Abstract
Background:
Use of electronic seizure diaries (e-diaries) by caregivers of children with epileptic spasms is not well understood. We describe the demographic and seizure-related information of children with epileptic spasms captured in a widely used e-diary, and explore potential biases in how caregivers report these data.
Methods:
We analyzed children with epileptic spasms in an e-diary, Seizure Tracker™, from 2007-2018. We described variables including sex, time of seizure, percentage of spasms occurring as individual spasms (versus in clusters), cluster duration, and number of spasms per cluster. We compared seizure characteristics in the e-diary cohort to published cohorts to identify biases in caregiver-reported epileptic spasms. We also reviewed seizure patterns in a small cohort of children with epileptic spasms monitored on overnight vEEG.
Results:
There were 314 children in the e-diary cohort and 9 children in the vEEG cohort. The e-diary cohort was more likely than expected to report counts divisible by five. The e-diary cohort had a lower proportion of nighttime spasms than expected based on data from published cohorts. The e-diary cohort had a significantly lower percentage of spasms as individual spasms, a greater number of spasms per cluster, and a greater cluster duration relative to the vEEG cohort.
Conclusions:
Caregivers using e-diaries for epileptic spasms may miss individual spams, be more likely to report long clusters, round counts to the nearest five, and underreport nighttime spasms. Clinicians should be aware of these reporting biases when using e-diary data to guide care for children with epileptic spasms.
Keywords: epilepsy, infantile, west syndrome, electroencephalogram, reporting bias, mobile app
Introduction
Infantile spasms (West Syndrome) is an epilepsy syndrome characterized by clusters of epileptic spasms that typically occurs between 4 and 8 month of age and is often associated with poor outcomes, such as lifelong treatment-resistant epilepsy.1 Some but not all children with infantile spasms also have hypsarrhythmia on electroencephalogram (EEG) and/or developmental delay at presentation. Early successful treatment of spasms can improve long-term neurodevelopmental outcomes.1-3 Thus, it is important for both caregivers and physicians to monitor response to treatment.
Electronic seizure diaries (e-diaries) have been used to document multiple seizures types.4 Thus e-diary data collected by caregivers of children with epileptic spasms are a potential source of information both for clinical care and research. Given increasing use of e-diaries by caregivers and people with epilepsy there is a need to study these systems, particularly because there may be biases in the ways users report seizures.4 For instance, adults with epilepsy tend to underreport the number of seizures they experience.5 Biases in the pediatric population are less well studied—it is unclear how caregivers use e-diaries for their children, including for epileptic spasms. Knowledge of such biases can allow physicians to more effectively interpret seizure diary reports and make better clinical decisions for children.
Seizure Tracker™ is an electronic system that allows users to track seizures and related information for him/herself or for a child on multiple devices.6 The system provides a valuable window into patient-reported seizure outcomes given that there are thousands of active users.4 The system also incorporates specific options for reporting infantile spasms.7 Therefore, analysis of Seizure Tracker™ data may provide insight into the demographic information and reporting habits of caregivers who use e-diaries to document epileptic spasms.
Here, we describe the demographic and seizure-related characteristics of epileptic spasms as reported by users of the e-diary. We also compare the e-diary cohort to published cohorts8,9 as well as to a sample of children analyzed on overnight video EEG to better understand how to interpret e-diary data provided by caregivers, including any potential reporting biases. Such knowledge will ultimately inform clinical decision making for patients with epileptic spasms.
Methods
Study Design
This study included a retrospective longitudinal study of e-diary data and a retrospective chart review of children admitted to the hospital to monitor epileptic spasms with video-electroencephalography (vEEG). The Institutional Review Board (IRB) at Weill Cornell Medicine reviewed and approved the study (IRB #19-06020360).
Data Sources
Seizure Tracker ™ (hereafter referred to as the e-diary) is a system available on mobile devices and via web browsers.10 From the website, caregivers can create a profile for their child and enter relevant demographic information. Seizure entries can be made via the website, mobile devices, wearable devices, or via Amazon Alexa (Amazon; Seattle, WA). Three-quarters of all seizures in the e-diary are recorded on a mobile device.11
We received access to the e-diary data through a data use agreement with Seizure Tracker LLC (Springfield, VA), facilitated by the International Seizure Diary Consortium. We reviewed demographic and seizure-related data from 2007-2018. The data was de-identified except for self-reported date of birth.
The vEEG sample consisted of children with a documented diagnosis of epileptic spasms (ICD10 G40.82x) in 2019 at NewYork-Presbyterian Weill Cornell Medical Center. This sample included children diagnosed with spasms before 2019.
Inclusion / Exclusion Criteria
For the e-diary cohort, we included all users with reported seizure episodes in the category of “infantile spasms.” There was no specific option for “epileptic spasms” in the e-diary. In the vEEG cohort we included children diagnosed with epileptic spasms on overnight vEEG. We focused on the first diagnostic vEEGs for each child so as to include children who were naïve to anti-seizure medication. VEEG files prior to 2017 had been trimmed at our center to minimize long time storage requirements - we excluded children with incomplete vEEG files.
Measurement
Sex, Age, and Medications
In the e-diary, profiles include sex, date of birth, and information about anti-seizure medications. Age of spasms onset was calculated as the difference in days between the first spasms episode and the reported date of birth. We excluded individuals with a negative calculated age from analyses of age. Medication brand names and alternative names were recoded with generic names. We excluded medication entries missing either the name or the dosage.
For the vEEG cohort, we recorded sex, age at the time of vEEG, and any medications active during hospitalization.
Underlying Medical Conditions
In each e-diary profile, users could specify a number of underlying medical conditions related to the child’s seizures, which have previously been described.7
For the vEEG cohort we reviewed the chart of each child and coded the presence of any medical conditions in line with the selections available in the e-diary system.7
Epileptic Spasm Episode Characteristics
E-diary cohort.
When reporting a seizure episode (i.e., an individual spasm or a cluster of spasms) from the e-diary mobile app, the date and time of the episode was pre-populated with the current date and time (though adjustable for retrospective input/edits). Users entered the number of spasms in the episode and the severity of the episode on a subjective scale ranging from one to five, with five being the most severe. A cluster of spasms was defined as two or more spasms per episode. Users could also enter the duration of the episode in hours, minutes, and seconds. Episodes with a duration of zero seconds were excluded from analyses of cluster duration.
vEEG cohort.
For each child admitted for overnight monitoring, the vEEG was reviewed by one child neurologist with fellowship training in clinical neurophysiology (MB). Coding included the beginning and end times of vEEG recording as well as the time of each spasm. An event was considered an epileptic spasm when a clinically consistent event on video was accompanied by an EEG correlate (diffuse slow wave followed by an electrodecrement and increased low amplitude fast activity). A cluster was defined as two or more spasms with a maximum of two minutes between consecutive spasms. The duration of a cluster was calculated as the elapsed time between the first and last spasm in that cluster.
Analysis
E-diary Analysis
We described the duration and number of spasms in each cluster. We used median statistics to address non-Gaussian distributions and log-transformed data with significant outliers. We examined the reported severity of clusters and evaluated correlations between the number of spasms per cluster and the duration of cluster with the reported severity of cluster. In addition, we investigated the reported time of day of epileptic spasms episodes to determine any diurnal reporting biases. Each spasm was assumed to occur at the time of the start of the associated spasm cluster.
Infantile spasms typically occurs in the first year of life; however, for many of the individuals in the e-diary dataset, the age of spasms onset was greater than one year. We were uncertain if this represented caregivers who used synthetic dates of birth to maintain anonymity, children who had epileptic spasms as part of a different epilepsy syndrome (i.e., Lennox-Gastaut Syndrome), or children with infantile spasms beginning after the first year. Thus, as a sensitivity analysis, we also reviewed a subgroup in which the age was less than one year.
To understand individual differences in e-diary usage, we classified users into three groups based on the frequency of reported seizure episodes: frequent reporters (over 100 episodes), moderate reporters (between 5 and 100 episodes), and infrequent reporters (fewer than 5 episodes). We performed chi-squared, analysis of variance (ANOVA), and Kruskal-Wallis tests to determine whether there were differences between the three groups in demographic and seizure-related variables, adjusting for multiple comparisons. We followed significant findings with Mann-Whitney U pairwise comparisons.
Comparison Between Cohorts
Published Cohorts.
Some studies have found a preference for spasms during the sleep-wake transition, indicating more spasms may be present at night, and others have shown that spasms have no nocturnal or diurnal preference, indicating they may be randomly distributed throughout the 24-hour interval.8,9 This means that at least 33% of spasms would be expected in an 8-hour nighttime interval. To examine for potential nighttime underreporting, we examined spasms occurring in an interval during which most parents are asleep. More specifically, we examined if the percentage of spasms in the e-diary cohort between 11pm-7am was lower than the minimum expected value of 33% using a binomial test. We also compared the number of spasms reported between 9pm and 9am to reports that 55% of spasms occur in a 12-hour night period.8
vEEG cohort.
We compared the e-diary cohort, including the subset under one year of age, to the vEEG cohort on seizure-related variables using chi-squared, Mann-Whitney U, and independent t-tests, adjusting for multiple comparisons.
To verify the diurnal findings from our comparison to published cohorts, we compared the e-diary cohort to the vEEG cohort on the time of day of spasms to examine for potential nighttime underreporting in the e-diary cohort. Unlike the e-diary cohort whereby seizures could be entered at any time of day, the children in the vEEG cohort were not analyzed for full 24-hour periods. We thus focused this analysis on a time period during which children from both cohorts could have potentially had a recorded seizure (i.e., between the latest vEEG start time and the earliest vEEG end time). We compared the cohorts on the percentage of spasms occurring between 11pm and 7am over this shortened time period.
Analysis Software
We used R software environment (v3.6.3)12 and the packages “ggplot2”13, “plyr”14, “dplyr”15, “lubridate”16, and “chron”17. Open source code is available on Github (https://github.com/brianlagrant/infantilespasmsVEEG).
Results
E-diary Demographic Characteristics
There were 314 unique children with epileptic spasms. For these 314 individuals, there were 24,675 total seizure episodes (median 6 [interquartile range (IQR) 2-34, range 1 to 5,448] episodes per child). The most common underlying condition was a “congenital” syndrome (38%), with Other (14%), Tuberous Sclerosis (10%), Aicardi Syndrome (6%) and Lennox Gastaut Syndrome (4%) being the most common subtypes. 209 users (67%) reported use of at least one medication – the median number of unique medications tried per individual was 2 [IQR 0-3]. Among those who used at least one medication, 87% reported a medication start date that was before their first reported spasm episode. The most common first-reported medications used were levetiracetam (21.5%) and vigabatrin (11.0%), whereas adrenocorticotropic hormone (2.9%) and prednisolone (0.5%) were used first less frequently. The median age of onset was 2.8 years [IQR 0.9-12.4 years] after excluding 12 individuals with a listed year of birth past 2018. 54 users (17%) had an age of spasms onset over 18 years of age. 27 percent of users had an age of spasms onset under one year of age. Demographic information for this subset under one year of age is shown in Table 1.
Table 1:
Demographic Breakdown of E-diary and vEEG Cohorts
| E-diary Cohort (N = 314) | E-diary Subset Under 1 Year (N = 86) |
vEEG Cohort (N = 9) | |
|---|---|---|---|
| Factor | n (%) | n (%) | n (%) |
| Sex | |||
| Female | 162 (51.6) | 41 (47.7) | 5 (51.6) |
| Male | 146 (46.5) | 45 (52.3) | 4 (44.4) |
| Undisclosed | 6 (1.9) | 0 (0) | 0 (0) |
| Age of onseta (years) | 2.8 [0.9-12.4] | 0.52 [0.40-0.75] | 0.6 [0.51-0.88] |
| Underlying Condition | |||
| Congenital Syndrome | 119 (37.9) | 36 (41.9) | 2 (22.2) |
| Other | 45 (14.3) | 10 (11.6) | 1 (11.1) |
| Tuberous Sclerosis | 30 (9.6) | 14 (16.3) | 0 (0) |
| Lennox-Gastaut | 13 (4.1) | 0 (0) | 1 (11.1) |
| Aicardi | 18 (5.7) | 9 (10.5) | 0 (0) |
| Rett | 4 (1.3) | 0 (0) | 0 (0) |
| Dravet | 3 (1.0) | 0 (0) | 0 (0) |
| Down | 2 (0.6) | 2 (2.3) | 0 (0) |
| Phelan-McDermid | 1 (0.3) | 0 (0) | 0 (0) |
| Hypothalamic Hamartoma | 1 (0.3) | 0 (0) | 0 (0) |
| Angelman Syndrome | 1 (0.3) | 0 (0) | 0 (0) |
| Sturge-Weber | 1 (0.3) | 1 (1.2) | 0 (0) |
| Stroke | 9 (2.9) | 4 (4.7) | 3 (33.3) |
| Metabolic Disorder | 5 (1.6) | 0 (0) | 0 (0) |
| Infection | 12 (3.8) | 1 (1.2) | 0 (0) |
| Perinatal Hypoxia | 7 (2.2) | 2 (2.3) | 3 (33.3) |
| Maternal Drug Abuse | 4 (1.3) | 0 (0) | 0 (0) |
| Brain Malformations | 39 (12.4) | 18 (20.9) | 0 (0) |
| Brain Trauma | 25 (8.0) | 6 (7.0) | 0 (0) |
| Electrolyte Disturbance | 1 (0.3) | 0 (0) | 0 (0) |
| Medication Usage b,c | |||
| Unique Meds per Childa | 2 [0-3] | 2 [0-3] | 0 [0-1] |
| Levetiracetam | 83 (26.4) | 24 (27.9) | 2 (22.2) |
| Topiramate | 59 (18.8) | 22 (25.6) | 0 (0) |
| Vigabatrin | 56 (17.8) | 30 (34.9) | 1 (11.1) |
| Valproic acid | 48 (15.3) | 5 (5.8) | 0 (0) |
| Clobazam | 48 (15.3) | 9 (10.5) | 0 (0) |
| Lamotrigine | 40 (12.7) | 1 (1.2) | 0 (0) |
These data are depicted as median [IQR]
These are the top six most commonly reported meds in the e-diary cohort
vEEG cohort data consists of neuroactive medications at time of epileptic spasms diagnosis
E-diary Seizure Characteristics
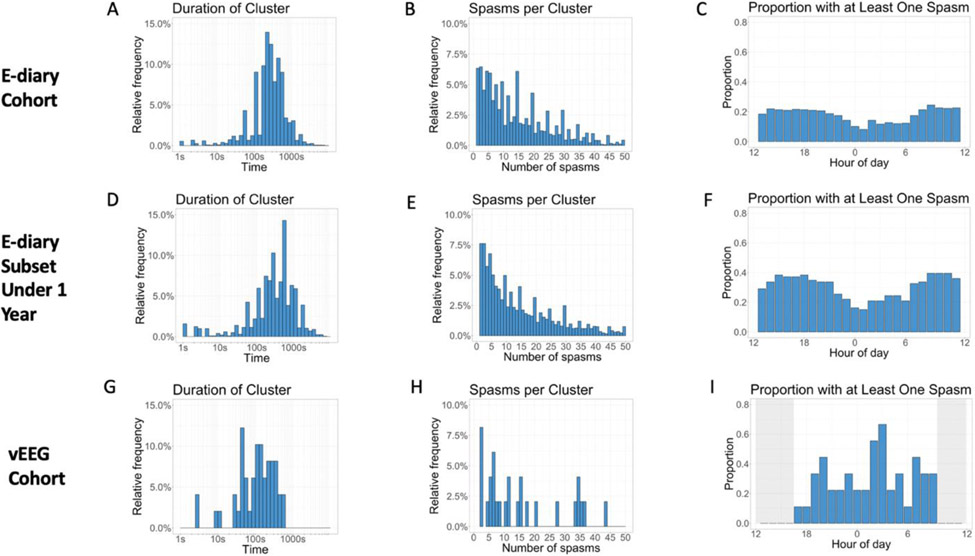
There were 15,225 seizure episodes with one or more reported spasms, with a total of 226,932 reported spasms. Of the 15,225 seizure episodes, 1,603 episodes (11% of episodes; 1% of all spasms) were individual spasms and 13,622 were seizure clusters (89% of episodes; 99% of all spasms). The median duration of a seizure cluster was 300 seconds [IQR 180-480 seconds] and the mean was 406 seconds (standard deviation [SD] = 735 seconds; Figure 1A). The subset under one year of age had a similar median cluster duration of 320 seconds [IQR 155-606 seconds] and a mean cluster duration of 580 seconds (SD = 963 seconds; Figure 1D). Of note, there were two individuals who appeared to be reporting clusters by reporting episodes of zero spasms in quick succession; they were not analyzed in our analysis of cluster duration. The median number of spasms per cluster was 12 [IQR 6-23] and the mean was 16.5 (SD = 14.9; Figure 1B). The subset under one year of age had a similar median number of spasms per cluster of 12 [IQR 5-24] and a similar mean value of 17.4 (SD = 16.8; Figure 1E). Visual review demonstrated a clustering of reported values around multiples of five. For instance, whereas 68 users reported clusters with 34 spasms and 48 users reported clusters with 36 spasms, 227 users reported clusters with 35 spasms (Figure 1b). There were weak correlations between (a) the number of spasms and the duration of the cluster (r = 0.22, p < 0.001) and (b) the number of spasms and the reported severity of the cluster (r = 0.19, p < 0.001).
Figure 1: Epileptic Spasms Characteristics of E-diary and vEEG Cohorts.
The vEEG cohort was not monitored for a full 24 hours. (2 column image)
Comparison of E-diary cohort to Published Cohorts
There were significantly fewer spasms at night than expected based on data from published cohorts. Between 11pm and 7am, instead of 33% of spasms, only 23.8% of spasms were reported in the whole cohort and 13.9% were reported in the subset under one year of age (p’s < 0.001; Table 2). Significantly fewer than 55%8 of spasms were reported between 9pm and 9am: 43.6% of spasms in the whole cohort and 30.1% in the subset under one year of age (p < 0.001 for both; Table 2). Figures 1C and 1F depict the proportion of e-diary users with a least one spasm at each hour of day.
Table 2:
Proportion of Nighttime Spasms in E-diary Cohorts Relative to Published Cohorts
| E-diary Cohort |
Published Cohorts |
p-value | E-diary Subset Under 1 Year |
Published Cohorts |
p-value | ||
|---|---|---|---|---|---|---|---|
| Percent of spasms over interval |
9pm-9am 43.6 |
12-hour
night period 55.28 |
< 0.001 |
9pm-9am 30.1 |
12-hour
night period 55.28 |
< 0.001 | |
| Percent of spasms over interval |
11pm-7am 23.8 |
11pm-7am 33.39 |
< 0.001 |
11pm-7am 13.9 |
11pm-7am 33.39 |
< 0.001 |
Comparisons between both e-diary cohorts to data from published cohorts were significant to p < 0.001.
Differences in E-diary Users by Frequency of Report
Forty-seven (15%) users were frequent reporters (over 100 episodes), 123 (39%) were moderate reporters (between 5 and 100 episodes), and 144 (46%) were infrequent reporters (fewer than 5 episodes). These groups were not significantly different in sex, age, or underlying medical conditions after Bonferroni correction (corrected alpha = 0.002; Table 3). Compared to moderate and infrequent reporters, frequent reporters had a significantly higher number of spasms per cluster, subjective cluster severity, and number of unique medications trialed per child (all p < 0.002). Frequent reporters had a significantly greater cluster duration relative to moderate reporters (p < 0.001). Moderate reporters had a greater number of spasms per cluster relative to infrequent reporters (p < 0.001).
Table 3:
Analysis of E-diary Cohort by Frequency of Report
| Infrequent N = 144 |
Moderate N = 123 |
Frequent N = 47 |
p-value (3-group comparison) |
|
|---|---|---|---|---|
| Median unique medications per child [IQR] | 2 [0, 3] | 1 [0, 3] | 3 [1.5, 6] | < 0.001 |
| Median spasms per cluster [IQR] | 5 [3, 10] | 8 [4, 17] | 13 [6, 23] | < 0.001 |
| Median duration of cluster, s [IQR] | 180 [60, 600] | 180 [73, 360] | 300 [180, 480] | < 0.001 |
| Mean reported seizure severity (SD) | 2.65 (1.29) | 2.57 (1.1) | 2.89 (0.91) | < 0.001 |
Only differences with a p-value under the corrected alpha of 0.002 are displayed in this table. Sex, age under one year, and underlying conditions were all non-significant. P-values represent a comparison between all three groups. IQR = interquartile range, SD = standard deviation, s = seconds.
vEEG Cohort Demographic Characteristics
The vEEG cohort consisted of nine children diagnosed with epileptic spasms on overnight vEEG (Table 1). Five (56%) were female. The median age at the time of vEEG was 7.2 months [range 5.1-31.3 months]. Three had a history of perinatal hypoxia, three had a history of stroke, one had Lennox-Gastaut Syndrome, one had a causative genetic mutation (undisclosed for anonymity), and one had an unknown cause. Four (44%) were on anti-seizure medications at the time of vEEG.
vEEG Cohort Seizure Characteristics
There were 93 total seizure episodes among the nine children, corresponding to 535 total spasms. Of the 93 episodes, 44 were individual spasms (47% of episodes; 8% of all spasms) and 49 were clusters of spasms (53% of episodes; 92% of all spasms). The median cluster duration was 125 seconds [IQR 48-217 seconds] and the mean was 166 seconds (SD = 142 seconds; Figure 1G). The median number of spasms per cluster was 4 [IQR 2-12] and the mean was 10 (SD = 11.7; Figure 1H). Figure 1I depicts the proportion of children with at least one spasm during each hour recorded on vEEG.
Comparison Between E-diary and vEEG Cohorts
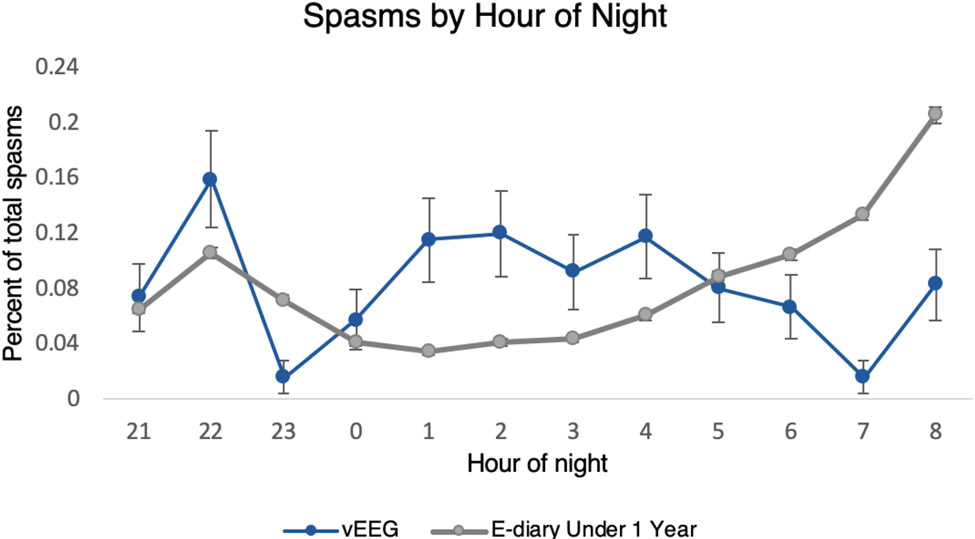
The vEEG cohort had a lower mean and median cluster duration, a lower mean and median number of spasms per cluster, and a greater percentage of individual spasms relative to both the whole e-diary cohort and the e-diary subset under one year of age (all p’s < 0.001). When examined over the hours during which all children could potentially have a recorded seizure (i.e., between 9:19pm and 9:00am), the vEEG cohort overall had a higher percentage of spasms at nighttime (i.e., between 11pm and 7am) relative to the whole e-diary cohort and the subset under one year of age (p’s < 0.001). Figure 2 illustrates the percentage of spasms that occur at each hour over the 9:19pm to 9:00am window. These comparisons all remained significant after Bonferroni correction (corrected alpha = 0.004; Table 4).
Figure 2: Density Plot of Spasms Between 9:19pm and 9:00am.
95% confidence intervals represented by error bars. Some error bars are narrow and thus not visible. (1.5 column image)
Table 4:
Differences in Seizure Characteristics Between Cohorts
| E-diary Cohort (N = 314) |
vEEG Cohort (N =9) |
p-value | E-diary Subset Under 1 Year (N = 86) |
vEEG Cohort (N =9) |
p-value | ||
|---|---|---|---|---|---|---|---|
| Cluster duration (s): | n = 9,045 | n = 49 | n = 2,032 | n = 49 | |||
| Median [95% CI] | 300 [180-480] | 125 [48-217] | < 0.001 | 320 [155-606] | 125 [48-217] | < 0.001 | |
| Mean (SD) | 406.1 (735.3) | 166 (141.5) | < 0.001 | 580 (962.7) | 166 (141.5) | < 0.001 | |
| Spasms per cluster: | n = 13,622 | n = 49 | n = 4,676 | n = 49 | |||
| Median [95% CI] | 12 [6-23] | 4 [2-12] | < 0.001 | 12 [5-24] | 4 [2-12] | < 0.001 | |
| Mean (SD) | 16.5 (14.9) | 10 (11.7) | < 0.001 | 17.4 (16.8) | 10 (11.7) | < 0.001 | |
| Percent of total spasms as individual spasms (%) | n = 226,932 | n = 535 | n = 82,167 | n = 535 | |||
| 0.7 | 8.2 | < 0.001 | 1.0 | 8.2 | < 0.001 | ||
| Percent of spasms between 11pm-7am over 9:19pm-9am interval (%) | n = 95,454 | n = 433 | n = 23,361 | n = 433 | |||
| 55.8 | 66.7 | < 0.001 | 48.9 | 66.7 | < 0.001 |
The total number of values used in the calculation for each variable is represented by n. Of note, cluster durations with a value of 0 seconds were excluded from analyses of cluster duration, specifically 4,577 inputs in the whole e-diary cohort and 2,644 in the e-diary subset under one year. These inputs of 0 seconds came from 23 unique users.
SD = standard deviation, CI = confidence interval, s = seconds.
Discussion
Our analysis indicates there may be several biases in how caregivers report epileptic spasms in an e-diary: 1) caregivers may underreport individual spasms relative to clusters, 2) caregivers may report longer cluster episodes, 3) caregivers may underreport spasms occurring at night, and 4) caregivers may round the number of spasms per cluster.
Several demographic characteristics of the e-diary cohort add face validity to the sample. There was a roughly equal proportion of females to males, similar to our vEEG cohort. Previous clinical cohorts have found a marginally increased proportion of males to females.18,19 Over one-third of children in the e-diary had a reported underlying “congenital syndrome” and 32% reported other known causes, in agreement with previous studies indicating roughly two thirds of infantile spasms are due to a known cause.18,19
Other demographics characteristics were different than expected. For example, median age of infantile spasms onset was 2.8 years whereas infantile spasms typically present between 4 and 8 months of age.1,19 This may be due to caregivers mistakenly putting their own year of birth, especially given that 17 percent had an age over 18 years. This older age of onset may also be attributed to miscoding of the dates of spasms or due to caregivers intentionally providing a synthetic date of birth to protect privacy. It is noteworthy that levetiracetam was the most common first-reported medication. It is unclear if this indicates poor compliance with the recommendation to use hormonal therapies or vigabatrin as first-line treatment for infantile spasms.20 Vigabatrin was the most common first-reported medication in the subset under one year of age, which is consistent with the finding that roughly one-sixth of this subset reported a diagnosis of tuberous sclerosis, for which vigabatrin is the preferred therapy.20
There are several characteristics of the reported seizure descriptions that agree with previous research. First, the wide ranges in cluster duration seen in both the e-diary and vEEG cohorts (several seconds to 15 minutes) are consistent with what is reported by studies using vEEG (27 seconds to 10 minutes).21 Similarly, there was variability in the number of spasms per cluster in both groups, ranging from two to over 50, as previously described.8,22 The average cluster in our e-diary cohort had about 17 spasms, while the average cluster in the vEEG cohort had about 10 spasms. These values are in line with those of several clinical studies in which the typical number of spasms per cluster ranges from 10-21.21-23
There were individual differences between e-diary users by frequency of report, specifically that more frequent reporters tended to have greater cluster duration, number of spasms per cluster, number of trialed anti-seizure medications, and subjective cluster severity relative to less frequent reporters. The most likely explanation for these findings is that the frequent reporter group represents a group of infants with more treatment-resistant disease. It is unclear whether a greater seizure cluster duration and number of spasms per cluster correlate with treatment-resistant disease, though one study showed these variables were unrelated to short-term prognosis.18 It is also possible that the frequent reporter group represents caregivers who more intensely monitor their children or who utilize the e-diary app more consistently relative to the less frequent reporters.
We found four important biases in how caregivers report epileptic spasms in an e-diary. First, individual spasms made up 47% of all seizure episodes in the vEEG cohort, whereas individual spasms were only 11% of all seizure episodes in the e-diary cohort. Two studies of infantile spasms recorded on vEEG found that roughly half of seizure episodes were individual spasms, thus supporting the findings in our vEEG cohort.21,24 Together, this indicates that caregivers may not recognize or report a large number of individual spasms. Therefore, it may be useful for clinicians to directly ask caregivers whether they have witnessed any individual quick, sudden movements that they did not report in the diary.
A second bias was a predisposition for caregivers to report longer clusters relative to what was seen clinically. The mean cluster duration was 6.5 minutes in the e-diary cohort but was 2.5 minutes in the vEEG cohort. The e-diary value is on the upper limit of what is typically reported in previous research, including one study in which the average cluster lasted 3.5 minutes.18 This may be because caregivers are more likely to witness clusters with a longer duration. Of note, the magnitude of this reporting bias may have been diminished in this study given that the majority of the patients in the e-diary cohort were taking anti-seizure drugs, and thus may have overall had less severe clusters, whereas the majority of patients in the vEEG cohort were medication-naïve, and thus may have overall had more severe clusters.
A third bias seen in e-diary users was underreporting of spasms at night relative to data from published cohorts.8,9 Our findings suggest some caregivers may miss spasms occurring at night, though more comprehensive 24-hour vEEG data is warranted to further validate this claim. This underreporting likely represents seizures missed by sleeping caregivers.7,8 Asking caregivers about the child’s sleep patterns may point towards nocturnal spasm activity. Clinicians should admit a patient for overnight vEEG if there is any concern for nighttime episodes.
A fourth bias noted in the report of seizure characteristics was the rounding of the number of spasms per cluster to the nearest multiple of five. This finding was not surprising, as caregivers may not always count the exact number of spasms. This kind of rounding has been noted in other aspects of seizure reporting and among patients self-reporting the number of cigarettes smoked per day.7,25 The clinical implications of this rounding bias are uncertain, as the number of spasms per cluster is unrelated to short-term prognosis.18
Our study has several limitations. First, we could not verify the demographic and clinical information of e-diary users, such as medications, medical history, and dates of birth. Second, caregivers may vary in comfort with e-diary usage, leading to potential errors in seizure documentation. For the future, physician curation and EHR integration could help mitigate such errors. Third, our vEEG cohort was small; however, the goal of this study was to find a representative sample of children with epileptic spasms for comparison rather than to definitively describe the characteristics of spasms, which have been described elsewhere.8,9,21 Regardless, our seizure estimates were in line with many previous studies, strengthening the validity of our vEEG cohort estimates.18,21 Fourth, we could not account for many potential confounding variables between the cohorts such as the amount of time on medication, which may have influenced the results. One potential approach for future studies would be to have caregivers electronically document spasms that they witness in an epilepsy monitoring unit and compare their reported findings to what is captured on vEEG. Additionally, our video-EEG recordings did not include a full 24 hours, so it was not possible to compare the full range of times between our two cohorts. We mitigated this by comparing our e-diary cohort to historical data. Fifth, this study did not capture many other aspects of e-diaries that clinicians, patients, and families find valuable, including accessibility, real-time video recording, integration with the electronic medical record, and tracking of seizure triggers.4,26 Finally, our study did not focus on the standard of care outcome for epileptic spasms, which is the all-or-none response.1 Though the exact number of spasms in a cluster or the precise duration of a cluster may not be clinically relevant, we believe a better understanding of how caregivers tend to report spasms can allow clinicians to more effectively interpret the plethora of data presented in an e-diary, especially given the increasing use of e-diaries by patients and caregivers.4,18 More importantly, in this study we demonstrated how caregivers are potentially missing individual spasms and spasms occurring at night. This could lead to caregivers incorrectly reporting the resolution of clinical spasms when in fact they are continuing, though infrequently. These findings highlight the importance to include extended EEG monitoring to confirm clinical resolution of spasms.
Conclusion
Based on the comparison with historical and video-EEG data, there appear to be multiple biases in how caregivers report epileptic spasms in e-diaries, including underreporting of individual spasms, underreporting of spasms at night, a predisposition to report longer clusters, and rounding of the number of spasms.
Acknowledgements
The authors thank Natasha Basma and Meghan Joline for assistance with the project. Use of data was facilitated by the International Seizure Diary Consortium (https://sites.google.com/site/isdchome/). Brian LaGrant conducted this research as part of the Areas of Concentration (AOC) Program of the Weill Cornell MD curriculum.
Footnotes
Declaration of Interest
DMG is an advisor for Magic Leap and Epilepsy AI, and has received grants from BIDMC and NIH. REM is the co-founder of Seizure Tracker™. ZMG receives research funding from the Pediatric Epilepsy Research Foundation, Weill Cornell Medicine, the Orphan Disease Center, Clara Inspired, and the Morris and Alma Schapiro Fund. ZMG also performs consulting work for Alpha Insights, Bio-Pharm Solutions (South Korea), and Epilog. The other authors have no conflicts of interest.
References
- 1.Shields W. Infantile Spasms: Little Seizures, BIG Consequences. Epilepsy Curr 2006;6:63–69. 10.1111/j.1535-7511.2006.00100.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lux A, Edwards S, Hancock E, Johnson A, Kennedy C, Newton R, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol 2005;4:712–717. 10.1016/S1474-4422(05)70199-X [DOI] [PubMed] [Google Scholar]
- 3.O’Callaghan F, Edwards SW, Alber FD, Borja MC, Hancock E, Johnson AL, et al. Vigabatrin with hormonal treatment versus hormonal treatment alone (ICISS) for infantile spasms: 18-month outcomes of an open-label, randomised controlled trial. Lancet Child Adolesc Health 2018;2:715–725. 10.1016/S2352-4642(18)30244-X [DOI] [PubMed] [Google Scholar]
- 4.Fisher R, Blum D, DiVentura B, Vannest J, Hixson J, Moss R, et al. Seizure diaries for clinical research and practice: Limitations and future prospects. Epilepsy Behav 2012;24:304–310. 10.1016/j.yebeh.2012.04.128 [DOI] [PubMed] [Google Scholar]
- 5.Blachut B, Hoppe C, Surges R, Elger C, Helmstaedter C. Subjective seizure counts by epilepsy clinical drug trial participants are not reliable. Epilepsy Behav 2017;67:122–127. 10.1016/j.yebeh.2016.10.036 [DOI] [PubMed] [Google Scholar]
- 6.Moss R. Seizure Tracker™ - Your comprehensive resource for tracking and sharing seizure information, 2019. Available at: https://www.seizuretracker.com/. Accessed September 27, 2019. [Google Scholar]
- 7.Ferastraoaru V, Goldenholz DM, Chiang S, Moss R, Theodore WH, Haut SR. Characteristics of large patient-reported outcomes: Where can one million seizures get us? Epilepsia Open 2018;3:364–373. 10.1002/epi4.12237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellaway P, Hrachovy R, Frost J, Zion T. Precise characterization and quantification of infantile spasms. Ann Neurol 1979;6:214–218. 10.1002/ana.410060306 [DOI] [PubMed] [Google Scholar]
- 9.Pavone P, Striano P, Falsaperla R, Pavone L, Ruggieri M. Infantile spasms syndrome, West syndrome and related phenotypes: What we know in 2013. Brain Dev 2014;36:739–751. 10.1016/j.braindev.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 10.Casassa C, Levit ER, Goldenholz DM. Opinion and Special Articles: Self-management in epilepsy: Web-based seizure tracking applications. Neurology 2018;91:e2027–e2030. 10.1212/WNL.0000000000006547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss R. Personal communication Apr 14 2020. [Google Scholar]
- 12.R Core Team. R: A language and environment for statistical computing. In Editor (Ed)^(Eds) Book R: A language and environment for statistical computing., R Foundation for Statistical Computing; 2018. [Google Scholar]
- 13.Wickham H. ggplot2: Elegant Graphics for Data Analysis. In Editor (Ed)^(Eds) Book ggplot2: Elegant Graphics for Data Analysis, Springer-Verlag; New York; 2016. [Google Scholar]
- 14.Wickham H. The Split-Apply-Combine Strategy for Data Analysis. Journal Stat Softw 2011;40:1–29. https://doi.org/ [Google Scholar]
- 15.Wickham H, Francois R, Henry L, Muller K. dplyr: A Grammar of Data Manipulation. In Editor (Ed)^(Eds) Book dplyr: A Grammar of Data Manipulation; 2018. [Google Scholar]
- 16.Grolemund G, Wickham H. Dates and times made easy with lubridate. J Stat Softw 2011;40:1–25. 10.18637/jss.v040.i03 [DOI] [Google Scholar]
- 17.James D, Hornik K. chron: Chronological objects which can handle dates and times. 2020. [Google Scholar]
- 18.Haga Y, Watanabe K, Negoro T, Aso K, Kasai K, Ohki T, et al. Do ictal, clinical, and electroencephalographic features predict outcome in West syndrome? Pediatr Neurol 1995;13:226–229. 10.1016/0887-8994(95)00157-b [DOI] [PubMed] [Google Scholar]
- 19.Brna PM, Gordon KE, Dooley JM, Wood EP. The Epidemiology of Infantile Spasms. Can J Neurol Sci 2001;28:309–312. 10.1017/s0317167100001517 [DOI] [PubMed] [Google Scholar]
- 20.Riikonen R. Infantile spasms: Outcome in clinical studies. Pediatr Neurol 2020;108:54–64. 10.1016/j.pediatrneurol.2020.01.015 [DOI] [PubMed] [Google Scholar]
- 21.King D, Dyken PR, Spinks IL, Murvin AJ. Infantile spasms: Ictal phenomena. Pediatr Neurol 1985;1. 10.1016/s0887-8994(85)80002-3 [DOI] [PubMed] [Google Scholar]
- 22.Fusco L, Vigevano F. Ictal Clinical Electroencephalographic Findings of Spasms in West Syndrome. Epilepsia 1993;34:671–678. 10.1111/j.1528-1157.1993.tb00445.x [DOI] [PubMed] [Google Scholar]
- 23.Lee Y-J, Berg AT, Nordli D. Clinical spectrum of epileptic spasms in children. Brain Dev 2015;37:37–48. 10.1016/j.braindev.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 24.Plouin P, Dulac O, Jalin C, Chiron C. Twenty-Four-Hour Ambulatory EEG Monitoring in Infantile Spasms. Epilepsia 1993;34:686–691. 10.1111/j.1528-1157.1993.tb00447.x [DOI] [PubMed] [Google Scholar]
- 25.Klesges R, Debon M, Ray JW. Are self-reports of smoking rate biased? Evidence from the Second National Health and Nutrition Examination Survey. J Clin Epidemiol 1995;48:1225–1233. 10.1016/0895-4356(95)00020-5 [DOI] [PubMed] [Google Scholar]
- 26.Yoo S, Lim K, Baek H, Jang S-K, Hwang G-Y, Kim H, et al. Developing a mobile epilepsy management application integrated with an electronic health record for effective seizure management. Int J M Inform 2020;134:104051. 10.1016/j.ijmedinf.2019.104051 [DOI] [PubMed] [Google Scholar]