Abstract
M1/M2 macrophage polarization plays a pivotal role in the development of acute lung injury (ALI). The hypoxia-inducible factor-1α/pyruvate kinase M2 (HIF-1α/PKM2) axis, which functions upstream of macrophage polarization, has been implicated in this process. The function of HIF-1α is known to be tightly regulated by SUMOylation. Upregulation of SUMO-specific peptidase 3 (SENP3), a deSUMOylation enzyme, is induced by reactive oxygen species (ROS), which are abundantly produced during ALI. To explore the links between SENP3, macrophage polarization, and lung injury, we used mice with Senp3 conditional knockout in myeloid cells. In the lipopolysaccharide (LPS)-induced ALI model, we found that in vitro and in vivo SENP3 deficiency markedly inhibited M1 polarization and production of pro-inflammatory cytokines and alleviated lung injury. Further, we demonstrated that SENP3 deficiency suppressed the LPS-induced inflammatory response through PKM2 in a HIF-1α-dependent manner. Moreover, mice injected with LPS after PKM2 inhibitor (shikonin) treatment displayed inhibition of M1 macrophage polarization and reduced lung injury. In summary, this work revealed that SENP3 promotes M1 macrophage polarization and production of proinflammatory cytokines via the HIF-1α/PKM2 axis, contributing to lung injury; thus, SENP3 may represent a potential therapeutic target for ALI treatment.
Keywords: Macrophage, acute lung injury, SENP3, SUMOylation, HIF-1α, PKM2
Introduction
Sepsis, a common complication in patients with infections, is a leading cause of mortality worldwide [1]. The lungs are susceptible to sepsis, and acute lung injury (ALI) can occur early in sepsis[2]. Despite advances in our knowledge regarding the pathophysiology of ALI and supportive care, the fatality rate of ALI still reaches 20–40%[3]. The search for novel and effective pharmacological approaches to treat ALI is a biomedical research priority. According to an emerging view, ALI is thought to be a systemic inflammatory process, manifesting as an “inflammatory cytokine storm” in the early stage and as increased secretion of anti-inflammatory cytokines in the late stage[4].
Macrophages play a central role in restoring inflammatory homeostasis[5]. Macrophages are categorized into classically activated macrophages (M1 inflammatory macrophages) and alternatively activated macrophages (M2 anti-inflammatory macrophages)[6]. In ALI progression, M1 macrophage activation can drive dysfunctional inflammatory responses, the release of high levels of inflammatory factors, and oxidative stress-associated damage[7]. Therefore, balancing pro- and anti-inflammatory processes by altering the polarization of M1 and M2 macrophages may be a novel strategy for ALI treatment.
Hypoxic conditions directly impact macrophage polarization[8]. Hypoxia inducible factor-1 (HIF-1), a pivotal regulator of cellular adaptation to hypoxia, influences the function and phenotype of macrophages[9] and is involved in cell apoptosis, cell proliferation, tumor angiogenesis, inflammatory diseases, and infections[10,11]. HIF-1 exerts its main biological actions primarily through the α-subunit (HIF-1α). HIF-1α activation induces a marked upregulation of aerobic glycolytic metabolism (the Warburg effect) in macrophages[12]. Pyruvate kinase M2 (PKM2) is the crucial regulatory enzyme promoting the Warburg effect and downregulation of PKM2-mediated glycolysis has been associated with attenuated ALI[13]. Therefore, we speculated that HIF-1α/PKM2 axis-induced macrophage polarization might be the underlying pathological mechanism of ALI.
The importance of post-translational modifications in regulating HIF-1α activity and stability has been well established[14]. For example, ubiquitination prevents unwanted HIF-1α accumulation under normoxia. Small ubiquitin-like modifier (SUMO) proteins are similar to ubiquitin and are classed as members of the ubiquitin-like protein family. However, SUMOylation has the opposite effect of ubiquitination, inhibiting the degradation of HIF-1α by the proteasome[15]. SUMO2/3 can modify HIF-1α on residues K391 and K477 to cause a decrease in its transcriptional activity. SUMOylation is reversible and can be regulated by the SUMO-specific protease family, which removes SUMO proteins from target proteins[16]. SUMO-specific peptidase 3 (SENP3) is highly sensitive to oxidative stress and reactive oxygen species (ROS). During ALI development, ROS accumulation in macrophages[17] elevates the SENP3 levels by inhibiting its proteasomal degradation[18]. The elevated SENP3 levels stabilize HIF-1α by promoting its deSUMOylation. In a model of acute inflammation, SENP3 enhanced TLR4 signaling via deSUMOylation of MKK7 leading to the production of pro-inflammatory cytokines in macrophages[19]. However, it is unknown if there is another pathological mechanism underlying the action of SENP3 other than that involving MKK7.
Thus, we hypothesized that SENP3 might contribute to LPS-induced M1 macrophage polarization by enhancing the HIF-1α/PKM2 axis. To test this hypothesis, we used Senp3 flox/flox (fl/fl) Lyz2-cre mice (mice with Senp3 conditional knockout (cKO) in myeloid cells, hereafter named Senp3 cKO mice) to investigate the contributions of SENP3 to LPS-induced ALI in vivo. The results indicated that SENP3 deficiency attenuated the severity of LPS-induced ALI and M1 polarization of macrophages. In vitro results confirmed that SENP3 deficiency reduced PKM2 expression in a HIF-1α-dependent manner. The PKM2 inhibitor mitigated the LPS-induced lung injury and reduced M1 macrophage polarization in vitro and in vivo. Therefore, this study verified that SENP3 can promote LPS-induced M1 macrophage polarization by enhancing the HIF-1α/PKM2 axis.
Methods
Animals
Fifty-four C57BL/6 (8-week-old male) mice (Shanghai SLAC Laboratory Animal Co., Ltd, Shanghai, China) were maintained under specific pathogen-free conditions in a barrier-sustained facility and provided with sterile food and water. C57BL/6 Senp3 fl/fl and Senp3 cKO mice were generated as previously described[19]. Animal experiments were conducted in accordance with the regulations in the Guide for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of the People's Republic of China. The protocol was approved by the Institutional Animal Care & Use Committee of Shanghai Jiao Tong University School of Medicine (Permit Number: A-2016-023). Sodium pentobarbital was used to anesthetize animals during surgery, and all possible efforts were made to reduce suffering.
LPS-induced ALI model
Mice were anesthetized and placed in a supine position on the operating field, and neck dissection was performed with sterile surgical instruments to achieve exposure of the trachea. Eighteen mice (nine Senp3 fl/fl and nine Senp3 cKO mice) were injected intratracheally with LPS (5 mg/kg) derived from E. coli O55:B5 (Sigma-Aldrich, St Louis, MO, USA) diluted in PBS and another eighteen in the vehicle control group were injected with PBS. Eighteen wild-type (WT) mice were injected intraperitoneally every 8 h for up to 24 h with the PKM2 inhibitor shikonin (Millipore Corporation, Billerica, MA, USA) (8 mg/kg) or PBS. The first dose of shikonin or PBS was given to mice 6 h before LPS injection. The lungs and bronchoalveolar lavage fluid (BALF) were obtained 24 h post-LPS injection.
Histopathologic evaluation
Slides were stained with hematoxylin and eosin (HE) using standard methods and scores were calculated from five randomly chosen sections. Sections were scored using a previously described histological scoring system[4], using the formula: Score = [(20 × A) + (14 × B) + (7 × C) + (7 × D) + (2 × E)] / (number of fields × 100)], in which A represents neutrophils in the alveolar space, B represents neutrophils in the interstitial space, C represents hyaline membranes, D represents proteinaceous debris filling airspaces, and E represents alveolar septal thickening.
Lung wet-to-dry weight ratio and total protein concentration in BALF
The lungs were removed, and the wet weight was recorded. Next, each lung was blotted dry, weighed, and placed in an oven at 70°C for 48 h to obtain the dry weight. For each lung, the ratio of the lung wet weight to its dry weight was calculated. For BALF collection, 1 mL of PBS was slowly instilled into the lung and then removed. The obtained BALF was aliquoted and stored at −80°C. The total protein concentration in the BALF was measured using a BCA Protein Assay Kit (Thermo Scientific, MA, USA). Cells from BALF were used for flow cytometry analysis.
Flow cytometry
Cells obtained from BALF samples were stained with appropriate primary antibodies on ice in the dark for 30 min, followed by washing and analysis using an LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Antibodies specific for CD11b (566 416), CD80 (555 013), CD206 (551 135), F4/80 (743 280), Rat IgG2a (553387), and Rat IgG2b (566418) were purchased from BD Biosciences. Macrophages were classified by CD11b (+) and F4/80 (+). M1 macrophages were classified by CD80 (+) and CD206 (-). Mixed M1/M2-type macrophages were classified by CD80 (+) and CD206 (+) [20,21]. Unstained cells, and isotype controls were included for gating scheme. FACSDiva (version 6.1.1; BD Biosciences) and Prism 9.0 (GraphPad) were used for data analysis.
Bone marrow-derived macrophage (BMDM) culture
BMDMs were generated from Senp3 fl/fl Lyz2-Cre murine bone marrow. Briefly, after euthanasia, bones were collected and flushed under sterile conditions using RPMI 1640 (Hyclone, Logan, UT, USA) to collect the bone marrow, which was then subjected to filtration through a 40-μm filter and red blood cell lysis for 2 min in an appropriate Red Blood Cell Lysis Buffer (Thermo Scientific, MA, USA). For differentiation, freshly isolated bone marrow cells were plated in RPMI 1640 containing 2 mM L-glutamine, 10% FBS, 5% penicillin/streptomycin, and 50 ng/mL murine macrophage colony-stimulating factor (M-CSF) (Peprotech, Rocky Hill, NJ, USA). On day 3, additional fresh M-CSF was added to a final concentration of 50 ng/mL. On day 5, the media was replaced with fresh media, and cells were collected for use on day 7. Cells were stimulated with LPS (100 ng/mL) and the HIF-1α inhibitor PX-478 at the indicated time points.
qRT-PCR
Total RNA was isolated from cells and tissues using Trizol (Invitrogen, Waltham, MA, USA), and cDNA synthesis was performed using 0.5 μg of RNA with a reverse transcription kit (Takara-Bio, Kusatsu, Japan). Quantitative real-time PCR was conducted using TB Green (Roche, Basel, Switzerland) on the Roche Light Cycler 480 system. β-actin expression was used as the internal control. The primer sequences used are reported in Table 1.
Table 1.
The primer sequences used in this study.
| G6pdx-F | CACAGTGGACGACATCCG AAA |
|---|---|
| G6pdx-R | AGCTACATAGGA ATTACGGGC AA |
| Pkm2-F | GTGGCTCGGCTGAATTTCTCT |
| Pkm2-R | CACCGCAACAGGACGGTAG |
| Glut1-F | CAGTTCGGCTATAACACTGGTG |
| Glut1-R | GCCCCCGACAGAGAAGATG |
| HK1-F | TTCGAGAAGATGGTGAGCGG |
| HK1-R | GGAGAGTTCCCATCCCGTTT |
| HK2-F | TGATCGCTGCTTATTCACGG |
| HK2-R | AACCGCCTAGAA ATCTCCAGA |
| PGK-F | CTG TGG TAC TGA GAG CAG CAA GA |
| PGK-R | CAG GAC CAT TCC AAA CAA TCT G |
| Pgd-F | CGTAAGGCCCTCTATGCTTC |
| Pgd-R | TGAAGTTCTGGGTTTCGCTC |
| Actin-F | AGAGCTACGAGCTGCCTGAC |
| Actin-R | AGCACTGTGTTGGCGTACAG |
| TNF-α-F | TCGTAGCAAACCACCAAGTG |
| TNF-α-R | TTGTCCCTTGAAGAGAACCTG |
| IL-6-F | TGCAAGAGACTTCCATCCAG |
| IL-6-R | ATTTCCACGATTTCCCAGAG |
Immunoblotting
Immunoblotting was conducted as previously described[22]. Antibodies specific for SENP3 (5591) and PKM2 (4053) were used (Cell Signaling Technology, Danvers, MA, USA). The antibodies against HIF-1α (NB100-105) were purchased from Novus (Novus Biologicals, CO, USA).
ELISA
The levels of interleukin-6 (IL-6), tumor necrosis factor α (TNFα), IL-4 and IL-10 in BALF were determined using mouse ELISA kits (San Diego, CA, USA) according to the manufacturer's instructions. BALF was harvested by the slow installation and retraction of 1 mL PBS. BALF was aliquoted and stored at −80 ˚C until use.
Statistical analysis
GraphPad Prism (La Jolla, CA, USA) was used to analyze data. Statistical comparison of two groups was performed using either unpair student's t test or Mann–Whitney U test (when normality test is failed), comparison of three or more groups was analyzed by a nonparametric equivalent test (Kruskal-Wallis test). Values of p < 0.05 were considered statistically significant, and data marked with a one (*), two (**) and three (***) asterisks indicate p values of <0.05, < 0.01 and <0.001, respectively.
Results
SENP3 deficiency in macrophages alleviates LPS-induced ALI and lung inflammation
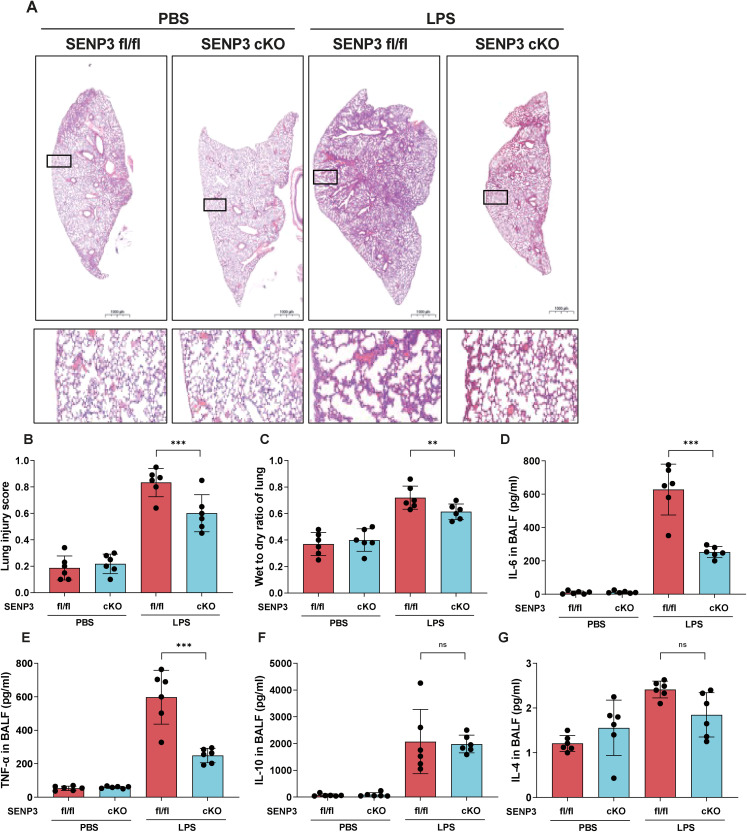
HE staining of lung tissues showed that lung injury was alleviated in Senp3 cKO mice, characterized by less alveolar thickening and leukocyte infiltration (Figure 1A), significantly lower lung injury scores compared to those of the control group (Figure 1B), and decreased levels of the lung wet-to-dry ratio (Figure 1C). Senp3 fl/fl mice exhibited higher levels of IL-6 and TNF-α than Senp3 cKO mice followed by LPS treatment (Figure 1D, E). However, the levels of anti-inflammatory cytokines, such as IL-10 and IL-4, did not differ between the two groups (Figure 1F, G). Thus, Senp3 cKO mice exhibited attenuated LPS-induced ALI and decreased pro-inflammatory cytokine production.
Figure 1.
SENP3 deficiency in macrophages alleviated LPS-induced acute lung injury and lung inflammation. Notes: Senp3 fl/fl (n = 12) and Senp3 cKO mice (n = 12) were intratracheally injected with LPS (5 mg/kg) for 24 h. (A) Hematoxylin and eosin staining. (B) Lung injury score (n = 6). (C) Wet-to-dry ratio assessment (n = 6). BALF (D) IL-6, (E) TNF-α, (F) IL-10, and (G) IL-4 levels were assessed using ELISA (n = 6). The graphs show the means ± SDs, and data shown in B–G are representative of three independent experiments. Values of p < 0.05 were considered statistically significant, and data marked with a one (*), two (**) and three (***) asterisks indicate p values of <0.05, < 0.01 and <0.001, respectively. SENP3, SUMO-specific peptidase 3; LPS, lipopolysaccharide; BALF, bronchoalveolar lavage fluid; IL, interleukin; TNF-α, tumor necrosis factor α.
SENP3 facilitates M1 alveolar macrophage polarization and expression of pro-inflammatory genes
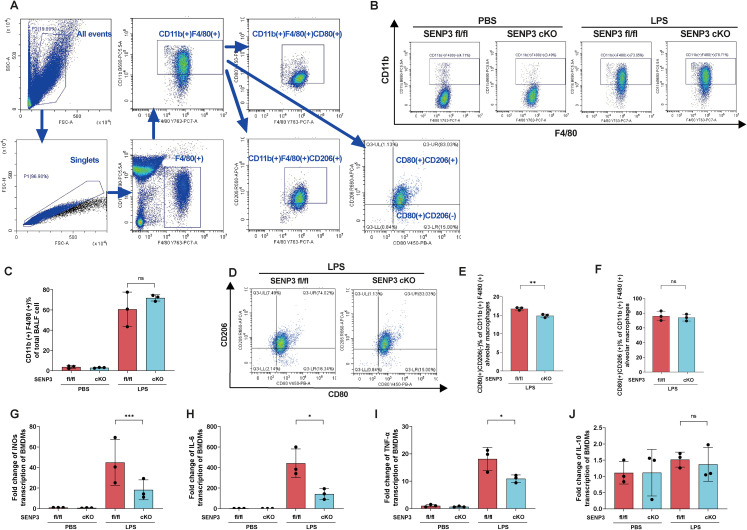
Macrophage polarization plays a key role in ALI prognosis[7,23]. To explore the effect of SENP3 knockout on macrophage polarization under LPS stimulation, we isolated alveolar macrophages from Senp3 fl/fl and Senp3 cKO mice and analyzed the percentages of M1 and M2 macrophages. Flow cytometry analysis showed that after LPS treatment, the percentage of CD11b (+) F4/80 (+) cells was significantly higher than that of PBS-treated controls, but no difference was observed between Senp3 fl/fl and Senp3 cKO mice (Figure 2B, C), confirming the differentiation of these monocytes into macrophages (Figure 2A). CD80 was an M1 macrophage marker and CD206 was an M2 macrophage marker. Typical M1 phenotype, characterized by induction of CD80 and downregulation of CD206. M1 phenotype macrophage exhibited lower expression in Senp3 cKO mice (Figure 2D, E). The percentage of cells expressing a mixed M1/M2-type phenotype coexpressing the CD80 and CD206 markers in Senp3 cKO mice was comparable with that of Senp3 fl/fl mice (Figure 2D, F). To determine the effect of SENP3 on the inflammatory response of macrophages, we assessed LPS-induced inflammation in BMDMs isolated from Senp3 fl/fl and cKO mice. qRT-PCR analysis showed that the expression levels of pro-inflammatory genes, including inducible nitric oxide synthase (iNOs), TNF-α, and IL-6, were significantly lower in BMDMs from Senp3 cKO mice when stimulated by LPS, compared to those from Senp3 fl/fl mice (Figure 2G-1). The expression of the anti-inflammatory IL-10 gene was comparable among groups upon LPS treatment (Figure 2J). Thus, SENP3 promoted M1 macrophage polarization and enhanced the expression of pro-inflammatory genes in vitro.
Figure 2.
SENP3 facilitates alveolar macrophage M1 polarization and expression of pro-inflammatory genes. Notes: Senp3 fl/fl (n = 6) and Senp3 cKO mice (n = 6) were intratracheally injected with LPS (5 mg/kg) and BALF macrophages were assessed using flow cytometry (A-G). (A) Gate strategy for alveolar macrophages in BALF. (B) CD11b (+) F4/80 (+) cells of the total BALF cells. (C) Frequency of CD11b (+) F4/80 (+) cells. (D) Gated cells were analyzed for CD80 and CD206 expression. Upper left quadrants: CD80 (-) CD206 (+) cells (M2 phenotype); lower right quadrants: CD80(+) CD206 (-) cells (M1 phenotype); upper right quadrants: CD80 (+) CD206 (+) cells (mixed M1/M2 phenotype); lower left quadrants: control. (E) Percentage of CD80 (+) CD206 (-) cells out of all CD11b (+) F4/80 (+) cells. (F) Percentage of CD80 (+) CD206 (+) cells out of all CD11b (+) F4/80 (+) cells. BMDMs were isolated from SENP3 fl/fl (n = 6 per group) and SENP3 cKO mice (n = 6 per group) and stimulated with LPS (100 ng/mL) for 24 h, and the transcription levels of (G) iNOs, (H) IL-6, (I) TNF-α, and (J) IL-10 were measured using qRT-PCR (n = 3 per group). The graphs show the means ± SDs, and the data shown in A–J are representative of three independent experiments. Values of p < 0.05 were considered statistically significant, and data marked with a one (*), two (**) and three (***) asterisks indicate p values of <0.05, < 0.01 and <0.001, respectively. SENP3, SUMO-specific peptidase 3; LPS, lipopolysaccharide; BALF, bronchoalveolar lavage fluid; IL, interleukin; TNFα, tumor necrosis factor α; iNOS, inducible nitric oxide synthase.
SENP3 deficiency dampens the LPS-induced inflammatory response through PKM2 in a HIF-1α dependent manner
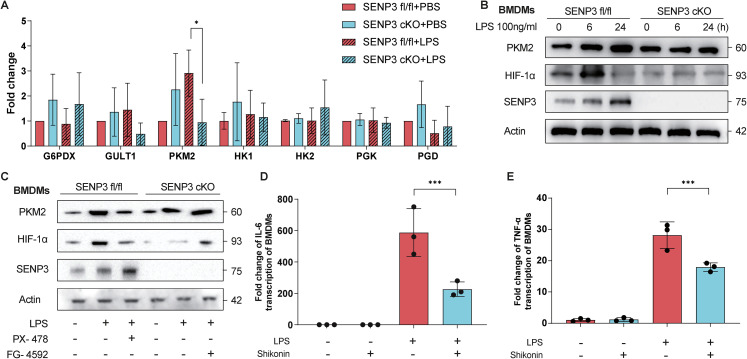
As glycolysis is important in macrophage activation and polarization[24], we examined if SENP3 affected key glycolytic enzymes. The LPS-stimulated BMDMs from Senp3 cKO mice had significantly lower relative PKM2 mRNA expression compared to those of Senp3 fl/fl mice (P < 0.05), while the remaining glycolytic enzymes did not differ between groups (Figure 3A). As PKM2 expression is regulated by HIF-1α in inflammatory conditions, we assessed the levels of PKM2 and HIF-1α proteins in BMDMs treated with LPS (100 ng/mL) at the indicated time, which were markedly decreased in BMDMs of Senp3 cKO mice compared to those of Senp3 fl/fl mice, with the most pronounced difference observed at 24 h (Figure 3B). To verify the regulatory effect of HIF-1α on PKM2, BMDMs were treated with the HIF-1α inhibitor PX-478 and agonist FG-4592 4 h before LPS stimulation. The PKM2 level was significantly reduced in BMDMs from Senp3 cKO mice, while the HIF-1α agonist prevented the decrease in PKM2 expression (Figure 3C), suggesting that HIF-1α activation was positively correlated with the PKM2 protein levels. Furthermore, the HIF-1α inhibitor PX-478 inhibited the LPS-induced increase in PKM2 levels (Figure 3C). Thus, SENP3 deficiency reduced PKM2 expression in a HIF-1α-dependent manner.
Figure 3.
SENP3 inhibited PKM2 transcription in a HIF-1α-dependent manner and the PKM2 inhibitor shikonin dampened the LPS-induced macrophage inflammatory response. Notes: BMDMs were isolated from Senp3 fl/fl (n = 6) and Senp3 cKO mice (n = 6) and stimulated with LPS (100 ng/mL) for the indicated time. (A) The mRNA expression of key glycolytic enzymes was analyzed using qRT-PCR. (B) SENP3, HIF-1α, and PKM2 levels were determined using immunoblotting at the indicated time points. (C) Effects of the HIF-1α inhibitor PX-478 and HIF-1α agonist FG-4592 on the PKM2 levels of BMDMs from Senp3 fl/fl and Senp3 cKO mice. (D, E) Cells were treated with the PKM2 inhibitor shikonin, after which the transcription levels of IL-6 and TNF-α were measured using qRT-PCR. The graphs show the means ± SDs, and the data shown in A–E are representative of three independent experiments. Values of p < 0.05 were considered statistically significant, and data marked with a one (*), two (**) and three (***) asterisks indicate p values of <0.05, < 0.01 and <0.001, respectively. SENP3, SUMO-specific peptidase 3; LPS, lipopolysaccharide; IL, interleukin; TNF-α, tumor necrosis factor α; HIF-1α, hypoxia-inducible factor-1α; BMDMs, bone marrow-derived macrophages; PKM2, pyruvate kinase M2.
We used qRT-PCR to detect the mRNA levels of TNF-α and IL-6 with and without shikonin treatment. Shikonin-treated cells exhibited a significant reduction in LPS-induced TNF-α and IL-6 mRNA expression compared to untreated cells (Figure 3D, E). Therefore, SENP3 promoted LPS-induced macrophage inflammatory responses through PKM2 in a HIF-1α-dependent manner.
Shikonin alleviates the LPS-induced macrophage M1 polarization and lung injury
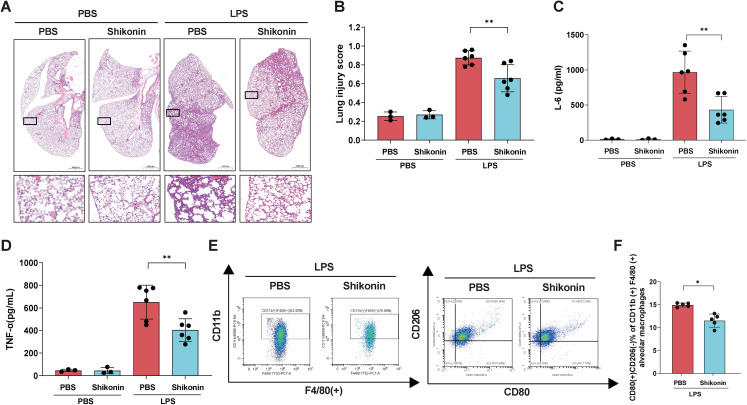
We demonstrated that SENP3 regulated the inflammatory response through HIF-1α/PKM2 signaling upon LPS stimulation in vitro. To validate the effect of the PKM2 inhibitor shikonin on M1 polarization and the lung injury profile in vivo, LPS-injected mice were treated with shikonin at the indicated time. Lung tissue sections were stained with HE (Figure 4A), and lung injury was assessed using a histological lung injury scoring system (Figure 4B). Compared with PBS-treated mice, those treated with shikonin exhibited less severe lung injury (Figure 4A) and significantly lower lung injury scores (Figure 4B). Furthermore, after shikonin treatment, ELISA analysis showed that the IL-6 and TNF-α levels were dramatically reduced compared with those of the PBS-treated mice (Figure 4C, D). We then investigated whether shikonin treatment regulated the activation of alveolar macrophages using flow cytometry. Our results ascertained that the frequency of CD80 (+) CD206 (-) cells of F4/80 (+) cells was significantly decreased after shikonin treatment (Figure 4E, F). Thus, in vivo treatment with shikonin inhibited M1 polarization in macrophages and reduced inflammatory cytokine production, ultimately attenuating LPS-induced lung injury.
Figure 4.
The PKM2 inhibitor shikonin alleviated the LPS-induced macrophage inflammation and lung injury. Notes: (A–F) Male C57BL/6 mice were administered shikonin (8 mg/kg) or PBS intraperitoneally every 8 h with a single dose of LPS (5 mg/kg) or PBS at 6 h after the first treatment (n = 6 per group). (A) Hematoxylin and eosin staining of lung tissues of the four groups was assessed 24 h after LPS injection. (B) Lung injury score assessment (n = 6, LPS injected group; and n = 3, control group). (C-D) The levels of IL-6 and TNF-α in BALF 24 h after LPS injection, as assessed via ELISA (n = 6, LPS injected group; and n = 3, control group). (E) CD80 (+) CD206 (-) cells of F4/80 (+) cells from BALF. (F) Percentage of CD80 (+) CD206 (-) cells out of all CD11b (+) F4/80 (+) cells. The graphs show the means ± SDs, and the data shown in A–F are representative of three independent experiments. Values of p < 0.05 were considered statistically significant, and data marked with a one (*), two (**) and three (***) asterisks indicate p values of <0.05, < 0.01 and <0.001, respectively. LPS, lipopolysaccharide; IL, interleukin; TNF-α, tumor necrosis factor α; BALF, bronchoalveolar lavage fluid; PKM2, pyruvate kinase M2.
Discussion
We report a previously uncharacterized role of SENP3 in the modulation of glycolysis and macrophage polarization in LPS-induced lung injury. Furthermore, we demonstrated that shikonin can alleviate pro-inflammatory cytokine production as well as ALI.
The lung is the organ most frequently damaged by sepsis[25], and its damage is correlated with the uncontrolled inflammatory reaction process[26]. Macrophages, which play important roles in ALI development, are the most important innate immune cells and their main responsibility is to rapidly respond to infections, injuries, or other pro-inflammatory stimuli. Furthermore, versatile macrophage phenotypes have been recognized as essential for repairing lung injury. Specifically, in the acute exudative phase of ALI/acute respiratory distress syndrome, lung macrophages are M1 polarized, releasing TNF-a, IL-1, NO, and ROS to induce a severe inflammatory response[10], as shown in Figure 2. M2 phenotype macrophages release anti-inflammatory molecules, including IL-10 and TNF-α, and inhibit pro-inflammatory pulmonary fibrosis, the late phase of ALI[27]. This was not observed in our experiments, probably due to the short time frame which did not allow us to observe M2 changes. Modulating the polarization of macrophages has therapeutic implications in multiple lung diseases, including asthma[28], lung cancer, and pulmonary fibrosis[29]. Likewise, targeting macrophage polarization has served as an effective and promising treatment for ALI in many preclinical studies[30,31].
Energy metabolism plays a vital role in the balance of macrophage polarization and the execution of immune functions[32]. M1 polarization is often accompanied by a shift in cells from oxidative phosphorylation to aerobic glycolysis for energy production. PKM2, acting as a key glycolytic enzyme activated by HIF-1α in response to LPS stimulation, is critical in M1/M2 differentiation[12]. Furthermore, pyruvate dehydrogenase kinase 1 was reported to participate in M1 macrophage polarization via HIF-1α, accounting for the pro-inflammatory responses[33]. We demonstrated that exposure of BMDMs to LPS led to enhanced HIF-1α levels, which were associated with Senp3 knockout.
HIF-1 is a key transcription factor induced by LPS stimulation that regulates the expression of numerous pro-glycolytic enzymes and pro-inflammatory cytokines. Numerous studies have shown that HIF-1α expression is mainly regulated through post-translational modifications, including phosphorylation[34], ubiquitination[35], acetylation[36], methylation[37], and SUMOylation[38]. Notably, SUMOylation is a highly dynamic process that can be reversed by SENPs (SUMO/sentrin-specific peptidases)[39]. Thus, together, SUMOylation and SENPs collectively determine the protein targets and subsequent function of these proteins.
SUMOylation of HIF-1α led to its ubiquitination and proteasomal degradation in the nucleus of ovarian cancer cells[40] after binding to VHL in a hydroxyl proline-independent manner. However, other studies revealed that SUMO conjugation to HIF-1α reduces its transcription activation function [41,42], consistent with our observation that the protein level of HIF-1α was increased in the presence of SENP3. This discrepancy might be due to the different functions of SUMO in different cellular processes. Recently, the same protective effect of reduced pro-inflammatory cytokine production has been observed in response to SENP3 deficiency[19]. They determined that SENP3-mediated deSUMOylation of MKK7 enhanced JNK phosphorylation and the release of downstream inflammatory factors. However, further investigation is required to determine if other mechanisms exist. We herein reveal the links between SENP3, glucose metabolism, and macrophage polarization.
Macrophage accumulation in the lung generates massive amounts of ROS that contribute to ALI development[43]. The accumulation of the redox-sensitive protease SENP3 in macrophages can be triggered by ROS in a concentration-dependent manner[19,44]. Using Senp3 cKO mice, we found that SENP3 deficiency was associated with alleviated lung injury and inflammatory responses. Several pharmacological treatments for clinical lung injury have been screened, but none were found to decrease mortality[2,3]. Despite being effective for ALI in vitro, targeting pro-inflammatory cytokines, including TNF-α and IL-6, did not prove to be a viable therapeutic option in human studies. Consequently, it would be better to specifically treat the upstream regulators of the inflammatory pathway rather than the downstream events.
Conclusions
Our study demonstrates that SENP3 facilitates the development of ALI via the HIF-1α/PKM2 axis, providing new insight into ALI development. Furthermore, it indicates that PKM2 is a potential pharmacological target in ALI treatment as its inhibition significantly alleviated the LPS-induced macrophage M1 polarization and lung injury.
However, our study has certain limitations. For example, we did not study the effects of SENP3 overexpression on macrophage polarization. Nonetheless, this study demonstrates that SENP3-mediated HIF-1α activity regulates PKM2 activation, and thus reveals a novel mechanism underlying the control of macrophage polarization mediated by the SUMO protease SENP3.
Supplemental Material
Supplemental material, sj-tif-1-ini-10.1177_17534259231166212 for SENP3 facilitates M1 macrophage polarization via the HIF-1α/PKM2 axis in lipopolysaccharide-induced acute lung injury by Shuangjun He, Chenyu Fan, Yiming Ji, Qian Su, Feng Zhao, Cuiying Xie, Xuelian Chen, Yang Zhang and Yi Chen in Innate Immunity
Footnotes
Abbreviations: ALI, acute lung injury; LPS, lipopolysaccharide; HIF-1, Hypoxia inducible factor-1; PKM2, Pyruvate kinase M2; SUMO, Small ubiquitin-like modifier; SENP3, SUMO-specific peptidase 3; ROS, reactive oxygen species; cKO, conditional knockout; BMDM, Bone marrow-derived macrophage; M-CSF, macrophage colony-stimulating factor; IL-6, interleukin-6; IL-10, interleukin-10;TNFα, tumor necrosis factor α.
Authorship confirmation statement: All authors agree to publish this article.
Authors’ contribution: Yang Zhang, Yi Chen: Conceptualization, investigation, and project administration.
Yiming Ji, Qian Su, Feng Zhao: Data curation, formal analysis, and writing – original draft.
Chenyu Fan: Experiments, visualization, and writing – original draft.
Cuiying Xie: Validation and writing – review & editing.
Xuelian Chen: Experiments and data curation.
All authors read and approved the final article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was funded by the Natural Science Project of Minhang District of Shanghai (No. 2020MHZ086) and the National Science Foundation of China (No. 82172157).
ORCID iD: Yi Chen https://orcid.org/0000-0002-1970-9322
Supplemental material: Supplemental material for this article is available online.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers 2019; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017; 377: 562–572. [DOI] [PubMed] [Google Scholar]
- 4.Root-Bernstein R. Innate receptor activation patterns involving TLR and NLR synergisms in COVID-19, ALI/ARDS and sepsis cytokine storms: a review and model making novel predictions and therapeutic suggestions. Int J Mol Sci 2021; 22: 2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain N, Moeller J, Vogel V. Mechanobiology of macrophages: how physical factors coregulate macrophage plasticity and phagocytosis. Annu Rev Biomed Eng 2019; 21: 267–297. [DOI] [PubMed] [Google Scholar]
- 6.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Tang J, Shuai Wet al. et al. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm Res 2020; 69: 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol 2014; 5: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda N, O'Dea EL, Doedens A, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev 2010; 24: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KE, Simon MC. Snapshot: hypoxia-inducible factors. Cell 2015; 163: 1288–1288.e1. [DOI] [PubMed] [Google Scholar]
- 11.Varga T, Mounier R, Horvath A, et al. Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J Immunol 2016; 196: 4771–4782. [DOI] [PubMed] [Google Scholar]
- 12.Palsson-McDermott EM, Curtis AM, Goel G, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 2015; 21: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong W-J, Yang H-H, Guan X-X, et al. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J Cell Physiol 2019; 234: 4641–4654. [DOI] [PubMed] [Google Scholar]
- 14.Albanese A, Daly LA, Mennerich Det al. et al. The role of hypoxia-inducible factor post-translational modifications in regulating its localisation, stability, and activity. Int J Mol Sci 2020; 22: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng J, Kang X, Zhang Set al. et al. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell 2007; 131: 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeh ETH. SUMOylation and De-SUMOylation: wrestling with life's processes. J Biol Chem 2009; 284: 8223–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008; 133: 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan S, Sun X, Xiang B, et al. Redox regulation of the stability of the SUMO protease SENP3 via interactions with CHIP and Hsp90. EMBO J 2010; 29: 3773–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lao Y, Yang K, Wang Z, et al. DeSUMOylation of MKK7 kinase by the SUMO2/3 protease SENP3 potentiates lipopolysaccharide-induced inflammatory signaling in macrophages. J Biol Chem 2018; 293: 3965–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cossarizza A, Chang HD, Radbruch A, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol 2017 Oct; 47: 1584–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raggi F, Pelassa S, Pierobon D, et al. Regulation of human macrophage M1-M2 polarization balance by hypoxia and the triggering receptor expressed on myeloid cells-1. Front Immunol 2017 Sep 7; 8: 1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K, Guo C, Lao Y, et al. A fine-tuning mechanism underlying self-control for autophagy: deSUMOylation of BECN1 by SENP3. Autophagy 2020; 16: 975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal NR, King LS, 'Alessio Det al. et al. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 2014; 306: L709–LL25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Li N, Zhang Xet al. et al. Mitochondrial metabolism regulates macrophage biology. J Biol Chem 2021; 297: 100904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol 2020; 11: 1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beasley MB. The pathologist's approach to acute lung injury. Arch Pathol Lab Med 2010; 134: 719–727. [DOI] [PubMed] [Google Scholar]
- 27.Arora S, Dev K, Agarwal Bet al. et al. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology 2018; 223: 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdelaziz MH, Abdelwahab SF, Wan J, et al. Alternatively activated macrophages; a double-edged sword in allergic asthma. J Transl Med 2020; 18: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Xu L, Xiang Z, et al. Microcystin-LR ameliorates pulmonary fibrosis via modulating CD206 M2-like macrophage polarization. Cell Death Dis 2020; 11: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tu G-W, Shi Y, Zheng Y-J, et al. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J Transl Med 2017; 15: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Li R, Peng Z, et al. GTS-21 reduces inflammation in acute lung injury by regulating M1 polarization and function of alveolar macrophages. Shock 2019; 51: 389–400. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L, Zhao Q, Yang Tet al. et al. Cellular metabolism and macrophage functional polarization. Int Rev Immunol 2015; 34: 82–100. [DOI] [PubMed] [Google Scholar]
- 33.Tan Z, Xie N, Cui H, et al. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol 2015; 194: 6082–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mylonis I, Chachami G, Samiotaki M, et al. Identification of MAPK phosphorylation sites and their role in the localization and activity of hypoxia-inducible factor-1alpha. J Biol Chem 2006; 281: 33095–33106. [DOI] [PubMed] [Google Scholar]
- 35.Tanimoto K, Makino Y, Pereira Tet al. et al. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J 2000; 19: 4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeong JW, Bae MK, Ahn MY, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell 2002; 111: 709–720. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y, Nam HJ, Lee J, et al. Methylation-dependent regulation of HIF-1α stability restricts retinal and tumour angiogenesis. Nat Commun 2016; 7: 10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Yang J, Yang K, et al. The biphasic redox sensing of SENP3 accounts for the HIF-1 transcriptional activity shift by oxidative stress. Acta Pharmacol Sin 2012; 33: 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulrich HD. SUMO protocols. Preface Methods Mol Biol 2009; 497: v–vi. [DOI] [PubMed] [Google Scholar]
- 40.Ao Q, Su W, Guo Set al. et al. SENP1 Desensitizes hypoxic ovarian cancer cells to cisplatin by up-regulating HIF-1α. Sci Rep 2015; 5: 16396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbings S, Elkins ND, Fitzgerald H, et al. Xanthine oxidoreductase promotes the inflammatory state of mononuclear phagocytes through effects on chemokine expression, peroxisome proliferator-activated receptor-{gamma} sumoylation, and HIF-1{alpha}. J Biol Chem 2011; 286: 961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kellner M, Noonepalle S, Lu Qet al. et al. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Adv Exp Med Biol 2017; 967: 105–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Lao Y, Yi Jet al. et al. SENP3 In monocytes/macrophages up-regulates tissue factor and mediates lipopolysaccharide-induced acute lung injury by enhancing JNK phosphorylation. J Cell Mol Med 2020; 24: 5454–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu J, Fan Y, Liu X, et al. SENP1 protects against myocardial ischaemia/reperfusion injury via a HIF1α-dependent pathway. Cardiovasc Res 2014; 104: 83–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-ini-10.1177_17534259231166212 for SENP3 facilitates M1 macrophage polarization via the HIF-1α/PKM2 axis in lipopolysaccharide-induced acute lung injury by Shuangjun He, Chenyu Fan, Yiming Ji, Qian Su, Feng Zhao, Cuiying Xie, Xuelian Chen, Yang Zhang and Yi Chen in Innate Immunity