Abstract
Background:
Cigarette smoking (CS) and opioid use disorder (OUD) significantly alter brain structure. Although OUD and cigarette smoking are highly comorbid, most prior neuroimaging research in OUD did not control for smoking severity. Specifically, the combined effect of smoking and OUD on the brain gray matter volume (GMV) remains unknown.
Objectives:
We used structural magnetic resonance imaging (sMRI) to examine: (1) the GMV differences between OUD and non-OUD individuals with comparable smoking severity; and (2) the differential effect of smoking severity on the brain GMV between individuals with and without OUD.
Methods:
We performed a secondary analysis of existing sMRI datasets of 116 individuals who smoked cigarettes daily, among whom 60 had OUD (CS-OUD; 37 male, 23 female) and 56 did not (CS; 31 male, 25 female). Brain GMV was estimated by voxel-based morphometry analysis.
Results:
Compared to the CS group, the CS-OUD group had a higher GMV in the occipital cortex and lower GMV in the prefrontal and temporal cortex, striatum, and pre/postcentral gyrus (whole-brain corrected-p<0.05). There was a significant interaction between group and smoking severity on GMV in the medial orbitofrontal cortex (whole-brain corrected-p<0.05), such that heavier smoking was associated with lower medial orbitofrontal GMV in the CS-OUD but not CS participants (r=–0.32 vs. 0.12).
Conclusions:
Our findings suggest a combination of independent and interactive effects of cigarette smoking and OUD on the brain gray matter. Elucidating the neuroanatomical correlates of comorbid opioid and tobacco use may shed the light on the development of novel interventions for affected individuals.
INTRODUCTION
Opioid use disorder (OUD) is a major public health problem that has reached epidemic proportion and carries substantial individual and societal burden, including disruption in family structure and increased risk of overdose-related fatalities (1). Morbidity and treatment outcomes in OUD are further compounded by frequent comorbidity with other substance use disorders. Tobacco cigarette smoking (CS) is highly comorbid with OUD. For example, in a sample of primary care patients, approximately 89.4% of individuals with OUD reported CS in the past year (2). Smoking increases the odds of OUD by >8 times (3). Conversely, OUD patients experience greater difficulties in smoking cessation (4). While opioid overdose is the most direct cause of mortality in OUD, the leading causes of death are pulmonary and cardiovascular disease and cancer, all of which can be directly or indirectly caused by CS.
Established OUD is mediated by neuroadaptations to repeated exogenous opioid exposure that evolve into an inability to resist drug seeking despite the negative consequences (1). Opioid toxicity may contribute to the chronicity of these neuroadaptations by altering the brain structure through such mechanisms as respiratory depression that leads to decreased brain oxygen levels. In rodents, heroin administration diminishes oxygen entry from brain arterial blood and results in hypoxia in the nucleus accumbens (5). In comparison to healthy controls, individuals with OUD exhibit hypoperfusion in the orbitofrontal cortex, superior temporal cortex, hippocampus, and putamen, and hyperperfusion in the anterior cingulate cortex and globus pallidus (6). Chronic cerebral hypoperfusion may lead to structural and functional alterations (7). Indeed, structural and functional magnetic resonance imaging (sMRI and fMRI) studies have identified lower frontotemporal gray matter volume (GMV) (8) and abnormal connectivity pattern in the prefrontal cortex (PFC), anterior cingulate cortex, striatum, insula, amygdala, and hippocampus (9,10) in OUD compared to non-OUD individuals. These regions are involved in multiple cognitive domains including impulse control (11), working-memory (12), and reward processing (9). Impairments in these domains may contribute to the perpetuation of OUD and poor treatment outcomes (13,14).
Cigarette smoking is also associated with brain structural abnormalities. One study reported significantly smaller total GMV in people who smoked vs. those who never smoked and a negative association between total GMV and smoking duration (15). Regional analysis of sMRI data using voxel-based morphometry (VBM) identified smaller PFC and cerebellar GMV in heavier smoking individuals (16). Despite extensive evidence supporting smoking-associated alterations in brain structure (17,18), the relationship between smoking and GMV may be confounded by co-occurrence of other substance use (19). A recent study showed that the negative association between smoking and brain GMV was limited to individuals with dual tobacco and alcohol use disorders (19). Given the extremely high comorbidity between smoking and OUD, dissociating their effects on the brain structure is critical for the field. However, little is known regarding the individual and combined effects of OUD and tobacco smoking on brain structural integrity.
To begin bridging this knowledge gap, we utilized sMRI and VBM to examine GMV differences between 60 OUD and 56 non-OUD individuals who smoked cigarettes and had comparable smoking severity. We further explored the interactive effects of OUD and smoking on GMV and examined the differential associations between smoking severity and GMV in individuals with and without OUD. To our knowledge, this study is the first to investigate the addictive and interactive effects of smoking and OUD on GMV.
MATERIAL AND METHODS
Participants
We analyzed baseline sMRI data collected by two previous studies that included individuals who smoked cigarettes on a daily basis. These individuals either had OUD (CS-OUD) (20) or did not have OUD (CS) (21). Inclusion criteria common for both studies were: 18 to 60 years old; good physical health; free of chronic psychiatric disorders other than substance use disorders and not receiving treatments that may affect the cerebrovascular system; free of contraindications for MRI (e.g., history of clinically significant head trauma); for female participants, not pregnant or breastfeeding. Inclusion criteria specific for the CS-OUD cohort (20) were: DSM-IV-TR diagnosis of opioid dependence; active opioid use confirmed by urine toxicology screen and self-reported daily heroin or prescription opioid use for more than 2 weeks in the past 3 months; urine toxicology screen negative for opioids after detoxification; free of contraindications for treatment with extended-release naltrexone. Inclusion criteria specific for the CS cohort (21) were: currently smoking at least 2 cigarettes daily; not enrolled in any smoking cessation program. For the purpose of the current analysis, we only included individuals who had sMRI data collected under the same MRI protocol and prior to anti-smoking intervention or OUD treatment. We also excluded one individual from the CS-OUD cohort who smoked fewer than 2 cigarettes per day. No individuals from the CS cohort had DSM-IV-TR diagnosis of opioid dependence. A total of 60 CS-OUD individuals and 56 CS individuals were included in the final analysis. All participants gave written informed consent to participate in the protocol approved by the University of Pennsylvania Institutional Review Board.
Assessments
DSM-IV-TR diagnosis of opioid dependence was established using the history and physical examination aided by medical records and the Mini International Neuropsychiatric Interview (MINI) (22,23). Current cigarette smoking severity was indexed by the number of cigarettes smoked per day during the week (CPD) before the scan, measured by the Timeline Follow-Back (TLFB) interview (24). The TLFB uses a calendar-based format to record participants’ cigarette smoking each day. CPD was calculated as the average of the past 7 days.
MRI scanning and processing
MRI was performed on a Siemens Tim Trio 3T system (Siemens USA, Malvern, PA, USA). MPRAGE T1-weighted structural images were acquired with the following parameters: repetition time/echo time=1510/3.71 ms, matrix=256×192, slice thickness/gap=1/0 mm, 160 slices, effective voxel resolution of 1×1×1 mm³, FA=9°. An oblique acquisition oriented along the anterior commissure – posterior commissure line, allowed coverage of the entire brain except for the lower cerebellum.
VBM analysis of MRI data was performed using MATLAB 2020a (MathWorks, Natick, MA) and SPM 12 (Wellcome Trust Centre for Neuroimaging, London, UK). The structural images of individual participants were segmented and transformed into gray matter, white matter, and cerebrospinal fluid probability maps in the Montreal Neurological Institute (MNI) stereotaxic space. Diffeomorphic anatomical registration through an exponentiated Lie (DARTEL) algebra was applied to the tissue probability maps to produce a sample-specific template (25). Individual gray matter probability maps were then normalized to the template, modulated by the Jacobian determinants and spatially smoothed by a Gaussian filter with full width at half-maximum set to 8 mm.
Statistical analysis
Whole-brain analysis of covariance (ANCOVA) was performed using the full factorial design module in SPM to examine the main effect of group (CS-OUD vs. CS), the main effect of CPD, and the group-by-CPD interaction while controlling for total intracranial volume, age, and sex. We also controlled for race because of a significant difference in racial composition between cohorts (see Table 1). In the regions that showed significant group-by-CPD interaction, we tested for CPD effect separately for each group. All variables were mean-centered, and CPD was subjected to log transformation beforehand due to high right-skewness (+1.30). Significant regions were determined using the threshold-free cluster-enhancement (TFCE) algorithm at cluster-level corrected p<0.05. For visualization purpose, we used a scatterplot to illustrate the group-specific associations between CPD and the GMV of brain region(s) that showed significant group-by-CPD interaction. Specifically, we extracted covariate-adjusted GMV from a spherical region centered at the peak voxel with a radius of 5 mm and plotted it against CPD using the ggplot2 package in R.
Table 1.
Participant characteristics [mean±SD or count (%)]
| Variable | CS-OUD group | CS group |
|---|---|---|
| N | 60 | 56 |
| Sex | 37 male, 23 female | 31 male, 25 female |
| Age (years) | 29.53±8.87 | 30.09±10.81 |
| Race | 53 Caucasian, 5 African American, 1 Asian, 1 Other | 23 Caucasian, 19 African American, 4 Asian, 10 Other |
| Ethnicity | 5 Hispanic | 7 Hispanic |
| Years of education | 13.40±2.03 | 13.81±2.29 |
| Number of cigarettes per day | 14.92±8.03 | 12.84±7.34 |
| Alcohol use disorder¹ | 9 (17.65%) | 11 (18.33%) |
| UDS positive for cocaine² | 4 (6.90%) | 3 (5.56%) |
| UDS positive for cannabis² | 22 (37.93%) | 23 (42.59%) |
Abbreviations: CS-OUD, individuals who smoked cigarettes daily and had opioid use disorder; CS, individuals who smoked cigarettes daily and did not have opioid use disorder; UDS, urine drug screening.
Diagnosis of AUD was missing in 5 CS participants.
Urine toxicology data were missing in 2 CS-OUD and 2 CS participants.
Additional exploratory analyses were performed to confirm the main findings while considering concurrent use of substances other than tobacco and opioids. Specifically, concurrent alcohol use disorder (AUD) was assessed by clinical interview in all except 5 CS participants. Concurrent cocaine, cannabis, and opioid use was assessed by urine drug screen (UDS) on the day of the MRI scan, in all except 2 CS-OUD and 2 CS participants. Brain regions that showed a significant effect in the above ANCOVA were defined as spherical regions of interest centered at the peak voxels with a radius of 5 mm. We first repeated the above ANCOVA of these regions of interest after excluding individuals with AUD or positive UDS results for cocaine or cannabis. We then examined the models that included all participants while controlling for the main effect of other substance use and its interaction with OUD status.
RESULTS
Demographics and clinical characteristics
The demographic characteristics of participants are summarized in Table 1. There was no difference between the CS-OUD and CS groups in sex (χ²(1)=0.48, p=0.49), age (t(114)=–0.30, p=0.76), Hispanic ethnicity (χ²(1)=0.54, p=0.46), or years of education (t(112)=–1.03, p=0.31). CPD ranged from 3 to 40 cigarettes per day and were comparable between the CS-OUD and CS participants (t(114)=1.45, p=0.15). Concurrent AUD and use of cocaine and cannabis were also comparable between CS-OUD and CS participants (χ²(1)=0.01, 0.09, & 0.25, p=0.93, 0.77, & 0.62, respectively). The two groups significantly differed in racial composition (χ²(3)=29.07, p<0.001), which was converted to three dummy variables (Caucasian, African American, Asian) and controlled for in subsequent analysis.
Group differences in GMV between CS-OUD and CS
Significantly higher GMV in the CS-OUD compared to CS participants was found in the precuneus and occipital cortex (see Figure 1a). We also found lower GMV in the CS-OUD compared to CS participants in the bilateral pre/postcentral gyrus, right dorsolateral prefrontal cortex, bilateral temporal cortex, and bilateral nucleus accumbens/caudate (see Figure 1b). A complete list of regions showing significant group effect is included in Table 2.
Figure 1.
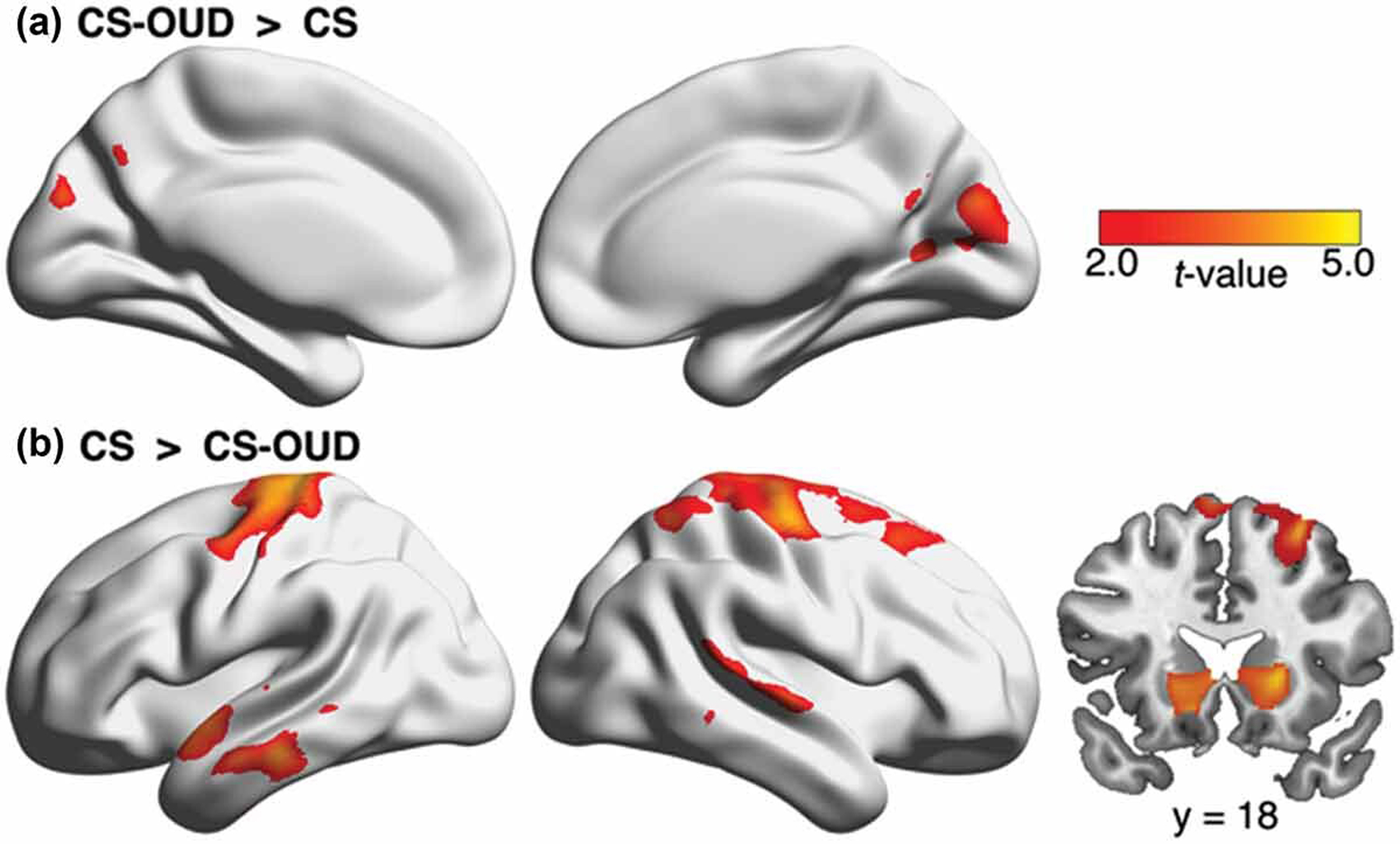
Higher occipital GMV and lower prefrontal, temporal, and striatal GMV in the CS-OUD group compared to the CS group. Abbreviations: GMV, gray matter volume; CS-OUD, individuals who smoked cigarettes daily and had opioid use disorder; CS, individuals who smoked cigarettes daily and did not have opioid use disorder.
Table 2.
Brain regions showing significant main effect of group.
| Region | Cluster size (mm³) | Z | MNI coordinates |
|---|---|---|---|
| CS-OUD > CS | |||
| Right cerebellum | 3205 | 3.86 | 26/–64/–37 |
| Precuneus | 10827 | 3.84 | 1/–74/33 |
| Cuneus | 3.48 | −1/–84/28 | |
| Left occipital cortex | 2170 | 3.45 | −23/–75/25 |
| Right occipital cortex | 862 | 2.96 | 20/–63/7 |
| CS > CS-OUD | |||
| Left middle temporal gyrus | 13513 | 5.08 | −68/–26/–21 |
| Left angular gyrus | 3.98 | −66/–53/13 | |
| Left inferior temporal gyrus | 3.79 | −62/–19/–32 | |
| Left superior temporal gyrus | 3.55 | −55/1/–7 | |
| Left pre/postcentral gyrus | 46212 | 4.87 | −20/–27/74 |
| Right pre/postcentral gyrus | 4.48 | 42/–15/62 | |
| Right middle frontal gyrus | 3.94 | 29/19/57 | |
| Right superior frontal gyrus | 3.84 | 21/–8/74 | |
| Right superior parietal lobule | 3.74 | 35/–56/60 | |
| Right nucleus accumbens/caudate | 2133 | 4.15 | 19/17/1 |
| Right middle temporal gyrus | 13939 | 3.80 | 71/–18/–3 |
| Right superior temporal gyrus | 3.76 | 71/–35/4 | |
| Left nucleus accumbens/caudate | 1797 | 3.71 | −13/20/–10 |
Abbreviations: MNI, Montreal Neurological Institute; CS-OUD, individuals who smoked cigarettes daily and had opioid use disorder; CS, individuals who smoked cigarettes daily and did not have opioid use disorder.
Interaction between group and CPD
Two clusters of the medial orbitofrontal cortex showed significant interaction between group and CPD (cluster extent=2154 mm³, Z=3.41, x/y/z=–9/25/–19; cluster extent=711 mm³, Z=3.28, x/y/z=–10/50/–20; see Figure 2a). Simple slope analysis of the interaction within the significant clusters showed that CPD was negatively associated with medial orbitofrontal GMV only in the CS-OUD group (cluster extent=1862 mm³, Z=3.07, x/y/z=–8/25/–20; cluster extent=609 mm³, Z=3.31, x/y/z=–11/49/–19; see Figure 2b).
Figure 2.
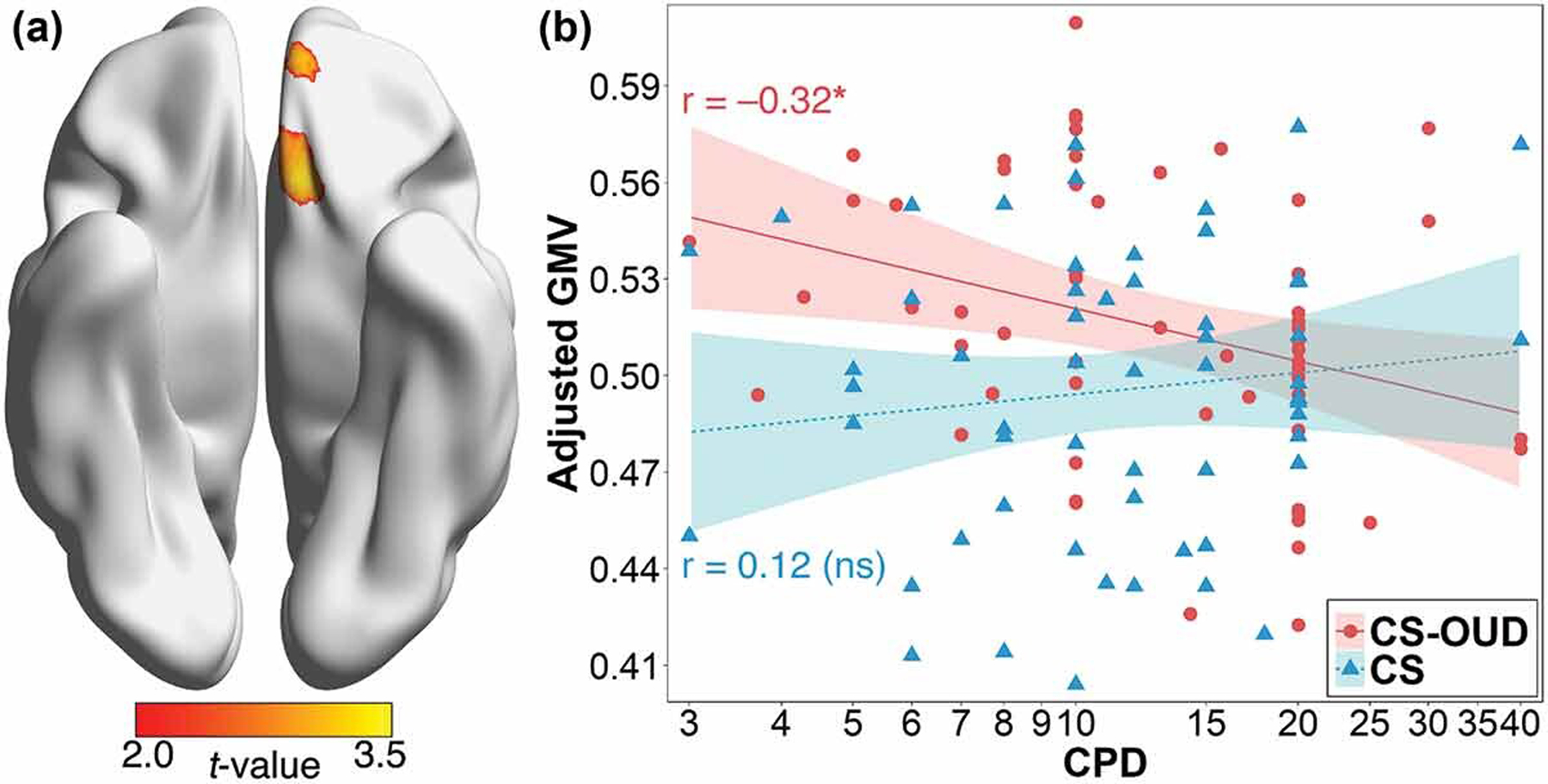
(a) Significant group-by-CPD interaction at the medial orbitofrontal cortex. (b) Negative association between CPD and covariate-adjusted medial orbitofrontal GMV (x/y/z=–9/25/–19) in the CS-OUD group, but not in the CS group. Shaded areas indicate 95% confidence interval. Abbreviations: CPD, number of cigarettes smoked per day; GMV, gray matter volume; CS-OUD, individuals who smoked cigarettes daily and had opioid use disorder; CS, individuals who smoked cigarettes daily and did not have opioid use disorder. *: p < .05.
Confirmatory analyses accounting for other substance use
One CS participant tested positive for opioids, but the study findings held after excluding this participant. Excluding individuals with AUD and those whose UDS was positive for cocaine did not change the results. However, after excluding those who had a positive UDS result for cannabis, the main effect of group was no longer significant for the GMV of the right occipital, left striatal, and right striatal regions (F(1,57)=2.54, 2.31, & 3.29, p=0.12, 0.13, & 0.075). This could be due to the reduced statistical power given a total of 45 cannabis users excluded (see Table 1) or potential interaction between group and cannabis use (see further analysis below).
Including the main effect of the use of other substances and their interaction with group in the ANCOVA models did not change the results. We found a significant interaction between group and AUD on precuneus GMV (F(1,99)=4.41, p=0.04), such that the difference between CS-OUD and CS participants was more pronounced in those with AUD (0.54 ± 0.02 vs. 0.44 ± 0.03, p<0.001) compared to those without (0.51 ± 0.02 vs. 0.47 ± 0.02, p=0.004). We also found a significant interaction between group and cannabis use in both left and right striatal GMV (F(1,100)=7.68 & 8.61, p=0.007 & 0.004). Specifically, the difference between CS-OUD and CS participants in striatal GMV was significant only among those who used cannabis (left, 0.24 ± 0.01 vs. 0.27 ± 0.01, p<0.001; right, 0.43 ± 0.02 vs. 0.49 ± 0.01, p<0.001), but not those who did not use cannabis (left, 0.25 ± 0.01 vs. 0.26 ± 0.01, p=0.14; right, 0.45 ± 0.01 vs. 0.46 ± 0.01, p=0.13). There was no other significant effect that involved AUD, cocaine, or cannabis use (ps>0.059).
DISCUSSION
The high comorbidity between smoking and OUD (2) makes dissociating their individual brain effects both challenging and important for understanding the nature of both disorders and improving treatment outcomes. Compared to controls with similar smoking severity, OUD patients had lower GMV in the prefrontal, sensorimotor and temporal cortices as well as the bilateral striatum. The results are largely consistent with those in previous reports (8). Furthermore, our data suggested interactive effects of smoking severity and opioid use, such that in OUD patients who also smoked cigarettes daily, higher smoking severity was associated with lower medial orbitofrontal GMV. Findings remained significant when controlling for concurrent use of substances other than opioids and tobacco.
Additionally, we found that alcohol use amplified the difference in precuneus GMV that was greater in the CS-OUD than CS participants. We also found that the effect of OUD status on striatal GMV was limited to individuals with concurrent cannabis use. Prior meta-analysis of existing sMRI studies showed that among common substances of abuse, alcohol had the strongest effect on brain structure (26). Specifically, individuals with AUD have lower GMV or cortical thickness compared to healthy controls across the bilateral frontal, parietal, and temporal cortices and the cortical midline structures (26). In addition, studies on cannabis use have shown structural abnormalities in the hippocampus, while its effect on other brain areas remains inconclusive (27). Our results complement the previous findings by showing that concurrent AUD and cannabis use may exacerbate the impact of OUD on brain regional GMV after controlling for smoking severity. Nevertheless, due to the small number of multi-substance users (see Table 1), these results should be considered preliminary.
The prefrontal cortex is involved in a myriad of higher-order cognitive processes, and disruption of normal prefrontal functioning in individuals with substance use disorders is associated with impairments in executive functioning, decision making, and incentive salience attribution (28). GMV, as measured by sMRI, is a robust marker of brain structural integrity and has been linked to neurocognitive functioning and behavioral outcomes (29). Meta-analysis of healthy adults showed that prefrontal GMV is positively associated with executive functioning (30). Among OUD patients, lower prefrontal GMV was linked to weaker impulse control (11). Here, we found lower dorsolateral prefrontal GMV in CS-OUD than CS individuals with comparable smoking severity, suggesting an independent OUD contribution to prefrontal structural abnormality.
CS-OUD individuals had lower GMV than CS individuals in the pre/postcentral gyrus and the lateral temporal lobe, regions that subserve sensorimotor functioning and complex audiovisual perception, respectively. We also found greater GMV in the occipital cortex in CS-OUD vs. CS individuals. While sensory and motor processes are not typically considered as key neuropsychological domains impacted by addiction, mounting evidence points to sensory and motor dysfunctions in patients with substance use disorders (31). In patients with OUD, opioid-related stimuli possess greater sensory salience than the neutral stimuli (32) and activate the bilateral middle/inferior temporal gyrus even after prolonged opioid abstinence (33). In addition, one study found impaired motor skills in a group of OUD patients receiving either partial agonist (buprenorphine) or antagonist (naltrexone) treatment (34).
The ventral and dorsal striatum are densely connected with the prefrontal cortex and play a central role in processing both natural and drug rewards (35). Attenuated frontal-striatal connectivity is observed in individuals with substance use disorders, including cocaine (36) and opioids (10). Prior literature has been inconsistent on the striatal structural alterations in OUD. Lower striatal GMV in OUD patients compared to controls was documented in some studies (37,38) but not in others (10). One possible cause for the inconsistency may lie in the differences in methodological approaches to handling potentially confounding variables such as comorbid polysubstance use disorder. Here, we showed lower GMV in the nucleus accumbens and caudate in CS-OUD compared to CS individuals who had comparable smoking severity, use of cocaine and cannabis, and AUD diagnosis. Low striatal GMV may be associated with dopaminergic dysfunction and contribute to anhedonia, dysphoria, and impaired impulse control in OUD (39).
The medial orbitofrontal cortex is a crucial node in the reward circuitry that, along with the striatum, shows heightened neural response to opioid-related stimuli in patients with OUD (40). Low orbitofrontal GMV has been previously reported in patients with substance use disorders and has been linked to poor decision-making (41). Specifically, meta-analytic evidence shows lower medial orbitofrontal GMV in people who smoke compared to those who do not smoke (42). Here, we found a negative correlation between smoking severity and medial orbitofrontal GMV only in individuals with OUD but not in those without OUD, suggesting that comorbid OUD may exacerbate smoking-related orbitofrontal gray matter abnormality.
Opioids and tobacco may elicit their effects on brain structure by altering cerebral blood flow (7). Chronic hypoperfusion induces reactive astrogliosis and neuronal death (43) and, consequently, leads to brain atrophy. Heroin administration results in frontal cortex hypoperfusion, which in turn is correlated with low frontal GMV (44,45). The detrimental effect of tobacco on brain perfusion is well-established: chronic cigarette smoking enhances arteriosclerosis, decreases global cerebral blood flow and, consequently, increases risk for stroke (46). Further examination of regional cerebral blood flow showed hypoperfusion in the orbitofrontal cortex and bilateral temporal cortex in people who smoked cigarettes (47). Future studies using multimodal imaging will be able to elucidate the association between perfusion and GMV in OUD patients who also smoke cigarettes.
The study is a secondary analysis of two existing datasets collected for different purposes, and therefore it has some inherent limitations. First, lack of neuropsychological assessments in the underlying studies limited our ability to interpret the behavioral consequences of GMV alterations. Specifically, nicotine has complex effects on cognitive functioning, ranging from protective (48) to deleterious (49). More comprehensive neuropsychological and clinical assessments would provide better insights into the interactive effects of polysubstance use on cognition. In addition, data regarding the amount and severity of substance use other than CPD were not collected, which limited the inference about dose-response effects. Second, the sample size of the current study was limited, and the CS-OUD and CS cohorts differed in racial composition. Although we included race as a covariate in the analysis, our findings need confirmation in a large and representative sample to rule out the potential confounding effects of race and other sociodemographic variables. Third, the cross-sectional study design did not allow us to distinguish between causality and correlation. It is possible that GMV alterations were a preexisting factor for developing substance use disorder (50). However, evidence of structural recovery following abstinence (51) and lower regional brain volumes following prenatal opioid exposure (52) suggests that OUD could be the cause of brain structural changes. Future longitudinal research is needed to determine whether brain structural alterations precede or follow OUD. Fourth, we found an unexpected higher occipital GMV in participants who smoked cigarettes and had OUD compared to those who smoked but did not have OUD. Interestingly, prior studies found that higher occipital GMV was associated with tobacco smoking (42,53). Further research is needed to disentangle the effects of opioid use and tobacco smoking on occipital morphology and visual perception. Lastly, alternative measures of smoking severity, such as the Fagerstrom Test for Nicotine Dependence (54) and smoking-related biomarkers [e.g., urine cotinine (55)], may be more informative than CPD. In addition, although all participants smoked >2 cigarettes daily and were likely to have met the diagnostic criteria for tobacco use disorder (TUD) (56), such diagnosis was not assessed in the current study. Future research is needed to confirm our findings in individuals with dual diagnosis of TUD and OUD compared to those with TUD only.
In conclusion, the current study shows that independent of smoking severity, OUD patients have lower GMV in the pre/postcentral gyrus, dorsolateral prefrontal cortex, lateral temporal cortex and striatum compared to non-OUD individuals. We also show that medial orbitofrontal GMV is inversely correlated with smoking severity only in those with OUD. These findings shed the light on the individual and combined effects of opioid and tobacco use on brain structure. Despite the limitations, such hypothesis-generating studies are a necessary prerequisite of future prospective longitudinal research that would be significantly more lengthy and costly. Our study provides such a starting point for an investigation of the mechanisms of concurrent smoking and OUD and a hope of alleviating this damaging comorbidity that has so far eluded public health interventions targeting the smoking population in general.
Acknowledgments
FUNDING
This work was supported by the Commonwealth of Pennsylvania CURE grant SAP#4100055577 (Childress) and the following National Institutes of Health grants: DA051709 (Shi), DA028874 (Childress), AA026892 (Wiers) and DA036028 (Langleben). The funding sources had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Disclosure: The authors report no relevant disclosures
DISCLOSURE STATEMENT
The authors report there are no competing interests to declare.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- (1).Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, Walsh SL. Opioid use disorder. Nat Rev Dis Primers 2020;6:3. [DOI] [PubMed] [Google Scholar]
- (2).John WS, Zhu H, Mannelli P, Subramaniam GA, Schwartz RP, McNeely J, Wu L-T. Prevalence and patterns of opioid misuse and opioid use disorder among primary care patients who use tobacco. Drug Alcohol Depend 2019;194:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Rajabi A, Dehghani M, Shojaei A, Farjam M, Motevalian SA. Association between tobacco smoking and opioid use: a meta-analysis. Addict Behav 2019;92:225–35. [DOI] [PubMed] [Google Scholar]
- (4).Parker MA, Weinberger AH, Villanti AC. Quit ratios for cigarette smoking among individuals with opioid misuse and opioid use disorder in the United States. Drug Alcohol Depend 2020;214:108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Solis E Jr., Cameron-Burr KT, Shaham Y, Kiyatkin EA Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response. eNeuro 2017;4. [DOI] [PMC free article] [PubMed]
- (6).Murray DE, Durazzo TC, Schmidt TP, Murray TA, Abe C, Guydish J, Meyerhoff DJ. Regional cerebral blood flow in opiate dependence relates to substance use and neuropsychological performance. Addict Biol 2018;23:781–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zonneveld HI, Loehrer EA, Hofman A, Niessen WJ, van der Lugt A, Krestin GP, Ikram MA, Vernooij MW. The bidirectional association between reduced cerebral blood flow and brain atrophy in the general population. J Cereb Blood Flow Metab 2015;35:1882–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Wollman SC, Alhassoon OM, Hall MG, Stern MJ, Connors EJ, Kimmel CL, Allen KE, Stephan RA, Radua J. Gray matter abnormalities in opioid-dependent patients: a neuroimaging meta-analysis. Am J Drug Alcohol Abuse 2017;43:505–17. [DOI] [PubMed] [Google Scholar]
- (9).Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, Zhang Y, Wang W, Wang Y, Li Q, et al. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett 2009;460:72–77. [DOI] [PubMed] [Google Scholar]
- (10).Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 2010;133:2098–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Qiu YW, Jiang GH, Su HH, Lv XF, Tian JZ, Li LM, Zhuo F-Z. The impulsivity behavior is correlated with prefrontal cortex gray matter volume reduction in heroin-dependent individuals. Neurosci Lett 2013;538:43–48. [DOI] [PubMed] [Google Scholar]
- (12).Bach P, Frischknecht U, Reinhard I, Bekier N, Demirakca T, Ende G, Vollstädt-Klein S, Kiefer F, Hermann D. Impaired working memory performance in opioid-dependent patients is related to reduced insula gray matter volume: a voxel-based morphometric study. Eur Arch Psychiatry Clin Neurosci 2021;271:813–22. [DOI] [PubMed] [Google Scholar]
- (13).Shi Z, Langleben DD, O’Brien CP, Childress AR, Wiers CE. Multivariate pattern analysis links drug use severity to distributed cortical hypoactivity during emotional inhibitory control in opioid use disorder. Neuroimage Clin 2021;32:102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Arias F, Arnsten JH, Cunningham CO, Coulehan K, Batchelder A, Brisbane M, Segal K, Rivera-Mindt M. Neurocognitive, psychiatric, and substance use characteristics in opioid dependent adults. Addict Behav 2016;60:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gray JC, Thompson M, Bachman C, Owens MM, Murphy M, Palmer R. Associations of cigarette smoking with gray and white matter in the UK Biobank. Neuropsychopharmacology 2020;45:1215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 2004;55:77–84. [DOI] [PubMed] [Google Scholar]
- (17).Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, Rao H, Childress AR. The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One 2014;9(8):e104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Yu R, Zhao L, Lu L. Regional grey and white matter changes in heavy male smokers. PLoS One 2011;6:e27440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Elbejjani M, Auer R, Jacobs DR Jr., Haight T, Davatzikos C, Goff DC Jr., Bryan RN, Launer LJ. Cigarette smoking and gray matter brain volumes in middle age adults: the CARDIA Brain MRI sub-study. Transl Psychiatry 2019;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wang AL, Shi Z, Elman I, Langleben DD. Reduced cigarette smoking during injectable extended-release naltrexone treatment for opioid use disorder. Am J Drug Alcohol Abuse 2020;46:472–77. [DOI] [PubMed] [Google Scholar]
- (21).Wang AL, Shi Z, Fairchild VP, Aronowitz CA, Langleben DD. Emotional salience of the image component facilitates recall of the text of cigarette warning labels. Eur J Public Health 2019;29:153–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatr 1998;59:22–33; quiz 4–57. [PubMed] [Google Scholar]
- (23).Kosten TA, Rounsaville BJ. Sensitivity of psychiatric diagnosis based on the best estimate procedure. Am J Psychiatry 1992;149:1225–27. [DOI] [PubMed] [Google Scholar]
- (24).Harris KJ, Golbeck AL, Cronk NJ, Catley D, Conway K, Williams KB. Timeline follow-back versus global self-reports of tobacco smoking: a comparison of findings with nondaily smokers. Psychol Addict Behav 2009;23:368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ashburner J A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- (26).Mackey S, Allgaier N, Chaarani B, Spechler P, Orr C, Bunn J, Allen NB, Alia-Klein N, Batalla A, Blaine S, et al. Mega-analysis of gray matter volume in substance dependence: general and substance-specific regional effects. Am J Psychiatry 2019;176:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Nader DA, Sanchez ZM. Effects of regular cannabis use on neurocognition, brain structure, and function: a systematic review of findings in adults. Am J Drug Alcohol Abuse 2018;44:4–18. [DOI] [PubMed] [Google Scholar]
- (28).Wollman SC, Hauson AO, Hall MG, Connors EJ, Allen KE, Stern MJ, Stephan RA, Kimmel CL, Sarkissians S, Barlet BD, et al. Neuropsychological functioning in opioid use disorder: a research synthesis and meta-analysis. Am J Drug Alcohol Abuse 2019;45:11–25. [DOI] [PubMed] [Google Scholar]
- (29).Whitwell JL. Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J Neurosci 2009;29:9661–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev 2014;42:180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Palffy Z, Farkas K, Csukly G, Keri S, Polner B. Cross-modal auditory priors drive the perception of bistable visual stimuli with reliable differences between individuals. Sci Rep 2021;11:16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lubman DI, Peters LA, Mogg K, Bradley BP, Deakin JF. Attentional bias for drug cues in opiate dependence. Psychol Med 2000;30:169–75. [DOI] [PubMed] [Google Scholar]
- (33).Li Q, Wang Y, Zhang Y, Li W, Zhu J, Zheng Y, Chen J, Zhao L, Zhou Z, Liu Y, et al. Assessing cue-induced brain response as a function of abstinence duration in heroin-dependent individuals: an event-related fMRI study. PLoS One 2013;8:e62911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Scott TM, Arnsten J, Olsen JP, Arias F, Cunningham CO, Rivera Mindt M. Neurocognitive, psychiatric, and substance use characteristics in a diverse sample of persons with OUD who are starting methadone or buprenorphine/naloxone in opioid treatment programs. Addict Sci Clin Pract 2021;16:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 2005;45:647–50. [DOI] [PubMed] [Google Scholar]
- (36).Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry 2015;72:584–92. [DOI] [PubMed] [Google Scholar]
- (37).Tolomeo S, Gray S, Matthews K, Steele JD, Baldacchino A. Multifaceted impairments in impulsivity and brain structural abnormalities in opioid dependence and abstinence. Psychol Med 2016;46:2841–53. [DOI] [PubMed] [Google Scholar]
- (38).Seifert CL, Magon S, Sprenger T, Lang UE, Huber CG, Denier N, Vogel M, Schmidt A, Radue E-W, Borgwardt S, et al. Reduced volume of the nucleus accumbens in heroin addiction. Eur Arch Psychiatry Clin Neurosci 2015;265:637–45. [DOI] [PubMed] [Google Scholar]
- (39).Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell 2015;162:712–25. [DOI] [PubMed] [Google Scholar]
- (40).Shi Z, Jagannathan K, Padley JH, Wang AL, Fairchild VP, O’Brien CP, Childress AR, Langleben DD. The role of withdrawal in mesocorticolimbic drug cue reactivity in opioid use disorder. Addict Biol 2021;26:e12977. [DOI] [PubMed] [Google Scholar]
- (41).Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry 2009;65:160–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Yang Z, Zhang Y, Cheng J, Zheng R. Meta-analysis of brain gray matter changes in chronic smokers. Eur J Radiol 2020;132:109300. [DOI] [PubMed] [Google Scholar]
- (43).Cechetti F, Pagnussat AS, Worm PV, Elsner VR, Ben J, da Costa MS, Mestriner R, Weis SN, Netto CA. Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull 2012;87:109–16. [DOI] [PubMed] [Google Scholar]
- (44).Denier N, Gerber H, Vogel M, Klarhöfer M, Riecher-Rossler A, Wiesbeck GA, Lang UE, Borgwardt S, Walter M. Reduction in cerebral perfusion after heroin administration: a resting state arterial spin labeling study. PLoS One 2013;8:e71461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Denier N, Schmidt A, Gerber H, Schmid O, Riecher-Rössler A, Wiesbeck GA, Huber CG, Lang UE, Radue E-W, Walter M, et al. Association of frontal gray matter volume and cerebral perfusion in heroin addiction: a multimodal neuroimaging study. Front Psychiatry 2013;4:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Rogers RL, Meyer JS, Shaw TG, Mortel KF, Hardenberg JP, Zaid RR. Cigarette smoking decreases cerebral blood flow suggesting increased risk for stroke. JAMA 1983;250:2796–800. [PubMed] [Google Scholar]
- (47).Durazzo TC, Meyerhoff DJ, Murray DE. Comparison of regional brain perfusion levels in chronically smoking and non-smoking adults. Int J Env Res Public Health 2015;12:8198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology 2010;210:453–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Chamberlain SR, Odlaug BL, Schreiber LR, Grant JE. Association between tobacco smoking and cognitive functioning in young adults. Am J Addict. 2012;21 Suppl 1:S14–9. [DOI] [PubMed] [Google Scholar]
- (50).Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol Rev 2010;20:398–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Wang X, Li B, Zhou X, Liao Y, Tang J, Liu T, Hu D, Hao W. Changes in brain gray matter in abstinent heroin addicts. Drug Alcohol Depend 2012;126:304–08. [DOI] [PubMed] [Google Scholar]
- (52).Hartwell ML, Croff JM, Morris AS, Breslin FJ, Dunn K. Association of prenatal opioid exposure with precentral gyrus volume in children. JAMA Pediatr 2020;174:893–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Ye Y, Zhang J, Huang B, Cai X, Wang P, Zeng P, Wu S, Ma J, Huang H, Liu H, et al. Characterizing the structural pattern of heavy smokers using multivoxel pattern analysis. Front Psychiatry 2020;11:607003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991;86:1119–27. [DOI] [PubMed] [Google Scholar]
- (55).Chang CM, Edwards SH, Arab A, Del Valle-Pinero AY Yang L, Hatsukami DK. Biomarkers of tobacco exposure: summary of an FDA-sponsored public workshop. Cancer Epidemiol Biomarkers Prev 2017;26:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Oliver JA, Foulds J. Association between cigarette smoking frequency and tobacco use disorder in US adults. Am J Prev Med 2021;60:726–28. [DOI] [PMC free article] [PubMed] [Google Scholar]


