Abstract
Epidemiological studies show an association between low body selenium and the risk of hypertension. However, whether selenium deficiency causes hypertension remains unknown. Here, we report that Sprague-Dawley rats fed a selenium-deficient diet for 16 weeks developed hypertension, accompanied with decreased sodium excretion. The hypertension of selenium-deficient rats was associated with increased renal angiotensin II type 1 receptor (AT1R) expression and function that was reflected by the increase in sodium excretion after the intrarenal infusion of the AT1R antagonist candesartan. Selenium-deficient rats had increased systemic and renal oxidative stress; treatment with the antioxidant tempol for 4 weeks decreased the elevated blood pressure, increased sodium excretion, and normalized renal AT1R expression. Among the altered selenoproteins in selenium-deficient rats, the decrease in renal glutathione peroxidase 1 (GPx1) expression was most prominent. GPx1, via regulation of NF-κB p65 expression and activity, was involved in the regulation of renal AT1R expression because GPx1 silencing or treatment with dithiocarbamate (PDTC), an NF-κB inhibitor, reversed the up-regulation of AT1R expression in selenium-deficient renal proximal tubule (RPT) cells. The up-regulation of AT1R expression with GPx1 silencing was restored by PDTC. Moreover, treatment with ebselen, a GPX1 mimic, reduced the increased renal AT1R expression, Na+-K+-ATPase activity, hydrogen peroxide (H2O2) generation, and the nuclear translocation of NF-κB p65 protein in selenium-deficient RPT cells. Our results demonstrated that long-term selenium deficiency causes hypertension, which is due, at least in part, to decreased urine sodium excretion. Selenium deficiency increases H2O2 production by reducing GPx1 expression, which enhances NF-κB activity, increases renal AT1R expression, causes sodium retention and consequently increases blood pressure.
Keywords: angiotensin II type 1 receptor, hypertension, kidney, oxidative stress, selenium deficiency
1. Introduction
Hypertension is one of the most important risk factors for coronary heart disease, heart failure, stroke, and other cardiovascular diseases [1]. The number of people with hypertension worldwide has doubled from 1990 to 2019 [2]. High systolic blood pressure was the third-leading health risk and cause of associated deaths in the United States in 2016 [3]. Thus, hypertension is a major public health problem [4].
The pathogenesis of essential hypertension is complex, involving genetics, epigenetics, behavior, and the environment [5]. It is well accepted that dietary factors such as high sodium, high fat, and refined carbohydrates are associated with hypertension [6]. In recent years, the importance of trace elements in the regulation of blood pressure has caught attention [7, 8]. Selenium, an essential trace element required for optimal human health, is incorporated into important amino acid derivatives, such as selenocysteine and selenomethionine, which are required for the synthesis selenoproteins [9]. Selenoproteins participate in various important physiological processes, including oxidative stress, inflammation, and immunity [10–12]. However, inadequate selenium may disrupt these processes which are involved in the pathogenesis of hypertension [13, 14].
Selenium deficiency is a serious problem worldwide [15]. Due to inadequate intake, 500 million to 1 billion people have selenium deficiency [16]. Approximately 51% of the soil in China is selenium deficient, and 39%—61% of the Chinese population have low daily selenium intake [17]. Selenium deficiency is associated with multiple cardiovascular diseases, including myocardial infarction, heart failure, coronary heart disease, and atherosclerosis [18]. There are a few human epidemiological studies showing a positive association between low serum selenium concentration and essential hypertension, and pregnancy-induced hypertension [19–22]. However, whether selenium deficiency causes hypertension remains unknown. Our present study determined the role and mechanism of selenium deficiency in the pathogenesis of hypertension.
2. Materials and methods
2.1. Animal preparation and treatment
Three-week-old Sprague-Dawley (SD) rats were obtained from the Animal Centre of The Third Military Medical University (Chongqing, China). The rats were maintained and treated in the Animal Centre of Daping Hospital. The protocols used in this study were approved by the Third Military Medical University Animal Use and Care Committee. All experiments conformed to the guidelines for the ethical use of animals, and all efforts were made to minimize animal suffering and reduce the number of animals used.
All rats were randomly divided into two groups fed with different diets: selenium-normal diet containing 0.180 mg/kg selenium and selenium-deficient diet containing 0.018 mg/kg selenium (TROPHIC Animal Feed High-tech Co., Jiangsu, China). The drinking water was deionized water. After feeding for 16 weeks, the rats, aged 19 weeks, were infused with candesartan, an angiotensin II type 1 receptor (AT1R) antagonist, via the right suprarenal artery (vide infra). The experimental protocol is shown in Flow diagram 1 (Supplemental Fig. 1).
In another set of experiments, the rats were treated with the antioxidant tempol, as described in our previous report [23]. In brief, after feeding the rats with the indicated diets for 12 weeks, selenium-normal diet-fed rats were randomly assigned into selenium-normal group (only selenium-normal diet) and selenium-normal+tempol group (selenium-normal diet+tempol). Selenium deficient diet-fed rats were also randomly assigned into selenium-deficient group (selenium-deficient diet) and selenium-deficient+tempol group (selenium-deficient diet+tempol). Treatment with tempol consisted with the addition of 1 mM tempol in deionized water (Sigma, St. Louis, MO, USA) for 4 weeks (twice a day). The diagram is shown in Flow diagram 2 (Supplemental Fig. 2).
2.2. Animal surgery for renal arterial perfusion
The detailed protocols of rat surgery for renal arterial perfusion were reported in our previous studies [23, 24]. In brief, the rats were anesthetized with the intraperitoneal injection of pentobarbital (50 mg/kg) and tracheotomized (PE-240). Anesthesia was maintained with the intravenous injection of pentobarbital (8 mg/kg body weight/h). To maintain a stable urinary volume, normal saline solution equal to 1 % body weight per hour was infused to replace the insensible water loss. Catheters (PE-50) were placed into the external jugular and femoral veins, for fluid replacement, and carotid artery, for monitoring systemic arterial pressure (Grass Instrument, Quincy, MA). After a laparotomy was performed, the right suprarenal artery, which originates from the right renal artery, was catheterized (PE-10); vehicle (saline) or an AT1R antagonist candesartan (MedChemExpress, New Jersey, USA) was infused at a rate of 40 μl/h. Both the right and left ureters were catheterized (PE-10) for urine collection. The duration of operation was about 60 minutes. After an equilibration period of 120 minutes, five consecutive 40-minute-urine volumes were collected. Vehicle alone was infused through the right suprarenal artery during the first period as basal state and the last collection as recovery. After the first period, candesartan (1, 5, 10 μg/kg/min) was infused during the next three (treatment) periods. The urine was stored at −80°C until use.
2.3. Blood pressure measurement and urine/blood analysis
The systolic-, diastolic- and mean blood pressures were measured at indicated weeks by a computerized noninvasive tail-cuff manometry system (BP-2010A; Softron Bio technology, Beijing, China). The five measured blood pressure values from each rat were averaged. To ensure the accuracy of blood pressure measurement, the rats were trained for one week before the experiments, in order for the rats to be acclimated to the procedure.
The rats were placed in metabolic cages for urine collection. The 24-hour-urine volumes and sodium excretions were obtained at the indicated times. The rats were acclimatized in the metabolic cages for at least 2 days before collecting the urine. Sodium concentrations in the urine samples were measured by Sodium Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In addition, serum selenium levels were measured by inductively coupled plasma mass spectrometry (Agilent 7900, Agilent Technologies, CA, USA).
2.4. Histopathology staining
Kidney tissues were fixed in 4% paraformaldehyde solution, embedded in paraffin, and sliced into 5-μm sections. The tissue samples were then stained with hematoxylin-eosin (Beyotime, Shanghai, China). After these staining, the sections were photographed using a microscope (BX53, Olympus Corporation, Tokyo, Japan).
2.5. Angiotensin II detection in renal cortex and serum
After feeding the rats with selenium-normal diet or selenium-deficient diet for 16 weeks, blood and renal cortex of SD rats were collected. The levels of angiotensin II (Ang II) in renal cortex and serum were quantified by a commercial ELISA Kit (mlbio, Shanghai, China), according to the manufacturer’s protocol.
2.6. Measurement of malondialdehyde levels
To assess the levels of systematic oxidative stress, the serum level of malondialdehyde (MDA) was measured using a commercially available MDA Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the reactivity of thiobarbituric acid (TBA). Briefly, MDA reacts with TBA at 90–100°C and acidic condition. The reaction produces a red MDA-TBA conjugate, which was measured spectrophotometrically at 532 nm.
2.7. Cell culture and treatment
Immortalized renal proximal tubule (RPT) cells from microdissected S1 segments of renal proximal tubules from 4- to 8-weeks-old normotensive Wistar-Kyoto (WKY) rats and Agtr1−/− mice were obtained from Dr. Ulrich Hopfer (Department of Physiology and Biophysics, Case Western Reserve School of Medicine, Cleveland, Ohio, USA), and cultured as previously decribed [23, 25, 26]. When the confluency of RPT cells reached 80–90%, they were cultured for 48 hours in serum-free medium (designated as selenium-deficient cells) or serum-free medium supplemented with 4 μM selenomethionine (SeMet, designated as selenium-replete cells).
The RPT cells with selenium-replete medium incubation were transfected with glutathione peroxidase 1 (GPx1) siRNA in Lipofectamine 2000 Reagent (Invitrogen Life Technologies, California, USA). Non-silencing scrambled siRNA was used as a negative control. After 48 hours, the RPT cells were harvested to determine the efficiency of siRNA silencing (Supplemental Fig. 3).
The RPT cells (80% confluent) were lysed in ice-cold lysis phosphate-buffered saline (PBS with 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin), sonicated, kept on ice for 1 hour, and centrifuged at 16,000g for 30 minutes. All samples were stored at −80°C until use.
2.8. Detection of reactive oxygen species production
Reactive oxygen species (ROS) in the kidney and RPT cells were measured by fluorescent dye dihydroethidium (DHE), as described in our previous reports [27, 28]. Sections of rat kidneys and RPT cells were incubated with fresh DHE (10 μM, 30 minutes) at 37°C in dark. After washing with PBS, images were obtained by fluorescence microscope (ECLIPSE Ti; Nikon, Tokyo, Japan). All samples were processed and shot with the same conditions. The fluorescence intensity was quantified by Image J software (National Institutes of health, Bethesda, MD, USA). In addition, the levels of hydrogen peroxide (H2O2) in the renal cortex and RPT cells were quantified by Hydrogen Peroxide Assay Kit (Jiancheng Bioengineering Institute, Nanjing, China).
2.9. Measurement of renal glutathione peroxidase activity
The activity of glutathione peroxidase (GSH-Px) in the kidney and RPT cells was determined by a commercially available kit (Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer’s protocol. The enzymatic reactions was activated by GSH-Px, which catalyzes glutathione (GSH) and H2O2 to oxidized glutathione (GSSG) and H2O. GSH conjugates with 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB) to produce yellow substances and the consumption of GSH, recorded spectrophotometrically at 412 nm, during the conversion of GSH to GSSG is indicative of GSH-Px activity.
2.10. Real-time quantitative PCR
Total RNA was isolated by TRIzol reagent and quantified spectrophotometrically (DU800, Beckman Coulter, Brea, CA). cDNA was synthesized from 2 μg of total RNA using reverse transcript reagents (Bio-Rad Laboratories, Hercules, CA). Gene expression was quantified by Bio-Rad CFX96 Touch™ Real-Time PCR Detection System and normalized by GAPDH mRNA expression. The PCR was performed under the following conditions: 95 °C for 3 minutes; 40 cycles of 95 °C for 10 seconds and 62 °C for 30 seconds; and 62 °C for 10 seconds. The primers used for PCR are listed in Supplemental Table 1.
2.11. Immunoblotting
The proteins (50 μg) were separated by SDS-PAGE (10% or 12% Tris-HCl polyacrylamide gels), and then transferred onto nitrocellulose membrane. Skim milk (5%) was used to block the nonspecific binding sites. The blots were incubated over night at 4 °C with the indicated primary antibodies (Supplemental Table 2). Then, the membranes were incubated with infrared-labeled secondary antibodies (1:10000, Li-Cor Biosciences, Lincoln, NE, USA) for 1 hour at room temperature. The bound complex was detected using the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). The band intensities were quantified by densitometry using Image J software (National Institutes of health, Bethesda, MD, USA).
2.12. Na+-K+-ATPase activity assay
The Na+-K+-ATPase activity in the crude membrane fraction of RPT cells was measured by a commercially available Na+-K+-ATPase Assay Kit (Solarbio, Beijing, China), according to the manufacturer’s protocol. Na+-K+-ATPase activity was corrected for protein concentration and expressed as μmol phosphate released per mg protein per min.
2.13. Electrophoretic mobility shift assay
The transcription factor NF-kappa B (NF-κB)-DNA binding activity in renal cortex was measured by electrophoretic mobility shift assay (EMSA). The detailed protocols have been reported in our previous studies [29, 30]. In brief, nuclear extracts from rat kidneys were prepared using the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Shanghai, China). A synthetic DNA double-stranded oligonucleotides probe containing the sequence of rat AT1R gene promoter (5′-AGTTGAGGGGACTTTCCCAGGC-3′, which contains a NF-κB site) was labeled with DyLight 800 in the 5′-end, and NF-κB mutant oligonucleotides (5′-AGTTGAGGGATCTTTCCCAGGC-3′) were synthesized. The nuclear extract was incubated with EMSA/Gel-Shift Binding Buffer (Beyotime, Shanghai, China) and infrared light probe at 20–25°C for 10–20 minutes and electrophoresed on 6% non-denaturant gel containing 0.5×Tris-Borate-EDTA buffer. The NF-κB-DNA bands were visualized using the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA).
2.14. Statistical analysis
Results are expressed as means ± standard error of the mean (SEM). Statistical significance among experimental groups (>2) was determined using the ANOVA with Newman-Keuls multiple test. Appropriate unpaired or paired t test was used when only two groups were compared. Statistical analysis was performed using GraphPad Prism version 5 (GraphPad Software, San Diego, CA). A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Selenium deficiency increased blood pressure and impaired renal sodium excretion in SD rats
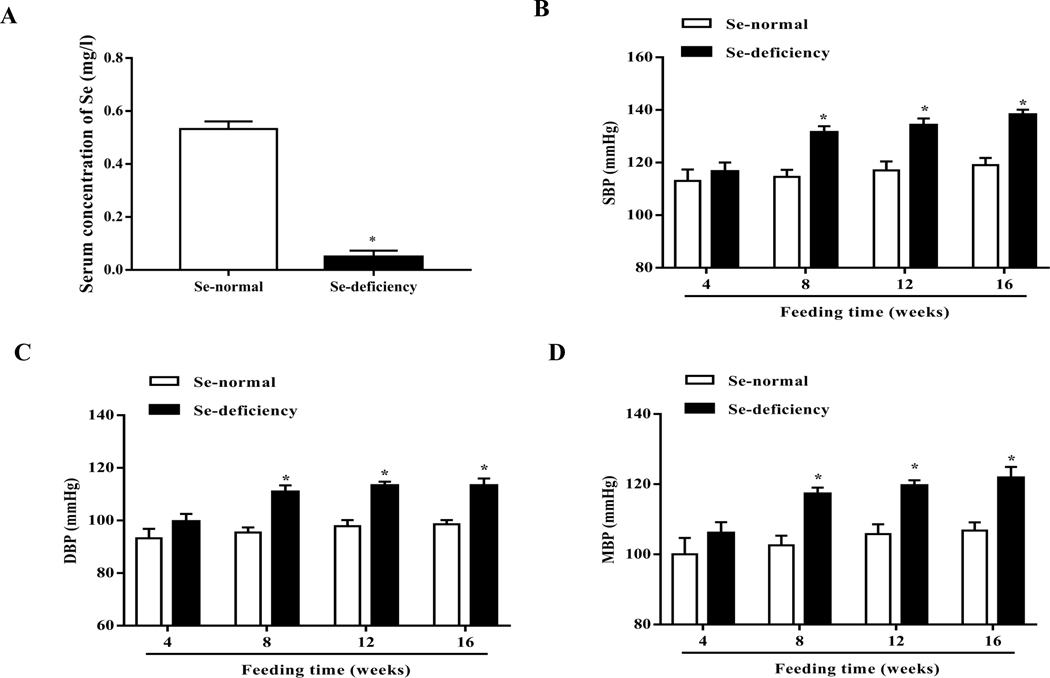
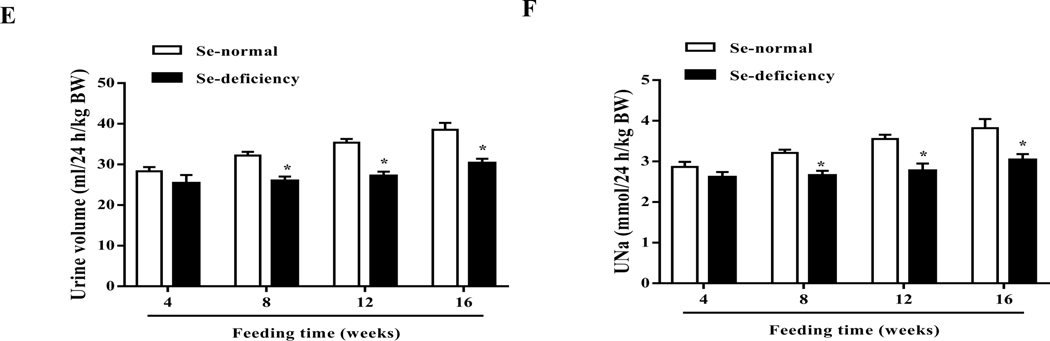
To determine the role of selenium in the regulation of blood pressure, SD rats were fed a selenium-deficient diet for 16 weeks; the serum selenium level (0.05±0.02 mg/l) was lower in SD rats fed a selenium-deficient diet than those fed a normal selenium diet (0.53±0.03 mg/l) (Fig. 1A). SD rats fed the selenium-deficient diet had higher systolic-, diastolic- and mean arterial blood pressures than those fed the normal selenium diet (Fig. 1B–D). The increase in blood pressure caused by the selenium-deficient was time-dependent; the increase was observed at 8 weeks and lasted at least 8 weeks.
Fig. 1. Effect of selenium deficiency in the regulation of blood pressure and sodium excretion in SD rats.
(A) The different serum selenium (Se) levels between SD rats fed a selenium-deficient diet and those fed a selenium-normal diet for 16 weeks (*P < 0.05 vs Se-normal, n=5/group). (B-D) Systolic-(SBP, B), diastolic-(DBP, C) and mean blood pressures (MBP, D) in SD rats fed the selenium-deficient diet for indicated time (*P < 0.05 vs Se-normal, n=5/group). (E, F) 24-hours urine volume (E) and sodium excretion (UNa, F) in SD rats fed the selenium-deficient diet for indicated time (*P < 0.05 vs Se-normal, n=5/group).
The kidney is one of the major organs involved in the long-term regulation of blood pressure by maintaining sodium homeostasis [31]. SD rats fed the selenium-deficient diet had lower 24-hours urine volume and sodium excretion than the SD rats fed the normal selenium diet (Fig. 1E–F). Consistent with the time-related increase in blood pressure with the selenium deficient diet, decreased urine volume and sodium excretion in SD rats fed the selenium-deficient diet also lasted at least 8 weeks. Selenium-deficient diet had no effect on the gross renal structure (Supplemental Fig. 4). There were also no differences in heart rate, and water and food intakes between the two groups; however, the body weight was slightly lower in selenium-deficient than selenium-normal rats (Supplemental Table 3).
3.2. Selenium deficiency increased renal AT1R expression and AT1R-mediated function in SD rats
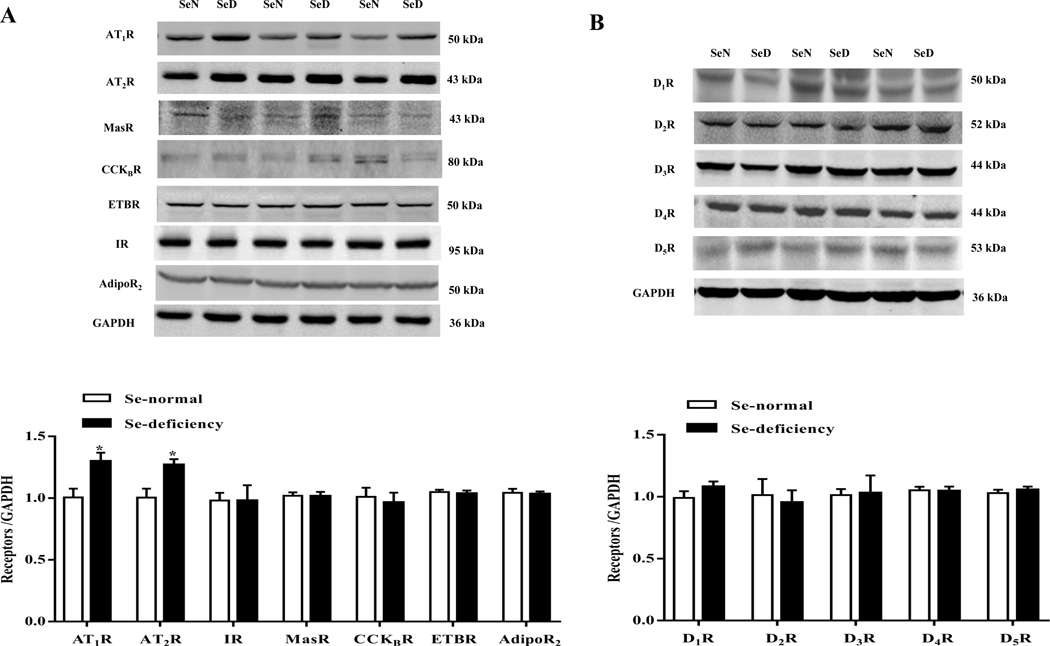
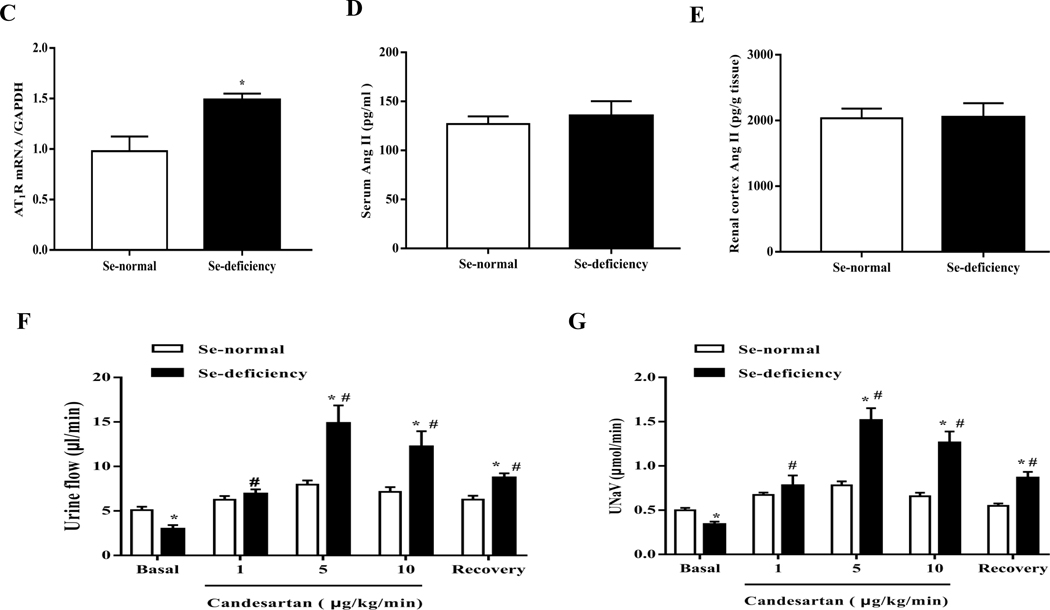
Urine volume and sodium are regulated by natriuretic and anti-natriuretic hormones and humoral factors, including dopamine and Ang II [32, 33]. After screening for the sodium excretion-related renal receptors, we found that the expression change of AT1R, a main receptor of Ang II, was the biggest one. Selenium-deficient diet increased AT1R protein expression (Fig. 2A–B). However, selenium deficiency did not affect renal cortical protein expressions of mas-related G protein-coupled receptor A (MasR), cholecystokinin B receptor (CCKBR), endothelin B receptor (ETBR), insulin receptor (IR), adiponectin receptor 2 (AdipoR2), and dopamine receptors (D1R-D5R) (Fig. 2A–B). Moreover, selenium deficient diet also increased renal cortical AT1R mRNA (Fig. 2C). AT2R, another receptor of Ang II, was also elevated in the renal cortex in SD rats fed the selenium-deficient diet (Fig. 2A), conceivably as a compensatory response. It should be noted that there were no differences in the serum and renal levels of Ang II, a ligand of AT1R, between selenium-deficient rats and selenium-normal rats (Fig. 2D–E).
Fig. 2. Effect of selenium deficiency on renal AT1R expression and AT1R-mediated function in SD rats.
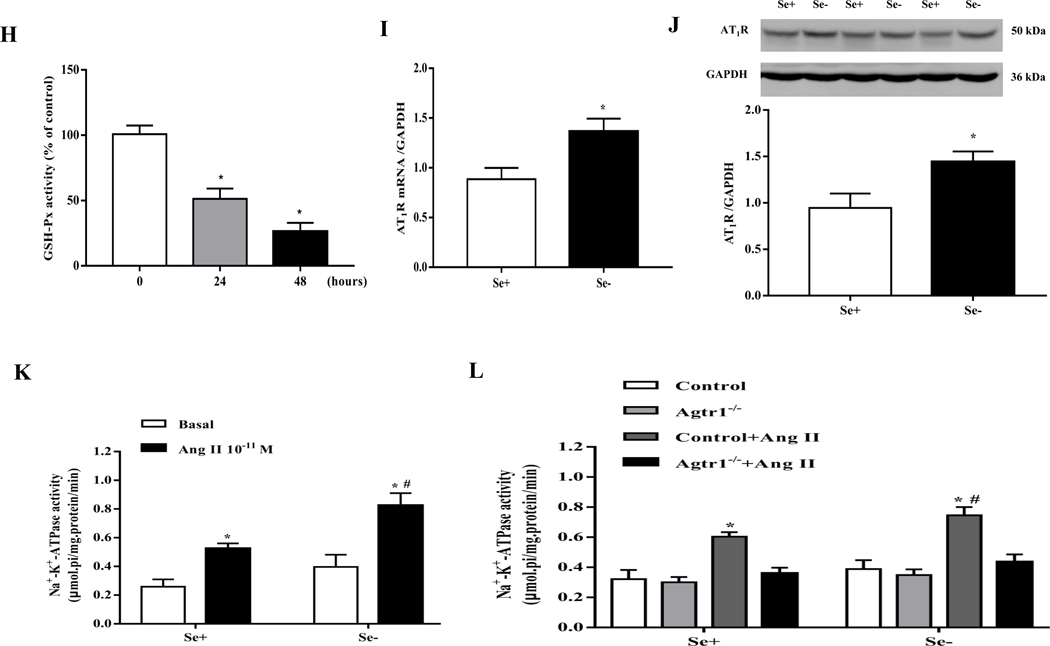
(A, B) Expression profiles of select receptors in the renal cortex of SD rats fed the selenium (Se)-deficient diet for 16 weeks. Protein expression was evaluated via immunoblotting for AT1R, AT2R, MasR, CCKBR, ETBR, IR, AdipoR2 (A) and dopamine receptors (B). The protein expression was normalized using GAPDH expression (*P < 0.05 vs Se-normal, n=5/group). (C) The mRNA expression of AT1R was determined by qt-PCR in the renal cortex of SD rats fed the selenium-deficient diet for 16 weeks. AT1R mRNA level was normalized using GAPDH expression (*P < 0.05 vs Se-normal, n=5/group). (D, E) The angiotensin II levels in serum (D) and renal cortex (E) in SD rats fed the selenium-deficient diet for 16 weeks (n=5/group). (F, G) Effect of candesartan (1, 5 or 10 μg/kg/min) on the urine flow (F) and urinary sodium excretion (G) in SD rats fed the selenium-deficient diet for 16 weeks (*P < 0.05 vs Se-normal, #P < 0.05 vs basal Se-deficiency, n=5/group). (H) The activity of GSH-Px in RPT cells with selenium-free incubation for 24 or 48 hours (*P < 0.05 vs the time 0, n=5/group). (I, J) The mRNA (I) and protein expression (J) of AT1R in selenium-free incubated RPT cells (Se-) and selenium-replete cells (Se+) for 48 hours. AT1R mRNA and protein levels were normalized using GAPDH (*P < 0.05 vs Se+, n=4–5/group). (K) Effect of Ang II on Na+-K+-ATPase activity in selenium-deficient RPT cells from normotensive WKY rats. Cells were incubated with selenium-free media for 48 hours and then treated with Ang II (10−11 M) for 30 minutes (*P < 0.05 vs basal, n=5; #P < 0.05 vs Ang II in Se+ group, n=5/group). (L) Effect of Ang II on Na+-K+-ATPase activity in selenium-deficient RPT cells from Agtr1−/ mice. Cells were incubated with selenium-free media for 48 hours and then treated with Ang II (10−11 M) for 30 minutes (*P < 0.05 vs control; #P < 0.05 vs Ang II in Se+ group, n=6/group).
The increase in renal AT1R expression, caused by selenium deficiency has pathophysiological significance, because the infusion of candesartan (5 or 10 μg/kg/min), an AT1R antagonist, via the right suprarenal artery, increased urine flow and sodium excretion to a greater extent in selenium-deficient than selenium-normal rats (Fig. 2F–G).
The increase in renal AT1R expression caused by selenium deficiency in vivo was confirmed in in vitro by studying the effect of selenium-free incubation media. The functional effect of selenium deficiency was proved by the reduced activity of GSH-Px, a selenium-dependent antioxidant enzyme, indicating the successful establishment of selenium-deficient cell model (Fig. 2H). As compared with cells incubated in selenium-replete medium, incubation of RPT cells in selenium-free medium for 48 hours increased both AT1R mRNA and protein expression (Fig. 2I–J). This was accompanied with enhanced AT1R-mediated function, proved by the increased stimulatory effect of Ang II (10−11 M, 30 minutes) on Na+-K+-ATPase activity in RPT cells from WKY rats in selenium-free medium, relative to RPT cells incubated in selenium-replete medium (Fig. 2K). Indeed in RPT cells from Agtr1−/− mice, Ang II was unable to increase Na+-K+-ATPase activity, regardless of the presence or absence of selenium in the incubating medium (Fig. 2L). These results suggested that the sodium retention and hypertension in selenium deficiency are related to the up-regulation of renal AT1R.
3.3. Decreased GPx1 expression is involved in the elevated renal AT1R expression and function induced by selenium deficiency
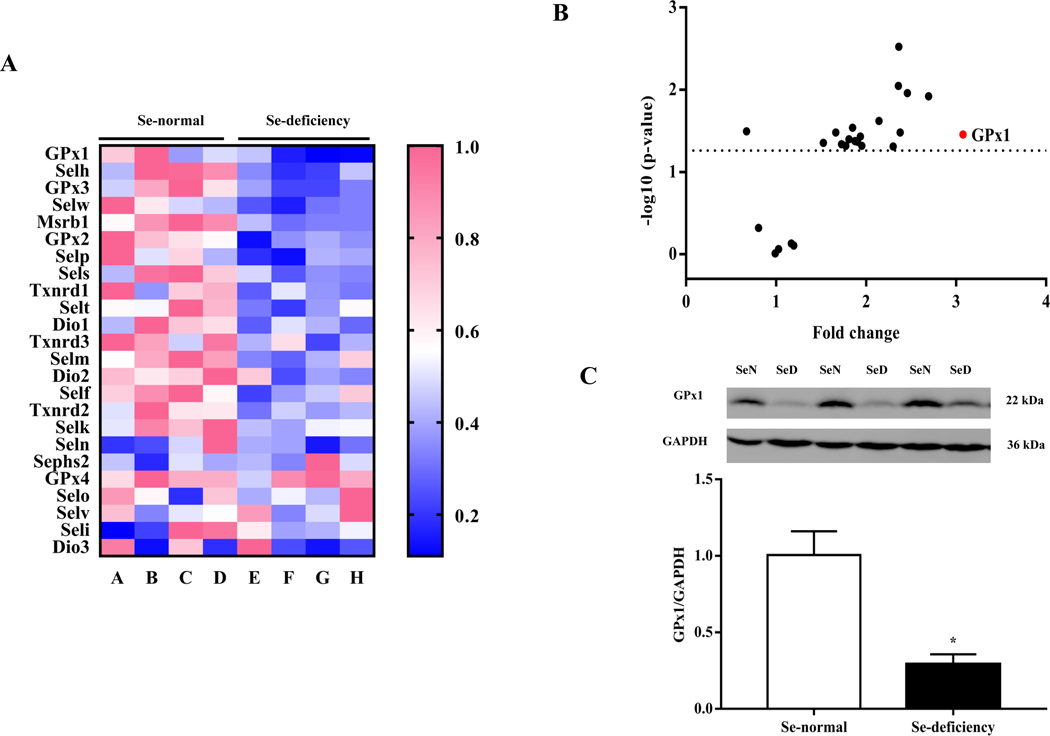
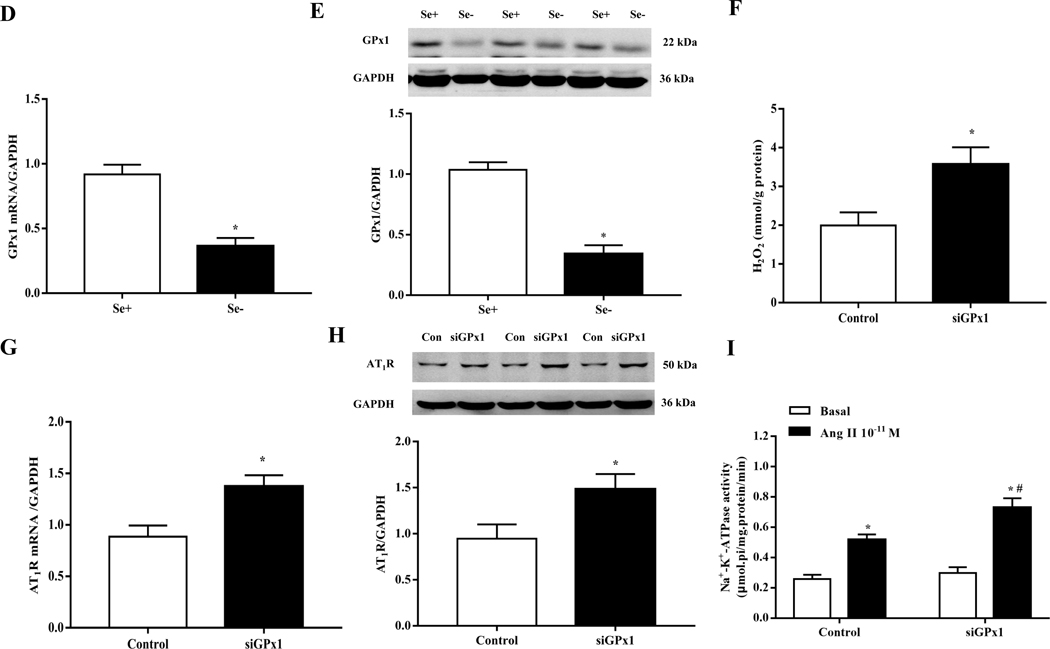
Selenium, an essential trace element, is mainly found in proteins such as selenocysteine, which is necessary for selenoproteins [9–11]. To explore the possible role of selenoproteins in the increase in renal AT1R expression induced by selenium deficiency, we investigated the mRNA expression of renal selenoproteins in SD rats fed diets with or without selenium. Among the selenoproteins that were affected by selenium deficiency, GPx1 mRNA expression had the greatest decrease in renal cortical expression (Fig. 3A–B). The decrease in GPx1 mRNA expression was accompanied by a decrease in protein expression, determined by immunoblotting (Fig. 3C). The effect of selenium deficiency on GPx1 expression was also seen in vitro. Incubation of RPT cells in selenium-free medium for 48 hours decreased both GPx1 mRNA and protein expression, as compared with cells incubated in selenium-replete medium (Fig. 3D–E).
Fig. 3. Role of GPxl in the increased renal AT1R expression in selenium-deficient rats.
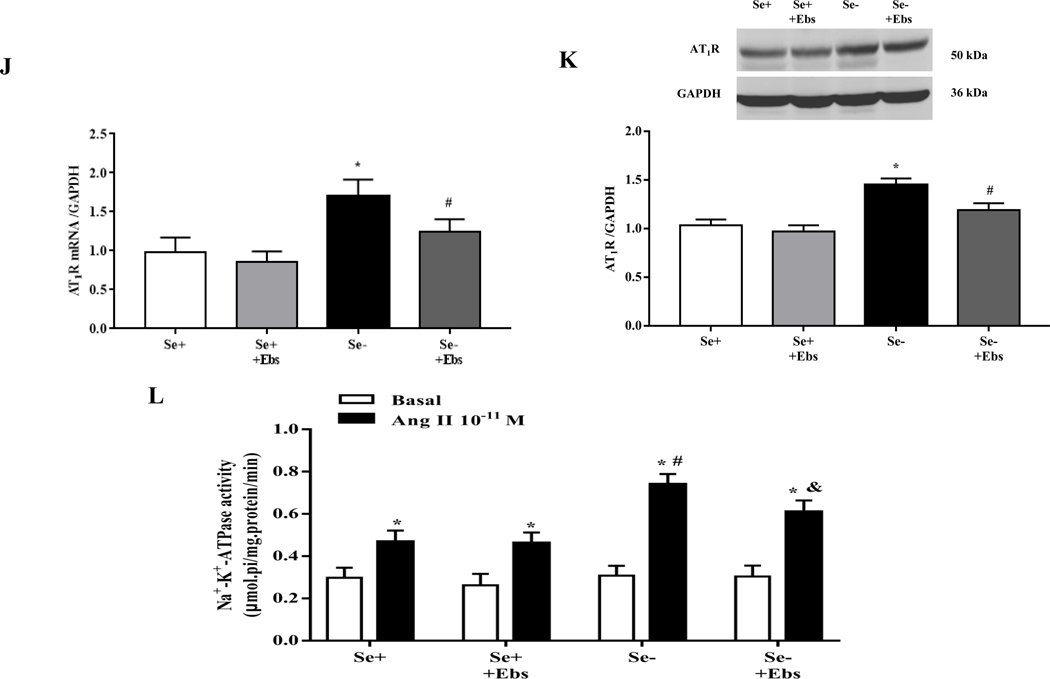
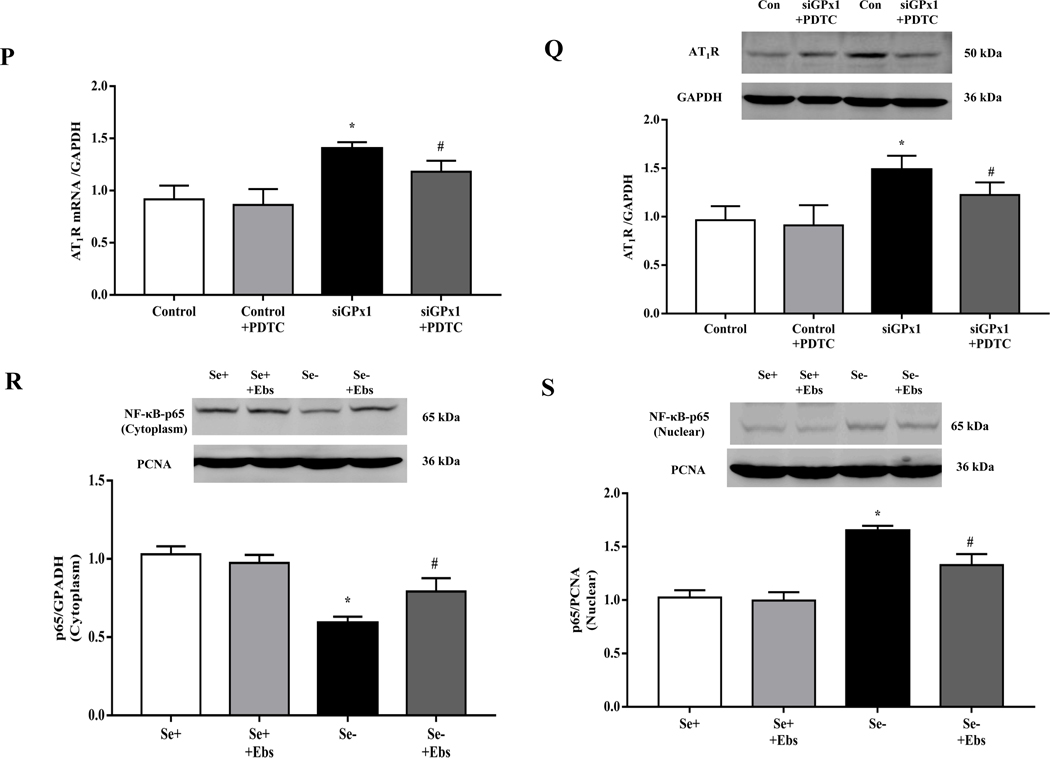
(A, B) Heatmap (A) and volcano plot (B) of 24 selenoproteins mRNA expression in the renal cortex of SD rats fed the selenium-deficient diet for 16 weeks. (C) The protein expression of GPx1 in the renal cortex of selenium-deficient rats (*P < 0.05 vs Se-normal, n=5/group). SeN, Se-normal; SeD, Se-deficiency. (D, E) The mRNA (D) and protein expression (E) of GPx1 in WKY RPT cells incubated with selenium-free media for 48 hours (*P < 0.05 vs Se+, n=4–5/group). Se-, selenium-deficient cells; Se+, selenium-replete cells. (F) The levels of hydrogen peroxide (H2O2) in selenium-replete RPT cells treated with GPx1 siRNA for 48 hours (*P < 0.05 vs control, n=5/group). (G, H) The mRNA (G) and protein expression (H) of AT1R in selenium-replete RPT cells treated with GPx1 siRNA (S) for 48 hours (*P < 0.05 vs control (C), n=4–5/group). (I) Effect of Ang II on Na+-K+-ATPase activity in RPT cells treated with GPx1 siRNA. RPT cells were incubated with GPx1 siRNA for 48 hours, and then treated with Ang II (10−11 M) for 30 minutes (*P < 0.05 vs basal; #P < 0.05 vs Ang II in control group, n=5/group). (J, K) The mRNA (J) and protein expression (K) of AT1R in RPT cells with selenium-free incubation and a GPX1 mimic ebselen (Ebs) treatment (30 μm) for 48 hours. AT1R mRNA and protein levels were normalized using GAPDH (*P < 0.05 vs Se+, n=4–5/group). (L) Effect of Ang II on Na+-K+-ATPase activity in selenium-deficient RPT cells treated with ebselen. Cells were incubated with selenium-free media and ebselen (30 μm) for 48 hours and then treated with Ang II (10−11 M) for 30 minutes (*P < 0.05 vs basal, n=5; #P < 0.05 vs Ang II in Se+ group, n=5/group).
Further study showed that GPx1 silencing by siRNA not only increased H2O2 level (Fig. 3F) but also increased AT1R mRNA and protein expression in RPT cells incubated with selenium-replete medium (Fig. 3G–H). This was accompanied with an augmented Ang II (10−11 M, 30 minutes)-mediated stimulation of Na+-K+-ATPase activity (Fig. 3I). Moreover, treatment with ebselen, a GPx1 mimic [34], reduced the increased AT1R expression and function in selenium-deficient RPT cells (Fig. 3J–L). These results suggested that decreased GPx1 expression induced by selenium deficiency causes the elevated renal AT1R expression and decreased sodium excretion.
3.4. Increased oxidative stress is the key signal in the increase in renal AT1R expression in selenium-deficient rats
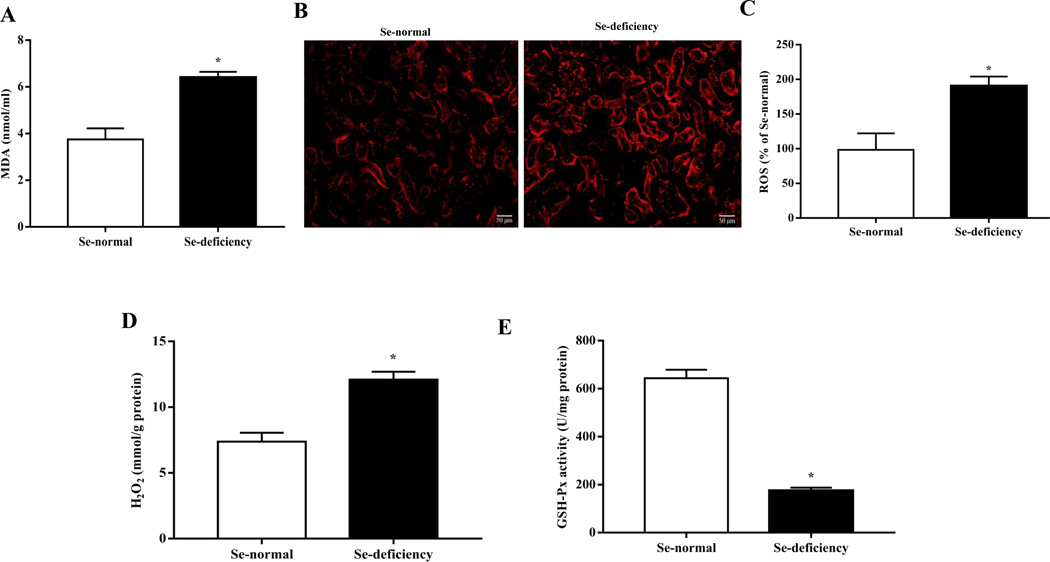
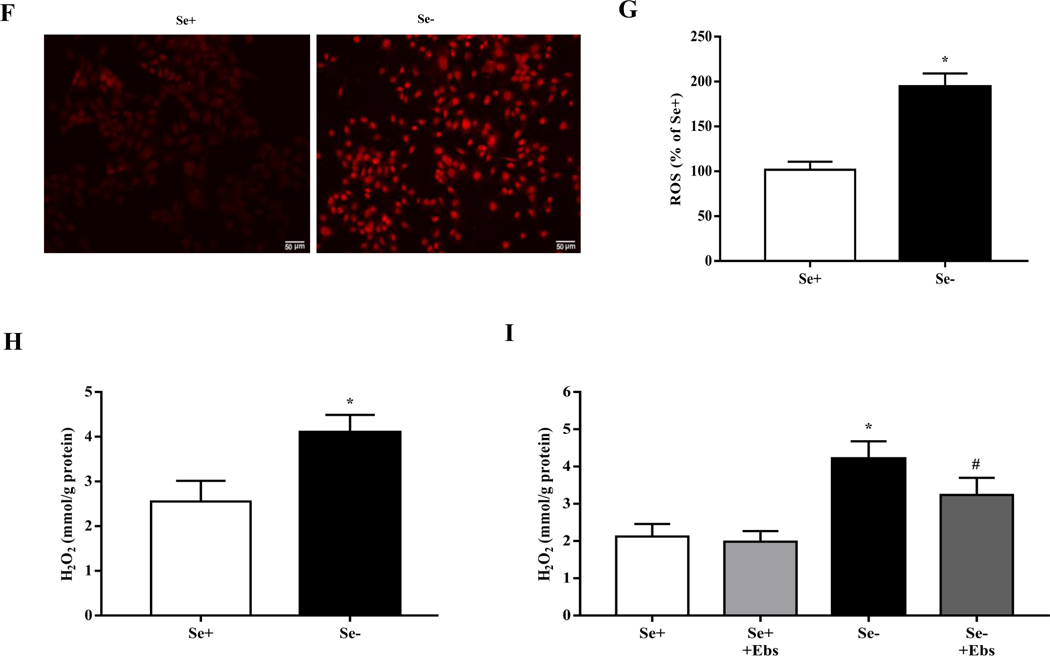
Because of the important role of GPx1 in the regulation of redox balance [35], we studied the state of oxidative stress in selenium-deficient rats. We found that selenium-deficient rats had increased serum levels of malondialdehyde (MDA), a lipid peroxidation product, and elevated renal ROS production and H2O2 level (Fig. 4A–D), accompanied with decreased activity of renal GSH-Px (Fig. 4E). In vitro studies also showed that compared with selenium-replete cells, ROS and H2O2 production were markedly elevated in selenium-deficient cells (Fig. 4F–H). We also found that treatment with the GPx1 mimic ebselen had no effect on H2O2 production in selenium-replete cells, but partially inhibited the increased H2O2 generation in selenium-deficient cells (Fig. 4I).
Fig. 4. Increased oxidative stress in selenium-deficient rats.
(A) Serum MDA levels in SD rats fed the selenium-deficient diet for 16 weeks (*P < 0.05 vs Se-normal, n=5/group). (B, C) Fluorescence microscopy images (B) and quantification (C) of renal ROS production in SD rats fed the selenium-deficient diet for 16 weeks (*P < 0.05 vs Se-normal, n=5/group). (D) The levels of renal hydrogen peroxide in the kidney of SD rats fed the selenium-deficient diet for 16 weeks (*P < 0.05 vs Se-normal, n=5/group). (E) The activity of renal GSH-Px in SD rats fed the selenium-deficient diet for 16 weeks (*P < 0.05 vs Se-normal, n=5/group). (F, G) Fluorescence microscopy images (F) and quantification (G) of ROS production in WKY RPT cells incubated with selenium-free media for 48 hours (*P < 0.05 vs Se+, n=5 /group). (H) The levels of hydrogen peroxide in RPT cells incubated with selenium-free media for 48 hours (*P < 0.05 vs Se+, n=5/group). (I) The levels of hydrogen peroxide in RPT cells incubated with selenium-free media and a GPX1 mimic ebselen (Ebs) treatment (30 μm) for 48 hours (*P < 0.05 vs Se+, n=5/group).
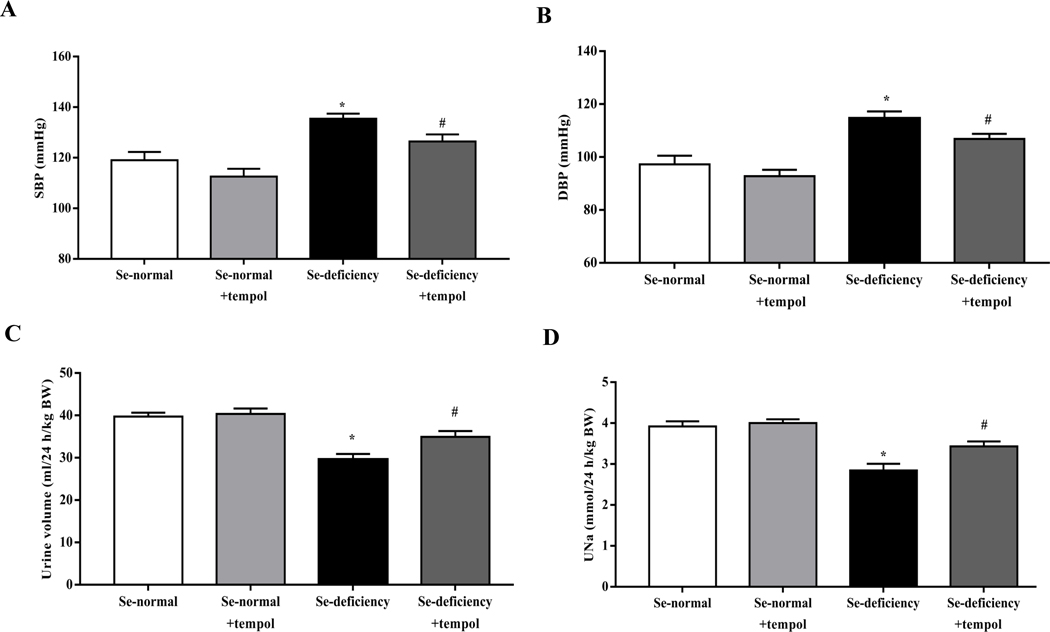
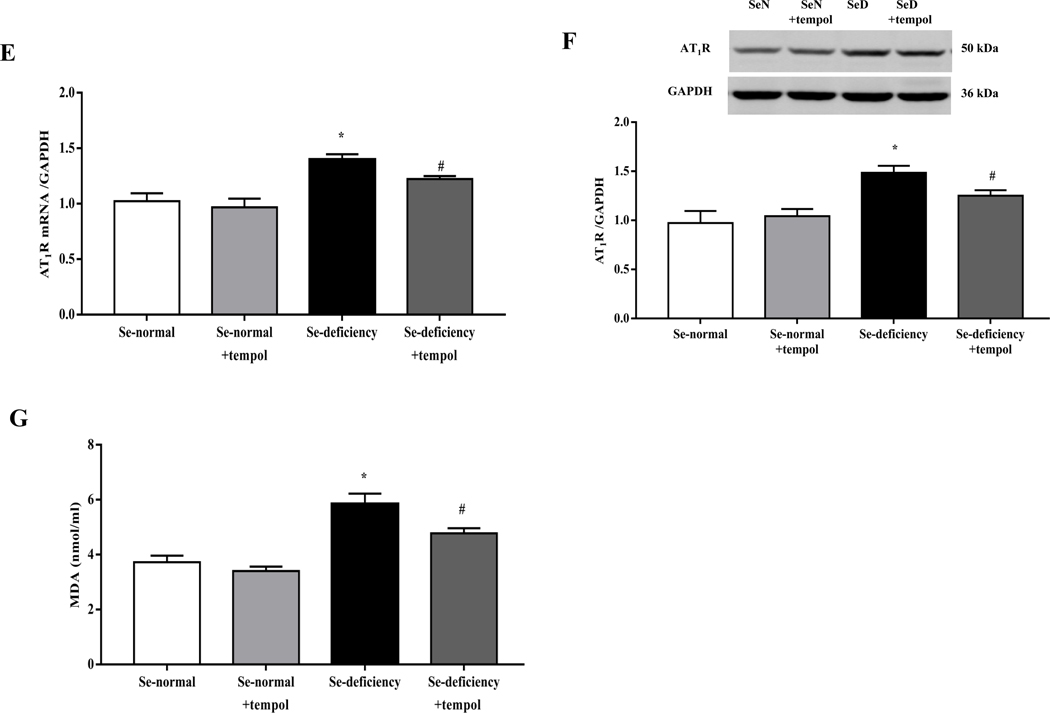
To confirm the role of oxidative stress in the development of hypertension in selenium-deficient rats, an SOD mimetic tempol was administered (Supplemental Table 4). Tempol (1 mM) added to the drinking water for 4 weeks decreased the elevated systolic- and diastolic blood pressures and increased 24-hours urine volume and sodium excretion in selenium-deficient rats (Fig. 5A–D). These were accompanied with a decrease in renal cortical AT1R mRNA and protein expressions (Fig. 5E–F). Tempol treatment also decreased the increased serum levels of MDA (Fig. 5G), authenticating the antioxidant effects of selenium.
Fig. 5. Effects of tempol in the regulation of blood pressure and sodium excretion in selenium-deficient rats.
After feeding the indicated diet for 12 weeks, selenium-deficient rats or selenium-normal rats were then treated with tempol (1 mM) for 4 weeks. (A, B) Systolic- (SBP, A) and diastolic blood pressures (DBP, B) in selenium-deficient rats treated with tempol (*P < 0.05 vs Se-normal; #P < 0.05 vs Se-deficiency, n=5/group). (C, D) 24-hours urine volume (C) and urine sodium excretion (UNa, D) in selenium-deficient rats treated with tempol (*P < 0.05 vs Se-normal; #P < 0.05 vs Se-deficiency, n=5/group). (E, F) The mRNA (E) and protein expression (F) of AT1R in selenium-deficient rats treated with tempol (*P < 0.05 vs Se-normal; #P < 0.05 vs Se-deficiency, n=5/group). (G) Effect of tempol in the serum MDA levels in selenium-deficient rats (*P < 0.05 vs Se-normal; #P < 0.05 vs Se-deficiency, n=5/group).
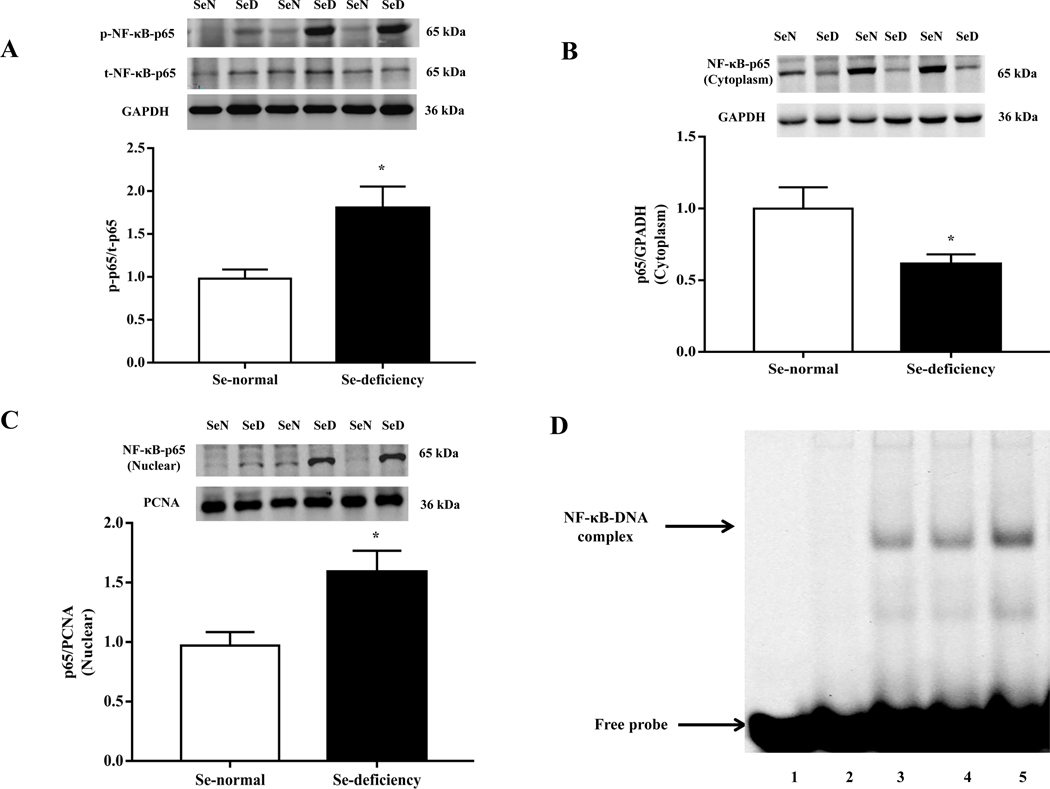
3.5. Selenium-deficiency, via GPx1 and ROS, increased renal NF-kappa B activity and AT1R expression
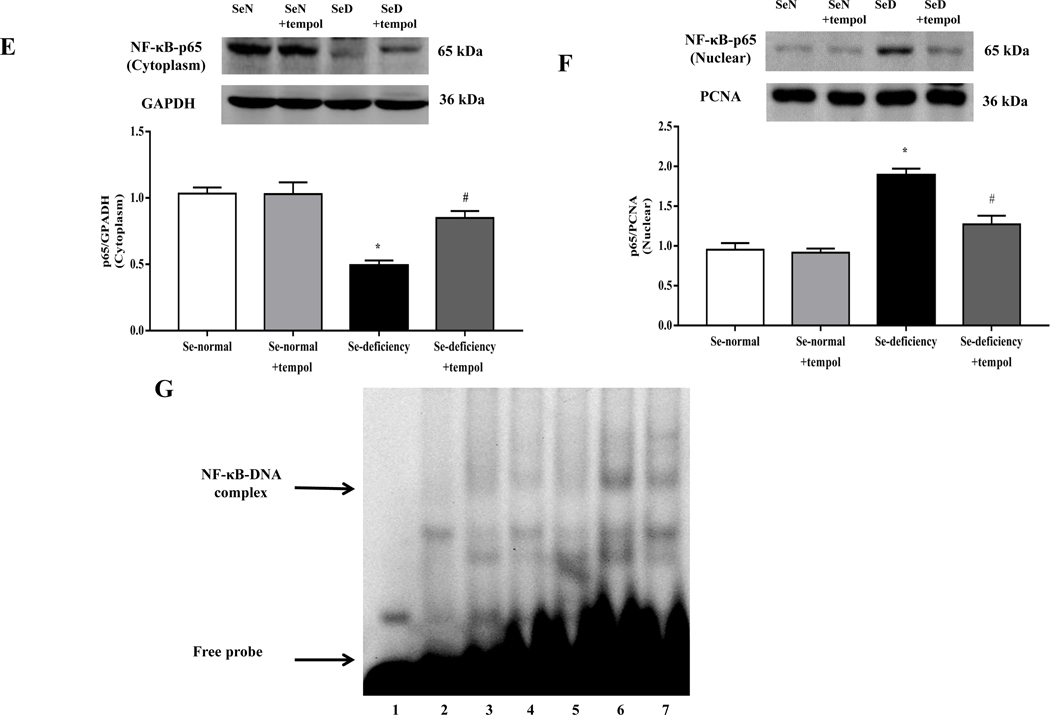
It is known that the ubiquitous transcription factor NF-κB is a major transcription factor for AT1R expression [36]. Since the NF-κB expression is regulated by ROS, we wondered whether or not NF-κB is the key signaling mediator of the increase in AT1R expression with selenium-deficiency. Our present study found that the phosphorylated expression of NF-κB p65, an NF-κB complex subunit, was increased in the renal cortex of selenium-deficient rats (Fig. 6A). Selenium-deficiency also increased NF-κB p65 translocation from the cytosol to nucleus in the renal cortex of selenium-deficient rats (Fig. 6B–C). EMSA showed that the amount of NF-κB bound to the promoters of AT1R gene was markedly higher in the renal cortex of selenium-deficient rats than selenium-normal rats (Fig. 6D). Studies using no nuclear extracts and 100 times of unlabeled probe eliminated this band, confirming the binding specificity. Moreover, a role of increased oxidative stress on the regulation of NF-κB activity in selenium-deficient rats was also found. Tempol (1 mM) added to the drinking water for 4 weeks partially reversed the decreased cytoplasmic expression and increased nuclear expression of p65 protein (Fig. 6E–F), and almost normalized the amount of NF-κB bound to AT1R DNA (Fig. 6G) in the renal cortex of selenium-deficient rats.
Fig. 6. Selenium-deficiency increased renal NF-κB activity by GPx1/ROS in selenium-deficient rats.
(A) The ratio of phosphorylated p65/total p65 expression in the renal cortex of selenium-deficient rats (*P < 0.05 vs Se-normal, n= 5/group). SeN, Se-normal; SeD, Se-deficiency. (B, C) The protein expression of NF-κB p65 in the cytoplasm (B) and nucleus (C) from the renal cortex of selenium-deficient rats (*P < 0.05 vs Se-normal, n= 5/group). (D) Effect of selenium deficiency on the binding of NF-κB at the AT1R gene promoter in the renal cortex of SD rats. The binding ability of the AT1R gene promoter, which contains a NF-κB site, was determined in nuclear protein from the renal cortex of selenium-normal (lane 4) and selenium-deficient (lane 5) rats by EMSA. No nuclear extracts (lane 1), 100 times of unlabeled probe (lane 2) or mutant probe (lane 3) were added to the reaction mixture as controls. (E, F) The cytoplasmic- (E) and nuclear (F) protein expression of NF-κB p65 in selenium-deficient rats treated with tempol (1 mM) for 4 weeks (*P < 0.05 vs Se-normal; #P < 0.05 vs Se-deficiency, n=5/group). (G) Effect of tempol on the binding of NF-κB at the AT1R gene promoter in the renal cortex of selenium-deficient rats. After treatment with tempol (1 mM) for 4 weeks, the binding ability of the AT1R gene promoter, which contains a NF-κB site, was determined in nuclear protein from kidney of selenium-normal and selenium-deficient rats by EMSA. Lane 4, selenium-normal rats; lane 5: selenium-normal rats treated with tempol; lane 6, selenium-deficient rats; lane 7, selenium-deficient rats treated with tempol. No nuclear extracts (lane 1), 100 times of unlabeled probe (lane 2) or mutant probe (lane 3) were added to the reaction mixture as controls. (H, I) The mRNA (H) and protein expression (I) of AT1R in selenium-deficient RPT cells treated with tempol (100 μM) for 48 hours (*P < 0.05 vs Se+; #P < 0.05 vs Se-, n=3–4/group). (J, K) The cytoplasmic- (J) and nuclear (K) protein expression of NF-κB p65 in selenium-deficient RPT cells treated with tempol (100 μM) for 48 hours (*P < 0.05 vs Se+; #P < 0.05 vs Se-, n=3/group). (L, M) The mRNA (L) and protein expression (M) of AT1R in selenium-deficient RPT cells incubated with PDTC (10 μM) for 48 hours (*P < 0.05 vs Se+; #P < 0.05 vs Se-, n=3–4/group). (N, O) The cytoplasmic- (N) and nuclear (O) protein expression of NF-κB p65 in selenium-replete RPT cells treated with GPx1 siRNA (Si) for 48 hours (*P < 0.05 vs control (C), n=5/group). (P, Q) The mRNA (P) and protein expression (Q) of AT1R in selenium-replete RPT cells incubated with PDTC (10 μM) for 30 minutes, and then treated with GPx1 siRNA for 48 hours (*P < 0.05 vs control; #P < 0.05 vs siGPx1, n=3–4/group). (R, S) The cytoplasmic- (R) and nuclear (S) protein expression of NF-κB p65 in RPT cells with selenium-free incubation and a GPX1 mimic ebselen (Ebs) treatment (30 μm) for 48 hours (*P < 0.05 vs Se+; #P < 0.05 vs Se-, n=5/group).
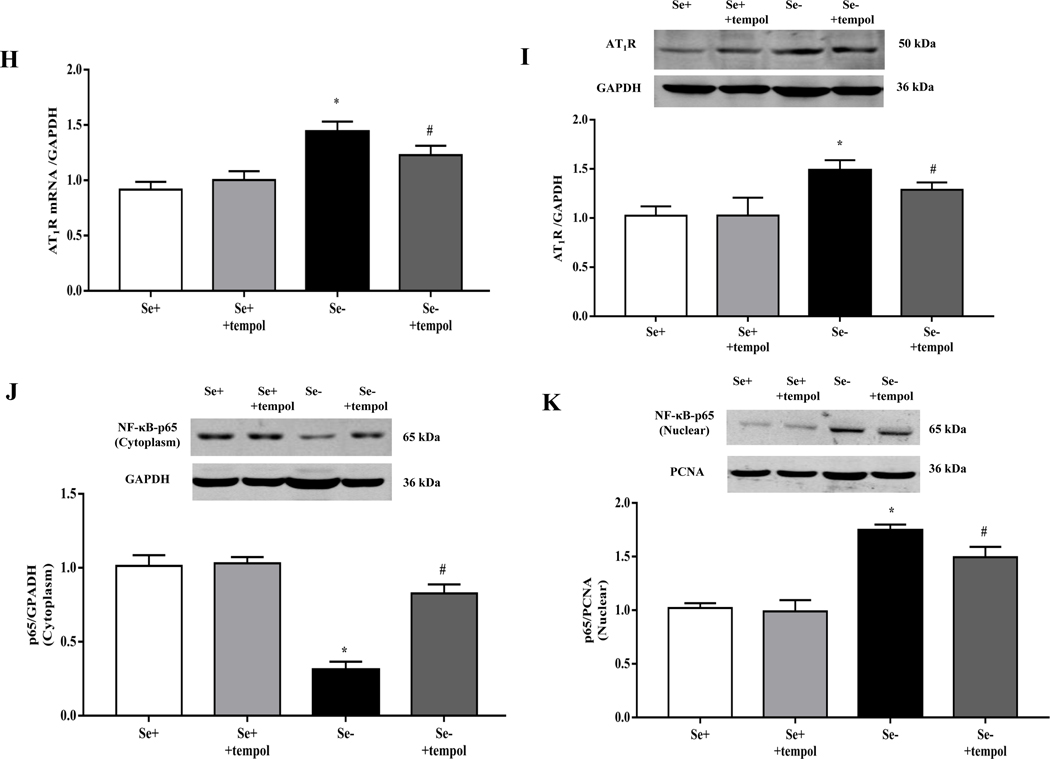
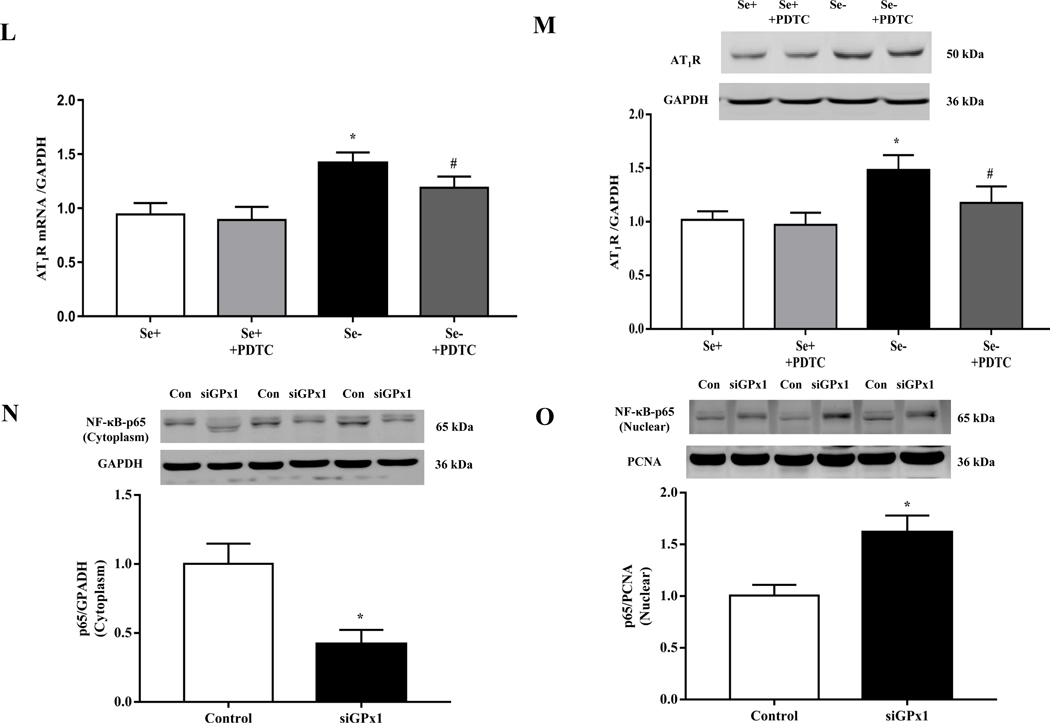
The above results in selenium-deficient rats were confirmed in vitro. Relative to selenium-replete cells, selenium-deficient cells had markedly higher mRNA and protein expression of AT1R (Fig. 6H–I), confirming the results shown in Fig. 3G–H. Moreover, incubation of RPT cells with selenium-deficient medium decreased NF-κB p65 expression in the cytoplasm and increased the translocation of NF-κB p65 from cytosol to nucleus (Fig. 6J–K). The effect of selenium-deficiency on AT1R expression and NF-κB p65 nuclear translocation were partially reversed by treatment with the antioxidant tempol (Fig. 6H–K). We also found that the increased expression of AT1R in selenium-deficient cells was partially reversed by treatment with pyrrolidine dithiocarbamate (PDTC), an NF-κB inhibitor (Fig. 6L–M). Moreover, in RPT cells incubated in selenium-normal medium, GPx1 silencing caused the translocation of p65 protein from cytosol to nucleus (Fig. 6N–O) and increased AT1R expression, that was partially lowered to the normal level by the treatment with PDTC (Fig. 6P–Q). In addition, treatment with the GPx1 mimic ebselen partially reduced the nuclear translocation of p65 protein in RPT cells incubated in selenium-deficient medium (Fig. 6R–S). These results indicated that GPx1 reduction-induced oxidative stress, via activation of NF-κB, increased AT1R mRNA and protein expression in selenium-deficient rats.
4. Discussion
Selenium is a trace element usually ingested either in its organic form via food or in its inorganic form through nutritional supplements [37]. It is essential for some synthesis of proteins; there are 25 selenoprotein genes in the human genome although there are 24 selenoproteins in rodents [38]. GPx6 has been identified as a selenoprotein only in humans but not in rats or mice because its mouse and rat orthologs have cysteine in place of selenocysteine and the corresponding genes lack selenocysteine insertion sequence elements [39]. Studies have shown the association between selenium and cardiovascular diseases [40–42]. Over the last decade, epidemiological studies have shown the association between selenium and the risk of hypertension. Low serum selenium level increases the risk of hypertension [43, 44]. Supplement with selenium prevents binge drinking-induced increased systolic blood pressure in adolescent rats [45]. A 20-year-prospective cohort study that had 13,668 Chinese participants showed that selenium intake is inversely associated with the risk of hypertension in those living in the low selenium zone; high selenium intake may be a protective factor for blood pressure in the low-selenium region [46]. However, opposite results are also reported. Selenium supplementation induces hypertension in male Wistar rats [47]. Tan et al reported that there is a U-shaped relationship between serum selenium concentrations and all-cause or cardiovascular mortality in hypertensive patients [48]. Therefore, there is still lack of clear evidence of the contribution of selenium in the pathogenesis of essential hypertension. In the present study, selenium-deficient rats were generated by feeding them with selenium-deficient diet for 16 weeks. We found that compared with control rats, selenium-deficient rats had increased blood pressure, which was accompanied with decreased urine flow and sodium excretion. These indicated that selenium-deficient feeding leads to hypertension, which may be caused by impaired sodium excretion.
The renin-angiotensin system (RAS) plays a key role in the development and maintenance of hypertension [49]. Its effector peptide Ang II exerts its effects, via its receptors including AT1R and AT2R. AT1R mediates the vast majority of the renal actions of Ang II, including renal tubular sodium transport [50]. We found that selenium-deficient rats had higher renal AT1R expression, which was reflected by the increased urine flow and sodium excretion induced by the intrarenal arterial infusion of candesartan, an AT1R antagonist. This result is consistent with a previous report, which showed that selenium supplementation decreases the expression of AT1R in the lung tissue during chemotherapy [51]. We also found that NF-κB, a transcription factor for AT1R, is regulated by selenium-deficiency; NF-κB is bound to AT1R promoter area, which subsequently elevated AT1R expression. These indicate that the increased renal AT1R expression may be involved in the selenium-deficient induced impaired renal sodium excretion and hypertension.
Selenium has antioxidant properties and selenium deficiency leads to oxidative stress [52, 53]. In patients with arterial hypertension, low serum selenium levels impair total antioxidant status and increase the thickness of the carotid intima media [54]. In the present study, systematic and renal oxidative stress were increased in selenium-deficient rats. This was confirmed by administration with an antioxidant tempol, which decreased the elevated blood pressure and increased sodium excretion, and also partially decreased the elevated renal AT1R expressions in selenium-deficient rats. After investigating the expression profile of all 24 selenoproteins, we found that GPx1 ranked first among the decreased selenoproteins in the kidney of selenium-deficient rats. GPx1, the first identified mammalian selenoprotein, is a selenium-dependent enzyme that reduces intracellular H2O2 and lipid peroxides [55]. Selenium deficiency reduces GPx1 expression and activity in the liver [56, 57]; selenium supplementation suppresses ROS generation by enhancing GPx1 expression in myocytes exposed to advanced glycation end products and improves its-induced cardiac functions [58]. Our in vitro studies showed that GPx1 silencing increased the mRNA and protein expressions of AT1R and Na+-K+-ATPase activity in selenium-replete cells. These suggested that decreased GPx1 expression induced by selenium deficiency, via increasing oxidative stress, causes the elevated renal AT1R expression and its-mediated antidiuretic effect.
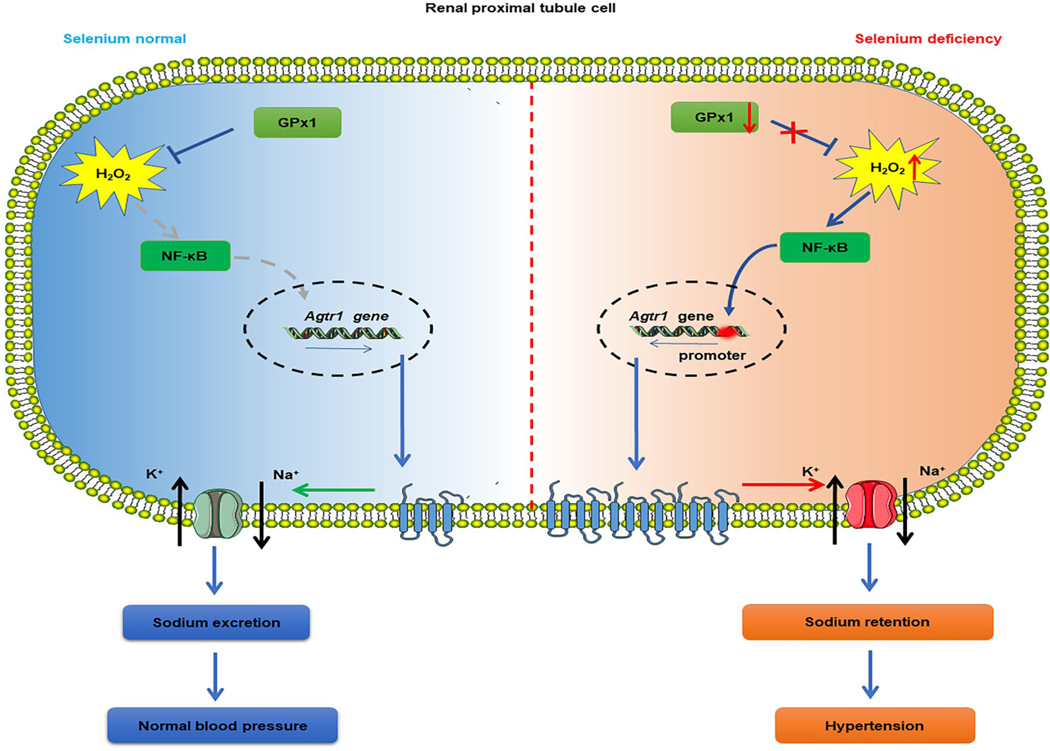
In summary in Fig. 7, we have demonstrated that long-term selenium deficiency causes hypertension in SD rats, which is, at least in part, due to the decreased urine volume and sodium excretion. Selenium deficiency increases H2O2 production, in part by reducing GPx1 expression, which enhances NF-κB activity, increases AT1R expression and impairs sodium exretion, and consequently increases blood pressure.
Fig. 7.
Long-term selenium deficiency causes hypertension in SD rats, which is, at least in part, due to the decreased urine volume and sodium excretion. Selenium deficiency elevates H2O2 production, partially via reducing GPx1 expression, enhances NF-κB activity, aggravates AT1R expression and its-mediated renal function, causing urinary sodium retention and consequently increasing blood pressure.
Supplementary Material
Highlights.
Long-term selenium deficiency causes hypertension.
Selenium deficiency increases renal AT1R expression and sodium retention.
Selenium deficiency elevates NF-κB activity via GPx1/H2O2 pathway.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (82070442, 81770425), National Key R&D Program of China (2018YFC1312700), Selenium Research Fund of Chongqing Nutrition Society (2019004), Program of Chongqing Medical University for Youth Innovation in Future Medicine (W0085), and the National Institutes of Health (P01HL074940, DK039308, and DK119652).
Abbreviations
- Ang II
angiotensin II
- AT1 R
angiotensin II receptor type 1 receptor
- DBP
diastolic blood pressure
- GPx1
glutathione peroxidase 1
- GSH-Px
glutathione peroxidase
- H2O2
hydrogen peroxide
- MBP
mean blood pressure
- MDA
malondialdehyde
- NF-κB
nuclear factor kappaB
- PDTC
pyrrolidine dithiocarbamate
- ROS
reactive oxygen species
- RPT
renal proximal tubule
- SBP
systolic blood pressure
- Se
selenium
- SeMet
selenomethionine
Footnotes
Availability of data and materials
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Competing Interests
The authors declared there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tsao CW, et al. , Heart disease and stroke statistics-2022 update: a report from the American Heart Association, Circulation. 145 (2022) 153–639. [DOI] [PubMed] [Google Scholar]
- [2].NCD Risk Factor Collaboration (NCD-RisC), Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants, Lancet. 398 (10304) (2021) 957–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mokdad AH, et al. , The state of US Health, 1990–2016: burden of diseases, injuries, and risk factors among US states, JAMA.319 (14) (2018) 1444–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou B, et al., Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension, Nat Rev Cardiol. 18 (11) (2021) 785–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Harrison DG, Coffman TM, Wilcox CS. Pathophysiology of hypertension: the mosaic theory and beyond, Circ Res. 128 (7) (2021) 847–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ozemek C, et al. , The role of diet for prevention and management of hypertension, Curr Opin Cardiol. 33 (4) (2021) 388–393. [DOI] [PubMed] [Google Scholar]
- [7].Williams CR, et al. , Zinc deficiency induces hypertension by promoting renal Na+ reabsorption, Am J Physiol Renal Physiol. 316 (4) (2019) 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Darroudi S, et al. , Association between hypertension in healthy participants and zinc and copper status: a population-based study, Biol Trace Elem Res. 190 (1) (2019) 38–44. [DOI] [PubMed] [Google Scholar]
- [9].Ye R, et al. , The role and mechanism of essential selenoproteins for homeostasis, Antioxidants (Basel). 11 (5) (2022) 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity, Nutrients. 10 (9) (2018) 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huang Z, Rose AH, Hoffmann RR, The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities, Antioxid Redox Signal. 16 (7) (2012) 705–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang Y, et al. , Role of selenoproteins in redox regulation of signaling and the antioxidant system: a review, Antioxidants (Basel). 9 (5) (2020) 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Touyz RM, et al. , Oxidative stress: a unifying paradigm in hypertension, Can J Cardiol. 36 (5) (2020) 659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Murray EC, et al. , Therapeutic targeting of inflammation in hypertension: from novel mechanisms to translational perspective, Cardiovasc Res. 117 (13) (2021) 2589–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jones GD, et al. , Selenium deficiency risk predicted to increase under future climate change, Proc Natl Acad Sci U S A. 114 (11) (2017) 2848–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shreenath AP, Ameer MA, Dooley J. Selenium Deficiency. StatPearls Publishing, Treasure Island (FL), 2022. [PubMed] [Google Scholar]
- [17].Dinh QT, et al. , Selenium distribution in the Chinese environment and its relationship with human health: a review, Environ Int. 112 (2017) 294–309.. [DOI] [PubMed] [Google Scholar]
- [18].Shimada BK, Alfulaij N, Seale LA. The impact of selenium deficiency on cardiovascular function, Int J Mol Sci. 22 (19) (2021) 10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nawrot TS, et al. , Blood pressure and blood selenium: a cross-sectional and longitudinal population study, Eur Heart J. 28 (5) (2007) 628–633. [DOI] [PubMed] [Google Scholar]
- [20].Afridi HI, et al., Interaction between essential elements selenium and zinc with cadmium and mercury in samples from hypertensive patients. Biol Trace Elem Res, 160 (2) (2014) 185–196. [DOI] [PubMed] [Google Scholar]
- [21].Lewandowska M, Sajdak S, Lubiński J. Serum selenium level in early healthy pregnancy as a risk marker of pregnancy induced hypertension, Nutrients. 11 (5) (2019) 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mazloomi S, et al. , Correlation of thioredoxin reductase (TrxR) and nitric oxide synthase (NOS) activities with serum trace elements in preeclampsia, Clin Exp Hypertens, 43 (5) (2021) 120–124. [DOI] [PubMed] [Google Scholar]
- [23].Luo H H, et al. , Exposure to maternal diabetes mellitus causes renal dopamine D1 receptor dysfunction and hypertension in adult rat offspring, Hypertension. 72 (4) (2018) 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Y, et al. , Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis, Hypertension. 62 (5) (2013) 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang Y, et al. , GRK4-mediated adiponectin receptor-1 phosphorylative desensitization as a novel mechanism of reduced renal sodium excretion in hypertension, Clin Sci (Lond). 134 (18) (2020) 2453–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Woost PG, et al. , Strategy for the development of a matched set of transport-competent, angiotensin receptor-deficient proximal tubule cell lines, In Vitro Cell Dev Biol Anim. 42 (7) (2006) 189–200. [DOI] [PubMed] [Google Scholar]
- [27].Wang S, et al. , Role of thioredoxin 1 in impaired renal sodium excretion of hD5RF173L transgenic mice, J Am Heart Assoc. 8 (8) (2019) 012192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen K, et al. , Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 9 (418) (2017) 6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen K, et al. , Role of GRK4 in the regulation of arterial AT1 receptor in hypertension, Hypertension. 63 (2) (2014) 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lan C, et al. , Progesterone, via yes-associated protein, promotes cardiomyocyte proliferation and cardiac repair, Cell Prolif. 53 (11) (2020) 12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hall JE, et al. , Obesity, kidney dysfunction and hypertension: mechanistic links, Nat Rev Nephrol. 15 (6) (2019) 367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang MZ, Harris RC. Antihypertensive mechanisms of intra-renal dopamine, Curr Opin Nephrol Hypertens. 24 (2) (2015) 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang N, et al., Dietary fructose enhances angiotensin II-stimulated Na+ transport via activation of PKC-α in renal proximal tubules, Am J Physiol Renal Physiol. 318 (6) (2020) 1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yun JW, et al. , Glutathione peroxidase-1 inhibits transcription of regenerating islet-derived protein-2 in pancreatic islets, Free Radic Biol Med. 134 (2019) 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Handy DE, Loscalzo J. The role of glutathione peroxidase-1 in health and disease, Free Radic Biol Med. 188 (2022) 146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cowling RT, et al. , Transcription factor NF-kappa B is necessary for up-regulation of type 1 angiotensin II receptor mRNA in rat cardiac fibroblasts treated with tumor necrosis factor-alpha or interleukin-1 beta, J Biol Chem. 277 (8) (2002) 5719–5724. [DOI] [PubMed] [Google Scholar]
- [37].Hadrup N, Ravn-Haren G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: a review, J Trace Elem Med Biol. 67 (2021) 126801. [DOI] [PubMed] [Google Scholar]
- [38].Rayman MP. Selenium and human health, Lancet. 379 (11) (2012) 1256–1268. [DOI] [PubMed] [Google Scholar]
- [39].Kryukov GV, et al. , Characterization of mammalian selenoproteomes, Science. 300 (5624) (2003)1439–1443. [DOI] [PubMed] [Google Scholar]
- [40].Zhang X, C, et al. , Selenium status and cardiovascular diseases: meta-analysis of prospective observational studies and randomized controlled trials, Eur J Clin Nutr. 70 (2) (2016) 162–169. [DOI] [PubMed] [Google Scholar]
- [41].Bomer N N, et al. , Selenium and outcome in heart failure, Eur J Heart Fail. 22 (8) (2020) 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Be, et al., Dietary and serum selenium in coronary heart disease and all-cause mortality: An international perspective, Asia Pac J Clin Nutr. 29 (4) (2020) 827–838. [DOI] [PubMed] [Google Scholar]
- [43].Swart R, et al. , Serum selenium levels, the selenoprotein glutathione peroxidase and vascular protection: the SABPA study, Food Res Int. 104 (2018) 69–76. [DOI] [PubMed] [Google Scholar]
- [44].Wang Z, et al. , A cross-sectional study of the distribution patterns and potential determinants in plasma selenium status among chinese adults with hypertension, Front Nutr. 9 (2022) 882309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ojeda ML, et al. , Selenium, a dietary-antioxidant with cardioprotective effects, prevents the impairments in heart rate and systolic blood pressure in adolescent rats exposed to binge drinking treatment, Am J Drug Alcohol Abuse. 47 (6) (2021) 680–693. [DOI] [PubMed] [Google Scholar]
- [46].Xie C, et al. , Regional difference in the association between the trajectory of selenium intake and hypertension: a 20-year cohort study, Nutrients. 13 (5) (2021) 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Grotto D, et al. , Long-term excessive selenium supplementation induces hypertension in rats, Biol Trace Elem Res. 182 (1) (2018) 70–77. [DOI] [PubMed] [Google Scholar]
- [48].Tan QH, et al. , A U-shaped relationship between selenium concentrations and all-cause or cardiovascular mortality in patients with hypertension, Front Cardiovasc Med. 8 (2021) 671618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Almeida LF, et al. , Role of the renin-angiotensin system in kidney development and programming of adult blood pressure, Clin Sci (Lond). 134 (6) (2020) 641–656. [DOI] [PubMed] [Google Scholar]
- [50].Li XC, et al. , Intratubular, intracellular, and mitochondrial angiotensin II/AT1 (AT1a) receptor/NHE3 signaling plays a critical role in angiotensin II-induced hypertension and kidney injury, Front Physiol. 12 (2021) 702797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yazici NG, et al. , An immunohistochemical study of the effects of various antioxidants on rat lung during chemotherapy, Biotech Histochem. 95 (6) (2020) 445–455.. [DOI] [PubMed] [Google Scholar]
- [52].Ruggeri RM, et al. , Selenium exerts protective effects against oxidative stress and cell damage in human thyrocytes and fibroblasts, Endocrine. 68 (1) (2020) 151–162. [DOI] [PubMed] [Google Scholar]
- [53].Li S, et al., Selenium deficiency-induced pancreatic pathology is associated with oxidative stress and energy metabolism disequilibrium, Biol Trace Elem Res. 199 (1) (2021) 154–165. [DOI] [PubMed] [Google Scholar]
- [54].Gać P, et al. , The total antioxidant status, serum selenium concentrations and the ultrasound assessment carotid intima media thickness in patients with arterial hypertension, Antioxidants (Basel). 10 (1) (2021) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities, Antioxid Redox Signal. 15 (7) (2011) 1957–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Muller AS, Pallauf J. Down-regulation of GPx1 mRNA and the loss of GPx1 activity causes cellular damage in the liver of selenium-deficient rabbits, J Anim Physiol Anim Nutr (Berl). 86 (9-10) (2002) 273–287. [DOI] [PubMed] [Google Scholar]
- [57].Cao JJ, Gregoire BR, Zeng H. Selenium deficiency decreases antioxidative capacity and is detrimental to bone microarchitecture in mice, J Nutr. 142 (8) (2012) 1526–1531. [DOI] [PubMed] [Google Scholar]
- [58].Zhu H, et al. , Selenium supplementation improved cardiac functions by suppressing DNMT2-mediated GPX1 promoter DNA methylation in AGE-induced heart failure, Oxid Med Cell Longev. (2012) 5402997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.