Abstract
Background:
In the Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery (PALACS) trial, posterior pericardiotomy was associated with a significant reduction in postoperative atrial fibrillation (POAF) after cardiac surgery. We aimed to investigate the mechanisms underlying this effect.
Methods:
We included PALACS patients with available echocardiographic data (n=387/420, 92%). We tested the hypotheses that the reduction in POAF with the intervention was associated with 1) a reduction in postoperative pericardial effusion and/or 2) an effect on left atrial size and function. Spline and multivariable logistic regression analyses were used.
Results:
Most patients (n=307, 79%) had postoperative pericardial effusions (anterior 68%, postero-lateral 51.9%). The incidence of postero-lateral effusion was significantly lower in patients undergoing pericardiotomy (37% vs 67%; p<0.001). The median size of anterior effusion was comparable between patients with and without POAF (5.0 [IQR 3.0–7.0] vs 5.0 [IQR 3.0–7.5] mm; p=0.42), but there was a non-significant trend towards larger postero-lateral effusion in the POAF group (5.0 [IQR 3.0–9.0] vs 4.0 [IQR 3.0–6.4] mm; p=0.06). There was a non-linear association between postero-lateral effusion and POAF at a cut-off at 10 mm (OR 2.70; 95%CI 1.13, 6.47; p=0.03) that was confirmed in multivariable analysis (OR 3.5, 95%CI 1.17, 10.58; p=0.02). Left atrial dimension and function did not change significantly after posterior pericardiotomy.
Conclusions:
Reduction in postero-lateral pericardial effusion is a plausible mechanism for the effect of posterior pericardiotomy in reducing POAF. Measures to reduce postoperative pericardial effusion are a promising approach to prevent POAF.
Keywords: intraoperative echocardiography, postoperative transesophageal echocardiography, postoperative atrial fibrillation, pericardial effusion, pericardiotomy
Graphical Abstract
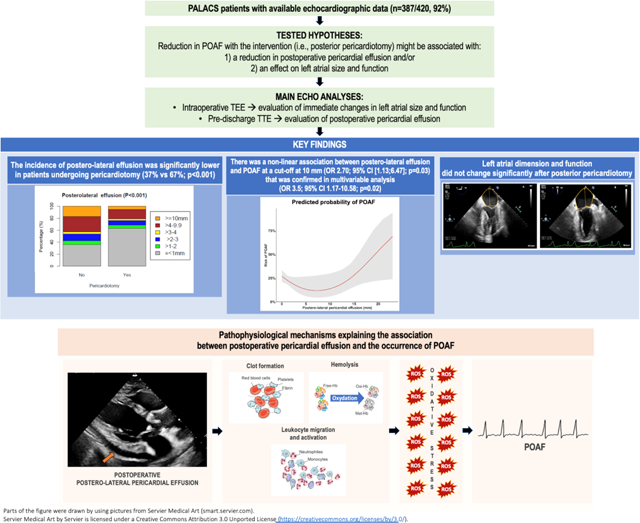
Pathophysiological mechanisms explaining the association between postoperative pericardial effusion and the occurrence of postoperative atrial fibrillation. Hb, hemoglobin; MetHb, methemoglobin; OxyHb, oxyhemoglobin; ROS, reactive oxygen species.
Introduction
The Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery (PALACS) randomized trial reported a large and statistically significant reduction in postoperative atrial fibrillation (POAF) in patients receiving posterior pericardiotomy, a surgical manoeuvre aimed at draining the pericardial sac into the left pleural cavity.[1] While the current evidence strongly suggests that posterior pericardiotomy is highly effective in reducing POAF,[2] the underlying mechanisms remain unclear. It is possible that the effect of posterior pericardiotomy on POAF is mediated by a reduction in postoperative pericardial effusion, a frequent finding after cardiac surgery that has been linked to POAF in experimental studies.[3–6] However, it is also possible that posterior pericardiotomy modifies atrial geometry and reduces atrial susceptibility to POAF triggers, but this has never been investigated.
We performed an explanatory analysis of prospectively collected clinical and echocardiographic data from the PALACS trial to test the hypotheses that the effect of posterior pericardiotomy on POAF is associated with 1) a reduction in postoperative pericardial effusion and/or 2) a modification of atrial size and function. We also investigated in detail the association between postoperative pericardial effusion and POAF.
Methods
The PALACS trial
The PALACS trial (NCT02875405) was approved by the Weill Cornell Medicine Institutional Review Board (#1502015867) and all patients consented to study participation and data usage. The protocol and the main results have been previously published.[1,7]
Briefly, the trial enrolled patients without a history of atrial fibrillation (AF) or other arrhythmias undergoing cardiac surgery for primary, elective interventions on the coronary arteries, aortic valve, or ascending aorta, or a combination of these. Patients were randomized to undergo either posterior left pericardiotomy (an incision in the posterior pericardium that drains the pericardial sac into the left pleural cavity) or no intervention. The primary outcome was in-hospital POAF, defined as the occurrence of an irregularly irregular rhythm without detectable P waves and lasting >30 seconds. Cardiac rhythm was continuously monitored during the entire postoperative in-hospital stay and all POAF episodes were adjudicated independently by a committee made of two cardiologists and a cardiac surgeon (blinded to patient level clinical and imaging data). In the main analysis, patients in the pericardiotomy arm had significantly lower risk of POAF (odds ratio [OR] 0.44; 95% Confidence interval [Cl] 0.27–0.70, p=0.0005).
Intraoperative transesophageal echocardiography
Each patient underwent a comprehensive intraoperative transesophageal echocardiography (TEE) study following a predefined protocol.[7] In the main analysis, preoperative and postoperative TEE data were used to evaluate immediate changes in left atrial (LA) size and function.
Exams were performed before skin incision (preoperative TEE) and after chest closure (postoperative TEE) using GE Vivid 7 ultrasound systems (GE Healthcare, Madison, WI) and Phillips iE33 and EPIQ ultrasound systems (Phillips Medical Systems, Andover, MA). All the intraoperative TEEs were performed according to a prospective dedicated echo protocol to optimize views of all structures of interest, including the LA. LA images were optimized before acquisition as follows: 1) in the mid-esophageal 4-chamber view, the LA was visualized using retroflexion when necessary to exclude the left ventricular outflow tract and biplane imaging was used to ensure the LA was visualized orthogonally and not obliquely to the imaging plane; 2) in the mid-esophageal 2-chamber view, the LA appendage was visualized to ensure accuracy and maximal area of the LA at approximately 90 degrees. Exams were performed by a select group of dedicated board-certified echocardiographers trained in the protocol to maximize the area of the LA that visualized in the echo field. These high-quality images allowed for near complete visualization of the entire atrial cavity (including left atrial posterior wall), where the LA volume was maximized (Supplementary Figure 1).
LA length was determined as the average distance from the mitral annulus to the posterior LA wall in the LA-focused mid-esophageal 4-chamber and 2-chamber views in end-systole. The LA maximum area was determined as the average of the area obtained by tracing the endocardium in LA-focused mid-esophageal 4-chamber and 2-chamber views in end-systole. The LA minimum area was determined as the average of the area obtained by tracing the endocardium in LA-focused mid-esophageal 4-chamber and 2-chamber views in end-diastole. [8,9] The LA reservoir function was determined by LA ejection fraction using the formula:[9]
The LA maximum area in the 4-chamber and 2-chamber views (LA1, LA2), and LA length in the 4-chamber and 2-chamber views (L1, L2) were used to quantify the LA volume (Supplementary Figure 1) using the biplane area/length method according to the equation:[10]
Postoperative transthoracic echocardiography
Each patient underwent pre-discharge transthoracic echocardiography (TTE) following a standardized protocol. As pericardial effusion and POAF develop in the days after surgery, pre-discharge TTE data were used in the main analysis to evaluate postoperative pericardial effusion.
Pericardial effusion was defined as any evidence of pericardial fluid and/or clot of any size and in any location (anterior, posterior, and lateral). Effusions were screened and size was quantified by linear measurements of the largest width of the effusion in end-diastole in each of the parasternal long axis, parasternal short-axis, apical 4-chamber, and subcostal views.[11] Effusions were classified as “anterior” if they were located 1) anterior to the left or right ventricle in the parasternal short-axis view, 2) next to the right atrium in the apical 4-chamber view, or 3) anterior to the right ventricle in the subcostal view. Effusions were classified as “lateral” if they were located lateral to the left ventricle in the parasternal short-axis view. Effusions were classified as “posterior” if they were posterior to the left ventricle in the parasternal short or long-axis views (Figure 1). For the analysis, posterior and lateral effusions were grouped together and classified as “postero-lateral” effusions.
Figure 1. Classification of pericardial effusion at postoperative transthoracic echocardiography.
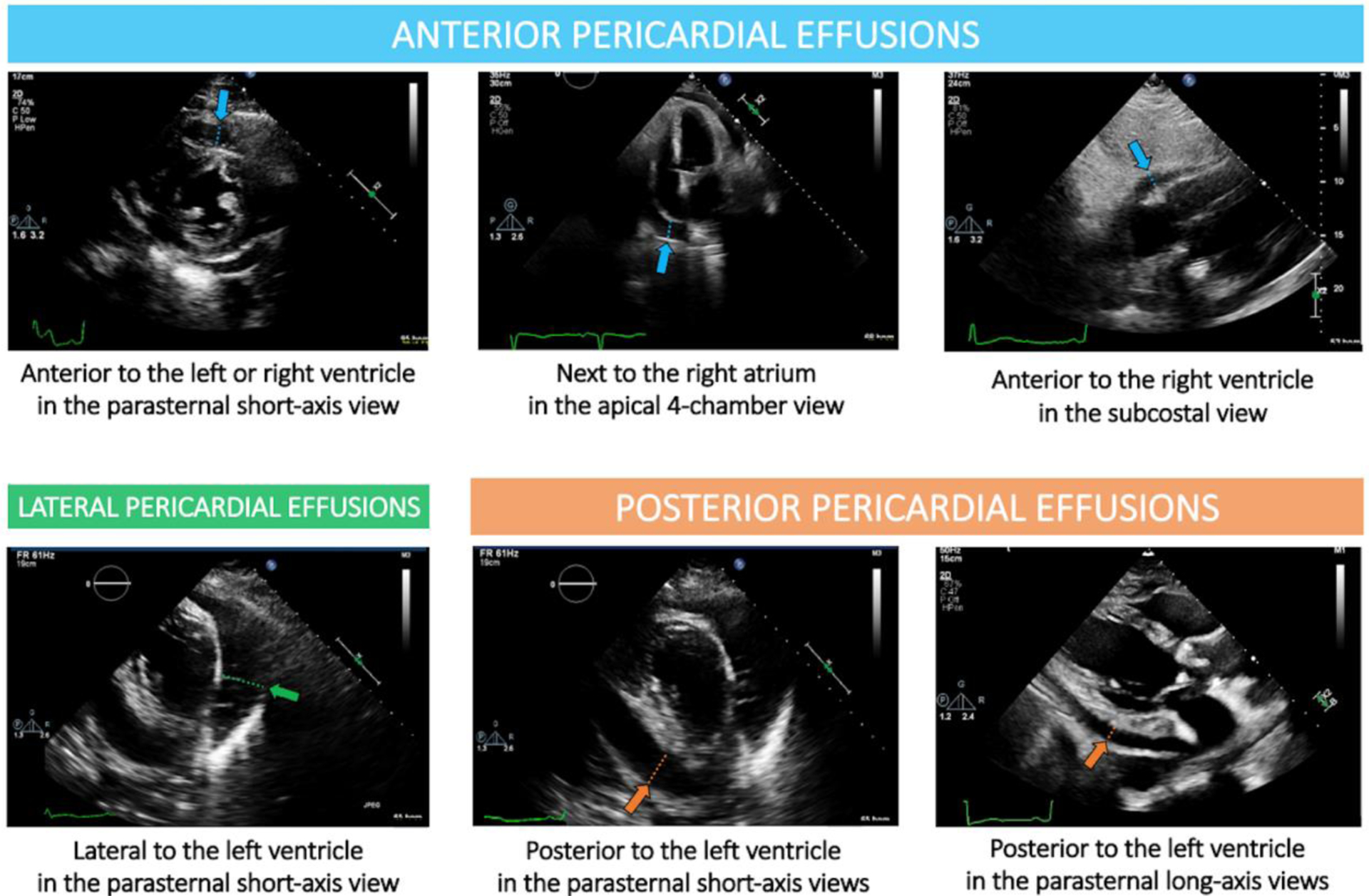
Effusions were classified as “anterior” if they were located 1) anterior to the left or right ventricle in the parasternal short-axis view, 2) next to the right atrium in the apical 4-chamber view, or 3) anterior to the right ventricle in the subcostal view. Effusions were classified as “lateral” if they were located lateral to the left ventricle in the parasternal short-axis view. Effusions were classified as “posterior” if they were posterior to the left ventricle in the parasternal short or long-axis views. For the analysis, posterior and lateral effusions were grouped together and classified as “postero-lateral” effusions.
All echocardiographic data were read and interpreted independently by two experienced investigators blinded to the intervention received within a high-volume laboratory, for which expertise and reproducibility for quantitative LA indices have been previously published. [8,9]
Statistical analysis
Shapiro-Wilk test was used to assess whether continuous variables were normally distributed. Normally distributed variables were reported as mean and standard deviation (SD) and compared using t-test, while non-normally distributed variables were reported as median and interquartile range (IQR) and compared using Mann-Whitney U test. Categorical variables were described as counts and proportions and compared using Pearson’s χ2 test.
Analysis of postoperative transthoracic echocardiographic pericardial effusion
Baseline and operative characteristics were compared between patients with and without evidence of pericardial effusion. Risk factors for pericardial effusion were assessed by means of a multivariable logistic model which included age, sex, race, body mass index (BMI), diabetes, New York Heart Association (NYHA) >2, preoperative hematocrit, EuroSCORE II, surgical procedure (coronary artery bypass grafting, aortic valve, vascular aortic), cardiopulmonary bypass and operative times.
Pericardial effusion characteristics (size and location) were then compared between patients with and without POAF and between interventions groups. To test the hypothesis of whether pericardial effusion affects the risk of POAF, we assumed that the relationship between pericardial effusion size and POAF was non-linear and used a restricted cubic splines model. This model was graphically interrogated to identify a threshold value for effusion size beyond which the risk of developing POAF increased. Based on this, a multivariable logistic model was built to test the independent association of pericardial effusion with POAF. The variables included in this model were the same as used for the adjusted main analysis of the PALACS trial[1] (age, sex, diabetes, LV ejection fraction, coronary artery bypass grafting, NYHA >2, chronic lung disease, EuroSCORE II, preoperative and postoperative use of beta-blockers), in addition to the pericardial effusion size threshold defined as above. A sensitivity analysis including key LA echocardiographic variables (preoperative area and reservoir function), CHA2DS2-VASc score, a clinical score which predicts the risk of POAF in cardiac surgery patients[12,13] and the pericardial effusion size threshold was also performed.
Analysis of LA indices
This analysis tested the hypotheses that:
Preoperative LA size and function are associated with POAF.
Posterior pericardiotomy affects LA size and function.
To test the first hypothesis, LA length, area, volume and reservoir function were compared between patients with and without POAF using univariate analysis and in a multivariable model that included LA dimensions and function and CHA2DS2-VASc score.[14] To test the second hypothesis, LA indices and function were compared in the posterior pericardiotomy and control group before and after surgery (based on the as treated principle).
Sensitivity analyses
To test the solidity of the results, several sensitivity analyses were performed:
In a sub-cohort of patients with available high quality preoperative TTE exams (n=118), the agreement between LA measurements (length and area) at preoperative TTE and preoperative TEE was evaluated using Bland-Altman plots with TTE as the reference.[15] The plots were created by calculating the paired difference between TTE and TEE measurements and estimating the related bias and the 95% limits of agreement. Correlation coefficients and plots were also produced to show the relationship between TTE and TEE measurements.
The presence of pericardial effusion on the postoperative TEE exam was compared in patients with and without POAF using Pearson’s Chi2 test. A multivariable model including the same variables as in the main analysis was then constructed to test the independent association of pericardial effusion at postoperative TEE with POAF.
To assess the relationship between preoperative TEE LA area and POAF, we used restricted cubic spline to model the odds of developing POAF as a function of the LA area. A threshold value for LA area was determined by identifying the point where there was a significant increase in the risk of POAF.
Inter- and intra-observer reproducibility for the evaluation of pericardial effusion and LA measurements (LA area and length) were assessed by means of intraclass correlation coefficient (ICC),[16] which measures the strength of agreement by comparing the variability in the ratings. The closer the value to 1, the better the agreement. Reproducibility was assessed in 24 patients for pericardial effusion and in 20 patients for LA measurements with paired measurements by two raters.
Results from the multivariable regression models are presented as ORs and corresponding 95%CI. Two-tailed p-value<0.05 was considered statistically significant without multiplicity adjustment. All statistical analyses were performed using R Statistical Software (version 3.2.3; R Foundation for Statistical Computing, Vienna, Austria).
Sources of funding
Dr Rong is funded by NIH NHLBI K23 HL153836. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
After excluding patients without available postoperative echocardiography data (n=33), 387 of the 420 patients included in the PALACS trial (92%) were included in this analysis. Excluded patients did not differ from included patients in any baseline characteristics (Supplementary Table 1). Pre-discharge TTEs were performed after a median of 5 days postoperatively (IQR 4–7). Of the 387 included patients, 96 (24.8%) were female and the median age was 62.0 [IQR 53.0–70.0] years (Table 1).
Table 1.
Baseline and operative characteristics in the overall cohort and by presence of postoperative pericardial effusion.
| Variable | Overall | No pericardial effusion | Pericardial effusion | p-value1 |
|---|---|---|---|---|
| Number of patients | 387 | 80 | 307 | |
| Age, years | 62.0 (53.0, 70.0) | 60.5 (51.8, 67.0) | 62.0 (54.0, 70.0) | 0.15 |
| Female sex | 96 (24.8) | 16 (20.0) | 80 (26.1) | 0.33 |
| Race | 0.27 | |||
| Asian | 15 (3.9) | 3 (3.8) | 12 (3.9) | |
| Black or African American | 23 (5.9) | 8 (10.0) | 15 (4.9) | |
| Others | 48 (12.4) | 12 (15.0) | 36 (11.7) | |
| White | 301 (77.8) | 57 (71.2) | 244 (79.5) | |
| Body mass index, kg/m2 | 27.8 (24.6, 30.4) | 29.0 (25.4, 31.0) | 27.4 (24.5, 30.2) | 0.10 |
| Hypertension | 268 (69.3) | 54 (67.5) | 214 (69.7) | 0.81 |
| Diabetes | 84 (21.7) | 13 (16.2) | 71 (23.1) | 0.24 |
| Smoking | 0.51 | |||
| • Never | 211 (54.5) | 46 (57.5) | 165 (53.7) | |
| • Current | 22 (5.7) | 6 (7.5) | 16 (5.2) | |
| • Previous | 154 (39.8) | 28 (35.0) | 126 (41.0) | |
| NYHA class III-IV | 30 (7.8) | 9 (11.2) | 21 (6.8) | 0.28 |
| Chronic lung disease | 12 (3.1) | 1 (1.2) | 11 (3.6) | 0.48 |
| Previous myocardial infarction | 52 (13.4) | 8 (10.0) | 44 (14.3) | 0.41 |
| Previous stroke | 13 (3.4) | 4 (5.0) | 9 (2.9) | 0.57 |
| Preoperative hematocrit | 39.7 (35.8, 43.2) | 40.1 (35.6, 44.1) | 39.7 (35.8, 43.1) | 0.69 |
| EuroSCORE II | 1.3 (0.9, 2.2) | 1.4 (1.0, 2.2) | 1.3 (0.9, 2.2) | 0.7 |
| Coronary artery bypass grafting | 174 (45.0) | 31 (38.8) | 143 (46.6) | 0.26 |
| Aortic valve procedures | 205 (53.0) | 46 (57.5) | 159 (51.8) | 0.3 |
| Aortic procedures | 165 (42.6) | 32 (40.0) | 133 (43.3) | 0.68 |
| Posterior pericardiotomy | 193 (49.9) | 58 (72.5) | 135 (44.0) | <0.001 |
| Cross-clamp time, minutes | 79.0 (61.8, 100.0) | 78.5 (60.8, 96.8) | 79.0 (62.0, 100.0) | 0.56 |
| Cardiopulmonary bypass time, minutes | 102.0 (83.0, 123.0) | 102.0 (84.0, 120.5) | 103.0 (82.0, 124.0) | 0.84 |
| Operative time, minutes | 301.0 (258.0, 356.0) | 326.5 (268.0, 368.3) | 298.0 (253.5, 351.5) | 0.11 |
NYHA, New York Heart Association.
Data are reported as median (IQR) and n (%)
Mann-Whitney U test; Pearson’s Chi-squared test
Analysis of pericardial effusion
Postoperative pericardial effusion was present in 307 (79%) patients and the median effusion width was 5.0 mm [IQR3.0–7.5]. Two-hundred sixty-two (67.7%) patients had anterior pericardial effusion with median width of 5.6 mm [IQR 3.0–9.8], and 201 (51.9%) patients had postero-lateral effusion with median width of 4.0 mm [IQR 2.5–5.0] (Table 2). Diabetes and aortic surgery were independently associated with postoperative pericardial effusion (Table 3).
Table 2.
Postoperative pericardial effusion of any size in patients with and without postoperative atrial fibrillation.
| Variable | Overall | No POAF | POAF | p-value1 |
|---|---|---|---|---|
| Number of patients | 387 | 291 | 96 | -- |
| Number of patients with any pericardial effusion | 307 (79.3) | 232 (79.7) | 75 (78.1) | 0.85 |
| Number of patients with any anterior pericardial effusion | 262 (67.7) | 196 (67.4) | 66 (68.8) | 0.89 |
| Number of patients with any postero-lateral pericardial effusion | 201 (51.9) | 156 (53.6) | 45 (46.9) | 0.30 |
| Median anterior pericardial effusion width (mm) | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.5) | 5.0 (3.0, 7.0) | 0.42 |
| Median postero-lateral pericardial effusion width (mm) | 4.5 (3.0, 7.0) | 4.0 (3.0, 6.4) | 5.0 (3.0, 9.0) | 0.06 |
POAF, postoperative atrial fibrillation.
Data are reported as median (IQR) and n (%)
Mann-Whitney U test; Pearson’s Chi-squared test
Table 3.
Risk factors for postoperative pericardial effusion.
| Variables | Odds ratio (95%CI) | p-value |
|---|---|---|
| Age (years) | 1.02 (1.00, 1.04) | 0.10 |
| Female sex | 1.56 (0.77, 3.14) | 0.22 |
| Body mass index (kg/m2) | 0.93 (0.88, 0.99) | 0.01 |
| Diabetes | 2.56 (1.15, 5.70) | 0.02 |
| NYHA class > II | 0.46 (0.19, 1.12) | 0.09 |
| Preoperative hematocrit (%) | 1.01 (0.97, 1.06) | 0.61 |
| EuroSCORE II (per point) | 0.94 (0.85, 1.04) | 0.24 |
| Surgery: Coronary artery bypass grafting | 2.04 (0.92, 4.51) | 0.08 |
| Surgery: Aortic valve procedures | 0.94 (0.50, 1.78) | 0.85 |
| Surgery: Aortic procedures | 2.33 (1.13, 4.80) | 0.02 |
| Cardiopulmonary bypass time (minutes) | 1.00 (0.99, 1.01) | 0.56 |
| Operative time (minutes) | 1.00 (0.99, 1.00) | 0.07 |
| Race (%) | ||
| White | Reference | |
| Asian | 0.97 [0.19, 4.86] | 0.97 |
| Black or African American | 0.35 [0.13, 0.94] | 0.03 |
| Other | 0.51 [0.23, 1.09] | 0.08 |
CI, confidence interval; NYHA, New York Heart Association.
The overall incidence of pericardial effusions was lower in patients undergoing posterior pericardiotomy (70% vs 89%, p<0.001). When analyzing the data based on the location, postero-lateral effusions were significantly less frequent in the pericardiotomy group (37% vs 67%, p<0.001), while the incidence of anterior effusions was not different between the two groups (63% vs 72%, p=0.08; Figure 2, panels A-B).
Figure 2. Bar plots showing the details of postoperative pericardial effusion anteriorly (A-C) and postero-laterally (B-D), according to intervention group (no intervention vs posterior pericardiotomy) and postoperative cardiac rhythm (no postoperative atrial fibrillation vs postoperative atrial fibrillation).
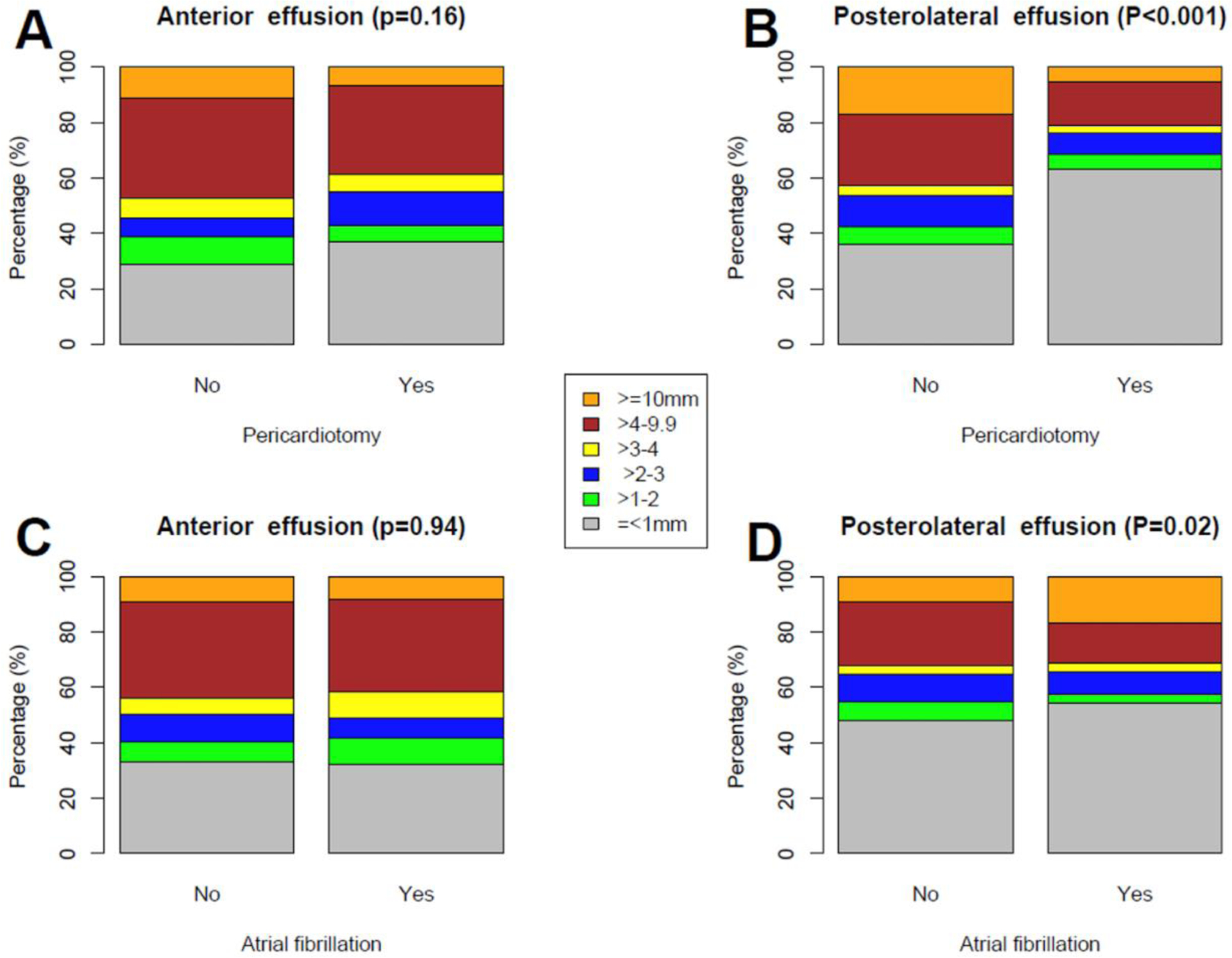
P-values from Pearson’s χ2 test comparing the proportion of pericardial effusions ≥10 mm with other groups.
The median width of anterior effusion was comparable between patients with and without POAF (5.0 mm [IQR 3.0–7.0] vs 5.0 mm [IQR 3.0–7.5], p=0.42), but there was a trend towards larger postero-lateral effusion in the POAF group (5.0 mm [IQR 3.0–9.0] vs 4.0 mm [IQR 3.0–6.4], p=0.06). There was also a significantly higher proportion of postero-lateral effusions ≥10 mm in the POAF group (10.4% vs 4.1%, p=0.02; Figure 2, panels C-D). At spline analysis, there was a non-linear association between postero-lateral pericardial effusion and POAF, with a cut-off at 10 mm (OR 2.7; 95%CI 1.13, 6.47; p=0.03; Figure 3). In the fully adjusted multivariable analysis, postero-lateral effusions ≥10 mm were significantly associated with POAF (OR 3.52; 95%CI 1.17, 10.58; p=0.02; Table 4); this result was confirmed in the multivariable model adjusted for LA area and reservoir function (OR 2.64; 95%CI 1.04, 6.68; p=0.04; Supplementary Table 2). The sensitivity analysis based on postoperative TEE confirmed the statistically significant association between pericardial effusion and POAF (Supplementary Tables 3 and 4).
Figure 3.
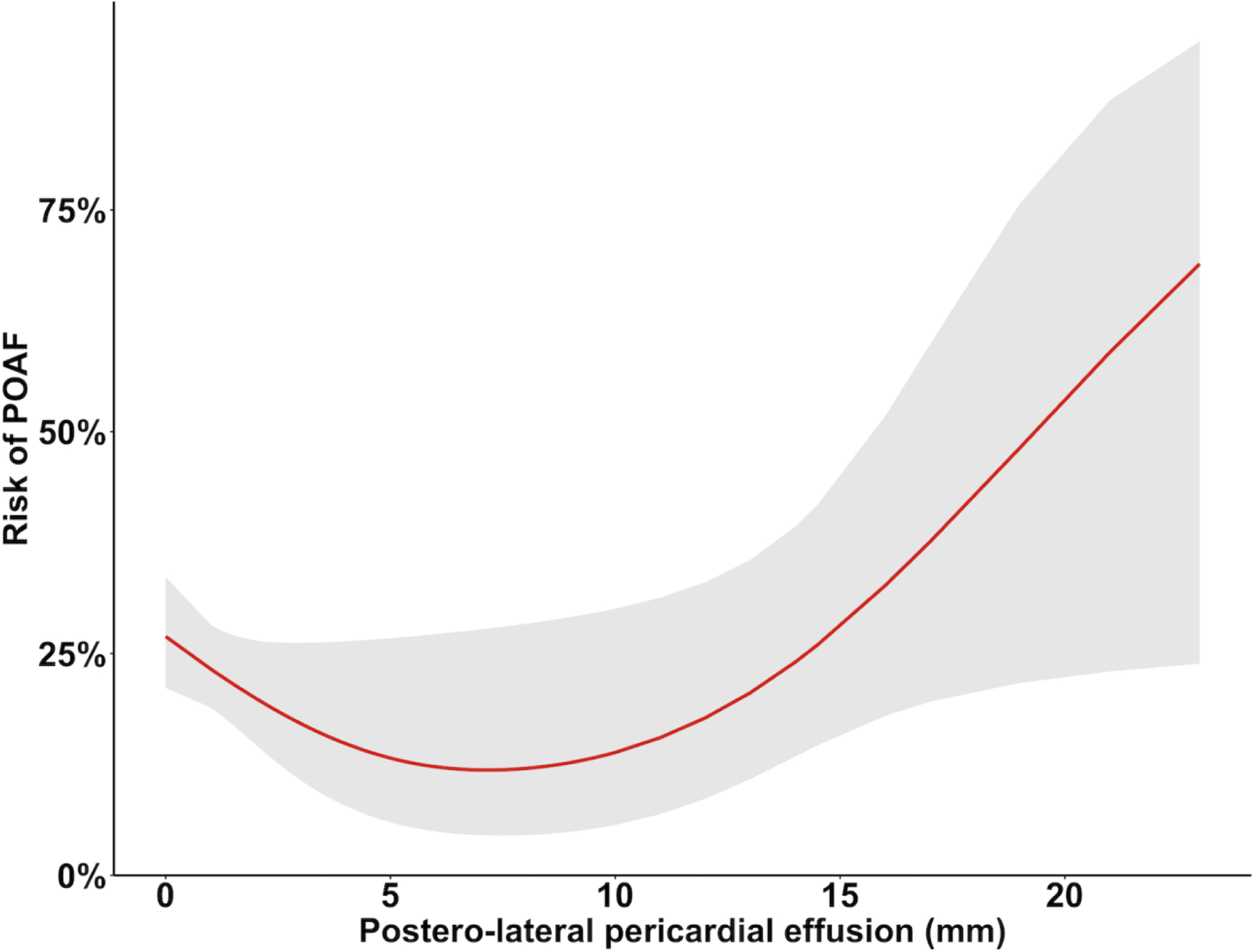
Non-linear (spline) model with 3 knots describing the relationship between postero-lateral pericardial effusion and postoperative atrial fibrillation (POAF).
Table 4.
Risk factors for postoperative atrial fibrillation.
| Odds ratio (95%CI) | p-value | |
|---|---|---|
| Postero-lateral effusion ≥10 mm | 3.52 (1.17, 10.58) | 0.02 |
| Age (years) | 1.08 (1.05, 1.12) | <0.001 |
| Female sex | 0.38 (0.18, 0.79) | 0.01 |
| Diabetes | 1.30 (0.63, 2.68) | 0.47 |
| Left ventricular ejection fraction (%) | 0.98 (0.95, 1.01) | 0.10 |
| Coronary artery bypass grafting | 0.75 (0.59, 0.94) | 0.01 |
| NYHA class > II | 1.16 (0.44, 3.08) | 0.76 |
| Chronic lung disease | 1.26 (0.27, 5.93) | 0.77 |
| EuroSCORE II (per point) | 1.09 (0.93, 1.27) | 0.30 |
| Preoperative use of beta-blockers | 1.65 (0.92, 2.96) | 0.09 |
| Postoperative use of beta-blockers | 0.12 (0.04, 0.33) | <0.001 |
CI, confidence interval; NYHA, New York Heart Association.
Analysis of LA size and function
Preoperative LA volume was significantly larger in patients who had POAF (Table 5). In multivariable analysis LA area was found to be independently associated with POAF (Supplementary Table 5). No differences in LA indices were found between patients who received posterior pericardiotomy vs no intervention (Supplementary Table 6). On sensitivity analysis, the spline curve demonstrated a steep progressive increase in risk of developing POAF for preoperative TEE LA area higher than 15 cm2 (Supplementary Figure 2).
Table 5.
Comparison of preoperative and postoperative transeophageal echocardiography (TEE) left atrial indices in patients with and without postoperative atrial fibrillation (POAF).
| Overall | No POAF | POAF | p-value1 | |
|---|---|---|---|---|
| Number of patients | 387 | 291 | 96 | |
| Preoperative LA indices | ||||
| Length, median (IQR) (cm) | 4.30 (3.65, 5.00) | 4.25 (3.60, 4.90) | 4.35 (3.66, 5.14) | 0.28 |
| Area, median (IQR) (cm2) | 15.55 (11.95, 19.05) | 15.40 (11.68, 18.60) | 15.93 (13.16, 20.20) | 0.09 |
| Volume, median (IQR) (ml) | 47.21 (34.27, 62.01) | 45.20 (32.79, 59.08) | 50.56 (38.79, 68.88) | 0.04 |
| Reservoir, median (IQR) (%) | 33.01 (26.45, 37.72) | 33.01 (27.36, 37.94) | 33.06 (26.07, 36.60) | 0.56 |
| Postoperative change in LA indices * | ||||
| Length, median (IQR) (cm) | −0.08 (−0.60, 0.40) | −0.05 (−0.59, 0.44) | −0.15 (−0.70, 0.16) | 0.34 |
| Area, median (IQR) (cm2) | −0.65 (−3.35, 1.80) | −0.50 (−3.40, 1.95) | −1.13 (−3.35, 1.20) | 0.44 |
| Volume, median (IQR) (ml) | −3.42 (−14.47, 6.06) | −2.72 (−14.78, 6.54) | −5.87 (−14.06, 3.72) | 0.32 |
| Reservoir, median (IQR) (%) | −2.58 (−8.34, 5.19) | −2.73 (−8.87, 5.21) | −2.09 (−7.44, 4.53) | 0.74 |
LA, left atrium.
Data are reported as median (IQR) and n (%)
Mann-Whitney U test
Calculated as postoperative – preoperative
Bland-Altman analyses confirmed the validity of TEE measurements, showing moderate offsets with no bias between LA length and LA area as measured from preoperative TTE vs preoperative TEE (Supplementary Table 7 and Supplementary Figure 3). Correlation analyses showed strong relations between preoperative TTE and preoperative TEE measurements of LA length and LA area (r=0.77 and r=0.83, respectively) (Supplementary Figure 4).
Analysis of intra-observer and inter-observer agreement
ICC showed high inter- and intra-observer reproducibility for both small (<10 mm) and larger (≥10 mm) pericardial effusions (Supplementary Tables 8 and 9). ICC also showed high inter- and intra-observer reproducibility for LA length and area (Supplementary Tables 10 and 11).
Discussion
In this explanatory analysis of the PALACS trial, we found that postero-lateral pericardial effusions ≥10 mm were associated with a statistically significant increase in the risk of POAF after cardiac surgery and that they were significantly reduced by posterior pericardiotomy. We also found that posterior pericardiotomy did not significantly modify LA dimensions or function in the immediate postoperative period and did not affect anterior pericardial effusions. To the best of our knowledge, this is the first study to directly show a significant association between pericardial effusion and POAF in the cardiac surgical population and to provide a potential mechanistic explanation of the effect of posterior pericardiotomy. Postoperative pericardial effusion is a very common finding after cardiac surgery; the exact incidence varies based on study design and assessment method used, but studies with prospective systematic echocardiographic follow-up report rates of 70 to 80%.[3–5] POAF affects 25 to 40% of patients and is the most common complication after cardiac surgery.[17] Historically, POAF has been considered a relatively benign condition but there is now evidence that it is associated with increased postoperative morbidity (e.g., cerebrovascular accidents), costs and resource utilization.[17–20] The estimated healthcare expenditure related to the burden of POAF in the United States is over 1 billion dollars annually.[17]
Different mechanisms for the development of POAF have been proposed. The majority of the available evidence suggests that neurohormonal activation and systemic inflammation may play a key role.[21] However, therapeutic strategies targeting these mechanisms, such as beta-blockers and anti-inflammatory drugs, have shown suboptimal effectiveness and are limited in their use by side effects.[21]
Indeed, there is growing interest in the role of local (pericardial) inflammation in triggering POAF. Blood in the pericardial space after surgery has been shown to exert a proinflammatory effect subsequent to the activation of the clotting cascade and the production of thrombin and fibrin.[22,23] Local inflammation could also be sustained by haemolysis, which releases haemoglobin that is rapidly oxidized into methaemoglobin, which in turn facilitates the diapedesis and activation of leukocytes.[24] The ultimate result is the recruitment and collection of activated white blood cells producing reactive oxygen species and prompting oxidative stress within the pericardial space. This pro-inflammatory and prooxidant environment has been shown to trigger POAF (Graphical Abstract).[6,25]
The association between pericardial effusion and POAF development is indirectly supported by clinical series. In a study enrolling 231 patients undergoing isolated aortic valve replacement, the incidence of POAF was the highest in patients undergoing surgical replacement (62%) as opposed to trans-apical (53%), trans-aortic (33%) and transfemoral (14%) transcatheter aortic valve replacement, suggesting that avoidance of exposure of the pericardial space to blood might reduce the incidence of POAF.[26] Other studies showed that the use of multi-drainage chest tubes with the aim to maintain a continuous effective drainage of the pericardial cavity was associated with a 2-to-3-fold reduction in the incidence of POAF.[27,28] A study investigating the incidence of chest tube clogging found that patients with clogged chest tubes had a higher incidence of POAF compared to patients with unblocked chest drainage (50 vs 21.9%; p=0.005).[29]
Posterior pericardiotomy provides an effective drainage of pericardial effusion and has been shown to be associated with a significant reduction in the risk of POAF. A meta-analysis of 10 randomized clinical trials including 1829 patients, found that posterior pericardiotomy was highly effective in reducing the incidence of POAF (RR 0.45; 95%CI 0.29, 0.64; p<0.0001) and postoperative pericardial effusions (RR 0.28; 95%CI 0.15, 0.50; p<0.05).[2]In the PALACS trial we reported that the incidence of POAF was significantly reduced in patients undergoing posterior left pericardiotomy (17% vs 32%, p=0.0007).[1] To date however the mechanisms of POAF reduction by pericardiotomy have not been rigorously investigated.
The summary of the current evidence and the results of this analysis suggest the existence of a potential causal link between postoperative pericardial effusion and POAF and that the effect of posterior pericardiotomy on POAF is mediated by a reduction of postero-lateral pericardial effusions. The finding that postoperative pericardial effusion on TEE performed at the end of surgery was independently associated with POAF suggests that pericardial effusion preceded POAF and strengthens the hypothesis of a causal association between the two. Also, the fact that only postero-lateral, not anterior, effusions were associated with POAF suggests that a local process (probably atrial inflammation) likely played a key role in POAF etiology in those patients. Further research will be needed to understand the causal mechanism which mediates the association between pericardial effusion and POAF, such as inflammation, mechanical compression, or otherwise. Mechanistic studies evaluating pro-inflammatory markers will therefore be a helpful first step to provide an answer to the pathophysiologic role of pericardial effusion in POAF.
It is interesting to note that the cutoff of postero-lateral pericardial effusions that we identified for POAF is consistent with the echocardiographic clinical cutoff in current guidelines.[11]
Our findings add to a growing body of evidence supporting the concept that strategies aimed at reducing postoperative pericardial effusion (including posterior pericardiotomy and active drainage)[1,30] reduce POAF occurrence.[6,31] Since such strategies might be more effective, have fewer side-effects, and lower costs compared to current treatments (e.g., prophylactic antiarrhythmic drugs, colchicine, steroids, magnesium, and statins, as well as postoperative overdrive atrial pacing),[31] clinicians should consider a more widespread adoption, especially in those patients at higher risk of developing POAF. This will be further encouraged if future data, such as the follow-up of the PALACS trial, will demonstrate a reduction in the risk of long-term cardiovascular events secondary to the reduction in the post-operative pericardial effusion.
Limitations
The results of this study should be interpreted within the context of its limitations. The PALACS trial cohort included patients at low risk of POAF, excluded patients undergoing mitral or tricuspid valve surgery and those with dilated LA. Also, the trial was performed at a single center and for all these reasons, our results may have limited generalizability. There also may be imaging limitations of the study. First, the limited quality of the transthoracic subcostal echocardiographic view in the postoperative period may have led to underestimation of pericardial effusions. Another imaging limitation is that LA size quantification may not be accurate on TEE. However, on sensitivity analysis, we found excellent correlation between LA measurements obtained at preoperative TEE compared to preoperative TTE. It is also possible that linear measurements of postoperative TTE effusion may have been imprecise for quantification of effusion size, especially for small pericardial effusions; however, we found good inter- and intra-observer reproducibility for all effusion measurements. Also, the TEE and TTE evaluation were limited to a single time point and may not be representative of the entirety of post-surgical time period. In addition, although the pericardial effusion cut-off of 10mm shown to be predictive of POAF in our dataset is supported by the current practice guidelines for patients with pericardial disease,[11] it lacks formal external validation and is an area for future research. Finally, this post-hoc analysis was not formally powered to detect differences in POAF according to the presence of pericardial effusion.
Conclusion
In conclusion, in this explanatory analysis of the PALACS trial, we found that postero-lateral pericardial effusions were associated with a statistically increased risk of POAF in patients undergoing cardiac surgery and that they were significantly reduced by posterior pericardiotomy. The reduction in postero-lateral pericardial effusions is the most plausible mechanism for the effect of posterior pericardiotomy in reducing POAF. Measures to reduce postoperative pericardial effusions are a promising approach to decrease POAF occurrence in cardiac surgical patients.
Supplementary Material
Highlights.
Posterior pericardiotomy is associated with lower rates of POAF after cardiac surgery
A plausible underlying mechanism is reduction in postero-lateral pericardial effusion
LA dimension/function did not change significantly after posterior pericardiotomy
Funding:
Dr Rong is funded by NIH NHLBI K23 HL153836-01A1
Abbreviations
- BMI
body mass index
- CI
confidence interval
- ICC
intraclass correlation coefficient
- IQR
interquartile range
- LA
left atrium
- NYHA
New York Heart Association
- OR
odds ratio
- PALACS
Posterior Left pericardiotomy for the prevention of postoperative Atrial fibrillation after Cardiac Surgery
- POAF
postoperative atrial fibrillation
- TTE
transthoracic echocardiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: Dr Di Franco has consulted for Novo Nordisk, Servier and is an Advisory Board Member for Scharper. The other authors have no disclosures.
Ethical approval: The PALACS trial (NCT02875405) was approved by the Weill Cornell Medicine Institutional Review Board (#1502015867) and all patients consented to study participation and data usage.
Data Sharing: Data collected for the study will be made available by the corresponding author upon reasonable request after publication.
References
- 1.Gaudino M, Sanna T, Ballman KV, et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single-centre, single-blind, randomised, controlled trial. The Lancet 2021;398:2075–83. doi: 10.1016/S0140-6736(21)02490-9 [DOI] [PubMed] [Google Scholar]
- 2.Xiong T, Pu L, Ma Y-F, et al. Posterior pericardiotomy to prevent new-onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis of 10 randomized controlled trials. J Cardiothorac Surg 2021;16:233. doi: 10.1186/s13019-021-01611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashikhmina EA, Schaff HV, Sinak LJ, et al. Pericardial Effusion After Cardiac Surgery: Risk Factors, Patient Profiles, and Contemporary Management. Ann Thorac Surg 2010;89:112–8. doi: 10.1016/j.athoracsur.2009.09.026 [DOI] [PubMed] [Google Scholar]
- 4.Pepi M, Muratori M, Barbier P, et al. Pericardial effusion after cardiac surgery: incidence, site, size, and haemodynamic consequences. Br Heart J 1994;72:327–31. doi: 10.1136/hrt.72.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikäheimo MJ, Huikuri HV, Airaksinen KE, et al. Pericardial effusion after cardiac surgery: incidence, relation to the type of surgery, antithrombotic therapy, and early coronary bypass graft patency. Am Heart J 1988;116:97–102. doi: 10.1016/0002-8703(88)90255-4 [DOI] [PubMed] [Google Scholar]
- 6.St-Onge S, Perrault LP, Demers P, et al. Pericardial Blood as a Trigger for Postoperative Atrial Fibrillation After Cardiac Surgery. Ann Thorac Surg 2018;105:321–8. doi: 10.1016/j.athoracsur.2017.07.045 [DOI] [PubMed] [Google Scholar]
- 7.Abouarab AA, Leonard JR, Ohmes LB, et al. Posterior Left pericardiotomy for the prevention of postoperative Atrial fibrillation after Cardiac Surgery (PALACS): study protocol for a randomized controlled trial. Trials 2017;18:593. doi: 10.1186/s13063-017-2334-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shore-Lesserson L, Moskowitz D, Hametz C, et al. Use of intraoperative transesophageal echocardiography to predict atrial fibrillation after coronary artery bypass grafting. Anesthesiology 2001;95:652–8. doi: 10.1097/00000542-200109000-00018 [DOI] [PubMed] [Google Scholar]
- 9.Leung JM, Bellows WH, Schiller NB. Impairment of left atrial function predicts post-operative atrial fibrillation after coronary artery bypass graft surgery. Eur Heart J 2004;25:1836–44. doi: 10.1016/j.ehj.2004.07.014 [DOI] [PubMed] [Google Scholar]
- 10.Jiamsripong P, Honda T, Reuss CS, et al. Three methods for evaluation of left atrial volume. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol 2008;9:351–5. doi: 10.1016/j.euje.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 11.Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013;26:965–1012.e15. doi: 10.1016/j.echo.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y-L, Zeng M, Liu Y, et al. CHA(2)DS(2)-VASc Score for Identifying Patients at High Risk of Postoperative Atrial Fibrillation After Cardiac Surgery: A Meta-analysis. Ann Thorac Surg 2020;109:1210–6. doi: 10.1016/j.athoracsur.2019.07.084 [DOI] [PubMed] [Google Scholar]
- 13.Chua S-K, Shyu K-G, Lu M-J, et al. Clinical utility of CHADS2 and CHA2DS2-VASc scoring systems for predicting postoperative atrial fibrillation after cardiac surgery. J Thorac Cardiovasc Surg 2013;146:919–926.e1. doi: 10.1016/j.jtcvs.2013.03.040 [DOI] [PubMed] [Google Scholar]
- 14.Lip GYH, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 15.Altman D, Bland J. Measurements in medicine: the analysis of method comparison studies. Statistician 1983;32:307–17. [Google Scholar]
- 16.Shrout P, Fleiss J. Intraclass Correlation: Uses in Assessing Rater Reliability. Psychol Bull 1979;86:420–8. [DOI] [PubMed] [Google Scholar]
- 17.Burrage PS, Low YH, Campbell NG, et al. New-Onset Atrial Fibrillation in Adult Patients After Cardiac Surgery. Curr Anesthesiol Rep 2019;9:174–93. doi: 10.1007/s40140-019-00321-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eikelboom R, Sanjanwala R, Le M-L, et al. Postoperative Atrial Fibrillation After Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann Thorac Surg 2021;111:544–54. doi: 10.1016/j.athoracsur.2020.05.104 [DOI] [PubMed] [Google Scholar]
- 19.Greenberg JW, Lancaster TS, Schuessler RB, et al. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardio-Thorac Surg 2017;52:665–72. doi: 10.1093/ejcts/ezx039 [DOI] [PubMed] [Google Scholar]
- 20.Benedetto U, Gaudino MF, Dimagli A, et al. Postoperative Atrial Fibrillation and Long-Term Risk of Stroke After Isolated Coronary Artery Bypass Graft Surgery. Circulation 2020;142:1320–9. doi: 10.1161/CIRCULATIONAHA.120.046940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Echahidi N, Pibarot P, O’Hara G, et al. Mechanisms, Prevention, and Treatment of Atrial Fibrillation After Cardiac Surgery. J Am Coll Cardiol 2008;51:793–801. doi: 10.1016/j.jacc.2007.10.043 [DOI] [PubMed] [Google Scholar]
- 22.CHEN D, DORLING A. Critical roles for thrombin in acute and chronic inflammation. J Thromb Haemost 2009;7:122–6. doi: 10.1111/j.1538-7836.2009.03413.x [DOI] [PubMed] [Google Scholar]
- 23.Jennewein C, Tran N, Paulus P, et al. Novel Aspects of Fibrin(ogen) Fragments during Inflammation. Mol Med 2011;17:568–73. doi: 10.2119/molmed.2010.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Spolarics Z. Methemoglobin is a potent activator of endothelial cells by stimulating IL-6 and IL-8 production and E-selectin membrane expression. Am J Physiol-Cell Physiol 2003;285:C1036–46. doi: 10.1152/ajpcell.00164.2003 [DOI] [PubMed] [Google Scholar]
- 25.Elahi MM, Flatman S, Matata BM. Tracing the origins of postoperative atrial fibrillation: the concept of oxidative stress-mediated myocardial injury phenomenon. Eur J Cardiovasc Prev Rehabil 2008;15:735–41. doi: 10.1097/HJR.0b013e328317f38a [DOI] [PubMed] [Google Scholar]
- 26.Tanawuttiwat T, O’Neill BP, Cohen MG, et al. New-onset atrial fibrillation after aortic valve replacement: comparison of transfemoral, transapical, transaortic, and surgical approaches. J Am Coll Cardiol 2014;63:1510–9. doi: 10.1016/j.jacc.2013.11.046 [DOI] [PubMed] [Google Scholar]
- 27.Ege T, Tatli E, Canbaz S, et al. The importance of intrapericardial drain selection in cardiac surgery. Chest 2004;126:1559–62. doi: 10.1378/chest.126.5.1559 [DOI] [PubMed] [Google Scholar]
- 28.Eryilmaz S, Emiroglu O, Eyileten Z, et al. Effect of posterior pericardial drainage on the incidence of pericardial effusion after ascending aortic surgery. J Thorac Cardiovasc Surg 2006;132:27–31. doi: 10.1016/j.jtcvs.2006.01.049 [DOI] [PubMed] [Google Scholar]
- 29.Karimov JH, Gillinov AM, Schenck L, et al. Incidence of chest tube clogging after cardiac surgery: a single-centre prospective observational study. Eur J Cardiothorac Surg 2013;44:1029–36. doi: 10.1093/ejcts/ezt140 [DOI] [PubMed] [Google Scholar]
- 30.Baribeau Y, Westbrook B, Baribeau Y, et al. Active clearance of chest tubes is associated with reduced postoperative complications and costs after cardiac surgery: a propensity matched analysis. J Cardiothorac Surg 2019;14:192. doi: 10.1186/s13019-019-0999-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudino M, Di Franco A, Rong LQ, et al. Pericardial Effusion Provoking Atrial Fibrillation After Cardiac Surgery: JACC Review Topic of the Week. J Am Coll Cardiol 2022;79:2529–39. doi: 10.1016/j.jacc.2022.04.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


