Abstract
BACKGROUND:
Microvascular measures of vascular dysfunction during acute mental stress may be important determinants of major adverse cardiovascular outcomes (MACE), especially among younger and middle-aged women survivors of an acute myocardial infarction (MI).
METHODS:
In the Myocardial Infarction and Mental Stress Study 2 (MIMS2), individuals who had been hospitalized for an MI in the past 8 months were prospectively followed for 5-years. MACE was defined as a composite index of cardiovascular death, and first/recurring events for nonfatal myocardial infarction, and hospitalizations for heart failure. Reactive hyperemia index (RHI) and flow-mediated dilation (FMD) were used to measure microvascular and endothelial function, respectively, before and 30 minutes after a public-speaking mental stress task. Survival models for recurrent events were used to examine the association between vascular response to stress (difference between post-stress and resting values) and MACE. RHI and FMD were standardized in analyses.
RESULTS:
Of 263 patients, the mean age was 51 years (range 25–61), 48% were women, and 65% Black/African American. During a median follow-up of 4.3 years, 64 patients with 141 adverse cardiovascular events (first and repeated). Worse microvascular response to stress (for each standard deviation decrease in RHI) was associated with 50% greater risk of MACE (HR: 1.50, 95% CI: 1.05, 2.13; p = 0.03) among women only (sex interaction: p = 0.03). Worse transient endothelial dysfunction in response to stress (for each standard deviation decrease in FMD) was associated with 35% greater risk of MACE (HR: 1.35; 95% CI: 1.07, 1.71; p-value: 0.01), and the association was similar in women and men.
CONCLUSIONS:
Peripheral microvascular dysfunction with mental stress was associated with adverse events among women but not among men. In contrast, endothelial dysfunction was similarly related to MACE among both men and women. These results suggest a female-specific mechanism linking psychological stress to adverse outcomes.
Keywords: Mental stress, Vascular Dysfunction, Myocardial Ischemia, Cardiovascular Outcomes, Women
Graphical Abstract
INTRODUCTION
Young and middle-aged women who have developed an acute myocardial infarction (MI) have greater morbidity, mortality, and worse quality of life relative to their male counterparts.1, 2 These disparities have not been explained by traditional cardiovascular risk factors and comorbidities.3 While coronary artery disease is often implicated as a pathophysiological substrate of MI in both women and men, there remains much to be learned about the mechanisms and sex differences leading to MI and worse outcomes for women with cardiovascular disease.
Emerging research has suggested that vascular function, an important determinant in the regulation of coronary blood flow, may play an important role in ischemic heart disease among women.4 Coronary microvascular dysfunction consists of dysregulation of small coronary arteriole through endothelium-dependent and/or endothelium-independent mechanisms. These alterations can lead to a higher microvascular resistance with reduced coronary blood flow that is associated with ischemia and adverse cardiovascular events.5 Microvascular coronary disease may be more important factors in the etiology of myocardial ischemia than plaque burden in women compared to men.6 Prior research has shown that up to two-thirds of women with stable ischemic heart disease have no obstructive coronary artery disease, and coronary vasomotor disorders or coronary microvascular dysfunction has been implicated.5
Sex differences in the regulation of coronary blood flow and vascular resistance may be especially important in regards to the physiological effects of acute mental stress on cardiovascular reactivity.7, 8 There is growing recognition that sex differences in the physiological response to stress are important in women’s cardiovascular vulnerability.9, 10 Several studies have suggested that women with coronary heart disease are at an increased risk of adverse cardiovascular outcomes than men in the presence of psychosocial exposures.11, 12 Also, compared to men, women with coronary heart disease are more likely to develop myocardial ischemia during mental stress,8–10 and have distinct vascular response mechanisms related to microvascular constriction.7, 8 Stress-induced coronary vascular reactivity in the epicardial and microvascular circulation has been implicated in mental stress-induced ischemia,13–17 and is thought to be especially common in women even in the case of non-obstructive coronary artery disease.18
These findings raise the possibility that acute mental stress may increase the risk of adverse cardiovascular events through microvascular mechanisms especially among women. To our knowledge, only one previous study has investigated sex differences in the association of vascular reactivity to mental stress with adverse cardiovascular outcomes, but the study sample was 95% male, and major adverse cardiovascular events were only recorded up to one year after the patients’ index event of acute coronary syndrome.19 Previous studies of coronary heart disease have also generally lacked adequate representation of racial minorities, which is a limitation given that Black patients, and Black women in particular, are a groups at disproportionate risk.20, 21 Understanding whether vascular reactivity to mental stress is related to cardiovascular risk is a critical issue and one that may shed new light on treatment and prevention strategies for patients with coronary heart disease, especially among women.
Thus, the overall objective of this study was to examine whether microvascular dysfunction in response to mental stress is associated with the incidence of major adverse cardiovascular events (MACE) in a diverse sample of post-MI patients with excellent representation of women and racial minorities; to explore whether these associations are heterogenous by sex; and to contrast the results with brachial artery endothelial dysfunction. We hypothesized that there would be sex differences in the association between microvascular dysfunction and MACE while the association between brachial artery endothelial dysfunction and MACE would be similar by sex. Also, since stress-induced coronary vascular reactivity in the epicardial and microvascular circulation may be explained by the occurrence of mental stress-induced ischemia, especially among women, we hypothesized that these results would be attenuated or explained by the occurrence of ischemia during mental stress.
MATERIALS AND METHODS
Study Design and Participants
The data that support the findings of this study are available from the principal investigators upon reasonable request. The Myocardial Infarction and Mental Stress 2 (MIMS2) study enrolled 313 patients with early onset MI from Emory affiliated hospitals (Atlanta, Georgia) between April 2012 and March 2017. MIMS2 is a prospective cohort study that was designed to examine sex differences in the prevalence, mechanisms, and consequences of mental stress-induced ischemia in young and middle-aged survivors of myocardial infarction (MI). MIMS2 was designed to include 50% women, and patients were included if they were hospitalized for a confirmed Type I MI within 8 months, and were between the ages of 18 and 60 years at the time of their MI. More detailed information on the objectives and study design of MIMS2 is described elsewhere.8
Of the 313 participants in MIMS2, 6 patients were lost-to-follow-up, and 44 were further excluded due to missing vascular measures at either baseline and post-stress time points. Thus, the analytic sample for this study included 263 patients. The Institutional Review Board at Emory University approved the MIMS2 research protocol, and all enrolled patients provided written informed consent.
Measurements
Mental Stress Procedure
As previously described, patients were tested using a standardized public speaking task after a 30-minute rest period, in a temperature controlled, quiet, and dimly lit room.7, 9, 10, 22, 23 Patients were asked to imagine a real-life stressful situation, in which a close relative had been mistreated in a nursing home and asked to make up a realistic story around this scenario. Patients were given two minutes to prepare a statement and then three minutes to present it in front of a video camera and an audience wearing white coats. Participants were told that their speech would be evaluated by the laboratory staff for content, quality, and duration. Cardiovascular medications, including beta-blockers, calcium-channel blockers, long-acting nitrates, and other anti-ischemic medications, as well as xanthine derivatives and caffeine-containing products were withheld for approximately 24 hours prior to stress testing. This mental stress protocol has been validated and widely used in patients with coronary heart disease,22–25 and hemodynamic, vascular and ischemic responses to this stress challenge are highly reproducible.7, 23
Myocardial Perfusion Imaging and Definition of Stress Induced Myocardial Ischemia
Myocardial perfusion imaging with 99m-Tc-sestamibi single-photon emission computed tomography (SPECT) was performed at rest and 30–60 minutes after mental stress using standard nuclear imaging protocols.26 Images were interpreted by an experienced reader without prior knowledge of patients’ medical history, demographic characteristics, or severity of coronary artery disease. Resting and post-stress images were then visually compared for perfusion defects using a 17-segment model. Each segment was scored from 0 to 4, with 0 being normal uptake, and 4 no uptake. Ischemia was defined as the presence of at least one new perfusion defect with a score of ≥2 in any segment, or as worsening of a pre-existing defect by at least 2 points if in a single segment, or by at least 1 point if in each of two or more contiguous segments, and as previously discussed.27, 28
Vascular Measures
Microvascular Function
Peripheral microvascular function was assessed using the reactive hyperemia index (RHI) from the Endo-PAT2000 (Itamar Medical, Caesarea, Israel), at both rest and 30 minutes after mental stress. The EndoPAT device measures finger pulse volume amplitude (PVA), reflecting peripheral blood volume changes using volume plethysmography technology.29, 30 For this test, PVA is obtained in resting condition and during reactive hyperemia, which is elicited by the release of an upper arm blood pressure cuff inflated to suprasystolic pressure for 5 minutes. The RHI is then calculated by a computer algorithm as the ratio of post-deflation to baseline pulse amplitude in the hyperemic finger divided by the ratio in the contra-lateral finger.31 RHI response was calculated as the difference between post-stress and resting values. Lower RHI values are indicative of more microvascular dysfunction. We and others have shown that the RHI is highly predictive of adverse cardiovascular events and in patients with coronary heart disease.32–34 A categorical measure of endothelial dysfunction was also defined as any decrease in RHI with mental stress (i.e., a post-stress RHI minus prestress RHI value <0).
Endothelial Function
Before and 30 minutes after the mental stress test, patients also underwent measurement of flow-mediated vasodilation (FMD) of the brachial artery using bi-mode ultrasound to assess endothelial function.35, 36 FMD was measured during the hyperemia phase along with the aforementioned RHI measurements using standard methodology.35, 36 More specifically, the artery diameter was measured from the anterior to the posterior intima line using edge-detection software, and the average of three diameter measurements was calculated. Then, the cuff was inflated to a pressure of 50 mmHg greater than the systolic blood pressure. After 5 minutes, the cuff was deflated, and ultrasound scans were acquired for post-occlusion diameter measurements. FMD was calculated as the percent change in brachial diameter during the first 60 seconds post cuff dilation. FMD measurements were repeated 30 minutes after mental stress testing, and the change in FMD with mental stress was calculated as FMD post-stress minus FMD at rest. Lower FMD values are indicative of more transient endothelial dysfunction.
Follow-Up and Cardiovascular Outcomes
MIMS2 patients were prospectively followed at their approximate 3- and 5-year anniversary from the initial baseline visit. At these follow-up periods, study staff collected information on the incidence of cardiovascular events and hospitalizations through direct patient contact, medical record review, and by querying the vital records and Social Security Death Index. If hospitalizations or procedures were reported, patients’ physicians were contacted, and hospital records obtained. First and recurrent events were adjudicated by study cardiologists blinded to other study data. For this study, we considered all recurrent nonfatal cardiovascular events to obtain a more accurate reflection of the true burden of disease.37 The main endpoint of major adverse cardiovascular events (MACE) was defined as a composite index of cardiovascular death, and first/recurring events for nonfatal MI, and hospitalizations for heart failure.
Other Study Measurements
During the baseline study visit, demographic information was obtained using standardized questionnaires. Race/ethnicity was self-reported. Previous medical history (dyslipidemia, diabetes, hypertension,) and medication use (e.g. aspirin, beta blockers) were obtained by study nurses or physicians through medical history, clinical examinations, and by reviewing medical records. Depressive symptoms were assessed with the Beck Depression Inventory-II,38 a reliable and valid self-report measure that has been widely used in cardiac as well as non-cardiac patients. Symptom scores of post-traumatic stress disorder (PTSD) were assessed with the PTSD Civilian Checklist.39, 40 Height and weight were objectively measured during the clinical exam and used to calculate body mass index (BMI, kg/m2). Angiographic data were obtained from the most recent coronary angiogram and severity of coronary artery disease was measured using the Gensini Score.41 Subjective ratings of distress from 0 to 100 (100 = highest level of distress) were obtained at baseline and after mental stress with the Subjective Units of Distress Scale (SUDS).42 All study measures were collected during the baseline study visit.
Statistical Analysis
We fitted multivariable hazards models that adjusted for pre-specified potential confounding or intervening factors in the association of vascular measures of microvascular function and endothelial function as continuous variables with MACE. As there were only a few participants who reported their race as neither being Black/African American or White, only two categories were used in the analysis, Black/African American and Non-Black/African American, consistent with previous reports.20, 28 Since we were interested in the repeated occurrence of events over time, we used the Wei-Lin-Weissfeld (WLW) model for recurrent events,43 which allows a separate underlying hazard for each event, and is more flexible for modeling correlation of repeated events than other methods. To better contrast results of vascular measures with different units of measure, we standardized both RHI and FMD in regression analyses. Since lower RHI and lower FMD values are indicative of more microvascular dysfunction and endothelial dysfunction, respectively, hazard ratios for vascular response can be interpreted as the hazards for MACE per 1 standard deviation decrease in the vascular variable. All analyses were conducted before and after adjusting for possible confounding factors considered a priori in sequential models that included the main vascular measure of interest (model 1). We added demographic variables including age, sex, race, and education in model 2, followed by the addition of lifestyle and clinical risk factors, including ever smoking, body mass index (BMI), history of hypertension, diabetes mellitus, and left ventricular ejection fraction in model 3. A subsequent model (model 4) adjusted for medication use, including beta blockers, statins, angiotensin converting enzyme inhibitors, and aspirin. Model 5 adjusted for psychological variables, including depression (Beck Depression Inventory) and symptom scores of post-traumatic stress disorder (PTSD). A final model (model 6), adjusted for mental stress induced myocardial ischemia. Additionally, since brachial artery diameter differs by sex and is an important determinant of FMD used to measure endothelial function,35 all models with this vascular measure were adjusted for baseline arterial diameter.35 All models containing measures of vascular response to stress were also adjusted for the respective baseline value. To determine whether the association between vascular measures and MACE differed by sex, we included vascular measure-by-sex interactions in the hazards model. We then estimated linear combinations of the regression coefficients for sex using a similar modeling strategy as for the main effects. In supplemental analyses, we also repeated the above hazards models using dichotomized variables for microvascular dysfunction (i.e., a lower post-stress FMD than pre-stress FMD) to examine whether results were sensitive to change. We also tested potential confounding of the results by SUDS, comparing effect estimates from our main effects model (model 1), and a subsequent model that included this variable separately (results not shown). However, since effect estimates did not change by more than 10% and our results were unchanged, SUDS was not included in the final models. The significance level for main effects was set at p < 0.05; while the statistical significance of interaction effects was set at p < 0.10. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Of the 263 patients in the analytic sample, the mean age was 51 years (range: 25–61), 48% were women. The racial composition of the sample was 65% Black/African American, 29% White and 6% other race/ethnicity. Compared to men, women were more likely to be Black/African American, to have a higher body mass index (BMI), and to have a greater burden of psychosocial stressors including lifetime history of depression and higher symptoms scores for PTSD while men were more likely to have a higher Gensini Score (a measure of angiographic coronary artery disease severity) (Table 1). Women also had significantly lower values of RHI (indicative of more microvascular dysfunction) at rest and 30-minutes after mental stress while there were no significant sex differences in FMD (endothelial function) at rest or after mental stress. Nineteen women (15.3%) had ever used post-menopausal hormones while 69 (55.2%) reported that they were post-menopausal. Only 3 women had a past medical history of autoimmune disorders.
Table 1.
Characteristics of the MIMS2 Sample by Sex (n = 263).
| Women (n = 127) | Men (n = 136) | p-value | |
|---|---|---|---|
|
| |||
| Demographic Factors | |||
| Age, mean (SD) | 50.7 (7.2) | 51.2 (5.8) | 0.56 |
| Age > 50, n (%) | 78 (61.4) | 86 (63.2) | 0.76 |
| Black/African American, n (%) | 96 (75.6) | 76 (55.9) | 0.001 |
| Education > 12 years, n (%) | 75 (59.1) | 78 (58.2) | 0.89 |
| Cardiovascular Risk Factors | |||
| BMI, kg/m2, mean (SD) | 33.2 (8.9) | 30.3 (5.5) | 0.002 |
| Ever smoker, n (%) | 69 (54.3) | 74 (54.4) | 0.99 |
| History of hypertension, n (%) | 105 (82.7) | 109 (80.2) | 0.60 |
| History of dyslipidemia, n (%) | 104 (81.9) | 109 (80.2) | 0.72 |
| History of diabetes, n (%) | 46 (36.2) | 38 (27.9) | 0.15 |
| Psychosocial Risk Factors | |||
| Beck Depression Inventory, median (IQR) | 11.0 (5.0, 19.0) | 7.0 (4.0, 15.0) | 0.01 |
| Lifetime history of PTSD, n (%) | 23 (18.4) | 15 (11.3) | 0.11 |
| Current PTSD, n (%) | 15 (12.0) | 13 (9.8) | 0.57 |
| PTSD Symptom Checklist, median (IQR) | 28.5 (22.0, 43.0) | 26.0 (20.0, 38.0) | 0.06 |
| Perceived Stress Scale, median (IQR) | 17.0 (11.0, 24.0) | 15.0 (9.0, 22.0) | 0.08 |
| State Anxiety, median (IQR) | 34.5 (26.0, 45.0) | 32.0 (25.0, 45.0) | 0.39 |
| Trait Anxiety, median (IQR) | 37.0 (31.0, 48.0) | 35.0 (26.0, 48.0) | 0.12 |
| Subjective Units of Distress, mean (SD)* | 29.5 (35.3) | 23.7 (26.1) | 0.14 |
| Medications | |||
| Beta Blocker, n (%) | 109 (86.5) | 115 (84.6) | 0.65 |
| Statins, n (%) | 105 (83.3) | 118 (86.8) | 0.44 |
| Aspirin, n (%) | 103 (81.8) | 108 (79.4) | 0.63 |
| ACE Inhibitors, n (%) | 57 (45.2) | 65 (47.8) | 0.68 |
| Clinical Characteristics | |||
| History of congestive heart failure, n (%) | 15 (11.8) | 12 (8.8) | 0.43 |
| History revascularization, n (%) | 101 (79.5) | 111 (81.6) | 0.67 |
| LV ejection fraction, mean (SD) | 52.9 (12.6) | 48.4 (12.6) | 0.002 |
| Gensini CAD severity score, mean (SD) | 2.7 (1.5) | 3.2 (1.5) | 0.01 |
| Mental stress induced myocardial ischemia, n (%) | 27 (21.6) | 19 (14.1) | 0.06 |
| Vascular Function | |||
| Baseline RHI, mean (SD) | 1.70 (0.56) | 1.84 (0.54) | 0.04 |
| Stress RHI, mean (SD) | 1.64 (0.61) | 1.81 (0.58) | 0.02 |
| RHI Response, mean (SD) | −0.06 (0.54) | −0.03 (0.50) | 0.60 |
| RHI Response < 0, n (%) | 73 (57.5) | 65 (47.8) | 0.12 |
| Baseline FMD, mean (SD) | 4.00 (2.90) | 3.65 (2.58) | 0.29 |
| FMD Stress, mean (SD) | 2.24 (2.27) | 2.01 (2.39) | 0.41 |
| FMD Response, mean (SD) | −1.76 (2.02) | −1.63 (2.03) | 0.63 |
| FMD Response < 0, n (%) | 106 (83.5) | 118 (86.8) | 0.45 |
Difference between posttest and pretest values. A positive value indicates higher distress with mental stress.
Abbreviations: ACE: Angiotensin-converting enzyme; BMI: Body mass index; FMD: Flow mediated dilation; LV: Left ventricular; PTSD: Post-traumatic stress disorder; RHI: Reactive hyperemia index.
Patients were followed for a median of 4.3 years (Interquartile Range: 2.8 – 5.1 years) and commenced in February 2020. There were a total 141 events (first and repeated) that occurred among 64 patients (Table 2). Sixty-three of these events occurred among women while 78 occurred among men. A greater number of recurrent events were attributed to non-fatal MI for both women and men.
Table 2.
Number of First and Recurrent Cardiovascular Events by Sex (n = 263).
| Women n = 127 | Men n = 136 | |
|---|---|---|
| All-Cause Death | 9 | 9 |
| Cardiovascular Death | 6 | 9 |
| MI | ||
| Total Number of First Events | 15 | 13 |
| Total Number of Recurrent Events | 21 | 20 |
| Hospitalizations for Heart Failure | ||
| Total Number of First Events | 6 | 8 |
| Total Number of Recurrent Events | 16 | 17 |
| Cardiovascular death or MI | ||
| Total Number of First events | 22 | 21 |
| Total Number of Recurrent events | 28 | 33 |
| Cardiovascular death, MI, or Heart Failure | ||
| Total Number of First events | 30 | 34 |
| Total Number of Recurrent events | 63 | 78 |
Abbreviations: MI: Myocardial infarction.
Microvascular Dysfunction and Risk of MACE
In the total analytic sample, each standard deviation decrease in RHI after stress (indicating worse microvascular function in response to stress) was associated with a greater risk of MACE in models adjusting for pre-stress RHI values (HR: 1.35; 95% CI: 1.11, 1.63; p = 0.003) and when adjusting for demographic factors (HR: 1.28; 95% CI: 1.05, 1.58; p = 0.02) (Table 3). However, this association was attenuated in subsequent models and no longer statistically significant after adjusting for clinical risk factors, medications, and psychological factors (Table 3).
Table 3.
Vascular Responses to Stress and Risk of Major Adverse Cardiovascular Events (MACE).
| Total Sample | Women | Men | |||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | p-value Sex Interaction | |
| Per Standard Deviation Decrease in Microvascular Response to Stress Measured by RHI * | |||||||
| Model 1 | 1.35 (1.11, 1.63) | 0.003 | 1.85 (1.37, 2.48) | <.0001 | 1.10 (0.85, 1.42) | 0.47 | 0.01 |
| Model 2 | 1.28(1.05, 1.58) | 0.02 | 1.80 (1.33, 2.45) | 0.0002 | 0.99 (0.76, 1.30) | 0.95 | 0.002 |
| Model 3 | 1.06 (0.86, 1.32) | 0.57 | 1.41 (1.02, 1.94) | 0.04 | 0.85 (0.64, 1.33) | 0.25 | 0.01 |
| Model 4 | 1.06 (0.86, 1.32) | 0.58 | 1.44 (1.03, 2.02) | 0.03 | 0.83 (0.62, 1.11) | 0.21 | 0.01 |
| Model 5 | 1.08 (0.87, 1.35) | 0.49 | 1.41 (1.01, 1.96) | 0.046 | 0.87 (0.64, 1.17) | 0.34 | 0.02 |
| Model 6 | 1.14 (0.91, 1.44) | 0.26 | 1.50 (1.05, 2.13) | 0.03 | 0.93 (0.69, 1.26) | 0.66 | 0.03 |
| Per Standard Deviation Decrease in Transient Endothelial Response to Stress Measured by FMD † | |||||||
| Model 1 | 1.50 (1.22, 1.84) | 0.0001 | 1.30 (0.98, 1.73) | 0.07 | 1.64 (1.30, 2.06) | <.0001 | 0.14 |
| Model 2 | 1.54 (1.24, 1.91) | 0.0001 | 1.30 (0.95, 1.79) | 0.10 | 1.66 (1.31, 2.12) | <.0001 | 0.15 |
| Model 3 | 1.47 (1.17, 1.84) | 0.001 | 1.61 (1.13, 2.29) | 0.01 | 1.42 (1.11, 1.81) | 0.005 | 0.49 |
| Model 4 | 1.41 (1.12, 1.78) | 0.004 | 1.49 (1.03, 2.16) | 0.03 | 1.39 (1.09, 1.77) | 0.01 | 0.69 |
| Model 5 | 1.41 (1.11, 1.78) | 0.005 | 1.54 (1.05, 2.26) | 0.03 | 1.38 (1.08, 1.76) | 0.01 | 0.55 |
| Model 6 | 1.35 (1.07, 1.71) | 0.01 | 1.66 (1.12, 2.48) | 0.01 | 1.29 (1.01, 1.65) | 0.04 | 0.22 |
Model 1: Includes vascular measure; Sex specific models includes vascular measure, sex, and their interaction.
Model 2: Model 1 + age, sex, race, and education
Model 3: Model 2 covariates + clinical risk factors (ever smoking, BMI, hypertension, diabetes, dyslipidemia, ejection fraction)
Model 4: Model 3 covariates + medications (beta-blockers, statins, ACE inhibitors, and aspirin).
Model 5: Model 4 covariates + psychological risk factors (beck depression inventory, PTSD symptoms)
Model 6: Model 5 covariates + mental stress induced myocardial ischemia
All models adjusted for baseline RHI
All models also adjusted for brachial artery diameter and baseline FMD
Abbreviations: ACE: Angiotensin-converting enzyme; BMI: Body mass index; FMD: Flow mediated dilation; PTSD: Post-traumatic stress disorder; RHI: Reactive hyperemia index.
Significant sex differences were detected for microvascular response to stress across all models (Table 3) such that a worse microvascular response to stress was associated with an increased risk of MACE among women but not among men. More specifically, the risk of MACE was 41% greater among women (HR: 1.41; 95% CI: 1.01, 1.96; p-value = 0.05) for each standard deviation decrease in RHI in response to stress (indicating worse microvascular response to stress) after adjusting for pre-stress RHI, demographics, clinical risk factors, medications, and psychological factors in model 5. After adjusting for differences in the occurrence of mental stress induced myocardial ischemia (model 6), this risk increased (HR: 1.50; 95% CI: 1.05, 2.13; p-value = 0.03) (Table 3, Figure 1). Microvascular response to stress was not significantly associated with MACE among men, and the sex-based interaction was significant in all models. These sex differences were similar when RHI response to stress was dichotomized, where values less than 0 indicated a decline in microvascular function in response to stress (Supplementary Table S1). Women with a decline in microvascular function in response to stress had a statistically significant 105% (HR: 2.05, 95% CI: 1.10, 3.82; p-value = 0.03) higher risk of MACE compared to women without a decline in microvascular function over the full study period, after adjusting for pre-stress RHI, demographics, clinical risk factors, medications, and psychological factors in model 5. After adjusting for mental stress-induced myocardial ischemia, this risk increased to 131% (HR: 2.31; 95% CI: 1.22, 4.40; p-value: 0.01). This association was weaker among men, and was entirely explained by cardiovascular risk factors.
Figure 1. Forest Plot of Hazard Ratios for each unit decrease in Vascular Response to Stress and Major Adverse Cardiovascular Events.
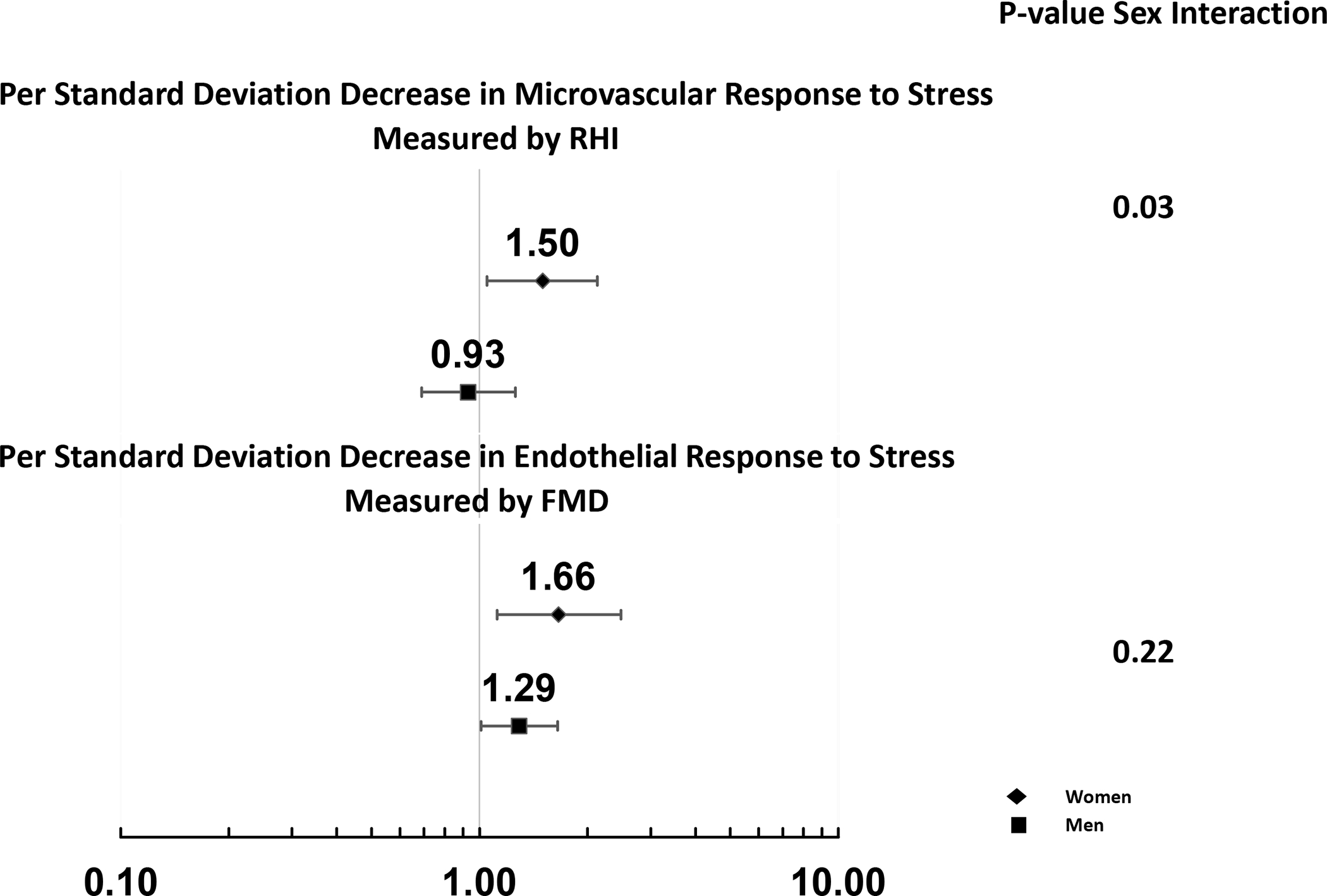
Estimates are from fully adjusted model (model 6).
Transient Endothelial Dysfunction and Risk of MACE
Across all models estimated for the total sample, greater endothelial dysfunction in response to stress was associated with an increased risk of MACE (Table 3). More specifically, each standard deviation decrease in FMD response to stress (indicating worse endothelial function) was associated with a 41% greater risk of MACE (HR: 1.41; 95% CI: 1.11, 1.78; p = 0.005) after adjusting for brachial artery diameter, demographics, clinical risk factors, medications, and psychological variables (model 5). Results were similar after adjusting for differences in the occurrence of mental stress induced myocardial ischemia (Table 3). There was no significant effect modification by sex suggesting that this association was not statistically different comparing women and men (Table 3, Figure 1).
DISCUSSION
In the first study to examine sex differences in vascular responses to mental stress and cardiovascular outcomes among a diverse sample of young and middle-aged survivors of an MI, peripheral microvascular dysfunction with mental stress, assessed by digital RHI, was associated with adverse events among women but not among men. In contrast, endothelial dysfunction with mental stress measured at the brachial artery was similarly related to MACE among both men and women. These results highlight the significance of microvascular disease as a mechanism linking psychological stress to adverse outcomes in women even in a population with a significant burden of coronary artery disease, such as post-MI patients.
In this same cohort we previously reported that, compared to men, women had a greater microvascular constriction (a lower RHI) after mental stress and a larger post-mental stress decline in RHI, indicating that women have a tendency towards enhanced microvascular dysfunction with mental stress.8 We also reported that a lower RHI with mental stress was associated with mental stress induced ischemia more in women than in men.7, 8 Herein, we extend these previous findings by showing that a greater microvascular constriction with mental stress is also associated with adverse outcomes more strongly in women than in men, and this effect is independent of the provocation of mental stress ischemia. As a whole, these data support an important pathophysiological role of microcirculatory dysfunction during mental stress among women with ischemic heart disease. While we assessed microvascular function peripherally, this measurement likely reflects what happens in the coronaries.44, 45 Prior research from our group44 found a significant correlation between peripheral microvascular responses to mental stress and coronary microvascular responses to stress measured during a coronary angiogram.
To our knowledge, only one other empirical study (Wildmer et al.)19 has investigated sex differences in microvascular dysfunction during mental stress and adverse cardiovascular outcomes, but the sample was 95% male, and cardiovascular events were only recorded up to one year.19 Although data stratified by sex were not reported due to the small sample of women, it was suggested that vascular reactivity to mental stress was associated with MACE in women, but not men.19
Our results support a stronger mechanistic link between microvascular responses to stress and outcomes among women than men, which could help elucidate a unique risk pathway for women. Emerging research has shown sexual dimorphisms in every level of cardiovascular physiology,46 which could be extended to the biological effects of mental stress on cardiovascular reactivity.7, 8 Several lines of research suggest that microvascular dysfunction could have a greater impact on women’s cardiovascular risk; our study suggests that this vulnerability could be accentuated during mental stress. Microvascular dysfunction is commonly seen in women with chest pain, even in the absence of significant epicardial coronary obstruction.47, 48 Repeated and cumulative exposure to stress-induced sympathetic activation could lead to microvascular dysfunction,49, 50 and these vascular effects could be accentuated in women given their higher levels of inflammation during mental stress.51, 52 While the exact mechanisms of these difference between men and women are not known, sex differences in autonomic and neuroendocrine pathways involved in homeostasis between microvascular constriction and dilation during stress could underlie differences in the risk of adverse cardiovascular outcomes associated with stress-induced microvascular resistance.44 Sex steroids could also be implicated as they can potentially affect the stress response. These pathways should be explored in detail in future studies.53
There are several strengths and limitations of our study, including a sizable number of women, a racially diverse sample, along with a well characterized population with comprehensive clinical and psychological measures, along with a long follow-up which enabled us to capture a substantial number of cardiovascular events. Also, since patients were recruited from within our university hospital network, attrition was minimal with only 6 patients lost-to-follow-up. Furthermore, cardiovascular events and causes of death were independently adjudicated by experienced cardiologists. Study limitations include those inherent with observational studies, including residual and unmeasured confounding and external validity, as this study was based on a single study center. Our study sample was 65% Black/African American and 29% White, with a small representation of other race/ethnic groups which limits generalizability of our study findings to other groups. Another limitation worth noting is the smaller number of adverse cardiovascular events in the study sample which may have limited the study power for some analyses and precluded the analyses in different age groups. However, our results point to a clear demarcation of associations between women and men, with statistically significant interactions which argue against a type II error. Also, we cannot rule out bias due to unmeasured confounders, such as perceptions of difficulty with the mental stress task (i.e. differences in engagement or effort). However, women and men did not differ in their emotional response to mental stress as measured by the SUDS in exploratory analyses and was not a confounder in our analyses. Thus, our results suggest that women and men have distinct vascular reactivity in response to mental stress that are not due to differences in perceived emotional response to stress. Finally, although cardiovascular medications and caffeine were withheld for 24 hours prior to mental stress testing, and we adjusted for medications in our models, we cannot rule out the possibility of sex differences in metabolic clearance and/or drug compliance which may have affected the results.
Conclusions
Among young and middle-aged survivors of an MI, peripheral microvascular dysfunction with mental stress, assessed by digital RHI, was associated with adverse events among women but not among men. These findings support the importance of psychological stress as a risk factor for women with coronary heart disease and suggest that a microvascular phenomenon may underlie this effect. Future studies should explore the mechanistic links underlying these results, including, for example, cardiovascular physiology/reactivity, sympathetic activation, inflammation, and neuroendocrine pathways.
Supplementary Material
HIGHLIGHTS.
A study to examine sex differences in vascular response to mental stress and cardiovascular outcomes among a diverse sample of young and middle-aged survivors of a myocardial infarction.
Peripheral microvascular dysfunction with mental stress was associated with adverse cardiovascular events among women but not among men.
These findings support the importance of psychological stress as a risk factor for women with coronary heart disease and suggest that a microvascular phenomenon may underlie this effect.
Sources of Funding:
This work was supported by the National Institutes of Health grant numbers: R01HL109413 (VV), R01HL109413-02S1 (VV), T32HL130025 (VV), U54AG062334 (AQ, SS), K12HD085850 (SS), UL1TR002378 (SS), K01HL149982 (SS), L30HL148912 (SS). The authors of this article are solely responsible for the content of this paper. The funding agency had no role in the design and conduct of this study, in the collection, analysis, interpretation of the data, or in the preparation, review or approval of this manuscript.
The authors of this article are solely responsible for the content of this paper. The funding agency had no role in the design and conduct of this study, in the collection, analysis, interpretation of the data, or in the preparation, review or approval of this manuscript.
Abbreviations and Acronyms:
- BMI
body mass index
- CAD
coronary artery disease
- FMD
flow-mediated vasodilation
- MACE
major adverse cardiovascular events
- MI
myocardial infarction
- MIMS2
Myocardial Infarction and Mental Stress 2 Study
- RHI
reactive hyperemia index
Footnotes
Disclosures: None.
Supplemental Materials
REFERENCES
- 1.Nabel EG. Heart Disease Prevention in Young Women: Sounding an Alarm. Circulation. 2015;132:989–991. [DOI] [PubMed] [Google Scholar]
- 2.Gebhard C Women and acute coronary syndromes: still up to no good. Eur Heart J. 2017;38:1066–1068. [DOI] [PubMed] [Google Scholar]
- 3.Wenger NK, Lloyd-Jones DM, Elkind MSV, et al. Call to Action for Cardiovascular Disease in Women: Epidemiology, Awareness, Access, and Delivery of Equitable Health Care: A Presidential Advisory From the American Heart Association. Circulation. 2022;145:e1059–e1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds HR, Bairey Merz CN, Berry C, Samuel R, Saw J, Smilowitz NR, de Souza A, Sykes R, Taqueti VR and Wei J. Coronary Arterial Function and Disease in Women With No Obstructive Coronary Arteries. Circ Res. 2022;130:529–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waheed N, Elias-Smale S, Malas W, Maas AH, Sedlak TL, Tremmel J and Mehta PK. Sex differences in non-obstructive coronary artery disease. Cardiovasc Res. 2020;116:829–840. [DOI] [PubMed] [Google Scholar]
- 6.Bugiardini R and Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA. 2005;293:477–484. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan S, Hammadah M, Al Mheid I, et al. Sex Differences in Hemodynamic and Microvascular Mechanisms of Myocardial Ischemia Induced by Mental Stress. Arteriosclerosis, thrombosis, and vascular biology. 2018;38:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaccarino V, Sullivan S, Hammadah M, et al. Mental Stress-Induced-Myocardial Ischemia in Young Patients With Recent Myocardial Infarction: Sex Differences and Mechanisms. Circulation. 2018;137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaccarino V, Shah AJ, Rooks C, et al. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom Med. 2014;76:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaccarino V, Wilmot K, Al Mheid I, et al. Sex Differences in Mental Stress-Induced Myocardial Ischemia in Patients With Coronary Heart Disease. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pimple P, Lima BB, Hammadah M, et al. Psychological Distress and Subsequent Cardiovascular Events in Individuals With Coronary Artery Disease. J Am Heart Assoc. 2019;8:e011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah AJ, Ghasemzadeh N, Zaragoza-Macias E, Patel R, Eapen DJ, Neeland IJ, Pimple PM, Zafari AM, Quyyumi AA and Vaccarino V. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc. 2014;3:e000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kop WJ, Krantz DS, Howell RH, Ferguson MA, Papademetriou V, Lu D, Popma JJ, Quigley JF, Vernalis M and Gottdiener JS. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: relationship with hemodynamic stress responses. J Am Coll Cardiol. 2001;37:1359–66. [DOI] [PubMed] [Google Scholar]
- 14.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS and Cannon RO, 3rd. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. Am J Cardiol. 1995;76:125–30. [DOI] [PubMed] [Google Scholar]
- 15.Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin-Glaser T, Zaret BL and Soufer R. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356:310–1. [DOI] [PubMed] [Google Scholar]
- 16.Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V and Quyyumi AA. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2013;2:e000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arri SS, Ryan M, Redwood SR and Marber MS. Mental stress-induced myocardial ischaemia. Heart. 2016;102:472–80. [DOI] [PubMed] [Google Scholar]
- 18.Bairey Merz CN and Pepine CJ. Syndrome X and microvascular coronary dysfunction. Circulation. 2011;124:1477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widmer RJ, Prasad M, Gomaa M, Sara JDS, Reriani MK, Lerman LO, Suwaidi JA and Lerman A. Vascular reactivity to mental stress is associated with poor cardiovascular disease outcomes in females following acute coronary syndrome. Coron Artery Dis. 2020;31:300–305. [DOI] [PubMed] [Google Scholar]
- 20.Garcia M, Almuwaqqat Z, Moazzami K, et al. Racial Disparities in Adverse Cardiovascular Outcomes After a Myocardial Infarction in Young or Middle-Aged Patients. J Am Heart Assoc. 2021;10:e020828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalinowski J, Taylor JY and Spruill TM. Why Are Young Black Women at High Risk for Cardiovascular Disease? Circulation. 2019;139:1003–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation. 1996;94:2402–9. [DOI] [PubMed] [Google Scholar]
- 23.Kim CK, Bartholomew BA, Mastin ST, Taasan VC, Carson KM and Sheps DS. Detection and reproducibility of mental stress-induced myocardial ischemia with Tc-99m sestamibi SPECT in normal and coronary artery disease populations. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2003;10:56–62. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS and Sheps DS. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol. 2006;47:987–91. [DOI] [PubMed] [Google Scholar]
- 25.Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–4. [DOI] [PubMed] [Google Scholar]
- 26.Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single Photon Emission Computed Tomography (SPECT) Myocardial Perfusion Imaging Guidelines: Instrumentation, Acquisition, Processing, and Interpretation. J Nucl Cardiol. 2018;25:1784–1846. [DOI] [PubMed] [Google Scholar]
- 27.Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17:941–73. [DOI] [PubMed] [Google Scholar]
- 28.Vaccarino V, Almuwaqqat Z, Kim JH, et al. Association of Mental Stress-Induced Myocardial Ischemia With Cardiovascular Events in Patients With Coronary Heart Disease. JAMA. 2021;326:1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan M, York KM, Li H, Li Q, Lucey DG, Fillingim RB and Sheps DS. Usefulness of Peripheral Arterial Tonometry in the Detection of Mental Stress-Induced Myocardial Ischemia. Clinical Cardiology. 2009;32:E1–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramadan R, Sheps D, Esteves F, Maziar Zafari A, Douglas Bremner J, Vaccarino V and Quyyumi AA. Myocardial Ischemia During Mental Stress: Role of Coronary Artery Disease Burden and Vasomotion. Journal of the American Heart Association. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris AA, Patel RS, Binongo JN, et al. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. Journal of the American Heart Association. 2013;2:e002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young A, Garcia M, Sullivan SM, et al. Impaired Peripheral Microvascular Function and Risk of Major Adverse Cardiovascular Events in Patients With Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2021;41:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO and Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–8. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzawa Y, Sugiyama S, Sumida H, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc. 2013;2:e000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 2002;39:257–65. [DOI] [PubMed] [Google Scholar]
- 36.Al Mheid I, Patel R, Murrow J, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. Journal of the American College of Cardiology. 2011;58:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon SD and Pfeffer MA. The Future of Clinical Trials in Cardiovascular Medicine. Circulation. 2016;133:2662–70. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA and Brown GK. Beck depression inventory : BDI-II : manual. San Antonio: The Psychological Corporation : Harcourt Brace & Company; 1996. [Google Scholar]
- 39.Blanchard EB, Jones-Alexander J, Buckley TC and Forneris CA. Psychometric properties of the PTSD checklist (PCL). Behavioral Research & Therapy. 1996;34:669–673. [DOI] [PubMed] [Google Scholar]
- 40.Bliese PD, Wright KM, Adler AB, Cabrera O, Castro CA and Hoge CW. Validating the primary care posttraumatic stress disorder screen and the posttraumatic stress disorder checklist with soldiers returning from combat. J Consult Clin Psychol. 2008;76:272–81. [DOI] [PubMed] [Google Scholar]
- 41.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. [DOI] [PubMed] [Google Scholar]
- 42.Wolpe J The practice of behavior therapy. New York, New York: Pergamon Press; 1969. [Google Scholar]
- 43.Therneau TM and Grambsch PM. The Cox Model. In: Modeling Survival Data: Extending the Cox Model. Statistics for Biology and Health Springer, New York, NY. 2000. [Google Scholar]
- 44.Hammadah M, Kim JH, Al Mheid I, et al. Coronary and Peripheral Vasomotor Responses to Mental Stress. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH and Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–74. [DOI] [PubMed] [Google Scholar]
- 46.Blenck CL, Harvey PA, Reckelhoff JF and Leinwand LA. The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ Res. 2016;118:1294–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N, Sharaf BL, Reis S, Kelsey SF and Pohost GM. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–35. [DOI] [PubMed] [Google Scholar]
- 48.Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41. [DOI] [PubMed] [Google Scholar]
- 49.Ersboll M, Al Enezi F, Samad Z, Sedberry B, Boyle SH, O’Connor C, Jiang W, Velazquez EJ and Investigators R. Impaired resting myocardial annular velocities are independently associated with mental stress-induced ischemia in coronary heart disease. JACC Cardiovasc Imaging. 2014;7:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kershaw KN, Lane-Cordova AD, Carnethon MR, Tindle HA and Liu K. Chronic Stress and Endothelial Dysfunction: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens. 2017;30:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rooks CR, Ibeanu I, Shah A, Pimple P, Murrah N, Shallenberger L, Pace T, Douglas Bremner J, Raggi P and Vaccarino V. Young women post-MI have higher plasma concentrations of interleukin-6 before and after stress testing. Brain Behav Immun. 2016;51:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan S, Young A, Hammadah M, et al. Sex differences in the inflammatory response to stress and risk of adverse cardiovascular outcomes among patients with coronary heart disease. Brain Behav Immun. 2020;90:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaccarino V and Bremner JD. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev. 2017;74:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


