Abstract
On February 2, 2022, President Biden and First Lady Dr. Biden reignited the Cancer Moonshot, setting a new goal to reduce age-standardized cancer mortality rates by at least 50% over the next 25 years in the U.S. We estimated trends in U.S. cancer mortality during 2000-2019 for all cancers and the six leading types (lung, colorectum, pancreas, breast, prostate, liver). Cancer death rates overall declined by 1.4%/year from 2000-2015 accelerating to 2.3%/year during 2016-19, driven by strong declines in lung cancer mortality (−4.7%/year, 2014-19). Recent declines in colorectal (−2.0%/year, 2010-2019) and breast cancer death rates (−1.2%/year, 2013-2019) also contributed. However, trends for other cancer types were less promising. To achieve the Moonshot goal, progress against lung, colorectal, and breast cancer deaths needs to be maintained and/or accelerated, and new strategies for prostate, liver, pancreatic, and other cancers are needed. We reviewed opportunities to prevent, detect and treat these common cancers that could further reduce population-level cancer death rates and also reduce disparities.
Introduction
Enormous progress has been made against cancer mortality since the passing of the National Cancer Act in 1971(1). Nevertheless, cancer remains the second leading cause of death in the U.S., responsible for approximately 600,000 deaths in 2019 (2). The U.S. government launched the Cancer Moonshot in 2016 to speed progress from cancer prevention to survivorship. In that year, Congress passed the 21st Century Cures Act whichdevoted $1.8 billion dollars over 7 years to Cancer Moonshot-driven research at the National Cancer Institute towards accelerating scientific discovery, fostering greater collaboration, and improving data sharing (3). On February 2, 2022, President Biden and First Lady Dr. Biden reignited the Cancer Moonshot, setting new goals to reduce age-standardized cancer mortality rates by at least 50% over the next 25 years and improve the experience of people living with and surviving cancer and their families, ending “cancer as we know it” (4).
In this manuscript, we examine trends in cancer mortality from 2000-2019 and project these trends forward 25 years, comparing the projected rate in 2047 with the goal of a 50% reduction. Reaching this goal requires consideration of specific approaches for each cancer type, as etiology, prevention, and treatment differ by site and type. Here, we focus on the trends in incidence, survival, and mortality (5) for the fifteen most common causes of cancer death in the U.S. and review the six most common cancers in greater detail, as substantial progress against these cancers will be required to reach the 50% goal. These six cancers (lung, colorectal, pancreatic, breast, prostate, and liver/intrahepatic bile duct (IHBD) cancer (i.e., “liver cancer” throughout the paper) explain 56% of cancer deaths in men and 57% in women (Supplemental Table 1). We then discuss some of the most promising, and realistic, opportunities to further reduce U.S. cancer death rates and disparities for these and other cancers over the next 25 years.
Our focus on these leading cancer types is not meant to diminish the importance of continued innovation to prevent deaths due to other cancer types, including rare tumors and pediatric cancers. Rather, we focus on these sites because they contribute the largest number of deaths such that progress against them is required to reach the 50% goal.
Results:
In 2019, there were nearly 600,000 cancer deaths in the U.S. (Supplemental Table 1). The largest number of cancer deaths were caused by cancers of the lung (n=139,601; 23.3%), colorectum (n=51,896; 8.7%), pancreas (n=45,885; 7.7%), female breast (n=42,280; 7.1%), prostate (n=31,636; 5.3%) and liver (n=27,958, 4.7%). To achieve a 50% decline in age-standardized mortality rates between 2022 and 2047, death rates need to decline an average of 2.7%/year overall.
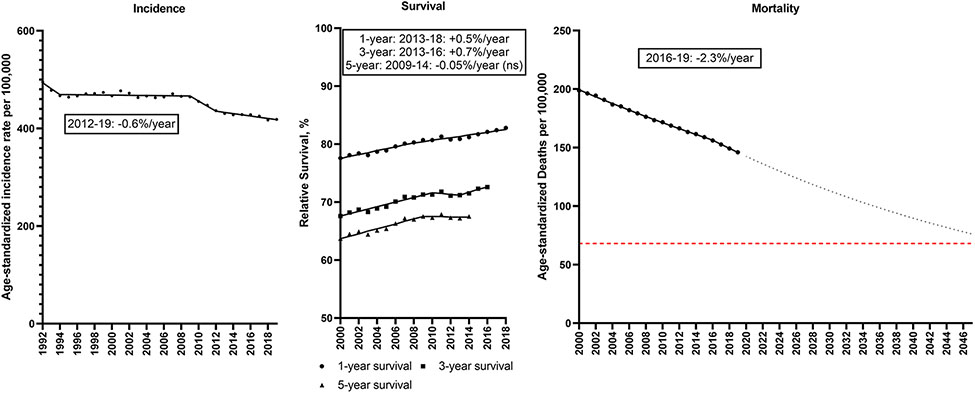
From 1992-2019, the overall age-standardized incidence of cancer decreased from 495.1 to 418.4/100,000 (Figure 1; Supplemental Table 2). Incidence rates were stable during 1994-2009 before declining during 2009-2019 (2009-12: −2.3%/year; 2012-19: −0.6%/year). Relative survival has improved (1-year relative survival: 77.6 to 82.8%; 5-year relative survival: 63.7 to 67.5%). The combination of declining incidence and increasing survival resulted in a decline in mortality rates from 198.8/100,000 in 2000 to 146.0/100,000 in 2019 and the rate of decline accelerated to −2.3%/year during 2016-19. If this trend continues, the cancer mortality rate will decline 44% from 136.1/100,000 in 2022 to 76.3/100,000 in 2047. To reach the Moonshot goal, however, the cancer death rate would need to decline further to fall below 68.1/100,000.
Figure 1. Total cancer.
Incidence rates (1992-2019), 1, 3 and 5-year relative survival (2000-2018, 2016, 2014) and mortality rates (2000-2019) for total cancer in the U.S. Points represent observed values and lines represent fitted estimates from Joinpoint models. For mortality rates, dashed lines represent projected mortality rates through 2047 based on extrapolating the data from the most recent trend. The red dashed line represents a 50% decline in age-standardized death rates from 2022. Estimates provided are annual percentage changes in rates for the most recent time period. ns: not statistically significant
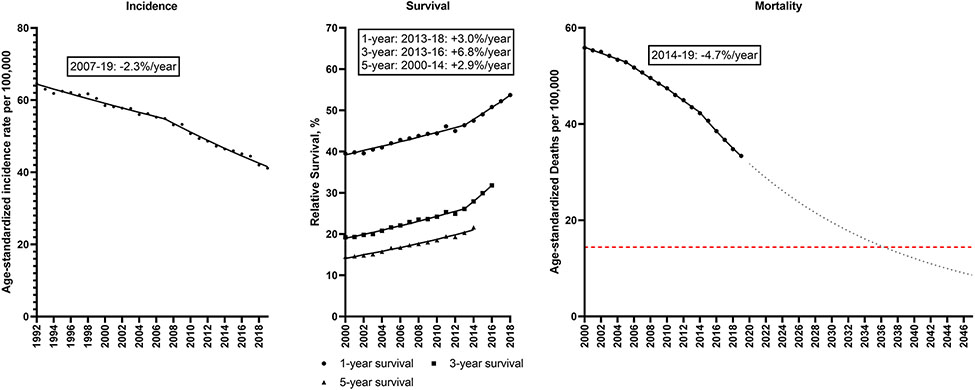
A major driver of these overall trends was lung cancer, as incidence declined from 64.7 to 41.2/100,000 during 1992-2019, with a decline of −2.3%/year during 2017-19 (Figure 2; Supplemental Table 3). Relative survival also improved over time, (1-year relative survival: 39.5 to 53.7%; 5-year relative survival: 14.5 to 21.6%). Rapid increases in survival were observed starting in 2013 (3.0%/year for 1-year survival; 6.8%/year for 3-year survival). Lung cancer mortality rates, therefore, declined significantly from 55.9 to 33.4/100,000, with the rate of decline accelerating to −4.7%/year during 2014-19. If this trend continues, the lung cancer mortality rate will further decline 70% from 28.8/100,000 in 2022 to 8.6/100,000 in 2047.
Figure 2. Lung cancer.
Incidence rates (1992-2019), 1, 3 and 5-year relative survival (2000-2018, 2016, 2014) and mortality rates (2000-2019) for lung cancer in the U.S. Points represent observed values and lines represent fitted estimates from Joinpoint models. For mortality rates, dashed lines represent projected mortality rates through 2047 based on extrapolating the data from the most recent trend. The red dashed line represents a 50% decline in age-standardized death rates from 2022. Estimates provided are annual percentage changes in rates for the most recent time period.
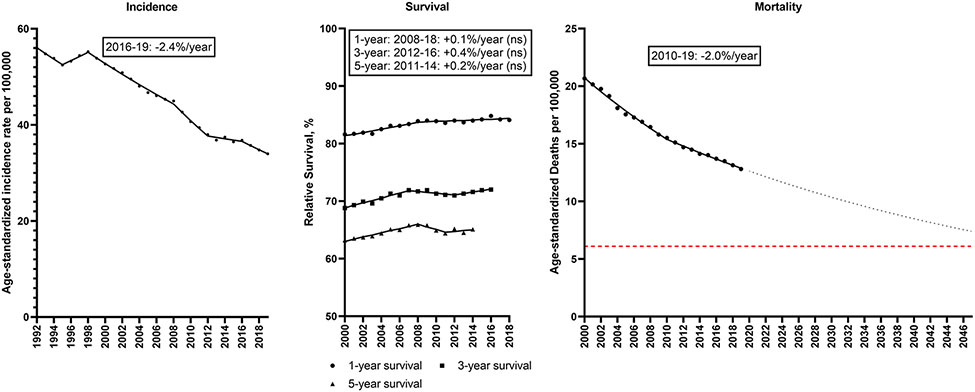
Colorectal cancer incidence decreased from 56.0 to 34.0/100,000 during 1992-2019 (Figure 3; Supplemental Table 4). Incidence declined intermittently across the study period, with rates declining −2.4%/year during 2016-19. Small gains in relative survival occurred during 2000-2019 (1-year relative survival: 81.6 to 84.1%; 5-year relative survival: 63.2 to 65.1%); however, relative survival has remained stable in the last decade. The major declines in incidence translated into declines in colorectal cancer mortality from 20.7 to 12.8/100,000, with a 2.0%/year decline during 2010-19. If this trend continues, the colorectal cancer mortality rate will decline 39% from 12.1/100,000 in 2022 to 7.4/100,000 in 2047.
Figure 3. Colorectal cancer.
Incidence rates (1992-2019), 1, 3 and 5-year relative survival (2000-2018, 2016, 2014) and mortality rates (2000-2019) for colorectal cancer in the U.S. Points represent observed values and lines represent fitted estimates from Joinpoint models. For mortality rates, dashed lines represent projected mortality rates through 2047 based on extrapolating the data from the most recent trend. The red dashed line represents a 50% decline in age-standardized death rates from 2022. Estimates provided are annual percentage changes in rates for the most recent time period. ns: not statistically significant
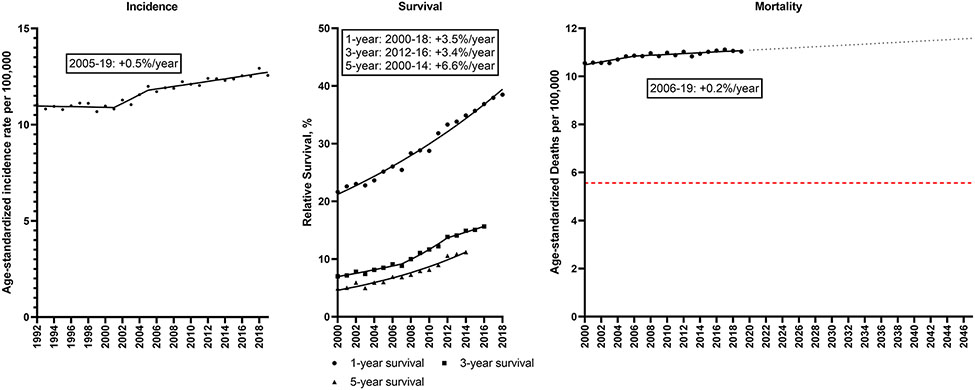
In contrast to declines in lung and colorectal cancer, pancreatic cancer incidence increased slightly from 1992 (11.1/100,000) to 2019 (12.6/100,000), with rates increasing 0.5%/year during 2005-19 (Figure 4; Supplemental Table 5). Increases in relative survival occurred over time, (1-year relative survival: 21.7 to 38.5%; 5-year relative survival: 4.9 to 11.2%), which corresponded to a 6.6%/year increase in 5-year survival during 2000-14, though survival remained poor. Pancreatic cancer mortality rates increased slightly from 10.5 to 11.0/100,000, with a 0.2%/year increase during 2006-19. If this trend continues, the pancreatic cancer mortality rate will increase 4.5% from 11.1/100,000 in 2022 to 11.6/100,000 in 2047.
Figure 4. Pancreas cancer.
Incidence rates (1992-2019), 1, 3 and 5-year relative survival (2000-2018, 2016, 2014) and mortality rates (2000-2019) for pancreatic cancer in the U.S. Points represent observed values and lines represent fitted estimates from Joinpoint models. For mortality rates, dashed lines represent projected mortality rates through 2047 based on extrapolating the data from the most recent trend. The red dashed line represents a 50% decline in age-standardized death rates from 2022. Estimates provided are annual percentage changes in rates for the most recent time period.
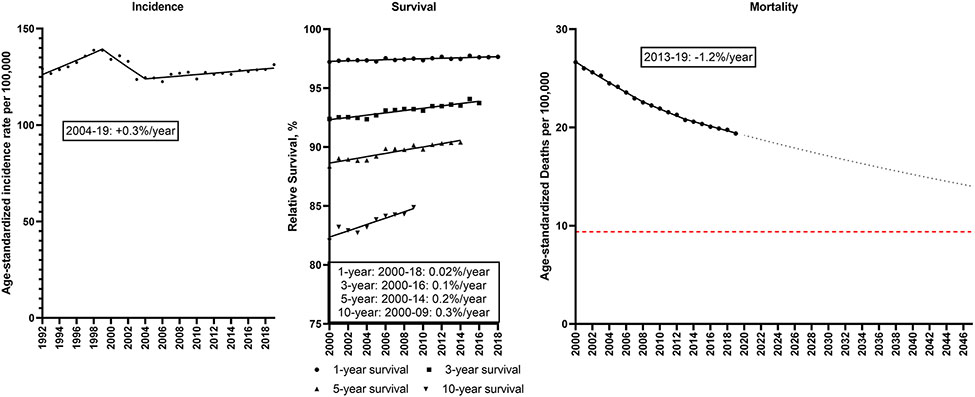
Breast cancer incidence among women has also increased slightly from 129.2 to 131.3/100,000 during 1992-2019 (Figure 5; Supplemental Table 6). Incidence rates increased 1.4%/year during 1992-99, decreased 2.3%/year during 1999-2004 and then increased 0.3%/year during 2004-19. Gains in the already high relative survival occurred over time (1-year relative survival: 97.2% to 97.7%; 5-year relative survival: 88.4 to 90.4%; 10-year relative survival: 82.1 to 84.9%). As a result of these changes, breast cancer mortality rates declined significantly from 26.6 to 19.4/100,000, with a −1.2%/year decline during 2013-19. If this trend continues, the breast cancer mortality rate will decline 23% from 18.8/100,000 in 2022 to 14.0/100,000 in 2047.
Figure 5. Female breast cancer.
Incidence rates (1992-2019), 1, 3, 5 and 10-year relative survival (2000-2018, 2016, 2014, 2009) and mortality rates (2000-2019) for female breast cancer in the U.S. Points represent observed values and lines represent fitted estimates from Joinpoint models. For mortality rates, dashed lines represent projected mortality rates through 2047 based on extrapolating the data from the most recent trend. The red dashed line represents a 50% decline in age-standardized death rates from 2022. Estimates provided are annual percentage changes in rates for the most recent time period.
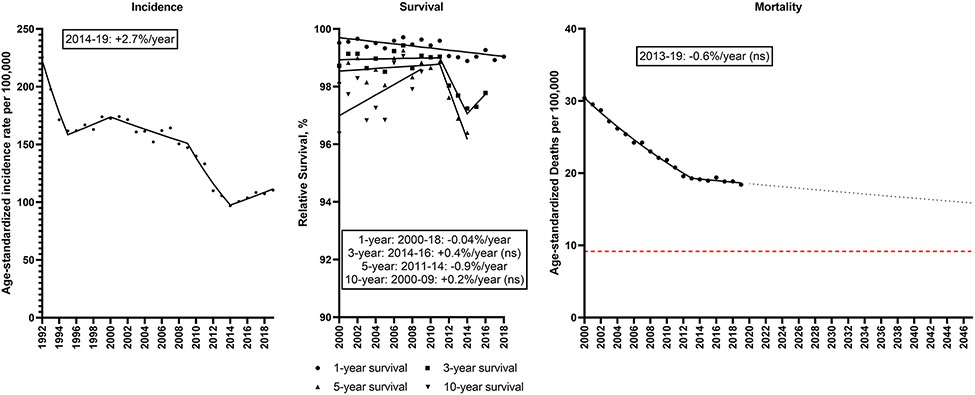
Although prostate cancer incidence among men declined from 1992-95 (−10.7%/year), it increased by 2.7%/year from 2014-2019 (Figure 6; Supplemental Table 7). Relative survival remained high during the time period. Prostate cancer mortality rates declined 3.4%/year from 2000-13, but plateaued from 2013-19, with an overall decline from 30.4 to 18.4/100,000. If this trend continues, prostate cancer mortality rates will decline 13% from 18.3/100,000 in 2022 to 15.9/100,000 in 2047.
Figure 6. Prostate cancer.
Incidence rates (1992-2019), 1, 3, 5 and 10-year relative survival (2000-2018, 2016, 2014, 2009) and mortality rates (2000-2019) for prostate cancer in men in the U.S. Points represent observed values and lines represent fitted estimates from Joinpoint models. For mortality rates, dashed lines represent projected mortality rates through 2047 based on extrapolating the data from the most recent trend. The red dashed line represents a 50% decline in age-standardized death rates from 2022. Estimates provided are annual percentage changes in rates for the most recent time period. ns: not statistically significant
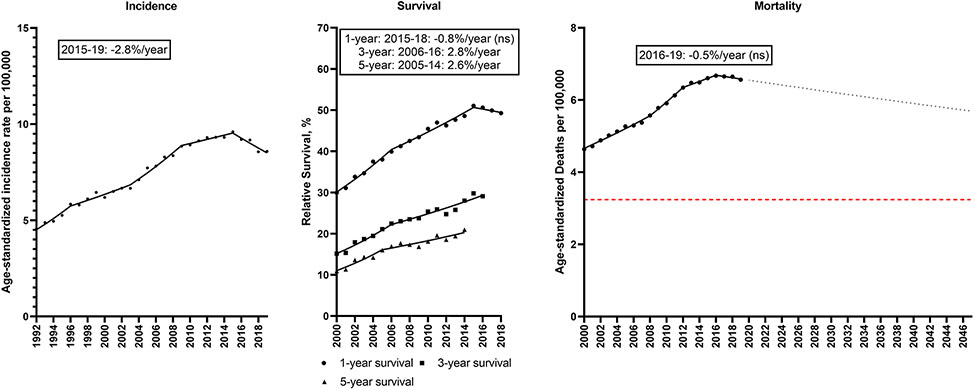
Liver cancer incidence increased from 4.6 to 8.6/100,000 during 1992-2019; however, rates declined 2.8%/year from 2015-2019 (Figure 7; Supplemental Table 8). Relative survival improved over time (1-year relative survival: 29.9 to 49.3%; 5-year relative survival: 10.9 to 21.0%). Liver cancer mortality rates increased from 4.6 to 6.6/100,000; however, mortality rates declined non-significantly 0.5%/year starting in 2016. If this trend continues, the liver cancer mortality rate will decline 14% from 6.5/100,000 in 2022 to 5.6/100,000 in 2047.
Figure 7. Liver cancer.
Incidence rates (1992-2019), 1, 3 and 5-year relative survival (2000-2018, 2016, 2014) and mortality rates (2000-2019) for liver and intrahepatic bile duct cancer in the U.S. Points represent observed values and lines represent fitted estimates from Joinpoint models. For mortality rates, dashed lines represent projected mortality rates through 2047 based on extrapolating the data from the most recent trend. The red dashed line represents a 50% decline in age-standardized death rates from 2022. Estimates provided are annual percentage changes in rates for the most recent time period. ns: not statistically significant
Mortality rates due to all other cancer types combined declined significantly from 80.7 to 63.9/100,000 during 2000-2019 with a decline of 1.7%/year during 2016-2019. Mortality trends for the next nine leading causes of cancer death are presented in supplemental table 9.
Discussion:
Substantial and rapid reductions in cancer mortality rates, averaging 2.7%/year, will be required to meet the Cancer Moonshot goal of a 50% reduction in overall age-standardized cancer mortality in the U.S. by 2047. We found large declines in cancer mortality during 2000-2019, with particularly rapid decreases of −2.3% per year from 2016-2019. These promising trends reflect substantial progress against lung cancer (−4.7%/year), as well as progress against deaths from colorectal (−2.0%/year) and breast cancer (−1.2%/year). However, trends for other cancer types were less promising. To achieve the Moonshot goal, progress against lung, colorectal, and breast cancer deaths needs to be maintained and/or accelerated, and new strategies for prostate, liver, pancreatic, and other cancers are needed.
We have focused the discussion below on the most promising and realistic opportunities to maintain progress and further reduce cancer death rates at the population level from the six most common causes of cancer mortality over the next 25 years (Table 1). For each site, we have highlighted potential interventions that could lead to acceleration in declining death rates in three areas – primary prevention, early detection, and treatment (see Figures 1-7). Our focus on these leading cancer types does not diminish the importance of continued innovation to prevent deaths due to other cancer types, including rare tumors and pediatric cancers. We focus on these sites because they contribute the largest number of deaths and relative reductions in death rates will have the largest population-level impact. Of note, we have not discussed treatments that have small survival benefits or are effective for only a small subset of patients. While tremendously important for individual patients, such treatments are unlikely to have an impact at the population level.
Table 1.
Opportunities for accelerating declines in in cancer death rates in the U.S.
| Cancer Type | Outcome | Potential intervention type | Opportunity? |
|---|---|---|---|
| All Cancers | Incidence | Modifiable risk factors | Reduce smoking, obesity, physical inactivity & alcohol consumption |
| Lung | Incidence | Modifiable risk factors | Increase smoking cessation; prevent initiation |
| Mortality | Screening (Early detection) | Increase low-dose CT uptake and reduce disparities in use | |
| Treatment | Reduce disparities in access to more effective treatments (targeted and immune-based therapies for NSCLC) | ||
| Colorectal | Incidence | Screening (Prevention) | Increase uptake of colonoscopy, flexible sigmoidoscopy, and FIT/FIT-DNA for hard-to-reach populations |
| Preventive treatment | Increase adherence to diagnostic follow-up and polyp removal | ||
| Mortality | Screening (Early detection) | Increase uptake of colonoscopy, flexible sigmoidoscopy; gFOBT and FIT with diagnostic follow-up | |
| Pancreas | Mortality | Treatment | Develop and evaluate new mutant KRAS inhibitors |
| Breast | Incidence | Preventive treatment | Evaluate efficacy of low-dose hormone therapies & improve risk-stratification |
| Mortality | Modifiable risk factors post-diagnosis | Evaluate strategies for increasing physical activity and decreasing obesity in survivors | |
| Screening (Early detection) | Increase mammography uptake amongst under-served populations | ||
| Treatment | Increase uptake/adherence to hormone therapy and chemotherapy especially in under-served populations | ||
| Prostate | Mortality | Screening (Early detection) | Evaluate risk-stratified PSA screening & improved diagnostic testing |
| Treatment | Evaluate strategies to further reduce over treatment and reduce disparities | ||
| Liver | Incidence | Modifiable risk factors | Increase uptake of HBV and HCV treatments, decrease smoking prevalence |
| Mortality | Screening (Early detection) | Increase cirrhosis diagnosis and screening uptake |
In the U.S., there are large disparities in cancer mortality by race and ethnicity, socioeconomic status, and geography. In 2019, Black men and women had the highest cancer death rates compared to each major racial/ethnic group (6). In addition, cancer death rates are significantly higher in the lowest income counties compared to the highest income counties (56% higher for 25-64-year-olds and 14% higher for ≥65-year-olds) (7). Addressing disparities in cancer prevention, early detection and treatment is intertwined with many of the opportunities discussed below and must be a key component in order to achieve sufficient reductions in cancer death rates to reach the 50% goal.
In addition to the specific recommendations below for each cancer site, there are several modifiable lifestyle risk factors that cause multiple types of cancer. In 2014, it was estimated that 28.8% of U.S. cancer deaths could be attributed to cigarette smoking, followed by 6.5% to excess body weight, 4.0% to alcohol and 2.2% to physical inactivity (8). In contrast to the dramatic reductions in smoking (see below under lung cancer), the prevalence of obesity in the U.S. grew from 30.5% in 1999-2000 to 41.9% in 2017-2020 (9). Also, recent increases in alcohol-related deaths suggest worrisome future increases for alcohol-associated cancers (10,11). Reversals of obesity, physical inactivity and alcohol trends are urgently needed. Of note, new obesity drugs have shown promise (12), and new guidelines from the American Academy of Pediatrics advocate for a more aggressive approach to address childhood obesity (13). Successful population-level efforts to increase physical activity, decrease obesity and alcohol consumption would lower deaths from cancer and other chronic diseases; however, it could take longer than the 25-year time frame to reverse the current trends in these exposures and observe an impact on cancer rates at the population-level.
Lung cancer
Despite substantial reductions in mortality over the past thirty years, lung cancer remains the most common cause of cancer-related mortality in men and women in the U.S. Declines in lung cancer mortality can largely be attributed to lower incidence, reflecting dramatic reductions in smoking prevalence and, to some degree, better lung cancer survival from improved treatments. Decades of tobacco control programs and policies have decreased the prevalence of cigarette smoking in youth and increased the prevalence of adult smokers who have successfully quit (14,15). As a result, the cigarette smoking prevalence among men fell from 37.6% in 1980 to 14.1% in 2020, and among women from 29.3% in 1980 to 11.0% in 2020 (16,17). The prevalence of cigarette smoking is particularly low among recent birth cohorts (e.g. 2.0% among high school students in 2022) (18). The proportion of the population that has never smoked also increased substantially, from 45.5% in 1980 to 65.3% in 2018 (17,19). Given the substantial lag time between changes in smoking prevalence and lung cancer incidence at a population level (20-22), and the current low smoking prevalence among younger generations, future declines in lung cancer incidence are likely.
Nevertheless, far greater gains are possible. The prevalence of smoking remains unacceptably high and there are considerable disparities (16). Increased adoption of population-level policies, such as tobacco product price increases and comprehensive smokefree policies, together with enhanced access to evidence-based smoking cessation treatment could accelerate cessation rates (15). In addition, regulatory efforts by the FDA play an important role. FDA has proposed new tobacco product standards that prohibit menthol as a characterizing flavor in cigarettes (23) and all characterizing flavors (other than tobacco) in cigars (24). Additionally, FDA announced that it planned to develop a tobacco product standard that would establish a maximum nicotine level to reduce the addictiveness of cigarettes and some other tobacco products (25). FDA scientists estimate that a potential nicotine product standard would reduce the cigarette smoking prevalence to 1.4% by the year 2060 (26). Given that tobacco smoking is estimated to cause 80% to 90% of lung cancer (27-29), further declines in prevalence would have a dramatic impact on lung cancer mortality. Attention is also needed on other tobacco products such as cigar, pipe, and smokeless tobacco that are known to cause cancer, as well as emerging tobacco products such as e-cigarettes and nicotine pouches.
Lung cancer survival improved from 2000-2018 with treatment of non-small cell lung cancer (NSCLC, ¾ of lung cancer cases in the U.S.), benefiting from targeted therapies against oncogenic driver mutations in the anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) genes and immune-based therapies (30). Likely as a result, declines in mortality rates for NSCLC were twice as fast as declines in incidence rates (30). Unfortunately, there are disparities in the use of these more effective, yet expensive treatments (31,32) and comparable advances have not occurred for small cell lung cancer. Lung cancer screening may also have contributed towards improved survival (33) in recent years. The U.S. Preventive Services Task Force (USPSTF) recommends (grade B) annual low-dose chest computed tomography (LDCT) screening for 50–80-year-olds who have a 20 pack-year smoking history and currently smoke or who have quit in the past 15 years (34). Unfortunately, LDCT is used by only a subset of eligible patients and there are notable disparities (35,36). Recent data from the Behavioral Risk Factor Surveillance System suggest that up to 20% of eligible patients received screening in 2017-2020, (37,38) although 2018 estimates from the American College of Radiology’s Lung Cancer Screening Registry were far lower (39). Increasing use of lung cancer screening would contribute to lower mortality rates, as would further improved treatments.
Colorectal cancer
Colorectal cancer incidence and mortality rates have declined steadily, and relative survival has remained fairly stable over the last decade. Randomized controlled trials of targeted drugs for metastatic colorectal cancer (both anti-EGFR and anti-vascular growth factor receptor [anti-VEGF] agents) have demonstrated minimal survival gains for the vast majority of patients (40). In the future, biomarker development may better identify patients that might benefit from therapies.
Declining colorectal cancer incidence and mortality rates have largely been attributed to screening and removal of precancerous adenomatous polyps (41). Stool-based screening tests, including the guaiac fecal occult blood test (gFOBT) and fecal immunochemical test (FIT), are non-invasive and when administered at two-year intervals reduce colorectal cancer mortality by 9-22% (42). Stool DNA tests have greater sensitivity to detect advanced adenomas than FIT tests; however, there is not yet direct evidence of effect on colorectal cancer mortality (42). Direct visualization screening tests that permit polyp removal include colonoscopy (68% mortality reduction with one round), and flexible sigmoidoscopy (26% reduction with screening every 3-5 five years) (42). While colonoscopy allows for evaluation of and removal of polyps from the entire colon, flexible sigmoidoscopy, which is less invasive with simpler bowel preparation, is used for examination of the distal colon. Computed tomography (CT) colonography is available and also sensitive to detect polyps, but direct evidence on mortality is lacking.
The USPSTF recommends that 45-75-year-olds be screened for colorectal cancer— annually with stool-based tests (or every 1-3 years with stool DNA tests), every ten years with colonoscopy, or every five years with flexible sigmoidoscopy or CT colonography (43). The USPSTF recommendations also include flexible sigmoidoscopy every ten years if accompanied by annual FIT testing.
Despite the demonstrated sensitivity and effectiveness of colonoscopy in particular, only 61% of 50-75-year-olds and 56% of 50-64-year-olds have received colonoscopy in the past ten years (44). In addition, only 9% received gFOBT or FIT, 3% stool DNA, 2% flexible sigmoidoscopy, and 1% CT colonography at recommended intervals (44). Documented racial disparities in screening rates between Black and White individuals have diminished since 2000 (41,45,46), but colorectal cancer incidence and mortality remain higher in Black individuals, perhaps in part due to lower screening rates prior to 2000 and the long-term impact of screening (47). Highlighting the importance of adherence, a recent pragmatic randomized trial drawn from population registries in Europe found that individuals who received an invitation to undergo a single colonoscopy had a non-significant 10% reduction in colorectal cancer mortality risk over 10 years compared to the usual-care (i.e., no invitation or screening) group; the per-protocol adjustment to assume all eligible participants were adherent with colonoscopy suggested that mortality could be reduced by up to 50% (48).
Moving forward, improved uptake of direct visualization screening (i.e., colonoscopy; flexible sigmoidoscopy) will be critical. Stool-based testing may provide opportunities for increased uptake in under-screened populations (49), but only if accompanied by diagnostic follow-up and polyp removal for screen-positive individuals. For a colorectal screening program to effectively reduce mortality, screen-positive (i.e., high-risk) individuals must receive direct visualization and polyp removal at rates higher than the 46% to 81% suggested by a recent study (50).
Pancreas
Age-standardized pancreatic cancer incidence rates in the U.S. have been increasing since 2001, although the increase has slowed in recent years (51). Approximately 90% of pancreatic cancers are pancreatic ductal adenocarcinomas and so most research (and this summary) is focused on this type.
Risk factors that have been consistently linked to pancreatic cancer risk include smoking, obesity, alcohol, diabetes and pancreatitis but all with relative risks generally below 2.0 (52). Only 19% of pancreatic cancers could be attributed to modifiable risk factors in a recent European study and only 14% if smoking was excluded (53). Thus, even substantial changes in known modifiable risk factors would have a relatively modest effect on pancreatic cancer mortality. Having a first-degree relative with pancreatic cancer is associated with a 6.8-fold increased risk (54). Variants of several inherited susceptibility genes have been associated with pancreatic cancer, including BRCA1, BRCA2, PALB2, ATM, P16/CNKN2A and Lynch syndrome genes, with approximately 10% of pancreatic cancer patients carrying a known inherited susceptibility gene variant (52).
Pancreatic cancer survival has improved over time, perhaps partially due to the increased detection of stage 1A disease (2.7% of cases in 2016); however, survival remains poor (55). The poor survival of pancreatic cancer is due to a failure to detect at an early stage and lack of effective treatments. Early symptoms are usually mild and non-specific, and there is no reliable general population screening tool or biomarker with sufficient sensitivity and specificity. 5-year relative survival is 44% among localized stage pancreatic cancers (vs. 3% among distant stage), but only 12% are diagnosed with localized stage disease ( https://seer.cancer.gov/statistics-network/explorer/; RRID:SCR_003293). Several organizations recommend screening with imaging (e.g., endoscopic ultrasound or MRI) for individuals with genetic or familial susceptibility to identify high grade intraepithelial neoplasia and early-stage pancreatic ductal adenocarcinoma. However, as only 10% of pancreatic cancers arise in high-risk individuals this would not have a material impact on overall pancreatic cancer mortality rates.
Surgery of early-stage pancreatic cancer is the only curative treatment, but only 10-20% of patients are identified at this stage and eligible for surgery (56). Currently, chemotherapy is the main form of treatment for unresectable tumors, most often gemcitabine. However, resistance is common, and treatment only increases overall survival by nine months (56). Immunotherapies, such as PD-1 or PD-L1 monoclonal antibodies, have been notably ineffective in treating pancreatic cancer (57).
An attractive target for pancreatic cancer therapies is mutant KRAS, because it is a driver mutation in 90% of pancreatic cancers. Mutant RAS proteins have proven to be difficult drug targets. However, sotorasib, a small molecule drug that specifically inactivates KRAS with the G12C mutation was recently approved for treatment of NSCLC (58). While relatively common in lung cancer, this mutation is found in only 2% of pancreatic cancers. The G12D, G12V, and G12R mutants are present in 38%, 38% and 14% of pancreatic cancers, respectively, and are therefore more attractive targets of pancreatic cancer therapies. Inhibitors for these KRAS mutants are under active development (59,60), and perhaps hold the greatest promise for increasing survival of a substantial fraction of pancreatic cancer patients in the future.
In summary, the prospects for decreasing pancreatic cancer rates substantially over the next 25 years are dim. Better screening tests could have a substantial impact on mortality, but only if they could identify early-stage cancers for surgical removal and could be applied to a broader high-risk segment of the population than current visual tests. New treatments that increase survival time after diagnosis (e.g., mutant KRAS inhibitors) can reasonably be expected in the coming decades.
Breast
The decline in breast cancer mortality rates over the last twenty years is driven by gains in survival, as incidence rates have increased slightly due to increases in estrogen receptor (ER)+ disease (61). Rates of ER− disease are decreasing in all racial/ethnic groups, although disparities remain (62). Although survival for ER− disease is lower than for ER+ disease, ER+ disease accounts for a larger fraction of breast cancer deaths overall (58.7% vs 32.5% for deaths in 2010-2019) and even among <50-year-olds (51%; Supplemental Tables 10 and 11).
Primary prevention trials using hormonal therapies such as tamoxifen or aromatase inhibitors in high-risk women found that treatment reduced ER+ breast cancer incidence by 50-65% (63-66). The USPSTF recommends these therapies be offered to women aged ≥35 years who are at increased risk of breast cancer. They concluded that those with at least a 3% risk for breast cancer in the next 5 years, are likely to derive more benefit than harm from risk-reducing medications (67). It is estimated that while >10 million women in the U.S. are eligible (68), <10% of eligible women receive these therapies (66,69). There are ongoing efforts to assess the efficacy of low-dose tamoxifen, which has reduced side-effects (70). Combined with improved risk stratification to identify women at greatest risk of breast cancer, this could improve the risk-benefit profile of hormonal therapy for primary prevention of ER+ disease.
Randomized trials and observational studies demonstrated that regular mammographic screening reduces breast cancer mortality by 10-30%, depending on age (71,72). Current U.S. survey data suggest that about 70% of eligible women are up to date with mammography (73). Uptake is similar across race/ethnic groups, but lower in women with lower education and income (about 60%) and without health insurance (about 40%) (73). Efforts to increase screening uptake in these populations are warranted.
Endocrine therapy, chemotherapy and targeted therapies improve overall survival in patients with metastatic cancer (74,75), but about two-thirds of breast cancer deaths are due to metastatic relapse after initial therapy. Neoadjuvant and adjuvant therapies, including endocrine therapy, HER2 targeted therapy, immunotherapy, and chemotherapy have significantly reduced mortality in patients initially diagnosed with early-stage disease. Five years of tamoxifen or aromatase inhibitors reduces breast cancer mortality by 30-35% and chemotherapy reduces the mortality by 20-30% (76-78). However, even in a HMO health care setting about 20% of eligible patients do not initiate endocrine treatment (79), and there are also racial/ethnic disparities in use (80). Additionally, studies have shown a higher likelihood of delays in initiation of chemotherapy treatment in Black women with breast cancer (81) which may contribute to worse outcomes (82,83). Therefore, efforts to increase uptake and adherence should further decrease breast cancer mortality.
Observational studies suggest that some lifestyle factors, particularly physical activity, could reduce breast cancer mortality among women with breast cancer, but confounding by treatment and tumor characteristics is difficult to rule out (84,85). Patients could be more motivated to change their behavior after a cancer diagnosis, therefore this warrants further investigation.
In summary, hormonal therapy is highly effective at both preventing and treating ER+ breast cancer, which cause approximately 60% of breast cancer deaths. Additionally, chemotherapy and targeted therapies have reduced mortality. Modeling studies suggest that treatment has had a greater impact than screening on mortality rates for ER+ disease, whereas for ER− disease the contributions were similar (86). Strategies to further increase uptake of hormonal therapy, particularly for prevention, and mammography screening in high-risk and under-served populations would likely have an impact on breast cancer mortality trends at the population level.
Prostate
Prostate cancer incidence and mortality rates declined significantly during 2000-2012, but subsequent declines have been modest. Prostate cancer is the most commonly diagnosed cancer in U.S. men; however, only about 10-15% of men with prostate cancer will die from the disease (87). The relatively low mortality is driven by many patients having low-grade tumors and/or competing causes of mortality in an older population. Among Black men, prostate cancer incidence is increased about 2-fold and mortality is increased 2-3-fold compared to White men (87,88).
There are no established modifiable risk factors for prostate cancer. Prevention studies using the 5α reductase inhibitors finasteride and dutasteride demonstrated a 20-25% reduction in incidence of low grade prostate cancers but a small increase (0.5-0.7%) in the risk of high grade prostate cancer, (89-91) and no reduction in prostate cancer-specific death or death from any cause (92). Thus, these drugs are not used for prostate cancer prevention. To successfully reduce incidence of prostate cancer, safe agents that could prevent high-grade disease among men at higher risk are required.
Prostate cancer mortality began declining in the 1990s, but in 2012 the rate of decline significantly attenuated. While the exact causes for these changes cannot be determined unequivocally, the decrease in the 1990s coincided with the FDA approval of PSA testing for early detection of prostate cancer in 1994. In 2002 and 2008 the USPSTF guidelines concluded that the evidence for PSA screening was insufficient to recommend for or against its use (93,94) and in 2012 they recommended against screening (95), leading to decreases in PSA screening (96). There has been an increase in metastatic prostate cancer diagnoses since 2012 (97-99), and also in localized and regional disease since 2014 (100). Whilst it is plausible that some of the attenuation in the prostate cancer mortality trend reflects the decrease in PSA screening (101), the underlying causes of the other stage-specific increases in prostate cancer incidence also need careful evaluation. Screening in 55-69-year-olds decreased from a peak of 42% in 2008 to 33% in 2013 and then plateaued (102). In 2018, the USPSTF released new guidance recommending shared decision making for screening in men aged 55-69 years (103). PSA screening is associated with significant morbidity including the risk of unnecessary biopsies and surgical or radiation treatment. Screening targeted to high-risk men, and the development of more sensitive and specific tests for high-risk prostate cancer could improve the balance of the risks and benefits.
Treatment of early stage and localized prostate cancer is primarily surgery alone or radiation therapy with or without androgen blockade depending on risk factors (104). This is highly effective in reducing recurrence and prostate cancer death with 10-year disease specific survival in excess of 95%. Active surveillance for patients with low-risk disease and selective patients with intermediate-risk disease significantly reduces the over-treatment of patients (105). The addition of androgen deprivation therapy to radiation in high-risk early-stage disease has been shown to increase overall survival (106). In the metastatic setting taxane-based chemotherapy (in metastatic hormone-sensitive cancer (mHSPC)) and second-generation androgen blockers (in metastatic castration-resistant prostate cancer, non-metastatic castration-resistant prostate cancer, and mHSPC) also significantly increase survival.
In summary, the flattening of the trends in prostate cancer mortality are a concerning development and could be related to reductions in PSA screening. Screening targeted to high-risk men, and the development of more sensitive and specific tests for high-risk prostate cancer could improve the balance of the risks and benefits of screening and reduce prostate cancer mortality.
Liver Cancer
Liver cancer death rates in the U.S. began to decline in 2016 after decades of increases. Roughly ¾ of deaths classified as liver/intrahepatic bile duct cancer deaths were due to liver cancers, which have declined in both incidence (since 2015) and mortality (since 2016) (Supplemental figure 1).
The etiology of liver cancer includes both viral (hepatitis B virus [HBV] and hepatitis C virus [HCV]), and non-viral causes (fatty liver disease, excessive alcohol consumption and cigarette smoking) (107). Excess body weight and alcohol intake combined cause over a half of all liver cancers in the U.S. (8); thus, progress against these important risk factors is critical to accelerate declines in liver cancer death rates. In the U.S., an estimated 7-8% of liver cancer cases are caused by HBV and an estimated 24-34% are caused by HCV (8,108). There are an estimated 1.5-2.4 million people with chronic HBV infection (109) and 2.5-4.7 million people with chronic HCV infection (110). In 2021, the White House unveiled The Viral Hepatitis National Strategic Plan for the United States: A Roadmap to Elimination (2021–2025) to eliminate hepatitis A, B and C as public health threats by 2030 (111).
Infant HBV vaccination started in 1991. However, only 30% of ≥19-year-olds and 19% of ≥50-year-olds reported prior HBV vaccination in 2018 (112). HBV-associated liver cancer risk in birth cohorts with a high prevalence of vaccination should decline in the coming years. As progression from acute to chronic HBV only occurs in 5-10% of healthy adults, adult HBV vaccination would have a limited impact on liver cancer prevention (113); however, currently available treatments for chronic HBV can reduce HCC risk, and have improved significantly over time (114).
There are no HCV vaccines; however, direct-acting antiviral agents (DAAs) to treat HCV were introduced in late 2014, and are highly effective in curing HCV infection and thereby reducing liver cancer risk (115), even among those with cirrhosis (116). DAAs may also provide a survival benefit among some liver cancer patients (116). However, uptake of timely DAA treatment has been low – 23% among those with Medicaid, 28% with Medicare and 35% with private insurance (117); significant disparities by state and race have been reported (117) . Further, in 2013-2016, just over half of persons infected with HCV were aware of their diagnosis (118). In 2020, the CDC expanded HCV testing recommendations to at least once in a lifetime for all adults and for women during each pregnancy, except in settings where the prevalence of HCV is <0.1% (119), though it is unclear if these new guidelines have improved diagnosis. Increased HCV testing and expanded access to curative HCV treatments could reduce future liver cancer incidence and mortality. It should also be noted that alcohol abstinence among those with alcohol-related cirrhosis may also reduce liver cancer risk (120).
There are substantial racial/ethnic disparities in liver cancer mortality rates. The highest death rates have been reported among American Indian/Alaska Native individuals; however, rates among Asian, Black, Latino, and Native Hawaiian/Pacific Islander individuals are also strongly elevated compared to White individuals (121). Given notable racial/ethnic differences in HCV and HBV prevalence (122), prevention and treatment of these viruses would additionally reduce disparities.
Liver cancer survival is poor, with the best prognosis found for early stage diagnoses when cure is possible with surgery or transplantation (123). There are no general population screening programs for liver cancer; however, cirrhosis precedes 80% of cases, and an estimated 69% of individuals in the U.S. with cirrhosis are unaware of their diagnosis (117). Surveillance of people with cirrhosis using ultrasound with or without alpha-fetoprotein measurements is recommended (124), and is associated with increased odds of having a transplant-eligible tumor (125). However, the utility of ultrasound varies by operator and is reduced among those who are obese (107).
In summary, there have been recent declines in liver cancer incidence and mortality rates, but more progress is needed against the three major causes – obesity, alcohol and viral hepatitis. New technologies focused on early detection may contribute to future mortality declines, as well as expanded surveillance of high-risk groups. Strategies to expand detection and treatment of HBV and HCV will be key to accelerate declines.
Other cancer sites and additional opportunities
We have focused on the six most common causes of cancer mortality that together account for 57% of cancer deaths. Deaths due to leukemia, non-Hodgkin lymphoma, myeloma, and cancers of the brain/CNS, bladder, esophagus, kidney/renal pelvis, ovary, and endometrium cause an additional 24% of cancer deaths. Death rates for each of these cancer types have declined significantly in recent years, with the exception of brain/CNS and uterine corpus cancers (Supplemental table 11). Cigarette smoking is estimated to cause 30% of all cancer deaths; thus, continued reductions in cigarette smoking will have a broad impact (8,126,127). HPV-associated cancer incidence and mortality are expected to decline substantially as HPV vaccinated adolescents reach the peak ages for these cancers. Future innovations that might accelerate progress against other cancers include multi-cancer screening technologies that are currently under development and being assessed in new large-scale studies (128). Therapeutic advances against less common cancers could also substantially benefit patients and contribute to further reductions in overall cancer mortality. Despite the difficulties, there is an urgent need for innovation to reduce the prevalence of obesity, physical inactivity and alcohol use. In particular, obesity rates have continued to increase and threaten progress against cancer. Effective population-level interventions would reduce risk of cancer mortality and many other chronic diseases in the years to come.
Unfortunately, cancer death rates have actually increased in recent years for some sites not highlighted here (129). Importantly, deaths rates due to cancer of the uterine corpus among women are increasing the most rapidly of any cancer type (129). These increases are driven by nonendometrioid uterine carcinomas, and there are significant racial/ethnic disparities (130). Interventions focused on prevention, early detection and treatment of these cancers could have an impact on future cancer mortality rates among women (130).
Limitations of our approach include the types of mathematical models used to project cancer mortality over the next 25 years, assumptions about the factors impacting the trends and the accuracy of death certificate coding. We used joinpoint regression models to estimate APCs in incidence, relative survival, and mortality based on recent data. While we can project recent APCs into the future, the method is agnostic to the underlying cause of such trends. Our projections rely on the assumption that recent changes will continue at the same rate for the designated time period, without factoring in the relative importance of exposures, prevention, and treatment embedded in the APC carried forward. This approach may be too optimistic if, for example, the prevalence of overweight and obesity accelerates in the coming years, increasing the number of cancers attributed to excess body weight. Another issue is the number of cancer deaths without a specified site (unknown primary), which continues to be 5% of cancer deaths. Some of these are likely misclassified and site-specific death rates will be slightly underestimated. Finally, it is important to note that a 50% decline in age-adjusted mortality rates does not correspond to a similar decline in the number of cancer deaths. The rapid growth of the older population in the U.S. (131), where cancer death rates are highest, means that a 50% reduction in age-adjusted cancer mortality rates will translate into a much smaller decline in the absolute number of cancer deaths over the next 25 years.
Microsimulation models can be used to predict the impact of specific prevention and treatment policies on incidence and mortality trends (132). For example, the relative harms and benefits of alternative cancer screening and management strategies have informed national guidelines for cancer prevention (133-136). However, there remains a lack of data on the age, period, and cohort effects— of carcinogens, of preventive methods, of therapeutic interventions— to fit models that reflect the cross-sectional U.S. population and the corresponding projections of policy impact on age-adjusted incidence and mortality.
We have focused here on the Moonshot goal of reducing cancer mortality by 50% over the next 25 years. Therefore, our recommendations focus on research and interventions that could impact this population-level goal in this timeframe. However, this does not diminish the importance of other parallel Moonshot priorities (4), including speeding progress against childhood and rare cancers; reducing environmental exposures; and supporting patients, caregivers, and survivors, all of which will contribute to “Ending cancer as we know it”.
In summary, we project that accelerating progress will be needed to reach the Moonshot goal of a 50% reduction in cancer mortality rates by 2047. In addition to continued innovation, substantial progress towards this goal could be accomplished by increasing use of what is already known to prevent, detect, and treat common cancers. Interventions including further reductions in the prevalence of cigarette smoking, increased uptake of colorectal cancer screening, increased use of hormonal therapy in breast cancer, and increased use of HBV and HCV therapy to reduce risk of liver cancer may allow U.S. to reach or even surpass the Moonshot goal of a 50% reduction in cancer mortality rates. Addressing underutilization of, and disparities in, access to prevention, screening, and treatment must play a central role as we work toward the goal of reducing cancer mortality for the entire U.S. population over the coming decades.
Methods:
We estimated trends in cancer incidence (1992-2019), survival (2000-2019) and mortality (2000-2019). Cancer incidence from 12 cancer registries and survival data from 17 cancer registries were obtained from the Surveillance, Epidemiology and End Results (SEER) Program and cancer mortality data were obtained from national death certificate data from the National Center for Health Statistics (NCHS). Incident cancers and cancer deaths were recorded into categories by the SEER program based on International Classification of Diseases for Oncology, third edition (ICD-O-3) and (ICD-10) codes.
Incidence and mortality rates are presented age-standardized to the 2000 U.S. population. 1-, 3-, 5- and 10-year relative survival was estimated for cancers diagnosed annually from 2000 through 2018, 2016, 2014 and 2009, respectively, with follow-up through 2019. Relative survival is commonly used in national surveillance reports (129), and is the ratio of the observed survival among individuals with cancer to the expected survival, based on survival estimates from the general U.S. population by race, sex, age and year. All analyses were carried out in SEER*Stat 8.4.0 (seer.cancer.gov/seerstat; RRID:SCR_003293).
Joinpoint regression (Joinpoint software 4.9.0.0) was used to estimate annual percent changes (APCs) in incidence, relative survival, and mortality estimates. Joinpoint identifies years where temporal trends change significantly. To demonstrate potential future cancer mortality rates under the assumption that recent trends will continue, we used the APC from the most recent time period, which varies by cancer type, to project cancer death rates forward until 2047. The projection assumes that cancer death rates will continue to change at the same trajectory as the most recent time period.
All data are publicly available: https://seer.cancer.gov/data-software/ and https://wonder.cdc.gov/ucd-icd10.html.
Supplementary Material
Statement of significance:
We reviewed opportunities to prevent, detect and treat common cancers, and show that to achieve the Moonshot goal, progress against lung, colon, and breast cancer deaths needs to be maintained and/or accelerated, and new strategies for prostate, liver, pancreatic, and other cancers are needed.
Acknowledgements
The authors would like to thank the following colleagues for providing a review of this manuscript: Michael Cook, Thomas O’Brien, Montserrat García-Closas, Michele Bloch, Robert Schoen, James Doroshow, Hilary Robbins, Phillip Castle, Howard Parnes, Ravi Madan, Jacqueline Vo and Anika Haque.
This work was funded by the Intramural Research Program of the National Cancer Institute.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Kratzer TB, Siegel RL, Miller KD, Sung H, Islami F, Jemal A. Progress Against Cancer Mortality 50 Years After Passage of the National Cancer Act. JAMA Oncology 2022;8(1):156 doi 10.1001/jamaoncol.2021.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Murphy S, Kochenek K, Arias E. Deaths: Final Deata for 2019. National Vital Statistics Reports 2021;70(8). [Google Scholar]
- 3.Sharpless NE, Singer DS. Progress and potential: The Cancer Moonshot. Cancer Cell 2021;39(7):889–94 doi 10.1016/j.ccell.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer DS. A new phase of the Cancer Moonshot to end cancer as we know it. Nat Med 2022;28(7):1345–7 doi 10.1038/s41591-022-01881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis L, Woods LM, Estève J, Eloranta S, Coleman MP, Rachet B. Cancer incidence, survival and mortality: Explaining the concepts. International Journal of Cancer 2014;135(8):1774–82 doi 10.1002/ijc.28990. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence WR, McGee-Avila JK, Vo JB, Luo Q, Chen Y, Inoue-Choi M, et al. Trends in Cancer Mortality Among Black Individuals in the US From 1999 to 2019. JAMA Oncol 2022;8(8):1184–9 doi 10.1001/jamaoncol.2022.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Withrow DR, Berrington de Gonzalez A, Spillane S, Freedman ND, Best AF, Chen Y, et al. Trends in Mortality Due to Cancer in the United States by Age and County-Level Income, 1999-2015. J Natl Cancer Inst 2019;111(8):863–6 doi 10.1093/jnci/djz123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68(1):31–54 doi 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. [cited 2023 16 February]. Available from: <https://www.cdc.gov/obesity/data/adult.html>.
- 10.White AM, Castle IP, Powell PA, Hingson RW, Koob GF. Alcohol-Related Deaths During the COVID-19 Pandemic. Jama 2022;327(17):1704–6 doi 10.1001/jama.2022.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spillane S, Shiels MS, Best AF, Haozous EA, Withrow DR, Chen Y, et al. Trends in Alcohol-Induced Deaths in the United States, 2000-2016. JAMA Netw Open 2020;3(2):e1921451 doi 10.1001/jamanetworkopen.2019.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prillaman M. The 'breakthrough' obesity drugs that have stunned researchers. Nature 2023;613(7942):16–8 doi 10.1038/d41586-022-04505-7. [DOI] [PubMed] [Google Scholar]
- 13.Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents With Obesity. Pediatrics 2023;151(2) doi 10.1542/peds.2022-060640. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Chronic Disease P, Health Promotion Office on S, Health. Reports of the Surgeon General. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [Google Scholar]
- 15.United States Public Health Service Office of the Surgeon G, National Center for Chronic Disease P, Health Promotion Office on S, Health. Publications and Reports of the Surgeon General. Smoking Cessation: A Report of the Surgeon General. Washington (DC): US Department of Health and Human Services; 2020. [Google Scholar]
- 16.Cornelius ME, Loretan CG, Wang TW, Jamal A, Homa DM. Tobacco Product Use Among Adults — United States, 2020. MMWR Morbidity and Mortality Weekly Report 2022;71(11):397–405 doi 10.15585/mmwr.mm7111a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giovino GA. Epidemiology of tobacco use in the United States. Oncogene 2002;21(48):7326–40 doi 10.1038/sj.onc.1205808. [DOI] [PubMed] [Google Scholar]
- 18.Park-Lee E, Ren C, Cooper M, Cornelius M, Jamal A, Cullen KA. Tobacco Product Use Among Middle and High School Students — United States, 2022. MMWR Morbidity and Mortality Weekly Report 2022;71(45):1429–35 doi 10.15585/mmwr.mm7145a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villarroel MA, Blackwell DL, Jen A. [cited 2023 16 February]. Available from: <https://www.cdc.gov/nchs/nhis/SHS/tables.htm>. [Google Scholar]
- 20.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008;100(23):1672–94 doi 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon J, Holford TR, Levy DT, Feuer EJ, Cao P, Tam J, et al. Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach. Ann Intern Med 2018;169(10):684–93 doi 10.7326/m18-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 2014;370(1):60–8 doi 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- 23.Administration FaD. [cited 2023 16 February]. Available from: <https://public-inspection.federalregister.gov/2022-08994.pdf>.
- 24.Food and Drug Administration. [cited 2023 February 16]. Available from: <https://www.federalregister.gov/documents/2022/05/04/2022-08993/tobacco-product-standard-for-characterizing-flavors-in-cigars>.
- 25.Food and Drug Administration. [cited 2023 16 February]. Available from: <https://www.federalregister.gov/documents/2018/03/16/2018-05345/tobacco-product-standard-for-nicotine-level-of-combusted-cigarettes>.
- 26.Apelberg BJ, Feirman SP, Salazar E, Corey CG, Ambrose BK, Paredes A, et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med 2018;378(18):1725–33 doi 10.1056/NEJMsr1714617. [DOI] [PubMed] [Google Scholar]
- 27.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-Century Hazards of Smoking and Benefits of Cessation in the United States. New England Journal of Medicine 2013;368(4):341–50 doi 10.1056/nejmsa1211128. [DOI] [PubMed] [Google Scholar]
- 28.Siegel RL, Jacobs EJ, Newton CC, Feskanich D, Freedman ND, Prentice RL, et al. Deaths Due to Cigarette Smoking for 12 Smoking-Related Cancers in the United States. JAMA Intern Med 2015;175(9):1574–6 doi 10.1001/jamainternmed.2015.2398. [DOI] [PubMed] [Google Scholar]
- 29.Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, Calle EE. Lung cancer death rates in lifelong nonsmokers. J Natl Cancer Inst 2006;98(10):691–9 doi 10.1093/jnci/djj187. [DOI] [PubMed] [Google Scholar]
- 30.Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. New England Journal of Medicine 2020;383(7):640–9 doi 10.1056/nejmoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ermer T, Canavan ME, Maduka RC, Li AX, Salazar MC, Kaminski MF, et al. Association Between Food and Drug Administration Approval and Disparities in Immunotherapy Use Among Patients With Cancer in the US. JAMA Netw Open 2022;5(6):e2219535 doi 10.1001/jamanetworkopen.2022.19535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osarogiagbon RU, Sineshaw HM, Unger JM, Acuna-Villaorduna A, Goel S. Immune-Based Cancer Treatment: Addressing Disparities in Access and Outcomes. Am Soc Clin Oncol Educ Book 2021;41:1–13 doi 10.1200/EDBK_323523. [DOI] [PubMed] [Google Scholar]
- 33.Potter AL, Rosenstein AL, Kiang MV, Shah SA, Gaissert HA, Chang DC, et al. Association of computed tomography screening with lung cancer stage shift and survival in the United States: quasi-experimental study. Bmj 2022;376:e069008 doi 10.1136/bmj-2021-069008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. Jama 2021;325(10):962–70 doi 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 35.Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in Lung Cancer Screening: A Review. Ann Am Thorac Soc 2020;17(4):399–405 doi 10.1513/AnnalsATS.201907-556CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sosa E, D'Souza G, Akhtar A, Sur M, Love K, Duffels J, et al. Racial and socioeconomic disparities in lung cancer screening in the United States: A systematic review. CA Cancer J Clin 2021;71(4):299–314 doi 10.3322/caac.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams RM, Li T, Luta G, Wang MQ, Adams-Campbell L, Meza R, et al. Lung cancer screening use and implications of varying eligibility criteria by race and ethnicity: 2019 Behavioral Risk Factor Surveillance System data. Cancer 2022;128(9):1812–9 doi 10.1002/cncr.34098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rustagi AS, Byers AL, Keyhani S. Likelihood of Lung Cancer Screening by Poor Health Status and Race and Ethnicity in US Adults, 2017 to 2020. JAMA Netw Open 2022;5(3):e225318 doi 10.1001/jamanetworkopen.2022.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedewa SA, Kazerooni EA, Studts JL, Smith RA, Bandi P, Sauer AG, et al. State Variation in Low-Dose Computed Tomography Scanning for Lung Cancer Screening in the United States. J Natl Cancer Inst 2021;113(8):1044–52 doi 10.1093/jnci/djaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet 2010;375(9719):1030–47 doi 10.1016/s0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 41.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66(4):683–91 doi 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 42.Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 2021;325(19):1978–98 doi 10.1001/jama.2021.4417. [DOI] [PubMed] [Google Scholar]
- 43.Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. Jama 2021;325(19):1965–77 doi 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro JA, Soman AV, Berkowitz Z, Fedewa SA, Sabatino SA, de Moor JS, et al. Screening for Colorectal Cancer in the United States: Correlates and Time Trends by Type of Test. Cancer Epidemiol Biomarkers Prev 2021;30(8):1554–65 doi 10.1158/1055-9965.Epi-20-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004;54(2):78–93 doi 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 46.Joseph DA, King JB, Dowling NF, Thomas CC, Richardson LC. Vital Signs: Colorectal Cancer Screening Test Use - United States, 2018. MMWR Morb Mortal Wkly Rep 2020;69(10):253–9 doi 10.15585/mmwr.mm6910a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 2019;16(12):713–32 doi 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 48.Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med 2022;387(17):1547–56 doi 10.1056/NEJMoa2208375. [DOI] [PubMed] [Google Scholar]
- 49.Tinmouth J, Lansdorp-Vogelaar I, Allison JE. Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut 2015;64(8):1327–37 doi 10.1136/gutjnl-2014-308074. [DOI] [PubMed] [Google Scholar]
- 50.Barlow WE, Beaber EF, Geller BM, Kamineni A, Zheng Y, Haas JS, et al. Evaluating Screening Participation, Follow-up, and Outcomes for Breast, Cervical, and Colorectal Cancer in the PROSPR Consortium. J Natl Cancer Inst 2020;112(3):238–46 doi 10.1093/jnci/djz137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Costa WL Jr., Oluyomi AO, Thrift AP. Trends in the Incidence of Pancreatic Adenocarcinoma in All 50 United States Examined Through an Age-Period-Cohort Analysis. JNCI Cancer Spectr 2020;4(4):pkaa033 doi 10.1093/jncics/pkaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol 2021;18(7):493–502 doi 10.1038/s41575-021-00457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naudin S, Viallon V, Hashim D, Freisling H, Jenab M, Weiderpass E, et al. Healthy lifestyle and the risk of pancreatic cancer in the EPIC study. Eur J Epidemiol 2020;35(10):975–86 doi 10.1007/s10654-019-00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brune KA, Lau B, Palmisano E, Canto M, Goggins MG, Hruban RH, et al. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst 2010;102(2):119–26 doi 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blackford AL, Canto MI, Klein AP, Hruban RH, Goggins M. Recent Trends in the Incidence and Survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results Analysis. J Natl Cancer Inst 2020;112(11):1162–9 doi 10.1093/jnci/djaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sally Á, McGowan R, Finn K, Moran BM. Current and Future Therapies for Pancreatic Ductal Adenocarcinoma. Cancers (Basel) 2022;14(10) doi 10.3390/cancers14102417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balachandran VP, Beatty GL, Dougan SK. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019;156(7):2056–72 doi 10.1053/j.gastro.2018.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conroy M, Cowzer D, Kolch W, Duffy AG. Emerging RAS-directed therapies for cancer. Cancer Drug Resist 2021;4(3):543–58 doi 10.20517/cdr.2021.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X, Allen S, Blake JF, Bowcut V, Briere DM, Calinisan A, et al. Identification of MRTX1133, a Noncovalent, Potent, and Selective KRAS(G12D) Inhibitor. J Med Chem 2022;65(4):3123–33 doi 10.1021/acs.jmedchem.1c01688. [DOI] [PubMed] [Google Scholar]
- 60.Kemp SB, Cheng N, Markosyan N, Sor R, Kim IK, Hallin J, et al. Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer. Cancer Discov 2023;13(2):298–311 doi 10.1158/2159-8290.CD-22-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg PS, Barker KA, Anderson WF. Estrogen Receptor Status and the Future Burden of Invasive and In Situ Breast Cancers in the United States. J Natl Cancer Inst 2015;107(9) doi 10.1093/jnci/djv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis Lynn BC, Chernyavskiy P, Gierach GL, Rosenberg PS. Decreasing Incidence of Estrogen Receptor-Negative Breast Cancer in the United States: Trends by Race and Region. J Natl Cancer Inst 2022;114(2):263–70 doi 10.1093/jnci/djab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90(18):1371–88 doi 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 64.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 2005;97(22):1652–62 doi 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 65.Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011;364(25):2381–91 doi 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 66.Crew KD, Albain KS, Hershman DL, Unger JM, Lo SS. How do we increase uptake of tamoxifen and other anti-estrogens for breast cancer prevention? NPJ Breast Cancer 2017;3:20 doi 10.1038/s41523-017-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Force USPST, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Medication Use to Reduce Risk of Breast Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2019;322(9):857–67 doi 10.1001/jama.2019.11885. [DOI] [PubMed] [Google Scholar]
- 68.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst 2003;95(7):526–32 doi 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 69.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol 2010;28(18):3090–5 doi 10.1200/jco.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L, et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J Clin Oncol 2019;37(19):1629–37 doi 10.1200/jco.18.01779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson HD, Cantor A, Humphrey L, Fu R, Pappas M, Daeges M, et al. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Screening for Breast Cancer: A Systematic Review to Update the 2009 US Preventive Services Task Force Recommendation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. [PubMed] [Google Scholar]
- 72.Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med 2016;164(4):244–55 doi 10.7326/m15-0969. [DOI] [PubMed] [Google Scholar]
- 73.Sabatino SA, Thompson TD, White MC, Shapiro JA, de Moor J, Doria-Rose VP, et al. Cancer Screening Test Receipt - United States, 2018. MMWR Morb Mortal Wkly Rep 2021;70(2):29–35 doi 10.15585/mmwr.mm7002a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344(11):783–92 doi 10.1056/nejm200103153441101. [DOI] [PubMed] [Google Scholar]
- 75.Swain SM, Miles D, Kim S-B, Im Y-H, Im S-A, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. The Lancet Oncology 2020;21(4):519–30 doi 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 76.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365(9472):1687–717 doi 10.1016/s0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 77.Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379(9814):432–44 doi 10.1016/s0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol 2022;23(3):382–92 doi 10.1016/s1470-2045(21)00758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bowles EJA, Ramin C, Buist DSM, Feigelson HS, Weinmann S, Veiga LHS, et al. Endocrine therapy initiation among women with stage I-III invasive, hormone receptor-positive breast cancer from 2001-2016. Breast Cancer Res Treat 2022;193(1):203–16 doi 10.1007/s10549-022-06561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farias AJ, Du XL. Racial Differences in Adjuvant Endocrine Therapy Use and Discontinuation in Association with Mortality among Medicare Breast Cancer Patients by Receptor Status. Cancer Epidemiol Biomarkers Prev 2017;26(8):1266–75 doi 10.1158/1055-9965.EPI-17-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Green AK, Aviki EM, Matsoukas K, Patil S, Korenstein D, Blinder V. Racial disparities in chemotherapy administration for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 2018;172(2):247–63 doi 10.1007/s10549-018-4909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Melo Gagliato D, Lei X, Giordano SH, Valero V, Barcenas CH, Hortobagyi GN, et al. Impact of Delayed Neoadjuvant Systemic Chemotherapy on Overall Survival Among Patients with Breast Cancer. Oncologist 2020;25(9):749–57 doi 10.1634/theoncologist.2019-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gagliato Dde M, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 2014;32(8):735–44 doi 10.1200/JCO.2013.49.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fortner RT, Brantley KD, Tworoger SS, Tamimi RM, Rosner B, Farvid MS, et al. Physical activity and breast cancer survival: results from the Nurses' Health Studies. JNCI Cancer Spectr 2023;7(1) doi 10.1093/jncics/pkac085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aune D, Markozannes G, Abar L, Balducci K, Cariolou M, Nanu N, et al. Physical Activity and Health-Related Quality of Life in Women With Breast Cancer: A Meta-Analysis. JNCI Cancer Spectr 2022;6(6) doi 10.1093/jncics/pkac072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst 2014;106(11) doi 10.1093/jnci/dju289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72(1):7–33 doi 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 88.Pietro GD, Chornokur G, Kumar NB, Davis C, Park JY. Racial Differences in the Diagnosis and Treatment of Prostate Cancer. Int Neurourol J 2016;20(Suppl 2):S112–9 doi 10.5213/inj.1632722.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349(3):215–24 doi 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 90.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010;362(13):1192–202 doi 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 91.Theoret MR, Ning YM, Zhang JJ, Justice R, Keegan P, Pazdur R. The risks and benefits of 5α-reductase inhibitors for prostate-cancer prevention. N Engl J Med 2011;365(2):97–9 doi 10.1056/NEJMp1106783. [DOI] [PubMed] [Google Scholar]
- 92.Thompson IM Jr., Goodman PJ, Tangen CM, Parnes HL, Minasian LM, Godley PA, et al. Long-term survival of participants in the prostate cancer prevention trial. N Engl J Med 2013;369(7):603–10 doi 10.1056/NEJMoa1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harris R, Lohr KN, Beck R, Fink K, Godley P, Bunton AJ. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Screening for Prostate Cancer. Rockville (MD): Agency for Healthcare Research and Quality (US); 2002. [PubMed] [Google Scholar]
- 94.Lin K, Lipsitz R, Janakiraman S. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Benefits and Harms of Prostate-Specific Antigen Screening for Prostate Cancer: An Evidence Update for the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. [PubMed] [Google Scholar]
- 95.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157(2):120–34 doi 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 96.Shoag J, Halpern JA, Lee DJ, Mittal S, Ballman KV, Barbieri CE, et al. Decline in Prostate Cancer Screening by Primary Care Physicians: An Analysis of Trends in the Use of Digital Rectal Examination and Prostate Specific Antigen Testing. J Urol 2016;196(4):1047–52 doi 10.1016/j.juro.2016.03.171. [DOI] [PubMed] [Google Scholar]
- 97.Butler SS, Muralidhar V, Zhao SG, Sanford NN, Franco I, Fullerton ZH, et al. Prostate cancer incidence across stage, NCCN risk groups, and age before and after USPSTF Grade D recommendations against prostate-specific antigen screening in 2012. Cancer 2020;126(4):717–24 doi 10.1002/cncr.32604. [DOI] [PubMed] [Google Scholar]
- 98.Jeong SH, Raman JD. Impact of the evolving United States Preventative Services Task Force policy statements on incidence and distribution of prostate cancer over 15 years in a statewide cancer registry. Prostate Int 2021;9(1):12–7 doi 10.1016/j.prnil.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bryant AK, Lee KM, Alba PR, Murphy JD, Martinez ME, Natarajan L, et al. Association of Prostate-Specific Antigen Screening Rates With Subsequent Metastatic Prostate Cancer Incidence at US Veterans Health Administration Facilities. JAMA Oncol 2022;8(12):1747–55 doi 10.1001/jamaoncol.2022.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cook MB, Hurwitz LM, Geczik AM, Butler EN. An Up-to-date Assessment of US Prostate Cancer Incidence Rates by Stage and Race: A Novel Approach Combining Multiple Imputation with Age and Delay Adjustment. Eur Urol 2021;79(1):33–41 doi 10.1016/j.eururo.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burgess L, Aldrighetti CM, Ghosh A, Niemierko A, Chino F, Huynh MJ, et al. Association of the USPSTF Grade D Recommendation Against Prostate-Specific Antigen Screening With Prostate Cancer-Specific Mortality. JAMA Netw Open 2022;5(5):e2211869 doi 10.1001/jamanetworkopen.2022.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berkowitz Z, Li J, Richards TB, Marcus PM. Patterns of Prostate-Specific Antigen Test Use in the U.S., 2005-2015. Am J Prev Med 2017;53(6):909–13 doi 10.1016/j.amepre.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. Jama 2018;319(18):1901–13 doi 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 104.National Comprehensive Cancer Network. [cited 2023 16 February]. Available from: <https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459>.
- 105.Fascelli M, George AK, Frye T, Turkbey B, Choyke PL, Pinto PA. The role of MRI in active surveillance for prostate cancer. Curr Urol Rep 2015;16(6):42 doi 10.1007/s11934-015-0507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009;373(9660):301–8 doi 10.1016/s0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 107.London WTP JL; McGlynn KA Chapter 33. Liver Cancer. In: Thun M, Linet MS, Cerhan JR, Haimain CA, Schottenfeld D, editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 2018. [Google Scholar]
- 108.de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology 2015;62(4):1190–200 doi 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wong RJ, Brosgart CL, Welch S, Block T, Chen M, Cohen C, et al. An Updated Assessment of Chronic Hepatitis B Prevalence Among Foreign-Born Persons Living in the United States. Hepatology 2021;74(2):607–26 doi 10.1002/hep.31782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015;62(5):1353–63 doi 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.U.S. Department of Health and Human Services. [cited 2023 16 February]. Available from: <https://www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf>.
- 112.Lu PJ, Hung MC, Srivastav A, Grohskopf LA, Kobayashi M, Harris AM, et al. Surveillance of Vaccination Coverage Among Adult Populations -United States, 2018. MMWR Surveill Summ 2021;70(3):1–26 doi 10.15585/mmwr.ss7003a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008;48(2):335–52 doi 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 114.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67(4):1560–99 doi 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology 2016;64(1):130–7 doi 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rich NE, Singal AG. Direct-Acting Antiviral Therapy and Hepatocellular Carcinoma. Clin Liver Dis (Hoboken) 2021;17(6):414–7 doi 10.1002/cld.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thompson WW, Symum H, Sandul A, Gupta N, Patel P, Nelson N, et al. Vital Signs: Hepatitis C Treatment Among Insured Adults — United States, 2019–2020. Morbidity and Mortality Weekly Report (MMWR) 2022;71:1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim HS, Yang JD, El-Serag HB, Kanwal F. Awareness of chronic viral hepatitis in the United States: An update from the National Health and Nutrition Examination Survey. J Viral Hepat 2019;26(5):596–602 doi 10.1111/jvh.13060. [DOI] [PubMed] [Google Scholar]
- 119.Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC Recommendations for Hepatitis C Screening Among Adults - United States, 2020. MMWR Recomm Rep 2020;69(2):1–17 doi 10.15585/mmwr.rr6902a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rodriguez M, Gonzalez-Dieguez ML, Varela M, Cadahia V, Andres-Vizan SM, Mesa A, et al. Impact of Alcohol Abstinence on the Risk of Hepatocellular Carcinoma in Patients With Alcohol-Related Liver Cirrhosis. Am J Gastroenterol 2021;116(12):2390–8 doi 10.14309/ajg.0000000000001399. [DOI] [PubMed] [Google Scholar]
- 121.Haque AT, Berrington de González A, Chen Y, Haozous EA, Inoue-Choi M, Lawrence W, et al. Cancer mortality rates by racial/ethnic groups in the United States, 2018–2020. Submitted 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Centers for Disease Control and Prevention. [cited 2023 16 February]. Available from: <https://www.cdc.gov/hepatitis/statistics/2020surveillance/index.htm>.
- 123.National Cancer Institute. [cited Available from: <https://seer.cancer.gov/statistics-network/explorer/application.html?site=1&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&hdn_stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0>.
- 124.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68(2):723–50 doi 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 125.Khalili K, Menezes R, Kim TK, Kochak Yazdi L, Jang HJ, Sharma S, et al. The effectiveness of ultrasound surveillance for hepatocellular carcinoma in a Canadian centre and determinants of its success. Can J Gastroenterol Hepatol 2015;29(5):267–73 doi 10.1155/2015/563893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Freedman ND, Abnet CC, Caporaso NE, Fraumeni JF Jr., Murphy G, Hartge P, et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int J Epidemiol 2016;45(3):846–56 doi 10.1093/ije/dyv175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jacobs EJ, Newton CC, Carter BD, Feskanich D, Freedman ND, Prentice RL, et al. What proportion of cancer deaths in the contemporary United States is attributable to cigarette smoking? Ann Epidemiol 2015;25(3):179–82.e1 doi 10.1016/j.annepidem.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 128.Minasian LM, Pinsky P, Katki HA, Dickherber T, Han PKJ, Harris L, et al. Study design considerations for trials to evaluate multicancer early detection assays for clinical utility. J Natl Cancer Inst 2023;115(3):250–7 doi 10.1093/jnci/djac218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cronin KA, Scott S, Firth AU, Sung H, Henley SJ, Sherman RL, et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer 2022;128(24):4251–84 doi 10.1002/cncr.34479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clarke MA, Devesa SS, Hammer A, Wentzensen N. Racial and Ethnic Differences in Hysterectomy-Corrected Uterine Corpus Cancer Mortality by Stage and Histologic Subtype. JAMA Oncol 2022;8(6):895–903 doi 10.1001/jamaoncol.2022.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vespa J, Armonstrong DM, Medina L. [cited 2023 16 February]. Available from: <https://www.census.gov/library/publications/2020/demo/p25-1144.html>. [Google Scholar]
- 132.Rutter CM, Zaslavsky AM, Feuer EJ. Dynamic microsimulation models for health outcomes: a review. Med Decis Making 2011;31(1):10–8 doi 10.1177/0272989x10369005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Knudsen AB, Rutter CM, Peterse EFP, Lietz AP, Seguin CL, Meester RGS, et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. Jama 2021;325(19):1998–2011 doi 10.1001/jama.2021.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim JJ, Burger EA, Regan C, Sy S. Screening for Cervical Cancer in Primary Care: A Decision Analysis for the US Preventive Services Task Force. Jama 2018;320(7):706–14 doi 10.1001/jama.2017.19872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mandelblatt JS, Stout NK, Schechter CB, van den Broek JJ, Miglioretti DL, Krapcho M, et al. Collaborative Modeling of the Benefits and Harms Associated With Different U.S. Breast Cancer Screening Strategies. Ann Intern Med 2016;164(4):215–25 doi 10.7326/m15-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meza R, Jeon J, Toumazis I, Ten Haaf K, Cao P, Bastani M, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: Modeling Study for the US Preventive Services Task Force. Jama 2021;325(10):988–97 doi 10.1001/jama.2021.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.