Abstract
Older adults typically report increased difficulty with language production, while its neural bases are less clear. The current study investigated the neural bases of age-related differences in language production at the word level and the modulating effect of task difficulty, focusing on task-based functional connectivity. Using an English phonological Go/No-Go picture naming task, task difficulty was manipulated by varying the proportion of naming trials (Go trials) and inhibition trials (No-Go trials) across runs. Behaviorally, compared to younger adults, older adults performed worse, and showed larger effects of task difficulty. Neurally, older adults had lower within language network connectivity compared to younger adults. Moreover, older adults’ language network became less segregated as task difficulty increased. These results are consistent with the Compensation-Related Utilization of Neural Circuits Hypothesis, suggesting that the brain becomes less specified and efficient with increased task difficulty, and that these effects are stronger among older adults (i.e., more dedifferentiated).
Keywords: Language production, Aging, Task difficulty, Functional connectivity
1. Introduction
Older adults often have increased difficulty with spoken language production (Burke & Shafto, 2008; Shafto et al., 2007; Zhang et al., 2019). For instance, they show increased word retrieval failures such as more frequent tip-of-the-tongue phenomena (e.g., Burke et al., 1991; Shafto et al., 2007), have more slips of the tongue and misspellings (MacKay & James, 2004; Taylor & Burke, 2000), and have more filled and unfilled pauses and omissions (Horton et al., 2010; Bortfeld et al., 2001; MacKay & James, 2004). Although these age-related differences in behavior have been studied widely, the neural bases of these age-related differences in language production are less clear. One factor that can contribute to age-related differences in cognition is task difficulty (Reuter-Lorenz & Cappell, 2008; Zhang et al., 2019), however this has been less frequently investigated in the realm of language. The current study used functional connectivity to investigate the effect of task difficulty on age-related behavioral and neural differences in spoken language production at the word level.
The neural bases of language has been well characterized through neuroimaging studies of younger adults and studies of clinical populations with language impairments (for a review, see Price, 2010). Language production, in particular, is characterized by a left-lateralized frontal-temporal brain network (Hickok & Poeppel, 2007; Indefrey & Levelt, 2004; Price, 2010). However, older adults often show broader patterns of activation, particularly in prefrontal regions, with decreased lateralization (Destrieux et al., 2012; Diaz et al., 2014; Diaz et al., 2019; Diaz et al., 2016; Nagels et al., 2012; Rizio et al., 2017; Wierenga et al., 2008; Zhang et al., 2019). For instance, older adults often show increased involvement of domain-general frontal-parietal regions that belong to the multiple-demand network (Grady et al., 2010; Hoffman & Morcom, 2018). Although these age-related neural differences have been consistently reported, it remains unclear whether such increases are compensatory (Cabeza, 2002; Davis et al., 2008; Reuter-Lorenz & Cappell, 2008) or whether they reflect neural decline, such as dedifferentiation (Ghisletta & Lindenberger, 2003; Li et al., 2001).
Compensatory accounts generally argue that age-related increases in activation, via the recruitment of additional regions, serve to compensate for neural declines elsewhere. For example, some evidence suggests that increased activation in right inferior frontal gyrus, which has been associated with executive function, helps older adults maintain performance during language processing (e.g., Baciu et al., 2016; Davis et al., 2014; Persson et al., 2004; Wierenga et al., 2008). Dedifferentiation accounts, on the other hand, argue that the aging brain becomes less efficient and organized, and age-related increases in activation reflect lower levels of inhibition and contribute to a noisier signal overall. Research in support of this perspective found no relationship between increased right hemisphere activation and performance (Diaz et al., 2014; Meinzer et al., 2009; Meinzer, Seeds, et al., 2012). However, a third factor may modulate these brain-behavior relationships: task difficulty. The CRUNCH model (Compensation-Related Utilization of Neural Circuits Hypothesis, Reuter-Lorenz & Cappell, 2008) proposes that as task demands initially increase, the recruitment of additional brain regions in older adults can help individuals maintain behavioral performance, and serve a compensatory function. However, as task demands begin to exceed cognitive resources, brain activation and behavioral performance may no longer correlate, indicating dedifferentiation. Despite evidence from other cognitive domains, only a handful of studies have tested the effect of task demands on neural activity in the domain of language processing (Haitas et al., 2021; Meinzer, Flaisch, et al., 2012; Persson et al., 2007; Zhang et al., 2019). These studies have reported age-related differences in neural activation related to task difficulty. For instance, the default mode network (DMN) activity reflects a number of off-task behaviors such as mind wandering, interoception, and internal dialogue, and often shows deactivation during task states (Raichle, 2015). Looking at DMN activity, Persson et al. (2007) found that older adults had weaker deactivation as a function of task demands compared to younger adults. This suggests that their deactivation response to task difficulty was less responsive than younger adults. Similarly, in one of our previous studies (Zhang et al., 2019), we found that older adults had disproportionately slower behavioral responses and were less neurally responsive to task difficulty manipulations during word production, supporting the CRUNCH model, which posits dedifferentiation or brain-behavior uncoupling at higher levels of task difficulty.
In addition to task-based functional activation, age-related differences in neural processing have also been found in the patterns of correlated brain activity (i.e., functional connectivity). When brain regions are concomitantly active, they are said to form functional networks. Most studies have focused on functional connectivity during rest (i.e., when there is no explicit task, Betzel et al., 2014; Cao et al., 2014; Chan et al., 2014; Geerligs et al., 2015; Onoda et al., 2012; Siman-Tov et al., 2017; Song et al., 2014; Tomasi & Volkow, 2012; Zhang, Bai, et al., 2021), and some studies have investigated connectivity during tasks (Andrews-Hanna et al., 2007; Geerligs et al., 2014; Steffener et al., 2012; Varangis, Razlighi, et al., 2019; Zhang, Gertel, et al., 2021). In general, these studies found that older adults have lower connectivity among brain regions within networks, and less segregated networks. Moreover, these age-related differences in network characteristics have been associated with worse behavioral performance across several different cognitive domains (King et al., 2018; Onoda et al., 2012; Sala-Llonch et al., 2015; Varangis, Habeck, et al., 2019; Wang et al., 2010), including language (Ferré et al., 2019; Gertel, Zhang, et al., 2020; Martin, Saur, et al., 2022; Martin, Williams, et al., 2022; Pistono et al., 2020; Zhang, Bai, et al., 2021). Although these studies looking at whole brain connectivity by and large support the dedifferentiation account, other functional connectivity studies that have examined more focal relationships sometimes find stronger regional functional connectivity among older adults that is associated with enhanced cognition, supporting a compensatory account (Gertel, Zhang, et al., 2020; Wang et al., 2010). Thus, there may be a distinction in the effects of age on functional connectivity when comparing whole brain patterns of connectivity and more regionally specific patterns. Moreover, most functional connectivity studies on aging have incorporated ‘resting state’ data. On the one hand, this is ideal for studies of aging because older adults can be more sensitive to task demands and ‘resting state’ data does not involve any task. However, there may be age-related differences in patterns of functional connectivity while relaxing (i.e., ‘rest’) and while performing a task (e.g., Zhang, Gertel, et al., 2021). Prior research suggests that age-related differences may be magnified by increases in task difficulty (i.e., CRUNCH). Yet, few studies have examined this dimension.
To reconcile compensation and dedifferentiation accounts of aging in the context of task difficulty, the current study investigated how task difficulty affects age-related differences in functional connectivity during word production. One study examined task difficulty through an implicit manipulation of lexical frequency (i.e., comparing picture naming of low frequency and high frequency items; Ferre et al., 2020). They found little evidence of age-related differences in the effects of task difficulty on functional connectivity (Ferré et al., 2020). Although behaviorally, older adults are generally sensitive to intrinsic lexical characteristics such as word frequency (Gertel, Karimi, et al., 2020; Gollan et al., 2008; LaGrone & Spieler, 2006; Newman & German, 2005), implicit manipulations of task difficulty, such as by manipulating item frequency, might be too subtle to elicit age differences in functional connectivity. More commonly, research that has focused on age-related task difficulty effects on functional activation incorporated explicit manipulations of task difficulty (Marsolais et al., 2014; Meinzer, Flaisch, et al., 2012; Persson et al., 2007; Reuter-Lorenz & Cappell, 2008). For instance, Persson et al. (2007) investigated age-related differences in cognitive control by comparing two levels of selection demands in a verb generation task compared to a reading baseline task. Indeed, cognitive control has been shown to contribute to language production (Novick et al., 2010; Nozari & Novick, 2017), and the extent to which it affects language production may be modulated by age (Britt et al., 2016; Higby et al., 2019). Therefore, the current functional connectivity study also employed an explicit manipulation of task difficulty. Specifically, we investigated this issue using a well-established paradigm that manipulated difficulty by varying cognitive control demands (i.e., Go/No-Go picture naming task). Given that previous fMRI studies have found that older adults showed reduced involvement of language-related regions, but increased engagement of domain-general regions, we focused on two, independently defined, networks: the language network and the multiple-demand network (MD network, Fedorenko et al., 2013; Fedorenko et al., 2010). We selected the networks that were defined by Fedorenko and colleagues because the regions were identified functionally, were replicable within subjects, had a clear correspondence across subjects, and manifested key signatures of different processes1. In this paper, we focused on age-related differences in functional connectivity, as behavioral and task-based fMRI results were previously published (Zhang et al., 2019).
Our previous published results showed that older adults performed worse and showed larger increases in reaction times to increasing task difficulty on a phonological Go/No-Go picture naming task compared to younger adults. In terms of functional connectivity, based on the CRUNCH model, we predicted that there would be a main effect of age in which older adults have less efficient networks compared to younger adults2. Less efficient networks would be reflected by weaker within network connectivity, or stronger connectivity between the two networks (i.e., less segregated networks), in combination with worse behavioral performance. Moreover, we hypothesized that functional connectivity efficiency would decrease as task difficulty increased, and that these effects would be larger for older adults. This reduced efficiency would be evidenced by lower within network connectivity, or less network segregation in both networks, along with worse behavioral performance as task difficulty increased. If, however, the higher involvement of the MD network (as reflected as higher between network connectivity, or less network segregation) is found to be associated with comparable or even better performance in older adults, this would be evidence of compensation3.
2. Method
2.1. Participants
Twenty younger adults (ages: 18–34, mean age = 22.65 years, 10 female) and 20 older adults (ages: 61–79, mean age = 67.45 years, 14 female) participated in the experiment. All participants were community-dwelling, right-handed, native American-English speakers who were not fluent in a second language. All participants had normal or corrected-to-normal vision, and reported no history of neurological, psychological, or major medical conditions (Christensen et al., 1992).
Participants were also screened for depression (Guerin et al., 2018; Sheikh & Yesavage, 1986), mild cognitive impairment, and dementia (MMSE, Folstein et al., 1975). Prior to the MRI session, each participant completed a battery of psychometric and neuropsychological tests to assess basic cognitive functions such as speed, executive function, memory, and language, details of which were previously reported in Zhang et al. (2019). To briefly summarize, participant groups did not differ in years of education, vocabulary, verbal fluency, or memory. Older adults were slower than younger adults in processing speed and executive function measures. All participants gave written, informed consent, and all procedures were approved by the Institutional Review Board at The Pennsylvania State University where the experiment was conducted.
2.2. Stimuli and procedure
Participants performed a phonological Go/No-Go picture naming task in the scanner, as previously reported in Zhang et al. (2019). Photographs (n=330, 110 per condition) were presented one at a time and participants were instructed to overtly name the photograph as quickly and accurately as possible. Task demands were manipulated via the proportion of trials that needed to be named or inhibited, constituting three conditions: All Go, Go Bias, No-Go Bias (Figure 1, reproduced with permission from Zhang et al., 2018). In the All Go condition, participants were instructed to name all of the photographs. In the Go Bias condition (75% Go trials, 25% No-Go trials), participants were required to name the photograph if the name of the photograph started with a consonant (i.e., Go trials, e.g., nose) and to withhold their response if the name started with a vowel (i.e., No-Go trials, e.g., apple). In contrast, in the No-Go Bias condition (25% Go trials, 75% No-Go trials), participants were instructed to name the photograph if the name started with a vowel (i.e., Go trials, e.g., orange) and to withhold their response if the name started with a consonant (i.e., No-Go trials, e.g., chair).
Figure 1.
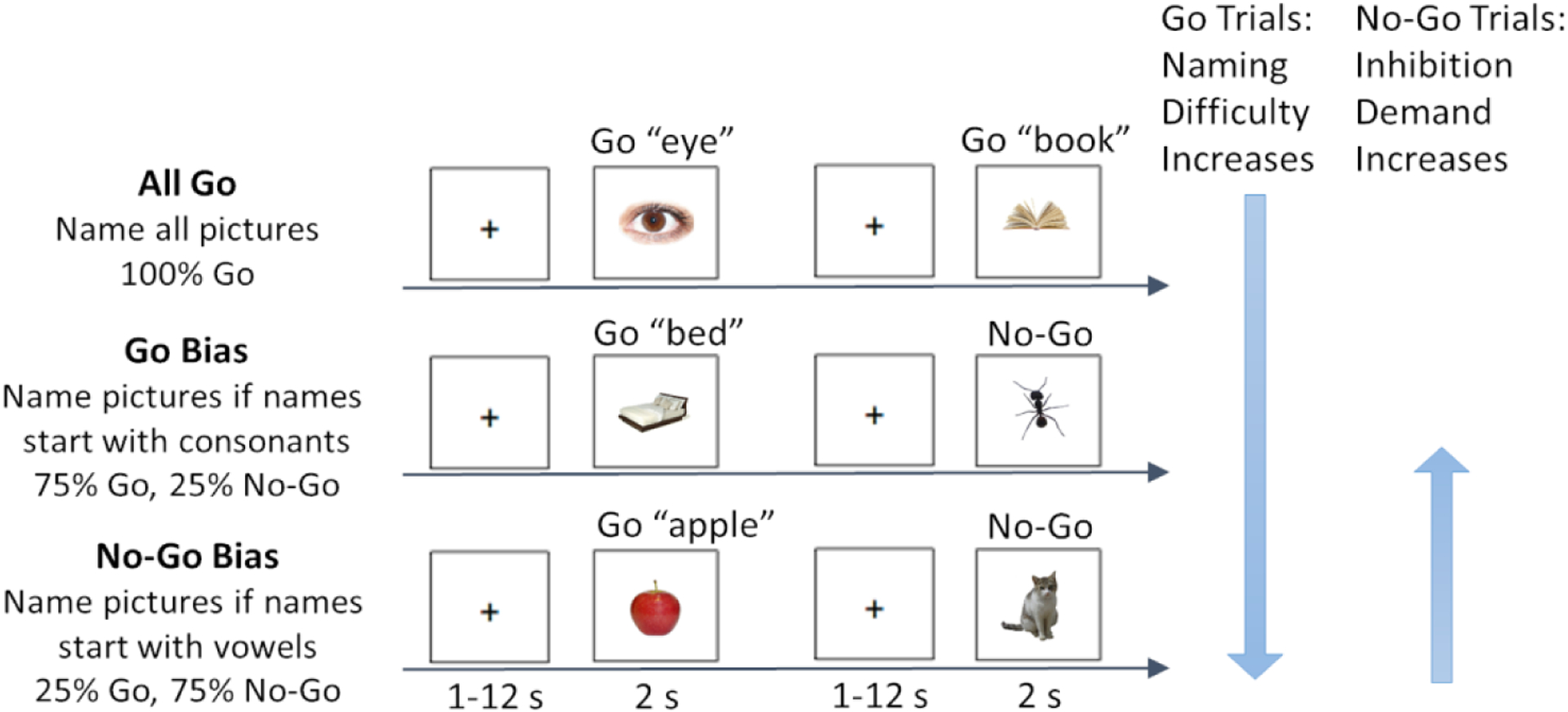
Task design (reprinted by permission from Springer Nature: Cognitive, Affective, & Behavioral Neuroscience, Zhang et al., 2018). An overview of the phonological Go/No-Go picture naming task is provided. Examples of Go trials and No-Go trials for each of the three conditions: All Go, Go Bias, and No-Go Bias. Correct names to the two No-Go trials are “ant”, and “cat”, respectively. Naming (Go trials) difficulty increased from the All Go condition to the Go Bias condition to the No-Go Bias condition. Inhibition (No-Go trials) demand increased from the No-Go Bias condition to the Go Bias condition.
Prior to scanning, participants practiced overt picture naming while minimizing head movement in a mock scanner. In the scanner, participants always performed the All Go condition first, prior to being informed about the Go/No-Go manipulation to avoid naming biases in this first run. After the All Go condition, participants underwent a practice run and then completed the Go Bias and the No-Go Bias conditions, whose order was counterbalanced across participants. Photographs were not repeated across practice runs or conditions to minimize possible priming effects.
Photographs were taken from two normed databases (Brodeur et al., 2014; Moreno-Martínez & Montoro, 2012). The final set of stimuli for the MRI experiment included 330 colored photographs, 110 unique items per condition. For the Go Bias and No-Go Bias conditions, these trials were further divided into 82 trials (75%) of the biased trial type (e.g., Go trials in the Go Bias runs) and 28 trials (25%) of the non-biased trial type (e.g., No-Go trials in the Go Bias runs). Linguistic characteristics (word length, frequency, number of phonemes, number of syllables) obtained from the English Lexicon Project (ELP, Balota et al., 2007), as well as the semantic categories for all of the stimuli, were matched across the three conditions.
In each trial, one color photograph (396 pixels × 396 pixels) was presented on a white background and participants were instructed to respond with the target name or withhold their response based on the condition requirements. Participants were also asked to limit their answers to only one word. Photographs (duration = 2 s) were presented with a variable inter-stimulus interval (range = 1–12 s, mean = 3.40 s) that was determined using the optseq2 program, as jittered presentations have been shown to optimize the hemodynamic response (Dale, 1999) and prevent participants from anticipating the onset of events. Participants completed 6 runs (2 runs per condition) in the scanner. During the task, overt verbal responses were recorded and filtered using an MR-compatible fiber optic microphone system (Optoacoustics Ltd., Or-Yehuda, Israel). To verify participants’ identification and naming of the photographs, after the scan they were asked to name all of the photographs from the Go Bias and the No-Go Bias conditions.
2.3. Acquisition of MRI data
MRI scanning was completed on a 3T Siemens Prisma Fit MRI scanner with a 20-channel head coil. Sagittal T1-weighted localizer images were collected and used to define a volume for subsequent data collection, higher-order shimming, and alignment to the anterior commissure and posterior commissure (AC-PC). T1-weighted anatomical images were collected using a magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence (repetition time [TR] = 2300 ms; echo time [TE] = 2.28 ms; Inversion Time [TI] = 900 ms; flip angle = 8°; echo spacing = 7 ms; acceleration factor = 2; field of view [FOV] = 256 mm2; voxel size = 1 × 1 × 1 mm; 160 contiguous slices).
Functional images were collected using an echo-planar imaging (EPI) sequence (TR = 2500 ms; TE = 25 ms; flip angle = 90°; echo spacing = 0.49 ms; FOV = 240 mm2; voxel size = 3 × 3 × 3 mm; 41 contiguous axial slices, parallel to the AC–PC, interleaved acquisition, 122 volumes (305 s) per run, 6 runs). Two additional volumes were acquired and deleted at the beginning of each functional run to reach steady state equilibrium.
2.4. Data Analyses
In this study, responses were coded based on both the recordings from the scanner session and the post-scan naming task. Details regarding how word production responses were coded have been reported in Zhang et al. (2019). In brief, correct trials included both correctly responded Go trials and correctly inhibited No-Go trials. Response errors included the following: 1) incorrect responses (e.g., Go trials: a response that did not match the picture, or had an incorrect onset category; No-Go trials: no response combined with not knowing the picture in the post-scan naming task or a response that had an incorrect onset category); 2) commission errors (failures to inhibit a response during a No-Go trial; and 3) omission errors (no response to a Go trial). Reaction times (RTs) to Go trials were calculated using customized PRAAT scripts. Only trials with correct responses and reaction times within 2.5 SDs were included in further analyses. Reaction time and error rates were analyzed using mixed-effect regression modeling, including Task Condition (3 levels: All Go, Go Bias, and No-Go Bias, and Age Group (2 levels: Younger vs Older), and their interaction as independent variables. R was used for all statistical analyses (R version 4.1.3, RStudio Team, 2022). The following R packages were used in the analyses, readxl (Wickham & Bryan, 2019), tidyverse (Wickham et al., 2019), lme4 (Bates et al., 2014), lmerTest (Kuznetsova et al., 2017), rstatix (Kassambara, 2019), gridExtra (Auguie et al., 2017), ggplot2 (Wickham, 2016), and ggpubr (Kassambara, 2020).
For fMRI data, the fBIRN QA tool was used to assess data quality (Glover et al., 2012, https://www.nitrc.org/projects/bxh_xcede_tools/), measuring the number of potentially clipped voxels, mean signal fluctuation to noise ratio (SFNR), and per-slice variation. Additionally, the anatomical and functional images were visually inspected for artifacts and signal drop-out. Preprocessing and first-level analyses were conducted using the CONN functional connectivity toolbox version 18.a (Whitfield-Gabrieli & Nieto-Castanon, 2012). First, functional realignment and unwarping were done to estimate and correct for participant motion, followed by the slice-timing correction, which corrected for maturation of the BOLD signal over time (Huettel et al., 2004). Functional outliers were detected with an ART (Artifact Detection Tools) based identification method in which outliers were defined using a conservative threshold (i.e., data points more extreme than the 97th percentile based on a normative sample). During registration, functional images were aligned to anatomical images and both were normalized to Montreal Neurological Institute (MNI) space. Segmentation was done on all anatomical images then applied to functional images to segment images into white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF). A smoothing kernel (6 mm) was used to increase the signal to noise ratio, as well as to reduce spurious activations of single voxels. During denoising, the representative noise signal from WM (5 components) and CSF (5 components) was extracted, and any signal correlated with these components was removed from the BOLD signal4. Additionally, the task effects were also regressed out to remove any changes in average activation that covaried with the task conditions since we were primarily interested in functional connectivity. To eliminate frequencies of less interest, a high-pass filter of 0.009 Hz was used. The following quality assurance parameters were also included as second level covariates during data preprocessing: number of outlier and non-outlier scans (outlier threshold = 0.5 mm), max and mean motion, and max and mean global BOLD signal changes (outlier threshold = global-signal z-value of 3). The total average numbers of invalid scans were 16.5 (SD = 35.87, 2.3% of total scans, 715.5 average remaining scans) for Younger Adults and 13.3 (SD = 22.22, 1.8% of total scans, 718.7 average remaining scans) for Older Adults, p = 0.74, and removed from analysis. The mean amount of motion was 0.18 mm (SD = 0.07 mm) for Younger Adults and 0.22 mm (SD = 0.06 mm) for Older Adults, p = .13. The analyses removing variance associated with all of the variables described above occurred in a single linear regression step, and the residualized BOLD signal was used for further statistical analyses. Because older and younger adults often differ in variability, we compared the whole brain standard deviation images derived from the preprocessed data to look for potential group differences using FSL’s randomize function. The groups did not significantly differ in terms of the amount of variation in any of the 3 conditions (All Go, Go Bias, and No-Go Bias, ts < 0.998, ns). Quality Control – Functional Connectivity (QC-FC) plots indicated that the similarity to the associated null-hypothesis (NH) distribution was significantly improved after denoising (before denoising = 72.6%; after denoising, 91.9%, with 95.2% for younger and 87.5% for older adults; Aquino et al., 2020; Ciric et al., 2017).
To investigate the network connectivity in the language network and the Multiple-Demand (MD) network, we first located 8 sphere language ROIs and 20 sphere MD ROIs (4mm radius) based on results previously reported by others (Fedorenko et al., 2013; Fedorenko et al., 2010). The coordinate information can be found in Table 1. Then we calculated the weighted within network connectivity in the language network, and the MD network. Fisher-transformed Z values were used as connectivity values. For any two ROIs that had a positive correlation, their connection was included in the analysis5. Within network connectivity was calculated as the mean node-to-node correlation of all ROIs in that network (i.e., diagonal blocks). For network connectivity between the two networks, we calculated the mean correlation value between each node in one network and all the nodes in the other network (i.e., off diagonal blocks). Finally, for each network, the network segregation was calculated as the difference between within connectivity for that network and between language-MD network connectivity divided by the within network connectivity of that network (i.e., [within-between]/within), as used in previous studies (Chan et al., 2014). All of the network measures were calculated at the individual level first before calculating group level results (i.e., each subject had one value for within language network connectivity, one value for between language-MD network connectivity, one value for within MD network connectivity, extending to one value for language network segregation, and one value for MD network segregation).
Table 1.
MNI coordinates for the Language and Multiple-Demand Network ROIs. ROIs were created using 4-mm radius sphere around these coordinates.
| Language Network | MD Network ROI | ||||||
|---|---|---|---|---|---|---|---|
| ROI | x | y | z | ROI | x | y | z |
| Lang_LH_pMTG | −56 | −38 | 0 | MD_LH_postParietal | −18 | −66 | 52 |
| Lang_LH_Angular_G | −53 | −53 | 12 | MD_LH_midParietal | −44 | −56 | 52 |
| Lang_LH_Temporal_Pole | −52 | 7 | −18 | MD_LH_antParietal | −48 | −38 | 48 |
| Lang_LH_aMTG | −55 | −13 | −13 | MD_LH_supFrontal | −30 | 0 | 58 |
| Lang_LH_Lateral_Occipital | −42 | −68 | 24 | MD_LH_Precentral_A_precG | −48 | 6 | 38 |
| Lang_LH_IFG_Pars opercularis | −52 | 21 | 18 | MD_LH_Precentral_B_IFGop | −50 | 12 | 22 |
| Lang_LH_Precentral_G | −44 | 0 | 50 | MD_LH_midFrontal | −42 | 30 | 30 |
| Lang_LH_IFG_Pars triangularis | −48 | 28 | −4 | MD_LH_midFrontalOrb | −34 | 54 | 12 |
| MD_LH_insula | −34 | 22 | −2 | ||||
| MD_LH_medialFrontal | −6 | 20 | 44 | ||||
| MD_RH_postParietal | 18 | −66 | 52 | ||||
| MD_RH_midParietal | 44 | −56 | 52 | ||||
| MD_RH_antParietal | 48 | 38 | 48 | ||||
| MD_RH_supFrontal | 30 | 0 | 58 | ||||
| MD_RH_Precentral_A_precG | 48 | 6 | 38 | ||||
| MD_RH_Precentral_B_IFGop | 50 | 12 | 22 | ||||
| MD_RH_midFrontal | 42 | 30 | 30 | ||||
| MD_RH_midFrontalOrb | 34 | 54 | 12 | ||||
| MD_RH_insula | 34 | 22 | −2 | ||||
| MD_RH_medialFrontal | 6 | 20 | 44 | ||||
Abbreviations: a/p MTG, anterior/posterior middle temporal gyrus; G, gyrus; IFG, inferior frontal gyrus; post, posterior; mid, middle; ant, anterior; sup, superior; precG, precentral gyrus; IFGop, opercular part of the inferior frontal gyrus; Orb, orbital.
All of our analyses on connectivity incorporated ANOVAs. Specifically, to investigate the effect of task difficulty on age-related differences in functional connectivity in both networks, we first conducted a three-way mixed ANOVA, with Connectivity Type (3 levels: Within Language, Within MD, Between Language-MD) and Task Condition (3 levels: All Go, Go Bias, and No-Go Bias) as within-subject variables, and Age Group (2 levels: Younger vs Older) as a between-subject variable. In addition, to look at more integrated network measurements, we also conducted two-way mixed ANOVAs on language network segregation and MD network segregation, with the Task Condition as the within-subject variable, and the Age Group as the between-subject variable. All multiple comparisons were Bonferroni corrected. All data and analysis scripts are available on https://osf.io/628qc/?view_only=44b3fe0ec8314644a69aa75a7421ff89.
3. Results
3.1. Behavioral Results
Statistical analyses and results on behavioral data including reaction time and accuracy were previously reported in Zhang et al. (2019). To summarize, a generalized linear mixed-effect model was conducted on Go trial RTs. Results showed a main effect of Task Condition (i.e., each of the two conditions had significantly different RTs; All Go vs Go Bias, β = 0.07, SE = 0.03, p = .01; All Go vs No-Go Bias, β = 0.30, SE = 0.04, p < .001; Go Bias vs No-Go Bias, β =0.22, SE = 0.04, p < .001), and a main effect of Age Group (i.e., older adults responded more slowly than younger adults in all three conditions; All Go: β = 0.07, SE = 0.03, p = .05; Go Bias: β = 0.12, SE = 0.05, p = .02; No-Go Bias: β = 0.23, SE=0.06, p = .002). More importantly, the interaction between Task Condition and Age Group was significant, β = 0.11, SE = 0.04, p = .008). To specify the interaction, a regression line was first fitted on reaction times as a function of task conditions for each participant (All Go < Go Bias < No-Go Bias), then an independent sample t-test was conducted on the subject-level regression coefficients between the two groups. Results indicated that the regression coefficients in older adults were significantly greater than younger adults (t (32.89) = 2.19, p = .04), indicating that older adults had larger increases in reaction times as naming demand increased (All Go < Go Bias < No-Go Bias). Additionally, a mixed logistic regression was conducted on the total number of errors. Only the main effect of Age Group was significant where older adults made more errors than younger adults in all three task conditions (All Go: β = 0.45, SE = 0.21, p = .03; Go Bias: β = 1.14, SE = 0.21, p < .001; No-Go Bias: β = 0.99, SE = 0.20, p < .001).
3.2. Functional Connectivity
First, a three-way mixed ANOVA was conducted on functional connectivity while including Connectivity Type (3 levels: Within Language, Within MD, Between Language-MD) and Task Condition (3 levels: All Go, Go Bias, and No-Go Bias) as within-subject variables, and Age Group (2 levels: Younger vs Older) as a between-subject variable (Figure 3). The main effect of Connectivity Type was significant (F (2, 76) = 49.48, p < .001). Further analysis showed that the within-network connectivity was significantly higher for the MD network compared to the language network (adjusted p < .001), which was significantly higher than the between language-MD network connectivity (adjusted p < .001). The main effect of Task Condition was marginally significant (F (2, 76) = 2.99, p = .06). Further analysis showed that network connectivity was significantly higher during the No-Go Bias condition than the Go Bias condition (adjusted p = .01). The main effect of Age Group was marginally significant (F (1, 38) = 3.35, p = .08), such that younger adults showed slightly higher connectivity compared to older adults. Additionally, the interaction between Connectivity Type and Age Group was significant (F (2, 76) = 6.67, p = .002). Further analyses showed that the effect of Age Group was only significant within the language network (F (1, 118) = 22.2, p < .001), but not significant within the MD network (F (1, 118) = 1.06, p = .31), or for the between language-MD network connectivity (F (1, 118) = .17, p = .68), suggesting that the age effect on connectivity was the strongest in the language network. Furthermore, the interaction between Connectivity Type and Task Condition was significant (F (4, 152) = 3.88, p = .005). Although explicit pairwise comparisons between conditions were not significant (ps > .1), a visual inspection of the results illustrated that the direction of the condition effect was different for the within MD network connectivity compared to the within language network connectivity, and between-network connectivity (Figure 3B). Within MD network connectivity was characterized by increases in connectivity as a function of task difficulty (i.e., No-Go Bias > Go Bias > All Go), whereas the Go Bias had the lowest connectivity strength in the other networks. Interactions between the other variables were not significant (ps > .1). Exploratory correlational analyses showed that there was no significant correlation between RT and connectivity (within language network connectivity, p = .63; within MD network connectivity, p = .11; between-network connectivity, p = .63).
Figure 3.
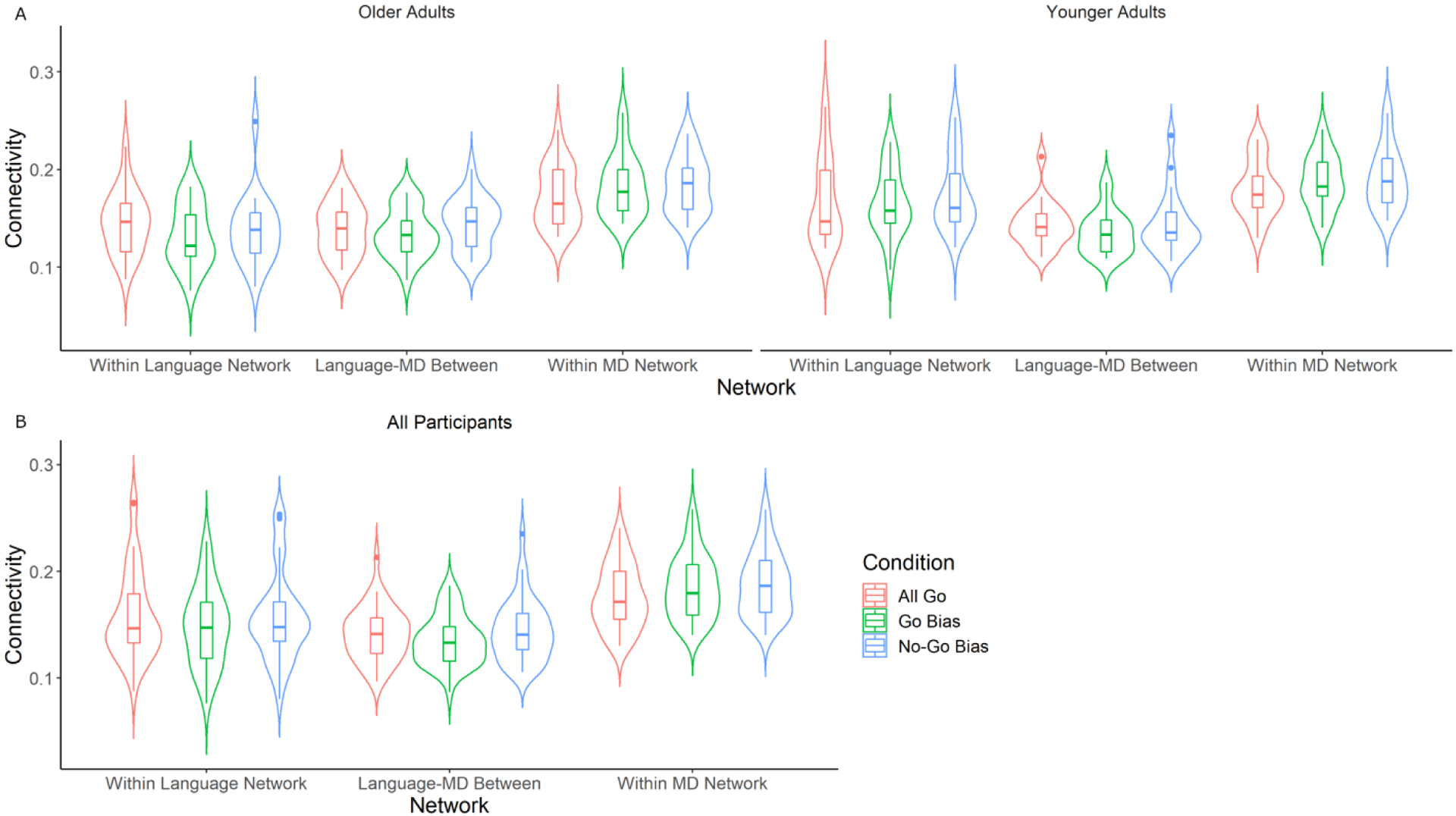
A) shows functional connectivity broken down by Age Group, Connectivity Type, and Task Condition. Significant main effects of Connectivity Type (Within MD > Within Language > Between Language-MD) and marginally significant main effects of Task Condition (No-Go Bias > Go Bias) and Age Group (Younger Adults > Older Adults) are shown. There was also a significant interaction between Connectivity Type and Age Group, indicating that the age effect on connectivity was only significant in the within language network connectivity. B) shows the significant interaction between Connectivity Type and Task Condition. The direction of the condition effect was different in the within MD network connectivity (No-Go Bias > Go Bias > All Go), compared to the within language and the between network connectivities (Go Bias being the lowest). Box plots represent median and quartile.
To explicitly look at network segregation, we combined within and between network connectivities to derive a composite measure of network segregation. Then we conducted two-way ANOVAs (Age × Condition) on language network segregation (Figure 4A), and MD network segregation (Figure 4B).
Figure 4.
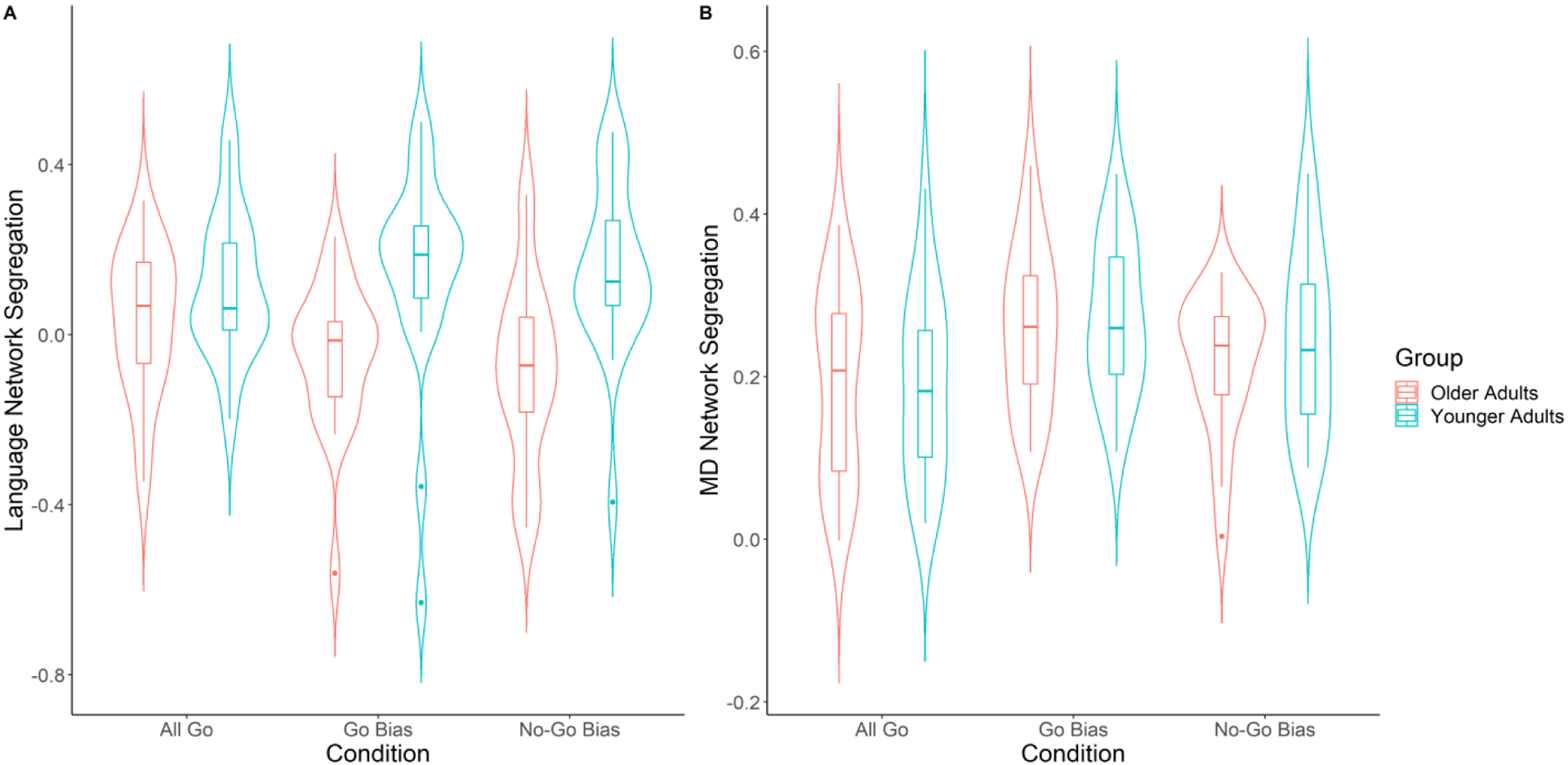
Effect of Task Condition and Age Group on network segregation. A) A significant main effect of Age Group and an interaction between Task Condition and Age Group on language network segregation is shown (Younger Adults in blue, Older Adults in red); Older adults showed a gradual decrease in segregation as task difficulty increased, whereas younger adults did not show significant task difficulty effect. Age differences in language network segregation were significant in the two Bias conditions, but not in the All Go condition. B) For MD network segregation, the main effect of Task Condition was significant (Go Bias > No-Go Bias > All Go). Box plots represent median and quartile.
In the language network (Figure 4A), the main effect of Age Group on language network segregation was significant (F (1, 38) = 11.16, p = .002). Specifically, younger adults showed higher language segregation than older adults, indicating that younger adults had more segregated language networks. Additionally, although the main effect of Task Condition was not significant (F (2, 76) = .68, p = .51), the interaction between Age Group and Task Condition was significant (F (2, 76) = 3.02, p = .05). Further analysis showed that, in older adults, there was a gradual decrease in language network segregation as task difficulty increased (All Go to No-Go Bias, F (2, 38) = 2.91, p = .07), while the effect of Task Condition in younger adults was not significant (F (2, 38) = .48, p = .63). Moreover, language network segregation between the two age groups was not significantly different during the All Go condition (p = .14), however younger adults had significantly higher language network segregation than older adults in both the Go Bias (p = .009) and the No-Go Bias (p < .001) conditions. An exploratory correlational analysis showed that there was no significant correlation between RT and language network segregation (p = .93).
For the MD network segregation (Figure 4B), only the main effect of Task Condition was significant (F (2, 76) = 8.79, p < .001). Further analyses showed that the MD network segregation in the Go Bias condition and the No-Go Bias condition were stronger than that in the All Go condition (Go Bias > All Go, adjusted p < .001; No-Go Bias > All Go, adjusted p = .09). The main effect of Age Group and its interaction with Task Condition were not significant (ps > .1). An exploratory correlational analysis showed that there was no significant correlation between RT and MD network segregation (p = .15).
4. Discussion
This study examined the effect of task difficulty on age-related differences in functional connectivity during word production using a Go/No-Go picture naming paradigm. Behaviorally (published in Zhang et al., 2019), in addition to the main effect of age (i.e., older adults showed longer RT and more errors than younger adults), older adults also showed greater increases in reaction times when task difficulty increased compared to younger adults, suggesting that older adults were affected more by the manipulation of task difficulty. In terms of functional connectivity, we hypothesized that older adults would show less efficient networks. Consistent with this prediction, we found that older adults had lower within language network connectivity, and less language network segregation, along with worse performance compared to younger adults. Moreover, we hypothesized that older adults’ neural networks would be more affected by the manipulation of task difficulty than younger adults. As predicted, older adults showed a decrease in language network segregation as task difficulty increased, consistent with the idea that task difficulty plays a key role in network efficiency among older adults. In contrast, younger adults showed similar network segregation across task difficulties. These results are consistent with the CRUNCH model (Reuter-Lorenz & Cappell, 2008), in that older adults were more sensitive to the manipulation of task difficulty both behaviorally and neurally and the language network’s efficiency decreased as task difficulty increased.
Expanding on these results, our finding that older adults showed reduced connectivity within the language network compared to younger adults is consistent with prior literature which has found lower within network connectivity for older adults in both resting-state (Betzel et al., 2014; Cao et al., 2014; Chan et al., 2018; Chan et al., 2014; Geerligs et al., 2015; Onoda et al., 2012; Siman-Tov et al., 2017; Song et al., 2014; Tomasi & Volkow, 2012) and task-related scans (Zhang, Gertel, et al., 2021). Also consistent with previous studies that look at resting-state network segregation (e.g., Chan et al., 2014), older adults in the current study showed less language network segregation than younger adults during the task state. Network segregation reflects functional specialization of a network because it measures the balance between within network connectivity and how that network interacts with other networks (Chan et al., 2014), in this case, interactions between the language and MD networks. Although age-related reductions in language network segregation, in and of itself, could be an adaptive brain response to ongoing anatomical and biochemical alterations, this does not seem to be the case here because the age-related reductions in language network segregation were accompanied by declines in behavioral performance (longer reaction time and more errors). Although the age effects on connectivity and behavior converged, a direct correlational link between brain and behavior was not observed. Therefore, we can only conservatively interpret the age-related differences in within language network connectivity and in language network segregation as indicating decreased functional specificity of the network. In other words, these reduced connectivities suggest that the language production network becomes less organized and efficient with increased age.
In addition to the main effect of age that we observed, the unique aspect of this study was to look at the dynamic effect of task difficulty on age-related differences in functional connectivity during word production. We found that only older adults showed a gradual decrease of language network segregation as task difficulty increased. It is even more striking that the language segregation value in the two Bias conditions in older adults became negative (Figure 4A), suggesting that the within language network connectivity became weaker than the connectivity between the language and MD networks in older adults when task difficulty increased. In contrast to older adults, younger adults did not show network segregation differences as a function of task difficulty since their network segregation values remained relatively constant across difficulty levels. Additionally, although the age difference in language network segregation was not significant in the All Go condition, it became significant in the Go Bias and the No-Go Bias conditions. This suggests two things: first, when task demands were minimal (i.e., when older adults were only required to name pictures with no inhibition demands), older adults’ language network segregation from the MD network was on par with younger adults. In other words, when the task demands were minimal, the age differences were minimal. Second, age-related differences were greater when the task was more difficult. Therefore, the age differences in language network segregation that we observed largely speak to the effect of task difficulty as opposed to general effects of age on language.
Recall that behaviorally, we found that older adults showed a greater decline in word production performance compared to younger adults as task difficulty increased. Together, these results suggest that as task difficulty increases, behavioral performance declines and neural efficiency is reduced. In contrast, although younger adults also showed behavioral effects of task difficulty, their language network segregation was well maintained with increased difficulty, suggesting a greater neural capacity to meet increased task difficulty. In other words, the extent to which younger adults needed to engage the MD network during word production was not affected as much by increased task difficulty. These age-related differences are consistent with the CRUNCH model (Reuter-Lorenz & Cappell, 2008). Specifically, the CRUNCH model proposes, that age differences can be minimal when task demands are low, however, older adults show steeper declines in performance, neural specificity, and efficiency, as task difficulty increases. Moreover, our observation of older adults’ reduced neural efficiency in the language network, along with their poorer behavioral performance when task demands were high, is consistent with neural dedifferentiation at the most difficult level. Finally, the changes in language network segregation as a function of task difficulty also suggest that brain networks are not static when engaged in a task. Rather, network interactions can change dynamically as a function of task difficulty, consistent with previous studies (Varangis, Razlighi, et al., 2019). Although this ability to dynamically adapt, may lessen with increasing age.
Interestingly, the age effects on network characteristics were only significant in the language network, but not the multiple-demand network. Language production often involves not only the language network, but also regions in the MD network – one of the key reasons that we examined both of these networks. Moreover, in fMRI studies with explicit language tasks, older adults often engage MD network regions to a greater extent than younger adults (Destrieux et al., 2012; Diaz et al., 2014; Diaz et al., 2019; Diaz et al., 2016; Nagels et al., 2012; Rizio et al., 2017; Wierenga et al., 2008; Zhang et al., 2019). Hence, age differences in the MD network were expected to be at least as salient as in the language network. Therefore, the lack of age effects on the MD network was unanticipated. However, it is worth noting that although the age effects were not significant in the MD network, there was a significant effect of task demands. Specifically, for all adults, MD network segregation, as calculated based on the within MD network connectivity and its between network connectivity with the language network, increased as task demands increased (i.e., from All Go to Go Bias), then started to decrease (i.e., from Go Bias to No-Go Bias, see Figure 4B). These results suggest that the MD network was sensitive to the manipulation of task difficulty during word production, although this effect was comparable across age groups. Future studies should look at a task that specifically recruits the MD network (e.g., fluid intelligence task) to see if the age-related patterns we observed within the language network would replicate in the MD network using a task more directly aligned with MD network functions. Additionally, while these results are promising and shed light on the interaction between the left-lateralized language network and the MD network, we only examined one network outside of the language network. Thus, our results speak most directly to the language network’s relationship with the MD network.
Looking at other networks, other studies have found that coordination between the language network, the default mode network, and the executive-control networks is important for older adults’ verbal fluency performance, however there was no younger adult comparison group, so the exact effects of aging are unclear (Muller et al., 2016). On the other hand, others have found that the stronger communication between different domain general networks (multiple demand and default mode networks) only benefited younger adults’ semantic memory performance (Martin, Saur, et al., 2022; Martin, Williams, et al., 2022). Therefore, whether the crosstalk among different networks was beneficial or not for different age group requires more systematic investigation.
In conclusion, we found that increased task difficulty during word production was associated with larger behavioral declines in performance and larger neural declines in language network segregation for older adults compared to younger adults. These results are consistent with the CRUNCH model which suggests that the brain becomes less efficient as task difficulty increases.
Figure 2.
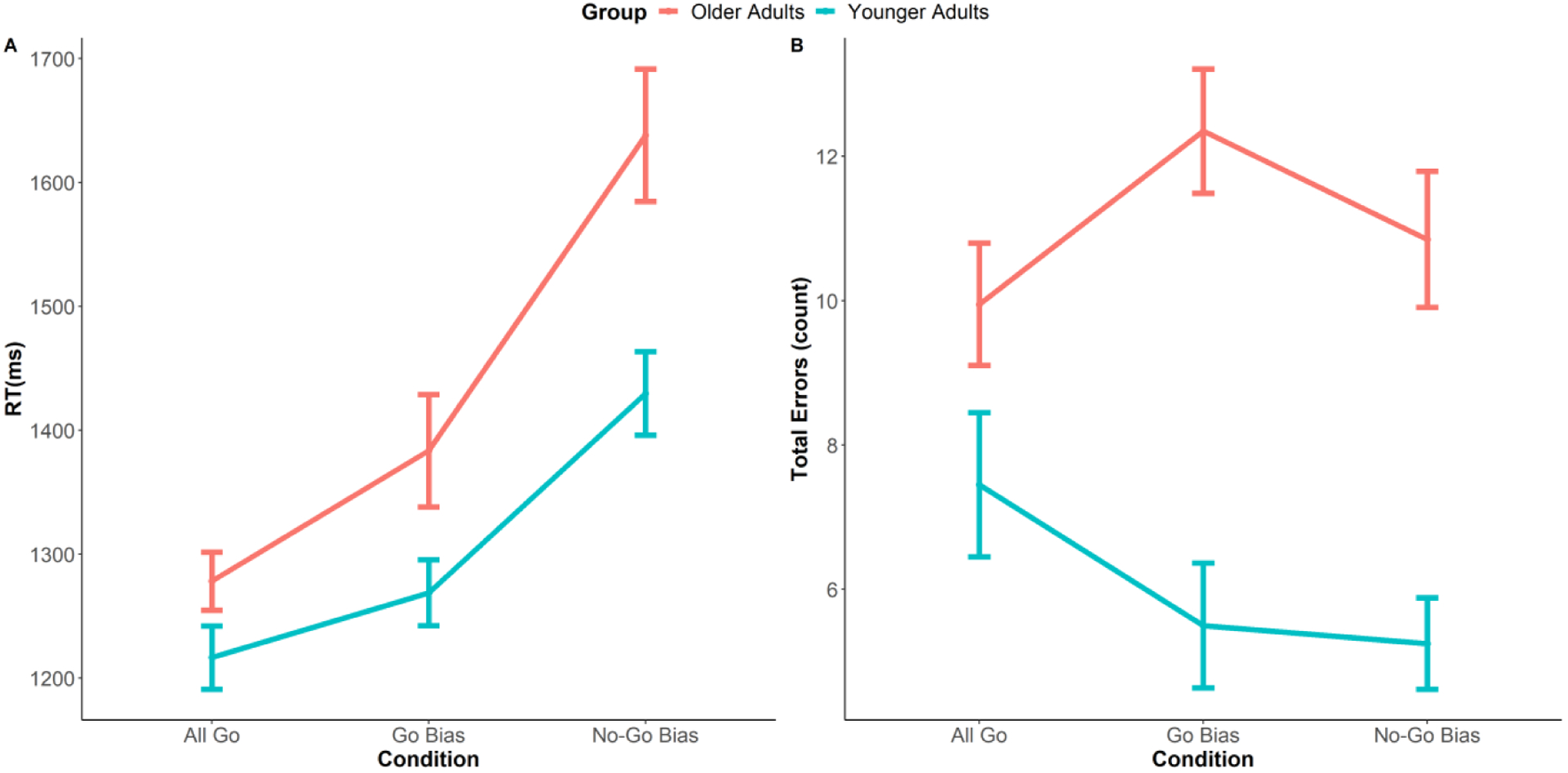
Reaction Times (RTs, A) for Go trials and Total Errors (B) during the Go/No-Go picture naming task (reprinted by permission from Elsevier: Neuropsychologia, Zhang et al., 2019). Older adults named pictures more slowly and had more errors. Additionally, older adults had significantly greater increases in RT as difficulty increased compared to younger adults.
Highlights.
Older adults more behaviorally sensitive to task difficulty in word production.
Older adults had lower within language network connectivity compared to younger adults.
Older adults’ language network became less segregated as task difficulty increased.
Findings support the Compensation-Related Utilization of Neural Circuits Hypothesis.
Acknowledgements
This project was funded by the National Institutes of Health (NIH) National Institute on Aging (NIA) grant R01 AG034138 (MTD), the Social Sciences Research Institute, and the Department of Psychology at the Pennsylvania State University. The writing of this paper was supported by the National Natural Science Foundation of China (32200845), and the Start-up Research Grant (SRG2022-00003-ICI) and Multi-Year Research Grant (MYRG2022-00148-ICI) from the University of Macau to Haoyun Zhang. We thank the staff and scientists at the Social, Life, and Engineering Sciences Imaging Center (SLEIC) and the Center for Language Science (CLS) where the study was conducted, for their support. Scripts and data are available at: https://osf.io/628qc/?view_only=44b3fe0ec8314644a69aa75a7421ff89.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Disclosure: The authors report no conflict of interest.
Fedorenko and colleagues identified language-related brain regions by having participants read sentences and nonword sequences, using the sentences>nonwords contrast (Fedorenko et al., 2010). The MD network was identified using a spatial working memory task in which participants saw a 3×4 grid in which squares appeared in various locations sequentially. The harder>easier contrast was used to localize the multiple demand network brain regions (Fedorenko et al., 2013).
In network science, efficiency refers to the manner in which information is distributed within a network. It is related to connectivity among nodes and reflected in mathematical calculations such as path length, clustering coefficient, and small-worldness (e.g., Watts & Strogatz, 1998; Sporns, 2011). Calculating these metrics requires many more nodes of data, so in the present analyses we capture efficiency by comparing within network and between network connectivities.
Note that the current study only focuses on the left language network and the MD network, which are most relevant to older adults’ language production. Therefore, the between network measures are only indications of the relationships between these two specific networks not a general assessment of whole-brain between-network connectivity.
The CONN toolbox uses the CompCor approach, which extracts multiple signals from CSF and white-matter areas to capture motion and physiological artifacts while excluding neural signals which avoids introducing artifactual negative correlations in the connectivity measures (Chai et al., 2012; Liu et al., 2017; Liu et al., 2021).
Negative correlations were not included in further analysis due to uncertainty regarding the meaning of negative correlations. Negative correlations may be related to statistical artifacts, global signal regression, or N-methyl-D-aspartate [NMDA] action in cortical inhibition. Moreover, others have adopted a similar strategy, for example, Chan et al., 2014 set all negative nodes to 0. To be comparable with their findings, we adopted the same analysis strategy. For a full discussion about the meaning of negative correlations see Hallquist and Hillary, 2018.
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, & Buckner RL (2007). Disruption of large-scale brain systems in advanced aging. Neuron, 56(5), 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino KM, Fulcher BD, Parkes L, Sabaroedin K, & Fornito A (2020). Identifying and removing widespread signal deflections from fMRI data: Rethinking the global signal regression problem. NeuroImage, 212, 116614. [DOI] [PubMed] [Google Scholar]
- Auguie B, Antonov A, & Auguie MB (2017). Package ‘gridExtra’. Miscellaneous Functions for “Grid” Graphics. [Google Scholar]
- Baciu M, Boudiaf N. l., Cousin E, Perrone-Bertolotti M, Pichat C, Fournet N, Chainay H, Lamalle L, & Krainik A (2016). Functional MRI evidence for the decline of word retrieval and generation during normal aging. Age, 38(1), 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Hutchison KA, Cortese MJ, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, & Treiman R (2007). The English lexicon project. Behavior Research Methods, 39(3), 445–459. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2014). Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823. [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo X-N, & Sporns O (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage, 102, 345–357. [DOI] [PubMed] [Google Scholar]
- Britt AE, Ferrara C, & Mirman D (2016). Distinct effects of lexical and semantic competition during picture naming in younger adults, older adults, and people with aphasia. Frontiers in Psychology, 7, 813–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur MB, Guérard K, & Bouras M (2014). Bank of Standardized Stimuli (BOSS) phase II: 930 new normative photos. PloS One, 9(9), e106953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, & Wade E (1991). On the tip of the tongue: What causes word finding failures in young and older adults? Journal of Memory and Language, 30(5), 542–579. [Google Scholar]
- Burke DM, & Shafto MA (2008). Language and aging (Craik F & Salthouse T Eds.). Psychology Press. [Google Scholar]
- Cabeza R (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging, 17(1), 85–100. [DOI] [PubMed] [Google Scholar]
- Cao M, Wang J-H, Dai Z-J, Cao X-Y, Jiang L-L, Fan F-M, Song X-W, Xia M-R, Shu N, & Dong Q (2014). Topological organization of the human brain functional connectome across the lifespan. Developmental Cognitive Neuroscience, 7, 76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Na J, Agres PF, Savalia NK, Park DC, & Wig GS (2018). Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proceedings of the National Academy of Sciences, 115(22), E5144–E5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, & Wig GS (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences, 111(46), E4997–E5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KJ, Moye J, Armson RR, & Kern TM (1992). Health screening and random recruitment for cognitive aging research. Psychology and Aging, 7(2), 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Shinohara RT, Elliott MA, Eickhoff SB, & Davatzikos C (2017). Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage, 154, 174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM (1999). Optimal experimental design for event-related fMRI. Human Brain Mapping, 8(2–3), 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, & Cabeza R (2008). Que PASA? The posterior–anterior shift in aging. Cerebral Cortex, 18(5), 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Zhuang J, Wright P, & Tyler LK (2014). Age-related sensitivity to task-related modulation of language-processing networks. Neuropsychologia, 63, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Hommet C, Domengie F, Boissy J-M, De Marco G, Joanette Y, Andersson F, & Cottier J-P (2012). Influence of age on the dynamics of fMRI activations during a semantic fluency task. JOURNAL OF NEURORADIOLOGY, 39(3), 158–166. [DOI] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, & Madden DJ (2014). Age-related differences in the neural bases of phonological and semantic processes. Journal of Cognitive Neuroscience, 26(12), 2798–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Johnson MA, Burke DM, Truong T-K, & Madden DJ (2019). Age-related differences in the neural bases of phonological and semantic processes in the context of task-irrelevant information. Cognitive, Affective & Behavioral Neuroscience, 19(4), 829–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, Rizio AA, & Zhuang J (2016). The Neural Language Systems That Support Healthy Aging: Integrating Function, Structure, and Behavior. Language and Linguistics Compass, 10(7), 314–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences, 110(41), 16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Hsieh P-J, Nieto-Castañón A, Whitfield-Gabrieli S, & Kanwisher N (2010). New method for fMRI investigations of language: defining ROIs functionally in individual subjects. Journal of Neurophysiology, 104(2), 1177–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré P, Benhajali Y, Steffener J, Stern Y, Joanette Y, & Bellec P (2019). Resting-state and vocabulary tasks distinctively inform on age-related differences in the functional brain connectome. Language, Cognition and Neuroscience, 34(8), 949–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré P, Jarret J, Brambati SM, Bellec P, & Joanette Y (2020). Task-induced functional connectivity of picture naming in healthy aging: the impacts of age and task complexity. Neurobiology of language, 1(2), 161–184. [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Geerligs L, Maurits NM, Renken RJ, & Lorist MM (2014). Reduced specificity of functional connectivity in the aging brain during task performance. Human Brain Mapping, 35(1), 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, & Lorist MM (2015). A brain-wide study of age-related changes in functional connectivity. Cerebral Cortex, 25(7), 1987–1999. [DOI] [PubMed] [Google Scholar]
- Gertel VH, Karimi H, Dennis NA, Neely KA, & Diaz MT (2020). Lexical frequency affects functional activation and accuracy in picture naming among older and younger adults. Psychology and Aging, 35(4), 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertel VH, Zhang H, & Diaz MT (2020). Stronger right hemisphere functional connectivity supports executive aspects of language in older adults. Brain and Language, 206, 104771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P, & Lindenberger U (2003). Age-based structural dynamics between perceptual speed and knowledge in the Berlin Aging Study: direct evidence for ability dedifferentiation in old age. Psychology and Aging, 18(4), 696–713. [DOI] [PubMed] [Google Scholar]
- Glover GH, Mueller BA, Turner JA, Van Erp TG, Liu TT, Greve DN, Voyvodic JT, Rasmussen J, Brown GG, & Keator DB (2012). Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. Journal of Magnetic Resonance Imaging, 36(1), 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Montoya RI, Cera C, & Sandoval TC (2008). More use almost always means a smaller frequency effect: Aging, bilingualism, and the weaker links hypothesis. Journal of Memory and Language, 58(3), 787–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JA, Churchill N, & McIntosh AR (2010). A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cerebral Cortex, 20(6), 1432–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin JM, Copersino ML, & Schretlen DJ (2018). Clinical utility of the 15-item geriatric depression scale (GDS-15) for use with young and middle-aged adults. Journal of Affective Disorders, 241, 59–62. [DOI] [PubMed] [Google Scholar]
- Haitas N, Amiri M, Wilson M, Joanette Y, & Steffener J (2021). Age-preserved semantic memory and the CRUNCH effect manifested as differential semantic control networks: An fMRI study. PloS One, 16(6), e0249948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, & Hillary FG (2018). Graph theory approaches to functional network organization in brain disorders: A critique for a brave new small-world. Network Neuroscience, 3(1), 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. [DOI] [PubMed] [Google Scholar]
- Higby E, Cahana-Amitay D, Vogel-Eyny A, Spiro A III, Albert ML, & Obler LK (2019). The role of executive functions in object-and action-naming among older adults. Experimental aging research, 45(4), 306–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, & Morcom AM (2018). Age-related changes in the neural networks supporting semantic cognition: A meta-analysis of 47 functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 84, 134–150. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, & McCarthy G (2004). Functional magnetic resonance imaging (Vol. 1). Sinauer Associates; Sunderland, MA. [Google Scholar]
- Indefrey P, & Levelt WJ (2004). The spatial and temporal signatures of word production components. Cognition, 92(1), 101–144. [DOI] [PubMed] [Google Scholar]
- Kassambara A (2019). Comparing groups: Numerical variables. In: Datanovia. [Google Scholar]
- Kassambara A (2020). ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.4.0. https://CRAN.R-project.org/package=ggpubr.
- King B, Van Ruitenbeek P, Leunissen I, Cuypers K, Heise K-F, Santos Monteiro T, Hermans L, Levin O, Albouy G, & Mantini D (2018). Age-related declines in motor performance are associated with decreased segregation of large-scale resting state brain networks. Cerebral Cortex, 28(12), 4390–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RH (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. [Google Scholar]
- LaGrone S, & Spieler DH (2006). Lexical competition and phonological encoding in young and older speakers. Psychology and Aging, 21(4), 804–809. [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, & Sikström S (2001). Aging cognition: from neuromodulation to representation. Trends in Cognitive Sciences, 5(11), 479–486. [DOI] [PubMed] [Google Scholar]
- MacKay DG, & James LE (2004). Sequencing, speech production, and selective effects of aging on phonological and morphological speech errors. Psychology and Aging, 19(1), 93–107. [DOI] [PubMed] [Google Scholar]
- Marsolais Y, Perlbarg V, Benali H, & Joanette Y (2014). Age-related changes in functional network connectivity associated with high levels of verbal fluency performance. Cortex, 58, 123–138. [DOI] [PubMed] [Google Scholar]
- Martin S, Saur D, & Hartwigsen G (2022). Age-dependent contribution of domain-general networks to semantic cognition. Cerebral Cortex, 32(4), 870–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Williams K, Saur D, & Hartwigsen G (2022). Age-related reorganization of functional network architecture in semantic cognition. Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Seeds L, Harnish S, Antonenko D, Witte V, Lindenberg R, & Crosson B (2012). Same modulation but different starting points: performance modulates age differences in inferior frontal cortex activity during word-retrieval. PloS One, 7(3), e33631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Wilser L, Eulitz C, Rockstroh B, Conway T, Gonzalez-Rothi L, & Crosson B (2009). Neural signatures of semantic and phonemic fluency in young and old adults. Journal of Cognitive Neuroscience, 21(10), 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Seeds L, Flaisch T, Harnish S, Cohen ML, McGregor K, Conway T, Benjamin M, & Crosson B (2012). Impact of changed positive and negative task-related brain activity on word-retrieval in aging. Neurobiology of Aging, 33(4), 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Martínez FJ, & Montoro PR (2012). An ecological alternative to Snodgrass & Vanderwart: 360 high quality colour images with norms for seven psycholinguistic variables. PloS One, 7(5), e37527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AM, Mérillat S, & Jäncke L (2016). Older but still fluent? Insights from the intrinsically active baseline configuration of the aging brain using a data driven graph-theoretical approach. NeuroImage, 127, 346–362. [DOI] [PubMed] [Google Scholar]
- Nagels A, Kircher T, Dietsche B, Backes H, Marquetand J, & Krug A (2012). Neural processing of overt word generation in healthy individuals: the effect of age and word knowledge. NeuroImage, 61(4), 832–840. [DOI] [PubMed] [Google Scholar]
- Newman RS, & German D (2005). Life span effects of lexical factors on oral naming. Language and Speech, 48(2), 123–156. [DOI] [PubMed] [Google Scholar]
- Novick JM, Trueswell JC, & Thompson-Schill SL (2010). Broca’s area and language processing: Evidence for the cognitive control connection. Language and Linguistics Compass, 4(10), 906–924. [Google Scholar]
- Nozari N, & Novick J (2017). Monitoring and control in language production. Current Directions in Psychological Science, 26(5), 403–410. [Google Scholar]
- Onoda K, Ishihara M, & Yamaguchi S (2012). Decreased functional connectivity by aging is associated with cognitive decline. Journal of Cognitive Neuroscience, 24(11), 2186–2198. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, & Reuter-Lorenz PA (2007). Age differences in deactivation: a link to cognitive control? Journal of Cognitive Neuroscience, 19(6), 1021–1032. [DOI] [PubMed] [Google Scholar]
- Persson J, Sylvester C-YC, Nelson JK, Welsh KM, Jonides J, & Reuter-Lorenz PA (2004). Selection requirements during verb generation: differential recruitment in older and younger adults. NeuroImage, 23(4), 1382–1390. [DOI] [PubMed] [Google Scholar]
- Pistono A, Guerrier L, Péran P, Rafiq M, Gimeno M, Bézy C, Pariente J, & Jucla M (2020). Increased functional connectivity supports language performance in healthy aging despite grey matter loss. Neurobiology of Aging. [DOI] [PubMed] [Google Scholar]
- Price CJ (2010). The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences, 1191(1), 62–88. [DOI] [PubMed] [Google Scholar]
- Raichle ME (2015). The brain’s default mode network. Annual Review of Neuroscience, 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, & Cappell KA (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177–182. [Google Scholar]
- Rizio AA, Moyer KJ, & Diaz MT (2017). Neural evidence for phonologically based language production deficits in older adults: An fMRI investigation of age-related differences in picture-word interference. Brain and Behavior, 7(4), e00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team. (2022). RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA: URL http://www.rstudio.com/. [Google Scholar]
- Sala-Llonch R, Bartrés-Faz D, & Junqué C (2015). Reorganization of brain networks in aging: a review of functional connectivity studies. Frontiers in Psychology, 6, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Burke DM, Stamatakis EA, Tam PP, & Tyler LK (2007). On the tip-of-the-tongue: neural correlates of increased word-finding failures in normal aging. Journal of Cognitive Neuroscience, 19(12), 2060–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, & Yesavage JA (1986). Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging Mental Health. [Google Scholar]
- Siman-Tov T, Bosak N, Sprecher E, Paz R, Eran A, Aharon-Peretz J, & Kahn I (2017). Early age-related functional connectivity decline in high-order cognitive networks. Frontiers in Aging Neuroscience, 8, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Birn RM, Boly M, Meier TB, Nair VA, Meyerand ME, & Prabhakaran V (2014). Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connectivity, 4(9), 662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O (2016). Networks of the Brain. MIT press. [Google Scholar]
- Steffener J, Habeck CG, & Stern Y (2012). Age-related changes in task related functional network connectivity. PloS One, 7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, & Burke D (2000). Slips of the tongue: An examination of language production in old age. Poster presented at the Cognitive Aging Conference, Atlanta, [Google Scholar]
- Tomasi D, & Volkow ND (2012). Aging and functional brain networks. Molecular Psychiatry, 17(5), 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varangis E, Habeck C, Razlighi Q, & Stern Y (2019). The effect of aging on resting state connectivity of predefined networks in the brain. Frontiers in Aging Neuroscience, 11, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varangis E, Razlighi Q, Habeck CG, Fisher Z, & Stern Y (2019). Between-network functional connectivity is modified by age and cognitive task domain. Journal of Cognitive Neuroscience, 31(4), 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, LaViolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamäki M, Dickerson BC, & Sperling RA (2010). Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. NeuroImage, 51(2), 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, & Strogatz SH (1998). Collective dynamics of ‘small-world’networks. Nature, 393(6684), 440–442. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. [Google Scholar]
- Wickham H, Averick M, Bryan J, Chang W, McGowan LDA, François R, Grolemund G, Hayes A, Henry L, & Hester J (2019). Welcome to the Tidyverse. Journal of open source software, 4(43), 1686. [Google Scholar]
- Wickham H, & Bryan J (2019). readxl: Read Excel Files. R package version 1.3. 1. In: Recuperado de https://cran.r-project.org/package=readxl. [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein WM, Leonard CM, Rothi LJG, Conway T, Cato MA, Briggs R, & Crosson B (2008). Age-related changes in word retrieval: role of bilateral frontal and subcortical networks. Neurobiology of Aging, 29(3), 436–451. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bai X, & Diaz MT (2021). The intensity and connectivity of spontaneous brain activity in a language network relate to aging and language. Neuropsychologia, 154, 107784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Eppes A, Beatty-Martinez A, Navarro-Torres C, & Diaz MT (2018). Task difficulty modulates brain-behavior correlations in language production and cognitive control: Behavioral and fMRI evidence from a phonological go/no-go picture-naming paradigm. Cognitive, Affective & Behavioral Neuroscience, 18(5), 964–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Eppes A, & Diaz MT (2019). Task difficulty modulates age-related differences in the behavioral and neural bases of language production. Neuropsychologia, 124, 254–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gertel VH, Cosgrove AL, & Diaz MT (2021). Age-related differences in resting-state and task-based network characteristics and cognition: a lifespan sample. Neurobiology of Aging, 101, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]


