Abstract
Background:
Over the past 2 years of the several strategies recommended to help fight COVID-19, nirmatrelvir/ritonavir is a novel drug shown in the EPIC-HR phase 2 to 3 clinical trial to lower COVID-19-related death or hospitalization at day 28 when compared with placebo.
Objective:
Our study’s aim was to explore the reported adverse events (AEs) associated with nirmatrelvir/ritonavir use for COVID-19.
Method:
We conducted a retrospective analysis using the FDA Adverse Event Reporting System (FAERS) database for AEs, listing nirmatrelvir/ritonavir as the primary drug between January and June 2022. The primary outcome was the incidence of reported AEs associated with nirmatrelvir/ritonavir. The OpenFDA database was queried using Python 3.10 to collect the AEs and Stata 17 was used to analyze the database. Adverse events were analyzed by associated medication, with “Covid-19” excluded.
Results:
A total of 8098 reports were identified between January and June 2022. Most reported complaints in the AE system were COVID-19 and disease recurrence. The most common symptomatic AEs were dysgeusia, diarrhea, cough, fatigue, and headache. Event rates significantly rose between April and May. Disease recurrence and dysgeusia were the most commonly reported complaints for the top 8 concomitant drugs identified. Cardiac arrest, tremor, akathisia, and death were reported in 1, 3, 67, and 5 cases, respectively.
Conclusions and Relevance:
This is the first retrospective study done on reported AEs associated with nirmatrelvir/ritonavir use for COVID-19. COVID-19 and disease recurrence were the most reported AEs. Further monitoring of the FAERS database is warranted to periodically reassess the safety profile of this medication.
Keywords: COVID-19, FAERS, adverse events, antivirals, nirmatrelvir/ritonavir
Introduction
The coronavirus disease 2019 (COVID-19) outbreak has been an extraordinary threat to the global health care system. The underlying causative pathogen has been identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and it is transmitted primarily by respiratory droplets. 1 COVID-19 infection typically causes systemic symptoms, including fatigue, fever, cough, dyspnea, and myalgias. 2 For most individuals, the illness course is mild and self-resolves within a few weeks. 3 However, many patients have required hospitalization and developed severe respiratory failure that warrants mechanical ventilation. 4 While management consists of mainly supportive treatment, several antiviral agents have been granted emergency use authorization for COVID-19. 5
Nirmatrelvir/ritonavir is an oral antiviral drug that consists of a co-packaging of 2 separate medications to be taken together as part of the therapy for COVID-19 infection. 6 Nirmatrelvir is a peptidomimetic inhibitor of the SARS-CoV-2 main protease (Mpro) and ritonavir is a human immunodeficiency virus-1 (HIV-1) protease inhibition. 7 Dosing ritonavir with nirmatrelvir was found to increase plasma levels of nirmatrelvir. 8 In the EPIC-HR phase 2 to 3 clinical trial, it was found that the incidence of COVID-19-related death or hospitalization at day 28 was significantly lower in the nirmatrelvir/ritonavir group when compared with the placebo; however, adverse events (AEs) were noted to not be significantly different in comparison with placebo in the EPIC-SR, EPIC-HR and EPIC-PEP trials. 9 The risk of progression of COVID-19 to severe COVID-19 was 89% lower in the experimental nirmatrelvir group compared with the placebo group. 9 All deaths in the study occurred in the placebo group and it was found that the viral load was significantly lower at day 5 in the nirmatrelvir/ritonavir group compared with the placebo. 9 This poses an interesting and relevant question we wish to answer in this article: whether there are significant AEs and drug-drug interactions when assessed with a larger sample size, such as the FDA Adverse Event Reporting System (FAERS) database.
Although nirmatrelvir/ritonavir has shown strong efficacy in reducing hospitalization rates and associated mortality in COVID-19 patients, the safety profile of this medication has not been well described in the literature. 10 Nirmatrelvir/ritonavir has been demonstrated to strongly inhibit the cytochrome P450 (CYP3A4) system with the ritonavir component and has significant drug-drug interactions with other agents metabolized through this system.11,12 Some commonly reported adverse effects include disease recurrence, dysgeusia, diarrhea, hypertension, and myalgias.10,13-15 The goal of our study is to explore the increase in adverse effects associated with nirmatrelvir/ritonavir use that have been reported to the FDA from March 2022 to the present. We aim to highlight the growing incidence of these adverse effects and potential interactions by better describing the safety profile of nirmatrelvir/ritonavir.
Method
Search Strategy
A retrospective analysis was conducted using that FAERS system for cases reported listing nirmatrelvir/ritonavir as the primary drug between January 2022 and June 2022. Case reports were included if registered in the United States, included at least one patient-related AE, and had 3 or fewer concomitant over-the-counter or prescription medications associated with the report.
Data Collection and Analysis
The OpenFDA database was queried using Python 3.10 to collect AE data, and the results were stored in a database. 16 Stata 17 was used to analyze the organized database. 17 Duplicate reports were identified and removed. Cases were individually analyzed for completeness and reported events. Reports were examined using descriptive statistics.
Cases reported with a single concomitant medication were identified and separated for analysis. Second medications reported as “other” were excluded. A total of 302 cases reported met this inclusion criterion. Medications were then grouped according to their generic name, and HMG-CoA reductase inhibitors were combined into a single group, “statins.” Adverse events were tallied by associated medication and then plotted.
Results
A total of 8098 reports were identified between January 2022 and June 2022. COVID-19 and disease recurrence were the most commonly reported complaints in the AE system. Dysgeusia, diarrhea, cough, fatigue, and headache were the most common symptomatic AEs overall (Figure 1). Event rates were noted to increase in March 2022 and significantly rose between April and May 2022 (Figure 2).
Figure 1.
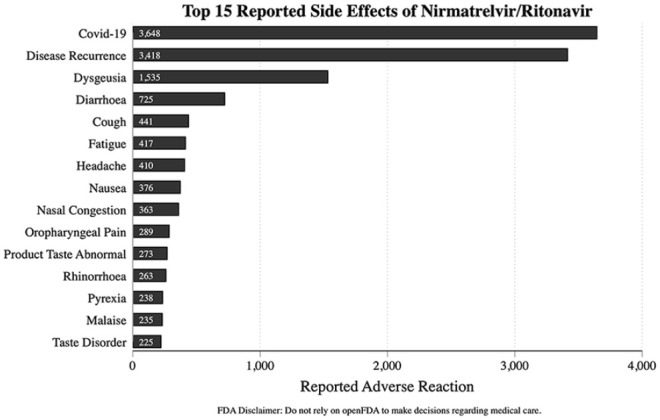
Top 15 reported side effects of nirmatrelvir/ritonavir.
Abbreviation: FDA, US Food & Drug Administration.
Figure 2.
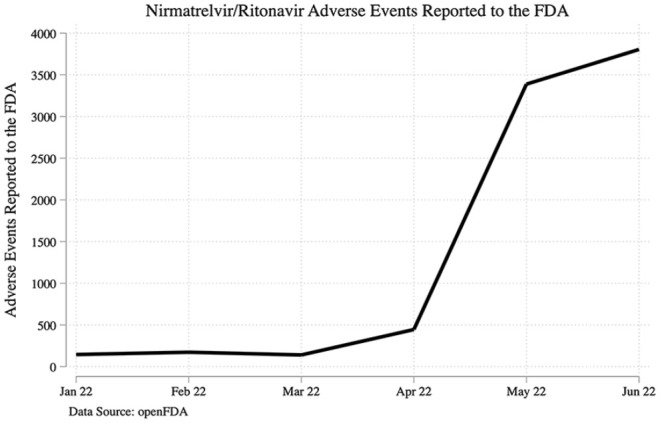
Timeline of reported events.
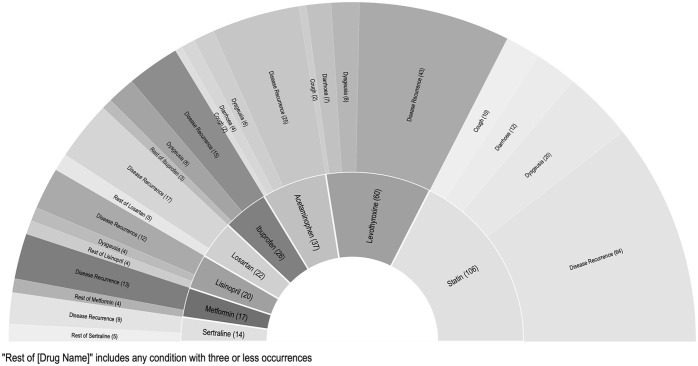
The use of a single second drug was identified in 302 cases. Disease recurrence and dysgeusia were the top 2 most commonly reported complaints for each of the top 8 concomitant drugs identified. Adverse events by comedication are presented in Figure 3. Death was reported in 5 cases. Cardiac arrest, anxiety, tremor, auditory hallucination, akathisia, and hyperhidrosis were reported in 1, 2, 3, 3, 67, and 73 cases, respectively.
Figure 3.
Reported events by single associated drug.
Abbreviation: FDA, US Food & Drug Administration.
Discussion
The US Food & Drug Administration (FDA) issued emergency use authorization of nirmatrelvir/ritonavir on December 22, 2021, for the treatment of mild to moderate COVID-19 infection in adults and children over the age of 12 and weighing over 40 kg who had positive SARS-CoV-2 viral testing and were also at higher risk of progression to hospitalization or death. 18 The FDA subsequently reissued the Letter of Authorization on March 17, 2022, April 14, 2022, and July 6, 2022, stating that there should be limitations applied to prescribing nirmatrelvir/ritonavir, including a review of recent medical records and labs, a list of the patient’s medications, and a referral for further evaluation if there is not enough information to assess renal and hepatic function or drug interactions according to the FDA fact sheet for providers. 19
Our analysis of the FAERS data demonstrated a significant increase in the number of AEs since April 2022 from 500 reported events a month to approximately 3500 reported events over the time span of 1 month. This is likely related to increasing with the increased availability and repeated letters of authorization for use by the FDA. COVID-19 was the most common report; however, this may have been an error due to the voluntary reporting system. The most common AEs reported were disease recurrence after treatment at 3418 reported events, dysgeusia with 1535 reported events, diarrhea with 725 instances, and 441 incidents with cough, concordant with other studies. A study by Wang et al 20 showed COVID-19 recurrence at 3.53% and 5.4%, respectively, at 7 and 30 days post-treatment. A meta-analysis by Wen et al 21 noted that the most frequent AEs were nausea, diarrhea, runny nose, and muscle pain; this was consistent among all COVID-19 antiviral medications.
Nirmatrelvir/ritonavir is a rapid and potent inhibitor of the cytochrome P450, especially 3A4. 22 This causes a wide array of drug-drug interactions and must be used carefully on patients with multiple comorbidities and on multiple medications. Most adverse reactions were in line with the most common overall AEs. Statins were found to have the highest incidence of AEs with disease recurrence having the highest frequency at 64 events. Levothyroxine had the second most events followed by acetaminophen and ibuprofen. The top AEs were similar for each drug. This is likely due to the virus or nirmatrelvir/ritonavir, not specifically drug-drug interactions. It is likely that these drugs were most commonly noted due to their frequent and ubiquitous use. Many anti-hypertensive medications and antidepressants were also noted to have similar interactions. Although no significant events in the FAERS database were noted for cross-reactivity with direct oral anticoagulants and corticosteroids, they are still medications clinicians should be aware of as they have known to have interactions with ritonavir. This was noted in Wang and Chan 23 pharmacokinetic modeling-based study noting the need for possible dose adjustment of direct oral anticoagulants due to drug-drug interactions in patients requiring antiviral therapy. Our study was limited by the events documented in the FAERS database being reported voluntarily, potentially missing AEs that were not reported. This could also be seen as the possibility of not reporting “rebound” COVID-19 as an AE but rather being managed as a separate clinical entity by physicians instead, thus not showing up in our analysis.
Conclusion and Relevance
Polypharmacy is commonly seen in the elderly population, who are also at more of a risk for more severe COVID-19 infections. Although the drug interactions of ritonavir have been studied in the past, the use of nirmatrelvir/ritonavir in patients with COVID-19 still requires a deeper understanding of DDI in this patient population for their appropriate risk-benefit analysis. Further analysis and monitoring of the FAERS data is warranted to periodically reassess the safety profile in the use of these antiviral agents as the use of these agents continues to rise.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Viraaj Pannu  https://orcid.org/0000-0001-6315-0186
https://orcid.org/0000-0001-6315-0186
References
- 1.Mohamadian M, Chiti H, Shoghli A, Biglari S, Parsamanesh N, Esmaeilzadeh A. COVID-19: virology, biology and novel laboratory diagnosis. J Gene Med. 2021;23(2):e3303. doi: 10.1002/jgm.3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133(7-8):377-382. doi: 10.1007/s00508-020-01760-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair PW, Brown DM, Jang M, et al. Ambulatory COVID study team. the clinical course of COVID-19 in the outpatient setting: a prospective cohort study. Open Forum Infect Dis. 2021;8(2):ofab007. doi: 10.1093/ofid/ofab007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brioni M, Meli A, Grasselli G. Mechanical ventilation for COVID-19 patients. Semin Respir Crit Care Med. 2022;43(3):405-416. doi: 10.1055/s-0042-1744305 [DOI] [PubMed] [Google Scholar]
- 5.Moshkovits I, Shepshelovich D. Emergency use authorizations of COVID-19-related medical products. JAMA Intern Med. 2022;182(2):228-229. doi: 10.1001/jamainternmed.2021.7257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb YN. Nirmatrelvir plus ritonavir: first approval. Drugs. 2022;82(5):585-591. doi: 10.1007/s40265-022-01692-5 [Erratum in: Drugs. 2022;82(9):1025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ullrich S, Ekanayake KB, Otting G, Nitsche C. Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir. Bioorg Med Chem Lett. 2022;62:128629. doi: 10.1016/j.bmcl.2022.128629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam C, Patel P. Nirmatrelvir/Ritonavir. StatPearls Publishing; 2022. Accessed August 18, 2022. https://www.ncbi.nlm.nih.gov/books/NBK585126/ [PubMed] [Google Scholar]
- 9.Hammond J, Leister-Tebbe H, Gardner A, et al. EPIC-HR investigators. oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397-1408. doi: 10.1056/NEJMoa2118542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis. 2022;76:e342-e349. doi: 10.1093/cid/ciac443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culas R, Nath S, Nath S. Safely prescribing nirmatrelvir and ritonavir—Avoiding drug-drug interactions. JAMA Intern Med. 2023;183:362-363. doi: 10.1001/jamainternmed.2022.6834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury . Paxlovid. National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Accessed January 31, 2022. https://www.ncbi.nlm.nih.gov/books/NBK577815/ [PubMed] [Google Scholar]
- 13.Chaplin S. Paxlovid: antiviral combination for the treatment of COVID-19. Prescriber. 2022;33(3-4):31-33. doi: 10.1002/psb.1979 [DOI] [Google Scholar]
- 14.Fact sheet for healthcare providers: emergency use authorization for paxlovid, September2022. https://www.fda.gov/media/155050/download
- 15.Wang Y, Chen X, Xiao W, Zhao D, Feng L. Rapid COVID-19 rebound in a severe COVID-19 patient during 20-day course of paxlovid. J Infect. 2022;85(5):e134-e136. doi: 10.1016/j.jinf.2022.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Rossum G. Python Tutorial (Technical Report CS-R9526). Centrum voor Wiskunde en Informatica; May1995. [Google Scholar]
- 17.StataCorp. 2021. Stata Statistical Software: Release 17. StataCorp LLC. [Google Scholar]
- 18.Parums DV. Editorial: current status of oral antiviral drug treatments for SARS-CoV-2 infection in non-hospitalized patients. Med Sci Monit. 2022;28:e935952. doi: 10.12659/MSM.935952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Food and Drug Administration. Coronavirus (covid-19) update: FDA authorizes pharmacists to prescribe paxlovid with certain limitations. July6, 2022. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pharmacists-prescribe-paxlovid-certain-limitations
- 20.Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R. COVID-19 rebound after paxlovid and molnupiravir during January-June 2022 [Preprint]. medRxiv; June22, 2022. doi: 10.1101/2022.06.21.22276724 [DOI] [Google Scholar]
- 21.Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54(1):516-523. doi: 10.1080/07853890.2022.2034936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzolini C, Kuritzkes DR, Marra F, et al. Recommendations for the management of drug–drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (paxlovid) and comedications. Clin Pharmacol Ther. 2022;112:1191-1200. doi: 10.1002/cpt.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Chan ECY. Physiologically-based pharmacokinetic modeling-guided dose management of oral anticoagulants when initiating nirmatrelvir/ritonavir (paxlovid) for COVID-19 treatment. Clin Pharmacol Ther. 2022;112(4):803-807. doi: 10.1002/cpt.2687PMC9349724 [DOI] [PMC free article] [PubMed] [Google Scholar]