (95%CI) | I2% | p-value (between groups) |
|
|---|---|---|---|---|---|---|
| General population | ||||||
| Overall effect | 6 | 29 | 119,121 | 1.05 (0.91, 1.21) | 68.4 | - |
| Region | ||||||
| Asia | 1 | 1 | 10,413 | 1.71 (1.20, 2.43) | - | 0.01 |
| Europe | 3 | 3 | 4,676 | 0.95 (0.78, 1.15) | 61.3 | |
| America | 1 | 1 | 1,764 | 1.87 (0.73, 4.80) | - | |
| Multiple regions combined | 1 | 24 | 102,268 | 1.02 (0.95, 1.10) | - | |
| Follow-up duration | ||||||
| < 10 years | 3 | 26 | 104,196 | 1.02 (0.96, 1.10) | 0.0 | 0.83 |
| ≥ 10 years | 3 | 3 | 14,925 | 1.06 (0.77, 1.48) | 85.5 | |
| Multiple studies* | ||||||
| Yes | 1 | 24 | 102,268 | 1.02 (0.95, 1.10) | - | 0.52 |
| No | 5 | 5 | 16,853 | 1.10 (0.86, 1.42) | 74.8 | |
| Adjustment for lipid-lowering medication | ||||||
| Yes | 1 | 1 | 2,739 | 0.77 (0.61, 0.97) | - | 0.008 |
| No | 5 | 28 | 116,382 | 1.10 (0.96, 1.25) | 59.6 |
* Some publications were conducted on more than one cohort study without reporting the separated results. HR: Hazard ratio; 95%CI: 95% confidence interval
Discussion
This is the first dose-response meta-analysis that provides a comprehensive overview of the associations between circulating Lp(a) and all-cause and cause specific mortality in the general population and in patients with chronic diseases.
The association of Lp(a) and mortality might be explained by some pathways. Several meta-analyses have reported the association between Lp(a) and cardiovascular disease/events [17, 87]. In addition, large genetic and epidemiological studies have indicated that higher levels of Lp(a) is a causal risk factor for myocardial infarction [2], coronary disease [4], atherosclerosis stenosis [88], aortic-valve calcification and aortic valve stenosis [3, 5, 89], which some mechanisms related to these diseases might mediate the association of higher Lp(a) levels and increased risk of all-cause and CVD mortality. Some other studies shed light on the prothrombotic effects [90] and anti-fibrinolytic roles [91] of Lp(a) as well. Lp(a) may interfere with plasminogen activity through molecular similarity. The homology between the fibrinolytic proenzyme, plasminogen, and Apolipoprotein (a) (apo(a)), a unique protein component of Lp(a) without any fibrinolytic activity, may slow down fibrinolysis and indirectly induce thrombosis [92]. Furthermore, elevated levels of Lp(a), particularly its apo(a) fragment, may induce vascular inflammation [93, 94], leading to the progression of atherosclerosis [94, 95], which may have been associated with increased risk of CVD and its related mortality either independently or interactively [96].
The significant increase of the Lp(a)-associated risk for CVD death in the general population in our study is in line with the results of a study conducted by the Emerging Risk Factors Collaboration including 72,683 individuals from 24 studies, found that a one standard deviation (SD) (3.5 fold) higher Lp(a) was associated with 14% increase in CVD-death, while no significant association was observed in risk of non-vascular mortality [8]. Findings of the BiomarCaRE consortium showed no significant association between Lp(a) and all-cause mortality for Lp(a) levels ≥ 90th percentile in comparison to Lp(a) levels in the lowest third which is in contrast to our findings [36]. Similarly, a large prospective study of 3,313 patients with established coronary heart disease found no significant associations between highest versus lowest tertile of Lp(a) and risk of all-cause and CVD mortality [82]. Two prospective cohorts on 2,308 patients with T2DM from Nurses’ Health Study and the Health Professional Follow-Up Study (n = 2308) reported no significant association of coronary heart disease, and CVD per 1-SD higher log-transformed Lp(a), while a marginally significant association was observed for CVD mortality [10].
This study has some limitations that should be considered while interpreting its results. Although we performed our analysis separately for studies in the general population, some studies in this population might not exclude patients with pre-existing diseases. Thus, this act may provide bias induced by pre-existing diseases. Residual confounding could have affected the results since we performed a meta-analysis of observational studies. However, there was little indication of heterogeneity between subgroups that adjusted for BMI or lipid-lowering medication, we could not perform subgroup analysis based on other confounding variables including hypertension status, blood lipid markers, physical activity and smoking as the majority of included studies presented these variables in their multivariable statistical models. Since most studies lacked multiple Lp(a) assessments, the reported relative risks in the original studies may have been subject to underestimation due to regression dilution bias and random measurement error [97]. We observed a high heterogeneity among the included studies which might be derived from variations in sample sizes or in concentration of Lp(a) in different populations (such as different races and ethnicities), Lp(a) assessment methods, and adjustments for confounding variables. Regarding the Lp(a) assessment methods it should be noted that there are both differences in assay methods between studies and also varieties within each assessment category that might be a reason of the large heterogeneity. Also, the majority of the studies did not take the variability of number of kringle-IV type 2 (KIV2)-like domains into account. Number of KIV2-like domains are associated with the size of apo(a) in structure of Lp(a), which means alleles with fewer repeats of KIV2 encode smaller apo(a) isoforms [98, 99], associating with higher plasma Lp(a) concentrations. This fact also might be a reason for heterogeneity among the studies. We were unable to perform sex-stratified analysis and also subgroup analysis for patients with CVD based on disease clinical presentation (acute and chronic coronary syndrome) owing to insufficient data. Lastly, only around half of the available studies could be included in the dose-response analyses, because many studies only reported dichotomous results. The dose-response analyses therefore need to be interpreted with some caution, and the high versus low analyses are therefore given more weight in the interpretation of the results.
The present study also has several strengths. Unlike the majority of systematic reviews and meta-analyses that only focused on the associations in the general population, we present all available evidence relating to the association of Lp(a) and mortality irrespective of health condition. Additionally, we systematically summarized the available literature presenting the information on the association between Lp(a) and mortality in univariate models or using descriptive/frequency results in our supplementary materials. The linear and non-linear dose-response analyses enabled us to clarify the shape of the dose-response relations and to provide clear insight into the quantitative evaluations of the associations. To identify sources of heterogeneity, we performed various subgroup analysis based on geographical region, follow-up duration, whether studies were conducted in multiple cohorts or a single cohort, adjustment for main confounders, and Lp(a) assessment methods. We transformed the relative risk estimates which were often reported differently by each study to top vs. bottom third of baseline Lp(a) distribution to enable a consistent approach for running the meta-analysis.
According to our findings, we have several suggestions for future studies. Research has indicated that Lp(a) concentrations are mainly determined by the number of KIV2 repeats genetically which inversely correlate with Lp(a) levels [100] and subsequently its function. However, apo(a) isoforms have not been taken into account in the majority of studies. Future studies should take this into consideration. Also, most of the included studies in the analyses were from the US, and some from European countries. Data are lacking for ethnic groups like Africans, which may have higher Lp(a) levels compared to Caucasians [36]. As it was shown in the results of this meta-analysis, regional differences in Lp(a) levels may lead to differences in Lp(a)-associated mortality. Thus, further studies are needed in other geographical regions to provide more clear insight into ethnicity-specific ranges since Lp(a) concentration varies by race/ethnicity [101, 102]. Also, our novel findings of dose-response association between Lp(a) and CVD mortality implicate that therapies which aim at lowering Lp(a) concentration might potentially decrease risk of CVD-death in the general population or in patients with CVD. Thus, this study emphasizes the need for running randomized clinical trials to provide more clear insight on the effects of Lp(a) lowering therapies to prevent all-cause and CVD mortality in populations with different health status. Any further studies should try to report dose-response relationships at more extreme levels of Lp(a) and perform sex-stratified analysis.
In conclusion, our meta-analysis suggests higher risks of all-cause and CVD mortality in both general population (HR: 1.09 for all-cause, HR: 1.18 for CVD mortality) and in patients with CVD (HR: 1.33 for all-cause, HR: 1.25 for CVD mortality) in third versus first tertile of Lp(a) distribution. Also, the linear dose-response analysis showed a higher risk of CVD mortality in both patients with CVD (HR: 1.15) and in the general population (HR: 1.31) per 50 mg/dL increase in Lp(a) concentration. According to 2019 ESC/EAS Guidelines, Lp(a) should be measured at least once in each adult person’s lifetime to identify those who have very high inherited Lp(a) levels > 180 mg/dL, who may have a lifetime risk of atherosclerotic cardiovascular disease which is approximately equivalent to the risk associated with heterozygous familial hypercholesterolemia [7]. Other previous guidelines also recommended Lp(a) measurement in selected cases at high risk, in patients with a family history of premature cardiovascular disease, and for reclassification in subjects with borderline risk [103, 104]. Our findings support these recommendations with regard to the associations of Lp(a) with CVD and all-cause mortality in the general population and in individuals with chronic diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
HR-D, AVW, and YW have received funding for their PhD trajectories by the Swiss National Science Foundation under the SPIRIT grant (SNSF IZSTZ0_190277), Jaap Schouten Foundation, and China Scholarship Council (CSC) (file number: 202008500141), respectively. The funding agencies had no role in the study (conceptualization, design, data collection, analysis, and writing). MA appreciates Dr. Amine Amiri for her valuable advice and consolations on this project.
Author contributions
MA, HR-D, TV contributed to the study conception. MA and WM.B performed the literature search. MA, HR-D, AV, YW, AVW, KB did the literature screening. MA and HR-D extracted data from included studies. MA, HR-D, and DA performed the data analysis. MA and HR-D wrote the manuscript. All authors acknowledge full responsibility for the analyses and interpretation of the report. All authors have read and approved the final manuscript. The corresponding author (TV) attests that all listed authors meet authorship criteria.
Funding
MA has awarded by Covidence Global Scholarship Program in 2022 for this systematic review and dose-response meta-analysis. The funding source had no role in the design of this study, its execution, analyses, interpretation, or decision to publish the results.
Data availability
All available data are provided within the manuscript and supplementary files.
Statements & declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study is systematic review and meta-analysis. No ethical approval is not applicable.
Consent to participate
This study is systematic review and meta-analysis. Consent to participate is not applicable.
Consent to publish
This study is systematic review and meta-analysis. Consent to publish is not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mojgan Amiri and Hamidreza Raeisi-Dehkordi have contributed equally to this systematic review and meta-analysis.
References
- 1.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844–53. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein (a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–9. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 3.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–12. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with lp (a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–28. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 5.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein (a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470–7. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57(11):1953–75. doi: 10.1194/jlr.R071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and european atherosclerosis society (EAS) Eur Heart J. 2020;41(1):111–88. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 8.Emerging Risk Factors C. Lipoprotein (a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. 2009. [DOI] [PMC free article] [PubMed]
- 9.Zewinger S, Kleber ME, Tragante V, et al. Relations between lipoprotein(a) concentrations, LPA genetic variants, and the risk of mortality in patients with established coronary heart disease: a molecular and genetic association study. Lancet Diabetes Endocrinol. 2017;5(7):534–43. doi: 10.1016/S2213-8587(17)30096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi Q, Workalemahu T, Zhang C, Hu FB, Qi L. Genetic variants, plasma lipoprotein(a) levels, and risk of cardiovascular morbidity and mortality among two prospective cohorts of type 2 diabetes. Eur Heart J. 2012;33(3):325–34. doi: 10.1093/eurheartj/ehr350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J. 2019;40(33):2760–70. doi: 10.1093/eurheartj/ehy902. [DOI] [PubMed] [Google Scholar]
- 12.Arsenault BJ, Pelletier W, Kaiser Y, et al. Association of Long-term exposure to elevated lipoprotein(a) levels with parental life Span, Chronic Disease-Free Survival, and mortality risk: a mendelian randomization analysis. JAMA Netw Open. 2020 doi: 10.1001/jamanetworkopen.2020.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Z, Li HL, Bei WJ, et al. Association of lipoprotein(a) with long-term mortality following coronary angiography or percutaneous coronary intervention. Clin Cardiol. 2017;40(9):674–8. doi: 10.1002/clc.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes LU, Gerdes C, Kervinen K, et al. The apolipoprotein ε4 allele determines prognosis and the effect on prognosis of simvastatin in survivors of myocardial infarction: a substudy of the scandinavian simvastatin survival study. Circulation. 2000;101(12):1366–71. doi: 10.1161/01.Cir.101.12.1366. [DOI] [PubMed] [Google Scholar]
- 15.Hernández C, Francisco G, Chacón P, Simó R. Lipoprotein(a) as a risk factor for cardiovascular mortality in type 2 diabetic patients: a 10-year follow-up study. Diabetes Care. 2005;28(4):931–3. doi: 10.2337/diacare.28.4.931. [DOI] [PubMed] [Google Scholar]
- 16.Genser B, Dias KC, Siekmeier R, Stojakovic T, Grammer T, Maerz W. Lipoprotein (a) and risk of cardiovascular disease–a systematic review and meta analysis of prospective studies. Clin Lab. 2011;57(3–4):143–56. [PubMed] [Google Scholar]
- 17.Wang Z, Zhai X, Xue M, Cheng W, Hu H. Prognostic value of lipoprotein (a) level in patients with coronary artery disease: a meta-analysis. Lipids Health Dis. 2019;18(1):1–9. doi: 10.1186/s12944-018-0950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willeit P, Ridker PM, Nestel PJ, et al. Baseline and on-statin treatment lipoprotein (a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. The Lancet. 2018;392(10155):1311–20. doi: 10.1016/S0140-6736(18)31652-0. [DOI] [PubMed] [Google Scholar]
- 19.Klingel R, Heibges A, Fassbender C. Lipoprotein(a) and mortality-a high risk relationship. Clin Res Cardiol Suppl. 2019;14(Suppl 1):13–9. doi: 10.1007/s11789-019-00095-3. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (clinical research ed.). 2009;339:b2535. [DOI] [PMC free article] [PubMed]
- 21.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Association: JMLA. 2016;104(3):240. doi: 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–45. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 25.Semnani-Azad Z, Khan TA, Mejia SB, et al. Association of major food sources of fructose-containing sugars with incident metabolic syndrome: a systematic review and meta-analysis. JAMA Netw open. 2020;3(7):e209993-e. doi: 10.1001/jamanetworkopen.2020.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chêne G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144(6):610–21. doi: 10.1093/oxfordjournals.aje.a008971. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj A, Damrauer SM, Anderson AH, et al. Lipoprotein(a) and risk of myocardial infarction and death in chronic kidney disease findings from the CRIC study (chronic renal insufficiency cohort) Arterioscler Thromb Vasc Biol. 2017;37(10):1971–8. doi: 10.1161/atvbaha.117.309920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27(7):954–70. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 29.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9(1):1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 30.Cheng SWK, Ting ACW. Lipoprotein (a) level and mortality in patients with critical lower limb ischaemia. Eur J Vasc Endovasc Surg. 2001;22(2):124–9. doi: 10.1053/ejvs.2001.1431. [DOI] [PubMed] [Google Scholar]
- 31.Iliescu EA, Marcovina SM, Morton AR, Lam M, Koschinsky ML. Apolipoprotein(a) phenotype and lipoprotein(a) level predict peritoneal dialysis patient mortality. Perit Dial Int. 2002;22(4):492–9. doi: 10.1177/089686080202200408. [DOI] [PubMed] [Google Scholar]
- 32.Koda Y, Nishi SI, Suzuki M, Hirasawa Y. Lipoprotein(a) is a predictor for cardiovascular mortality of hemodialysis patients. Kidney Int Suppl. 1999;56(71):251-S3. doi: 10.1046/j.1523-1755.1999.07167.x. [DOI] [PubMed] [Google Scholar]
- 33.Ohashi H, Oda H, Ohno M, Watanabe S, Sakata S. Lipoprotein(a) as a risk factor for coronary artery disease in hemodialysis patients. Kidney Int Suppl. 1999;56(71):242-S4. doi: 10.1046/j.1523-1755.1999.07164.x. [DOI] [PubMed] [Google Scholar]
- 34.Ariyo AA, Thach C, Tracy R. Lp(a) lipoprotein, Vascular Disease, and Mortality in the Elderly. New Engl J Med. 2003;349(22):2108–15. doi: 10.1056/NEJMoa001066. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldeyer C, Makarova N, Zeller T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the european population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38(32):2490–8. doi: 10.1093/eurheartj/ehx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088 – 101. [PubMed]
- 38.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 40.Madsen CM, Kamstrup PR, Langsted A, Varbo A, Nordestgaard BG. Lipoprotein (a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20% in secondary prevention: a population-based study. Arterioscler Thromb Vasc Biol. 2020;40(1):255–66. doi: 10.1161/ATVBAHA.119.312951. [DOI] [PubMed] [Google Scholar]
- 41.Rifai N, Ma J, Sacks FM, et al. Apolipoprotein (a) size and lipoprotein (a) concentration and future risk of angina pectoris with evidence of severe coronary atherosclerosis in men: the Physicians’ Health Study. Clin Chem. 2004;50(8):1364–71. doi: 10.1373/clinchem.2003.030031. [DOI] [PubMed] [Google Scholar]
- 42.Danik JS, Rifai N, Buring JE, Ridker PM. Lipoprotein (a), measured with an assay independent of apolipoprotein (a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296(11):1363–70. doi: 10.1001/jama.296.11.1363. [DOI] [PubMed] [Google Scholar]
- 43.Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein (a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117(2):176–84. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 44.Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. 2010;29(12):1282–97. doi: 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- 45.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 47.Hemingway H, Philipson P, Chen R, et al. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7(6):e1000286. doi: 10.1371/journal.pmed.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson A, Danesh J. Associations between apolipoprotein B, apolipoprotein AI, the apolipoprotein B/AI ratio and coronary heart disease: a literature-based meta‐analysis of prospective studies. J Intern Med. 2006;259(5):481–92. doi: 10.1111/j.1365-2796.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 49.Chowdhury R, Stevens S, Gorman D, et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ (Clinical research ed.). 2012;345. [DOI] [PMC free article] [PubMed]
- 50.Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160(6):398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 51.Guang-Da X, Xiang-Jiu Y, Lin-Shuang Z, Zhi-Song C, Yu-Sheng H. Apolipoprotein e4 allele and the risk of CAD death in type 2 diabetes mellitus with ischaemia electrocardiographic change. Diabetes Res Clin Pract. 2005;68(3):223–9. doi: 10.1016/j.diabres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Kollerits B, Drechsler C, Krane V, et al. Lipoprotein(a) concentrations, apolipoprotein(a) isoforms and clinical endpoints in haemodialysis patients with type 2 diabetes mellitus: results from the 4D study. Nephrol Dial Transplant. 2016;31(11):1901–8. doi: 10.1093/ndt/gfv428. [DOI] [PubMed] [Google Scholar]
- 53.Nestel PJ, Barnes EH, Tonkin AM, et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33(12):2902–8. doi: 10.1161/ATVBAHA.113.302479. [DOI] [PubMed] [Google Scholar]
- 54.Zhou H, Cui L, Zhu G, et al. Survival advantage of normal weight in peritoneal dialysis patients. Ren Fail. 2011;33(10):964–8. doi: 10.3109/0886022x.2011.615968. [DOI] [PubMed] [Google Scholar]
- 55.Wohlfahrt P, Jenča D, Melenovský V, et al. Very low lipoprotein(a) and increased mortality risk after myocardial infarction. Eur J Intern Med. 2021 doi: 10.1016/j.ejim.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Fleischmann EH, Bower JD, Salahudeen AK. Risk factor paradox in hemodialysis: better nutrition as a partial explanation. Asaio J. 2001;47(1):74–81. doi: 10.1097/00002480-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 57.Park SH, Rha SW, Choi BG, et al. Impact of high lipoprotein(a) levels on in-stent restenosis and long-term clinical outcomes of angina pectoris patients undergoing percutaneous coronary intervention with drug-eluting stents in asian population. Clin Exp Pharmacol Physiol. 2015;42(6):588–95. doi: 10.1111/1440-1681.12396. [DOI] [PubMed] [Google Scholar]
- 58.Akinyemiju T, Moore JX, Judd SE, et al. Pre-diagnostic biomarkers of metabolic dysregulation and cancer mortality. Oncotarget. 2018;9(22):16099–109. doi: 10.18632/oncotarget.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chien KL, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT. Lipoprotein(a) and cardiovascular disease in ethnic Chinese: the Chin-Shan community cardiovascular cohort study. Clin Chem. 2008;54(2):285–91. doi: 10.1373/clinchem.2007.090969. [DOI] [PubMed] [Google Scholar]
- 60.D’Angelo A, Ruotolo G, Garancini P, Sampietro F, Mazzola G, Calori G. Lipoprotein(a), fibrinogen and vascular mortality in an elderly northern italian population. Haematologica. 2006;91(12):1613–20. [PubMed] [Google Scholar]
- 61.Katzke VA, Sookthai D, Johnson T, Kühn T, Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective EPIC-Heidelberg cohort. BMC Med. 2017;15(1). doi:10.1186/s12916-017-0976-4. [DOI] [PMC free article] [PubMed]
- 62.Nomikos T, Panagiotakos D, Georgousopoulou E, et al. Hierarchical modelling of blood lipids’ profile and 10-year (2002–2012) all cause mortality and incidence of cardiovascular disease: the ATTICA study. Lipids Health Dis. 2015;14(1). doi:10.1186/s12944-015-0101-7. [DOI] [PMC free article] [PubMed]
- 63.Onat A, Can G, Çoban N, et al. Lipoprotein(a) level and MIF gene variant predict incident metabolic syndrome and mortality. J Invest Med. 2016;64(2):392–9. doi: 10.1136/jim-2015-000003. [DOI] [PubMed] [Google Scholar]
- 64.Patterson CC, Blankenberg S, Ben-Shlomo Y, et al. Which biomarkers are predictive specifically for cardiovascular or for non-cardiovascular mortality in men? Evidence from the Caerphilly prospective study (CaPS) Int J Cardiol. 2015;201:113–8. doi: 10.1016/j.ijcard.2015.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawabe M, Tanaka N, Mieno MN, et al. Low lipoprotein(a) concentration is associated with cancer and all-cause deaths: a population-based cohort study (the jms cohort study). PLoS ONE. 2012;7(4). doi:10.1371/journal.pone.0031954. [DOI] [PMC free article] [PubMed]
- 66.Séguro F, Bérard E, Bongard V, et al. Real life validation of the european atherosclerosis Society Consensus Panel lipoprotein(a) threshold of 50 mg/dL. Int J Cardiol. 2016;221:537–8. doi: 10.1016/j.ijcard.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Delaney JA, Quek RGW, et al. Cardiovascular Disease, Mortality Risk, and Healthcare costs by lipoprotein(a) levels according to low-density lipoprotein cholesterol levels in older high-risk adults. Clin Cardiol. 2016;39(7):413–20. doi: 10.1002/clc.22546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez-Cornago A, Fensom GK, Andrews C, et al. Examination of potential novel biochemical factors in relation to prostate cancer incidence and mortality in UK Biobank. Br J Cancer. 2020;123(12):1808–17. doi: 10.1038/s41416-020-01081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Welsh P, Welsh C, Celis-Morales CA, et al. Lipoprotein(a) and cardiovascular disease: prediction, attributable risk fraction, and estimating benefits from novel interventions. Eur J Prev Cardiol. 2020 doi: 10.1093/eurjpc/zwaa063. [DOI] [PubMed] [Google Scholar]
- 70.Solfrizzi V, Colacicco AM, D’Introno A, et al. All-cause mortality and competing risks of fatal and nonfatal vascular events in the italian longitudinal study on aging: impact of lipoprotein(a) Rejuvenation Res. 2009;12(6):395–402. doi: 10.1089/rej.2009.0865. [DOI] [PubMed] [Google Scholar]
- 71.Ahlbeckglader C, Slungabirgander L, Stenlund H, Dahlén GH. Is lipoprotein(a) a predictor for survival in patients with established coronary artery disease? Results from a prospective patient cohort study in northern Sweden. J Intern Med (GBR) 2002;252(1):27–35. doi: 10.1046/j.1365-2796.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 72.Gencer B, Rigamonti F, Nanchen D, et al. Prognostic value of elevated lipoprotein(a) in patients with acute coronary syndromes. Eur J Clin Invest. 2019;49(7). doi:10.1111/eci.13117. [DOI] [PubMed]
- 73.Golledge J, Rowbotham S, Velu R, et al. Association of serum lipoprotein (a) with the requirement for a peripheral artery operation and the incidence of major adverse Cardiovascular events in people with peripheral artery disease. J Am Heart Assoc. 2020;9(6):e015355. doi: 10.1161/jaha.119.015355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu HH, Cao YX, Jin JL, et al. Predicting Cardiovascular Outcomes by Baseline Lipoprotein(a) concentrations: a large cohort and long-term follow-up study on real-world patients receiving percutaneous coronary intervention. J Am Heart Assoc. 2020;9(3). doi:10.1161/jaha.119.014581. [DOI] [PMC free article] [PubMed]
- 75.Lundstam U, Herlitz J, Karlsson T, Lindén T, Wiklund O. Serum lipids, lipoprotein(a) level, and apolipoprotein(a) isoforms as prognostic markers in patients with coronary heart disease. J Intern Med (GBR) 2002;251(2):111–8. doi: 10.1046/j.1365-2796.2002.00937.x. [DOI] [PubMed] [Google Scholar]
- 76.O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk insights from the FOURIER trial. Circulation. 2019;139(12):1483–92. doi: 10.1161/circulationaha.118.037184. [DOI] [PubMed] [Google Scholar]
- 77.Roth C, Krychtiuk KA, Gangl C, et al. Lipoprotein(a) plasma levels are not associated with survival after acute coronary syndromes: an observational cohort study. PLoS ONE. 2020;15(1). doi:10.1371/journal.pone.0227054. [DOI] [PMC free article] [PubMed]
- 78.Shlipak MG, Simon JA, Vittinghoff E, et al. Estrogen and progestin, lipoprotein(a), and the risk of recurrent coronary heart disease events after menopause. JAMA. 2000;283(14):1845–52. doi: 10.1001/jama.283.14.1845. [DOI] [PubMed] [Google Scholar]
- 79.Stubbs PJ, Seed M, Lane D, Collinson P, Kendall F, Noble M. Lipoprotein(a) as a risk predictor for cardiac mortality in patients with acute coronary syndromes. Eur Heart J. 1998;19(9):1355–64. doi: 10.1053/euhj.1998.1043. [DOI] [PubMed] [Google Scholar]
- 80.Xu N, Jiang L, Xu L, et al. Impact of lipoprotein(a) on Long-Term (Mean 6.2 years) outcomes in patients with three-vessel coronary artery disease. Am J Cardiol. 2020;125(4):528–33. doi: 10.1016/j.amjcard.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 81.Zairis MN, Ambrose JA, Manousakis SJ, et al. The impact of plasma levels of C-reactive protein, lipoprotein (a) and homocysteine on the long-term prognosis after successful coronary stenting: the global evaluation of new events and restenosis after stent implantation study. J Am Coll Cardiol. 2002;40(8):1375–82. doi: 10.1016/s0735-1097(02)02267-2. [DOI] [PubMed] [Google Scholar]
- 82.Zewinger S, Kleber ME, Tragante V, et al. Relations between lipoprotein(a) concentrations, LPA genetic variants, and the risk of mortality in patients with established coronary heart disease: a molecular and genetic association study. Lancet Diabetes Endocrinol. 2017;5(7):534–43. doi: 10.1016/s2213-8587(17)30096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang M, Liu HH, Jin JL, Yan XN, Dong Q, Li JJ. Lipoprotein(a) and cardiovascular death in oldest-old (≥ 80 years) patients with acute myocardial infarction: a prospective cohort study. Atherosclerosis. 2020;312:54–9. doi: 10.1016/j.atherosclerosis.2020.08.033. [DOI] [PubMed] [Google Scholar]
- 84.Cao YX, Zhang HW, Jin JL, et al. Lipoprotein(a) and Cardiovascular Outcomes in patients with previous myocardial infarction: a prospective cohort study. Thromb Haemost. 2021 doi: 10.1055/a-1340-2109. [DOI] [PubMed] [Google Scholar]
- 85.Heinrich NS, von Scholten BJ, Reinhard H, et al. Lipoprotein(a)and renal function decline, cardiovascular disease and mortality in type 2 diabetes and microalbuminuria. J Diabetes Complications. 2020 doi: 10.1016/j.jdiacomp.2020.107593. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Jin JL, Cao YX, et al. Lipoprotein (a) predicts recurrent worse outcomes in type 2 diabetes mellitus patients with prior cardiovascular events: a prospective, observational cohort study. Cardiovasc Diabetol. 2020;19(1). doi:10.1186/s12933-020-01083-8. [DOI] [PMC free article] [PubMed]
- 87.Danesh J, Collins R, Peto R. Lipoprotein (a) and coronary heart disease: meta-analysis of prospective studies. Circulation. 2000;102(10):1082–5. doi: 10.1161/01.CIR.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 88.Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Genetic evidence that lipoprotein (a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(7):1732–41. doi: 10.1161/ATVBAHA.112.248765. [DOI] [PubMed] [Google Scholar]
- 89.Arsenault BJ, Boekholdt SM, Dubé M-P, et al. Lipoprotein (a) levels, genotype, and incident aortic valve stenosis: a prospective mendelian randomization study and replication in a case–control cohort. Circulation: Cardiovasc Genet. 2014;7(3):304–10. doi: 10.1161/CIRCGENETICS.113.000400. [DOI] [PubMed] [Google Scholar]
- 90.Martinez C, Rivera J, Loyau S, et al. Binding of recombinant apolipoprotein (a) to human platelets and effect on platelet aggregation. Thromb Haemost. 2001;85(04):686–93. doi: 10.1055/s-0037-1615654. [DOI] [PubMed] [Google Scholar]
- 91.Gardener H, Della Morte D, Elkind MS, Sacco RL, Rundek T. Lipids and carotid plaque in the Northern Manhattan Study (NOMAS) BMC Cardiovasc Disord. 2009;9(1):1–8. doi: 10.1186/1471-2261-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res. 2016;57(5):745–57. doi: 10.1194/jlr.R060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Valk FM, Bekkering S, Kroon J, et al. Oxidized phospholipids on lipoprotein (a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611–24. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pirro M, Bianconi V, Paciullo F, Mannarino MR, Bagaglia F, Sahebkar A. Lipoprotein (a) and inflammation: a dangerous duet leading to endothelial loss of integrity. Pharmacol Res. 2017;119:178–87. doi: 10.1016/j.phrs.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 95.Deb A, Caplice NM. Lipoprotein (a): new insights into mechanisms of atherogenesis and thrombosis. Clin Cardiol. 2004;27(5):258–64. doi: 10.1002/clc.4960270503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 97.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. Bmj. 2010;340. [DOI] [PubMed]
- 98.Hegele RA, Breckenridge WC, Brunt JH, Connelly PW. Genetic variation in factor VII associated with variation in plasma lipoprotein (a) concentration. Arteriosclerosis, thrombosis, and vascular biology. 1997;17(9):1701–6. [DOI] [PubMed]
- 99.Boerwinkle E, Menzel HJ, Kraft HG, Utermann G. Genetics of the quantitative lp (a) lipoprotein trait. Hum Genet. 1989;82(1):73–8. doi: 10.1007/BF00288277. [DOI] [PubMed] [Google Scholar]
- 100.Kettunen J, Demirkan A, Würtz P, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016;7(1):1–9. doi: 10.1038/ncomms11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen Y. The metabolic and molecular bases of inherited disease. Glycogen storage diseases. 2001:1521–51.
- 102.Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. J Lipid Res. 2016;57(7):1111–25. doi: 10.1194/jlr.R051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 104.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-81. doi:10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All available data are provided within the manuscript and supplementary files.