Abstract
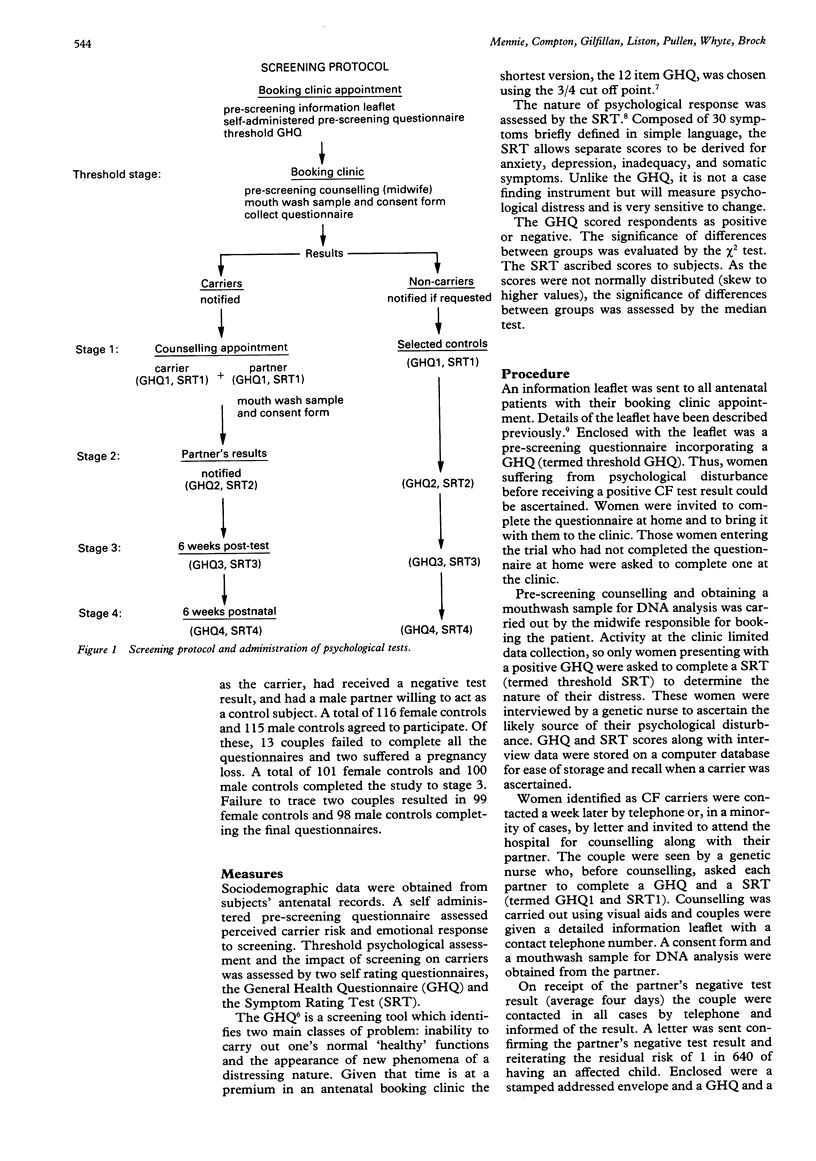
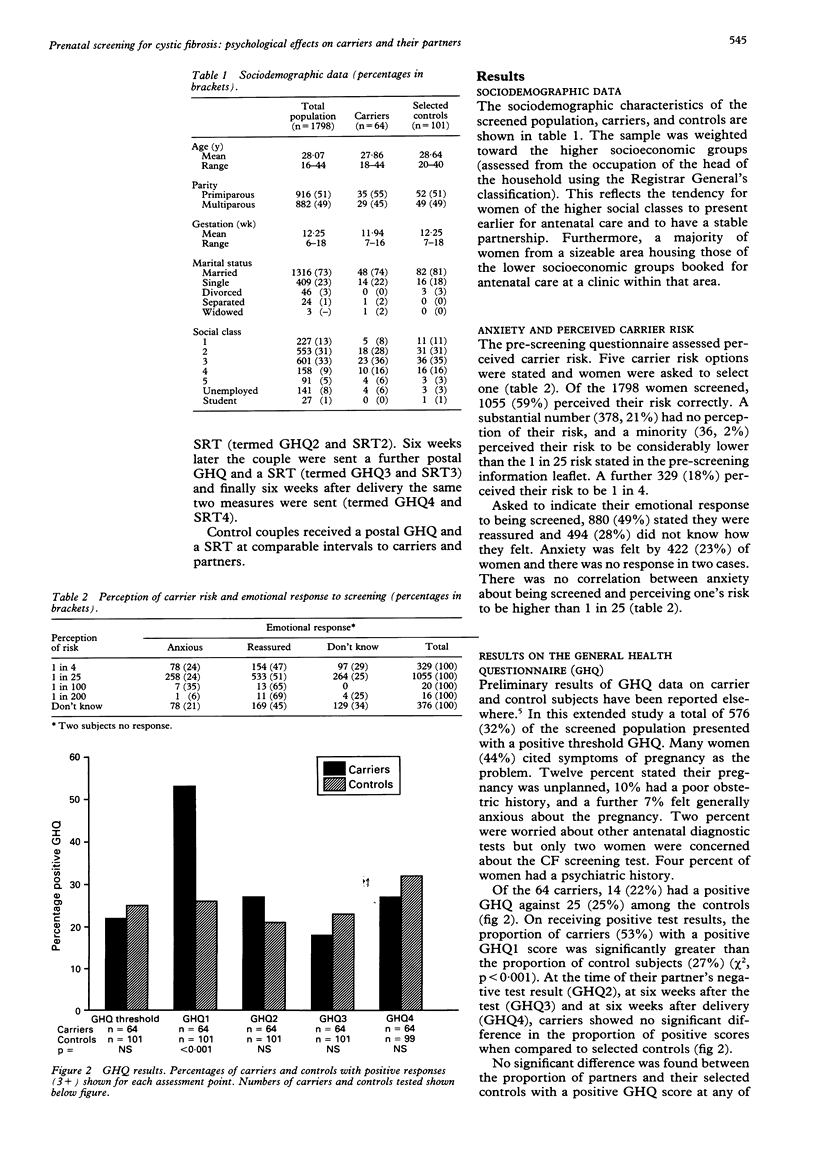
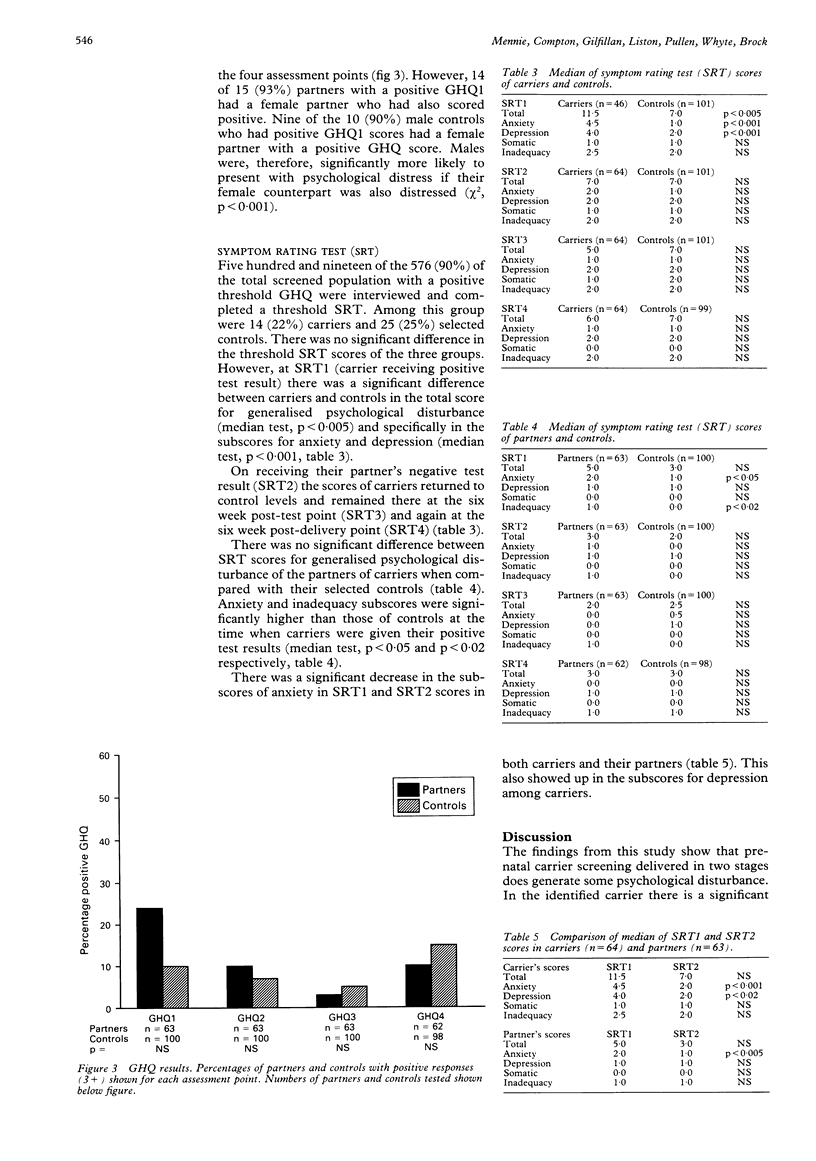
This study aimed to assess the psychological impact of screening for cystic fibrosis (CF) carrier status in a population of pregnant women. A cohort of 1798 women, who accepted the offer of testing before 18 weeks of pregnancy, filled in a self administered questionnaire seeking information on their perceived risk of carrier status and their emotional response, as well as a general health questionnaire (GHQ). Sixty-four women identified as CF carriers had partners who received a negative test result. This group and their partners were assessed, together with selected controls, on four further occasions: (1) on receiving the carrier's positive test result; (2) on receiving the partner's negative test result; (3) six weeks later; (4) six weeks after delivery. The instruments used were the GHQ and the Symptom Rating Test (SRT). When compared to control subjects, carriers showed a significant increase in generalised psychological disturbance which could be attributed specifically to symptoms of anxiety and depression during the period (average four days) that they awaited their partner's test result. On receiving a partner's negative test result, the carriers returned to control levels and maintained this equilibrium. Although there was no significant difference in generalised psychological disturbance between partners and their selected controls, partners did become significantly more anxious and manifested feelings of inadequacy while awaiting their own test result. Both male partners and male control subjects were more likely to become anxious if their partner was distressed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birch-Cox J. Design of diagnostic tests for congenital and acquired colour vision defects. Mod Probl Ophthalmol. 1976;17:196–201. [PubMed] [Google Scholar]
- Gouras P., Eggers H. M., MacKay C. J. Cone dystrophy, nyctalopia, and supernormal rod responses. A new retinal degeneration. Arch Ophthalmol. 1983 May;101(5):718–724. doi: 10.1001/archopht.1983.01040010718003. [DOI] [PubMed] [Google Scholar]
- Keunen J. E., van Everdingen J. A., Went L. N., Oosterhuis J. A., van Norren D. Color matching and foveal densitometry in patients and carriers of an X-linked progressive cone dystrophy. Arch Ophthalmol. 1990 Dec;108(12):1713–1719. doi: 10.1001/archopht.1990.01070140067031. [DOI] [PubMed] [Google Scholar]
- Reichel E., Bruce A. M., Sandberg M. A., Berson E. L. An electroretinographic and molecular genetic study of X-linked cone degeneration. Am J Ophthalmol. 1989 Nov 15;108(5):540–547. doi: 10.1016/0002-9394(89)90431-5. [DOI] [PubMed] [Google Scholar]
- Went L. N., Pronk N. The genetics of tritan disturbances. Hum Genet. 1985;69(3):255–262. doi: 10.1007/BF00293036. [DOI] [PubMed] [Google Scholar]
- de Vries-de Mol E. C., Went L. N., van Norren D., Pols L. C. Increment spectral sensitivity of hemizygotes and heterozygotes for different classes of colour vision. Mod Probl Ophthalmol. 1978;19:224–228. [PubMed] [Google Scholar]
- van Norren D., Went L. N. New test for the detection of tritan defects evaluated in two surveys. Vision Res. 1981;21(8):1303–1306. doi: 10.1016/0042-6989(81)90235-2. [DOI] [PubMed] [Google Scholar]
- van Schooneveld M. J., Went L. N., Oosterhuis J. A. Dominant cone dystrophy starting with blue cone involvement. Br J Ophthalmol. 1991 Jun;75(6):332–336. doi: 10.1136/bjo.75.6.332. [DOI] [PMC free article] [PubMed] [Google Scholar]


