Abstract
Aim: Pemafibrate is a highly selective agonist for peroxisome proliferator-activated receptor (PPAR)-α, a key regulator of lipid and glucose metabolism. We compared the efficacy and safety of pemafibrate with those of bezafibrate, a nonselective PPAR-α agonist.
Methods: In this randomized crossover study, 60 patients with hypertriglyceridemia (fasting triglyceride [TG] ≥ 150 mg/dL) were treated with pemafibrate of 0.2 mg/day or bezafibrate of 400 mg/day for 24 weeks. The primary endpoint was percent change (%Change) from baseline in TG levels, while the secondary endpoints were %Change in high-density lipoprotein cholesterol (HDL-C) and apolipoprotein A-I (Apo A-I) levels.
Results: The %Change in TG and Apo A-I levels was significantly greater with pemafibrate than with bezafibrate (−46.1% vs. −34.7%,p<0.001; 9.2% vs. 5.7%,p =0.018, respectively). %Change in HDL-C levels was not significantly different between the two treatments. %Change in liver enzyme levels was markedly decreased with pemafibrate than with bezafibrate. Creatinine levels significantly increased in both treatments; however, its %Change was significantly lower with pemafibrate than with bezafibrate (5.72% vs. 15.5%,p<0.001). The incidence of adverse events (AEs) or serious AEs did not differ between the two treatments; however, the number of patients with elevated creatinine levels (≥ 0.5 mg/dL and/or 25% from baseline) was significantly lower in the bezafibrate group than in the pemafibrate group (16/60 vs. 3/60,p =0.004).
Conclusion: Compared with bezafibrate, pemafibrate is more effective in decreasing TG levels and increasing Apo A-I levels and is safer regarding liver and renal function.
Keywords: Bezafibrate, Pemafibrate, Peroxisome proliferator-activated receptor (PPAR)-α, Renal function, Triglycerides, Comparison of pemafibrate and bezafibrate
See editorial vol. 30: 429-431
Introduction
Dyslipidemia is a major risk factor for atherosclerotic cardiovascular disease (ASCVD), and low-density lipoprotein cholesterol (LDL-C) is the primary target for preventing the development of atherosclerotic lesions 1) . Although the benefit of decreasing LDL-C levels for reducing ASCVD events is well known, important residual risks remain to be elucidated in patients with ASCVD, particularly in those with statin-treated coronary artery disease (CAD) with dyslipidemia 2) . A few clinical studies have shown that an increase in serum triglyceride (TG) levels is not only a biomarker of residual risk but also an independent risk factor for patients with ASCVD on LDL-C lowering therapy 2 , 3) . The serum level of TG is closely associated with those of other atherosclerogenic lipids, such as TG-rich lipoproteins and their remnants and small dense LDL-C 2 , 4 , 5) . Patients with increased TG levels often present with insulin resistance and metabolic disturbance, which are linked to visceral obesity and ectopic fat deposition 6) . In addition to reducing LDL-C levels, controlling and reducing serum TG levels are important measures to reduce the residual risk of ASCVD event occurrences.
Fibrates exhibit potent TG-lowering effects via the activation of peroxisome proliferator-activated receptor (PPAR)-α and have been recommended as TG-lowering drugs by both Japanese and foreign guidelines 7 - 9) . PPAR-α, a nuclear hormone receptor, is involved in lipid metabolism and is associated with the transcription of genes responsible for decreasing TG levels and increasing high-density lipoprotein cholesterol (HDL-C) levels in the serum 10) . Pemafibrate, the newly developed PPAR-α modulator, is a potent TG decreasing and HDL-C increasing drug with highly selective PPAR-α agonist activity 10) . Although a phase III clinical trial of pemafibrate demonstrated its efficacy and safety in patients with high TG and low HDL-C levels compared with those who were administered fenofibrate 11) , the usefulness of pemafibrate and other existing fibrates has not been compared well.
Bezafibrate was developed as an agonist for all three isoforms of human PPAR (PPAR-α, PPAR-γ, and PPAR-δ) and has been widely used in and outside Japan 12 , 13) . In this prospective crossover study, we enrolled patients with CAD receiving statin treatment and examined the efficacy and safety of pemafibrate (0.2 mg/day), a selective PPAR-α agonist, in comparison with bezafibrate (400 mg/day), a pan-PPAR agonist.
Aim
This study aimed to determine and compare the efficacy and safety of pemafibrate treatment with those of bezafibrate treatment in patients with statin-treated CAD with dyslipidemia.
Methods
Study Patients
Eligible patients were men and postmenopausal women aged 20–75 years with CAD and dyslipidemia treated with statin. The inclusion criteria were (1) a fasting serum TG level of ≥ 150 mg/dL and (2) an HDL-C level of <50 mg/dL in men or <55 mg/dL in women at entry.
The major exclusion criteria were as follows: (1) patients who required additional medication for dyslipidemia during the study period; (2) fasting serum TG level of >1,000 mg/dL; (3) type 1 diabetes mellitus (DM) or uncontrolled type 2 DM (hemoglobin A1c [HbA1c] level of ≥ 8.5%); (4) familiar hypercholesterolemia; (5) chronic kidney disease (CKD) showing a serum creatinine level of ≥ 1.5 mg/dL; (6) history or complication of gallbladder disease, cholelithiasis, pancreatitis, and malignant tumor; (7) aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation to a level more than two times the upper limit of normal (ULN) range; (8) uncontrolled hypertension (systolic blood pressure [BP] of ≥ 160 mmHg or diastolic BP of ≥ 100 mmHg); (9) hemoglobin level of <12 g/dL in men or <11 g/dL in women; (10) recent myocardial infarction or cerebrovascular disorder within 3 months before the study; (11) hospitalization for worsening heart failure within 3 months before the study; and (12) use of ezetimibe and supplements containing eicosapentaenoic acid, and/or docosahexaenoic acid within 3 months before the study. Patients were also prohibited from using agents that affect lipid metabolism, such as thiazolidinedione, insulin, oral corticosteroid, or protease inhibitor.
Patients with stable CAD were defined as cardiac ischemic patients who had a history of myocardial infarction, coronary artery bypass, percutaneous coronary intervention with or without stenting, or previous angiographically proved stenotic lesion ≥ 75% in a major epicardial coronary artery. They were also confirmed to be in a stable condition when chest pain was induced by exertion, resolved under nitrate therapy, and when its characteristics (frequency, severity, duration, time of onset, and precipitating factors) did not change over a period of 12 weeks before the study. Patients with metabolic syndrome (MetS) were defined according to the criteria in Japan: waist circumferences of ≥ 85 cm in men and ≥ 90 cm in women as an essential component and the presence of two or more metabolic abnormalities of three components, namely, (1) TG level of ≥ 150 mg/dL, HDL-C level of <40 mg/dL, and/or the use of medication for dyslipidemia; (2) BP of ≥ 130/85 mmHg and/or the use of antihypertensive medications; and (3) fasting plasma glucose (FPG) level of ≥ 110 mg/dL and/or the use of medication for DM 14) .
Study Design
This was a single-center, prospective, randomized, open-label, crossover study of patients with stable CAD and hypertriglyceridemia and was conducted from October 2019 to July 2021 at Iwate Prefectural Central Hospital, Iwate, Japan. The study protocol was approved by the local Ethics Committee (approval no. 1606) and conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent. This study was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN000047737).
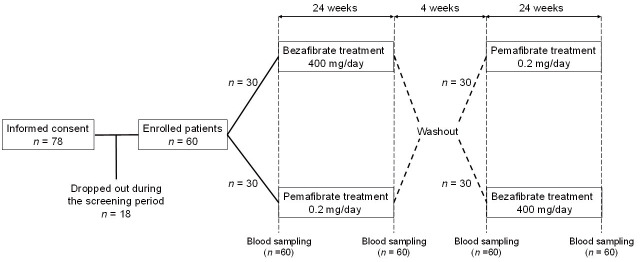
This crossover study consisted of two 24-week crossover treatment periods and a 4-week washout period. Patients were randomly assigned in a 1:1 ratio to two treatment groups: bezafibrate at a dose of 400 mg/day or pemafibrate at a dose of 0.2 mg/day for the first 24-week treatment period. If patients were under fibrate treatment for dyslipidemia, the study started after a 4-week washout period, which was followed by a 4-week observation period and a 24-week treatment period. Patients without fibrate treatment did not go through the washout period. In the second 24-week treatment period, the groups were reversed. There was a 4-week washout period between the two treatment phases in which neither group received bezafibrate or permafibrate. Laboratory tests were conducted before and after each 24-week treatment period ( Fig.1 ) .
Fig.1.
Flow diagram of patients through each stage of the study
Measurement of Biochemical Markers
Patients were instructed to avoid exercising or consuming food, caffeine, vitamins, or alcohol 12 hours before blood sampling. Blood samples were collected in the fasting state. Sera were separated immediately after blood collection by low-speed centrifugation at 3,000 rpm for 15 min at 4℃ and stored at −80℃ until processed. In this study, measurements of all biochemical markers were outsourced to a commercial laboratory (SRL Co., Tokyo, Japan). TG levels were measured by an enzymatic method (Kanto Chemical Co., Toyo, Japan); LDL-C and HDL-C levels by a direct method (Sekisui Medical Co., Tokyo, Japan) 15 , 16) ; lipoprotein(a) [Lp(a)] levels by a latex flocculation turbidimetric method (Sekisui Medical); remnant lipoprotein cholesterol (RemL-C) levels by an enzymatic method (Minaris Medical Co, Tokyo, Japan); apolipoprotein A-I (Apo A-I) and apolipoprotein B (Apo B) levels by an immunoturbidimetric method (Sekisui Medical); apolipoprotein B-48 (Apo B-48) by a chemiluminescence enzyme immunoassay (CLEIA, Fujirebio Inc., Tokyo, Japan); creatinine levels by an enzymatic method (Minaris Medical); AST, ALT, gamma-glutamyl transferase (γ-GT), and creatine kinase (CK) levels by the Japan Society of Clinical Chemistry transferable method (Kanto Chemical); and HbA1c levels by an enzymatic method (Minaris Medical). The estimated glomerular filtration rate (eGFR) was calculated based on the serum creatinine value: eGFRmale=194×creatinine−1.094×age−0.287; eGFRfemale=194×creatinine−1.094×age−0.287×0.739 17) . Patients with CKD were defined as those with baseline eGFR of <60 mL/min/1.73 m2.
In this study, the following markers were evaluated to assess insulin resistance or sensitivity: (1) serum insulin levels; (2) homeostasis model assessment of insulin resistance (HOMA-IR), which was calculated as FPG (mg/dL)×fasting plasma insulin (µIU/mL)/405] 18) ; and (3) serum adiponectin and leptin levels. At the SRL laboratory, the plasma glucose and insulin levels were determined by a hexokinase UV method (Kanto Chemical) and a CLEIA (Fujirebio), respectively; adiponectin levels by a latex immunoturbidimetric method (LSI Medience Co, Tokyo, Japan); and leptin levels by a double-antibody radioimmunoassay (Merck Millipore Co, MO, USA)
Efficacy Endpoints
The primary efficacy endpoint was a percent change (%Change) in the fasting serum TG levels from baseline to the study endpoint after the 24-week treatment with bezafibrate or pemafibrate. The baseline and study endpoint were defined as measurements before and after the 24-week treatment, respectively ( Fig.1 ) . %Change was calculated as follows: ([value after the 24-week treatment − value at baseline]/value at baseline)×100.
The secondary efficacy endpoints were %Change in the fasting serum HDL-C and Apo A-I levels from baseline to the study endpoint.
Other exploratory endpoints were also assessed, wherein the %Change from baseline to the study endpoint was evaluated for the following: (1) lipid markers, such as LDL-C, Apo B, RemL-C, Apo B-48, and Lp(a); (2) lipid and apolipoprotein ratios, such as LDL-C/Apo B and Apo B/Apo A-I; (3) glucose and insulin resistance-related markers, such as FPG, fasting serum insulin, HOMA-IR, adiponectin, and leptin; and (4) liver and kidney function parameters, such as AST, ALT, γ-GT, creatinine, and eGFR.
Safety Endpoints
The safety endpoints included adverse events (AEs)/serious AEs (SAEs), vital signs, and symptoms at each visit. The AEs of clinical interest were as follows: (1) consecutive elevations in the CK levels (>5×ULN); (2) consecutive elevations in the AST and/or ALT levels (>3×ULN); (3) elevations in the serum creatinine levels (≥ 0.5 mg/dL and/or 25% from baseline); and (4) symptoms related to rhabdomyolysis/myopathy and gallbladder, hepatic, pancreatic, and gastrointestinal diseases. In this study, the primary safety endpoint was the proportion of patients with AEs.
Statistical Analysis
We assumed a %Change in fasting serum TG levels of 46% and 21% with pemafibrate and bezafibrate treatment, respectively, based on the results of previous studies 11 , 13) . To use a two-sided test for differences between the two treatments, a minimal sample size of 57 patients was required to detect statistical differences in the %Change in fasting serum TG levels with a power of 80% and an α-type error of 5%. Allowing for a 5% dropout rate, the sample size was; thus, set at 60 patients in this study. All values are expressed as mean±standard deviation for continuous variables and as number and percentage for categorical variables. The within-group changes in the parameters or the between-treatment differences for continuous variables were assessed using the paired t-test or Wilcoxon signed-rank test, as appropriate, regardless of whether the variables were normally distributed. The chi-square test or Fisher’s exact test was used to examine categorical variables for between-treatment differences as appropriate. A two-sided p value of <0.05 was considered statistically significant. All statistical analyses were conducted using Excel (Microsoft, Redmond, WA, USA) with the add-in software Statcel4.
Results
Patient Characteristics
Of 78 patients initially screened, 60 were enrolled and randomized with 30 patients per treatment ( Fig.1 ) . All 60 patients completed the study and were included in both analysis sets. Table 1 summarizes the clinical characteristics of the enrolled patients. The mean age was 63 years, and 92% (n=55) of the patients were men. The mean body mass index was 27 kg/m2, and 77% (n=46) of the patients met the Japanese criteria for MetS 14) . Approximately 40% (n=24) of the patients had type 2 DM and 80% (n=48) had hypertension. Nearly half of the patients (n=31, 52%) received rosuvastatin as statin therapy.
Table 1. Clinical characteristics of enrolled patients (n = 60) .
| Clinical variables | |
|---|---|
| Age, years | 62.7±9.2 |
| Men sex, n (%) | 55 (92) |
| Height, cm | 167.7±7.6 |
| Weight, kg | 75.4±13.7 |
| BMI, kg/m2 | 26.6±3.4 |
| Waist circumference, cm | |
| Men | 95.5±8.1 |
| Women | 82.8±7.0 |
| Current smoker or ex-smoker, n (%) | 40 (67) |
| Drinking habit, n (%) | 27 (45) |
| Systolic blood pressure, mmHg | 129.5±13.4 |
| Diastolic blood pressure, mmHg | 79.7±9.1 |
| Fasting plasma glucose, mg/dL | 118.4±34.6 |
| HbA1c, % | 6.2±0.8 |
| TG, mg/dL | 248.5±145.3 |
| Type of statins used | |
| Pitavastatin/Atorvastatin/Rosuvastatin | 18 (30)/11 (18)/31 (52) |
| Type 2 diabetes mellitus, n (%) | 24 (40) |
| Hypertension, n (%) | 48 (80) |
| Metabolic syndrome, n (%) | 46 (77) |
Data are presented as mean±standard deviation or n (%). BMI, body mass index; HbA1c, hemoglobin A1c; TG, triglycerides.
Effects on Lipid Metabolism
Table 2 shows the change or %Change in lipid markers during the 24-week treatment with bezafibrate or pemafibrate. The results of the primary efficacy endpoint demonstrated significant decreases in fasting serum TG levels from baseline in both treatments. The %Change in serum TG levels was significantly greater with pemafibrate treatment than with the bezafibrate treatment (p<0.001). The results of the secondary efficacy endpoints demonstrated significant increases in serum HDL-C and Apo A-I levels in both treatments. The %Change in serum HDL-C levels was greater with pemafibrate treatment than with bezafibrate treatment, although this difference was not statistically significant (p=0.067). The %Change in serum Apo A-I levels was significantly greater with pemafibrate treatment than with bezafibrate treatment (p=0.018).
Table 2. Percent change in lipid markers from baseline to the study endpoint after the 24-week treatment with bezafibrate or pemafibrate.
| Bezafibrate treatment (n= 60) | Pemafibrate treatment (n= 60) | p value (%Change) | |||
|---|---|---|---|---|---|
| %Change | %Change | ||||
| TG (mg/dL) | |||||
| Baseline | 248.5±144.1 | 252.5±106.1 | |||
| 24 weeks | 154.5±83.9§ | −34.7±21.5 | 136.3±71.6§ | −46.1±14.4 | <0.001 |
| HDL-C (mg/dL) | |||||
| Baseline | 43.3±5.9 | 43.4±5.6 | |||
| 24 weeks | 49.5±11.6§ | 14.0±19.3 | 51.5±11.6§ | 18.4±19.6 | 0.067 |
| Apo A-I (mg/dL) | |||||
| Baseline | 134.2±14.9 | 132.3±15.1 | |||
| 24 weeks | 141.6±16.9§ | 5.7±8.3 | 143.6±12.3§ | 9.2±9.4 | 0.018 |
| LDL-C (mg/dL) | |||||
| Baseline | 91.5±25.7 | 92.1±20.7 | |||
| 24 weeks | 103.7±21.8§ | 18.7±31.8 | 102.4±24.0§ | 13.5±23.5 | 0.289 |
| Apo B (mg/dL) | |||||
| Baseline | 89.2±19.0 | 90.3±19.1 | |||
| 24 weeks | 85.3±17.3* | −3.1±15.9 | 82.1±14.7§ | −8.0±10.4 | 0.028 |
| RemL-C (mg/dL) | |||||
| Baseline | 11.0±10.8 | 11.4±9.6 | |||
| 24 weeks | 5.6±3.8§ | −36.8±27.8 | 4.9±3.3§ | −48.9±21.1 | <0.001 |
| Apo B-48 (μg/mL) | |||||
| Baseline | 9.1±7.7 | 10.7±9.7 | |||
| 24 weeks | 4.8±3.5§ | −31.8±48.1 | 5.5±5.4§ | −38.8±35.0 | 0.620 |
| Lp(a) (mg/dL) | |||||
| Baseline | 19.9±30.2 | 20.4±30.3 | |||
| 24 weeks | 21.0±26.2 | 33.3±74.2 | 19.1±23.9 | −17.8±59.5 | 0.125 |
Data are presented as mean±standard deviation. *p<0.05; §p<0.001 vs. baseline value. TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; Apo A-I, apolipoprotein A-I; LDL-C, low-density lipoprotein cholesterol; Apo B, apolipoprotein B; RemL-C, remnant-like particle cholesterol; Apo B-48, apolipoprotein B-48; Lp(a), lipoprotein(a).
The effects on other lipid parameters were as follows: serum LDL-C levels significantly increased in both treatments (p<0.001 in both), while the serum Apo B levels significantly decreased in both treatments (p=0.037 in the bezafibrate treatment and p<0.001 in the pemafibrate treatment). Although the %Change in serum LDL-C levels was not significantly different between the two treatments (p=0.289), that in the serum Apo B levels was significantly greater with pemafibrate treatment than with bezafibrate treatment (p=0.028). The LDL-C/Apo B ratio, which serves as a surrogate marker of a smaller LDL-particle size, significantly increased in both treatments (from 1.03±0.20 to 1.23±0.16 with bezafibrate treatment [p<0.001] and from 1.03±0.16 to 1.24±0.15 with pemafibrate treatment [p<0.001]), although this difference was not statistically significant (24.8%±40.0 % vs. 24.5%±30.1 %, p=0.930). The Apo B/Apo A-I ratio as an atherogenic index significantly decreased in both treatments (from 0.67±0.16 to 0.61±0.14 with bezafibrate treatment [p<0.001] and from 0.68±0.18 to 0.58±0.11 with pemafibrate treatment [p<0.001]), and the %Change was significantly greater with pemafibrate treatment than with bezafibrate treatment (−8.0%±15.6% vs. −14.9%±14.1%, p=0.002). Serum RemL-C levels significantly decreased in both treatments (p<0.001 in both), and the %Change was significantly greater with pemafibrate treatment than in the bezafibrate treatment (p<0.001). Serum Apo B-48 levels significantly decreased in both treatments (p<0.001 in both), but the %Change was not significantly different between the two treatments (p=0.620). No significant change in the serum Lp(a) levels was found between baseline and the study endpoint with bezafibrate and pemafibrate treatments.
Effects on Glucose–Insulin Metabolism
Table 3 shows the change or %Change in glucose and insulin resistance-related markers during the 24-week treatment with bezafibrate or pemafibrate. The plasma glucose and serum insulin levels and HOMA-IR significantly decreased in both treatments (glucose, p=0.003 and 0.010 in the bezafibrate and pemafibrate treatment, respectively; insulin, p=0.003 and 0.008 in the bezafibrate and pemafibrate treatment, respectively; HOMA-IR, p<0.001 and p=0.010 in the bezafibrate and pemafibrate treatment, respectively). These decreases were greater with bezafibrate treatment than with pemafibrate treatment, although none of these were statistically significant (glucose, p=0.470; insulin, p=0.860; HOMA-IR, p=0.877).
Table 3. Percent change in glucose- and insulin resistance-related markers from baseline to the study endpoint after the 24-week treatment with bezafibrate or pemafibrate.
| Bezafibrate treatment (n= 60) | Pemafibrate treatment (n= 60) | p value (%Change) | |||
|---|---|---|---|---|---|
| %Change | %Change | ||||
| Glucose (mg/dL) | |||||
| Baseline | 118.4±34.6 | 113.1±23.5 | |||
| 24 weeks | 111.1±31.0‡ | −4.7±14.6 | 108.6±20.8* | −3.1±11.2 | 0.470 |
| HbA1c (%) | |||||
| Baseline | 6.2±0.7 | 6.1±0.7 | |||
| 24 weeks | 6.2±0.8 | −0.3±5.0 | 6.2±0.8 | 1.3±6.0 | 0.101 |
| Insulin (μIU/mL) | |||||
| Baseline | 14.3±14.9 | 12.4±9.9 | |||
| 24 weeks | 9.8±8.1‡ | −10.1±55.3 | 9.9±6.9† | −6.5±49.3 | 0.860 |
| HOMA-IR | |||||
| Baseline | 4.4±4.7 | 3.7±3.8 | |||
| 24 weeks | 3.0±3.6§ | −12.0±59.8 | 2.7±2.1* | −7.8±53.3 | 0.877 |
| Adiponectin (μg/mL) | |||||
| Baseline | 6.4±2.7 | 6.4±2.8 | |||
| 24 weeks | 7.2±3.7§ | 12.3±18.1 | 6.3±2.6 | 3.2±35.2 | 0.042 |
| Leptin (ng/mL) | |||||
| Baseline | 16.1±10.9 | 16.3±11.7 | |||
| 24 weeks | 15.7±11.7 | 2.3±34.0 | 14.9±14.3† | −3.3±37.7 | 0.215 |
Data are presented as mean±standard deviation. *p<0.05; †p<0.01; ‡p<0.005; §p<0.001 vs. baseline value. HbA1c, hemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance.
To examine the effect of bezafibrate or pemafibrate on adipose tissue metabolism, serum adiponectin and leptin levels were determined before and after treatment. Serum adiponectin levels significantly increased with bezafibrate treatment (p<0.001) but not with pemafibrate treatment (p=0.496). The %Change in serum adiponectin levels was significantly greater with bezafibrate treatment than with the pemafibrate treatment (p=0.042). Serum leptin levels significantly decreased with pemafibrate treatment (p<0.01) but not with bezafibrate treatment (p=0.263). No significant difference in the %Change in serum leptin levels was found between the two treatments (p=0.215).
Effects on Liver and Kidney Function
As shown in Table 4 , serum ALT and γ-GT levels significantly decreased in both treatments (ALT, p=0.003 and p<0.001 in the bezafibrate and pemafibrate treatment, respectively; γ-GT, p<0.001 in both), and the %Change was significantly greater with pemafibrate treatment than with bezafibrate treatment (ALT, p=0.048; γ-GT, p=0.025). Although the serum creatinine levels significantly increased in both treatments (p<0.001 in both), the %Change was significantly lower with pemafibrate treatment than with bezafibrate treatment (p<0.001). The eGFR significantly decreased in both treatments (p<0.001), but the %Change was significantly lower with pemafibrate treatment than with bezafibrate treatment (p<0.001).
Table 4. Percent change in CK, liver enzymes, and parameters of kidney function from baseline to the study endpoint after the 24-week treatment with bezafibrate or pemafibrate.
| Bezafibrate treatment (n= 60) | Pemafibrate treatment (n= 60) | p value (%Change) | |||
|---|---|---|---|---|---|
| %Change | %Change | ||||
| CK (U/L) | |||||
| Baseline | 118.5±60.9 | 117.2±70.4 | |||
| 24 weeks | 128.2±95.0 | 13.7±72.2 | 114.2±69.7 | 8.6±59.2 | 0.672 |
| AST (U/L) | |||||
| Baseline | 25.7±8.7 | 27.1±10.2 | |||
| 24 weeks | 27.3±11.4 | 8.4±33.8 | 25.8±10.7 | −0.5±30.6 | 0.051 |
| ALT (U/L) | |||||
| Baseline | 29.7±15.0 | 32.0±15.2 | |||
| 24 weeks | 26.7±20.3‡ | −10.6±41.3 | 23.2±13.3§ | −21.9±36.5 | 0.048 |
| γ-GT (U/L) | |||||
| Baseline | 60.7±56.0 | 66.2±72.3 | |||
| 24 weeks | 39.7±45.7§ | −33.1±30.3 | 31.4±26.2§ | −43.5±17.2 | 0.025 |
| Creatinine (mg/dL) | |||||
| Baseline | 0.88±0.19 | 0.89±0.20 | |||
| 24 weeks | 1.02±0.27§ | 15.5±13.8 | 0.94±0.22§ | 5.72±11.3 | <0.001 |
| eGFR (mL/min/1.73 m2) | |||||
| Baseline | 70.1±15.1 | 69.5±16.6 | |||
| 24 weeks | 61.0±16.0§ | −13.3±11.5 | 65.6±15.6§ | −5.1±10.3 | <0.001 |
Data are presented as mean±standard deviation. ‡p<0.005; §p<0.001 vs. baseline value. CK, creatine kinase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transferase; eGFR, estimated glomerular filtration rate.
When the patients were divided into two groups according to the baseline eGFR (non-CKD group, ≥ 60 mL/min/1.73 m2, n=43; CKD group, <60 mL/min/1.73 m2, n=17), the %Change in eGFR was greater in the CKD group than in the non-CKD group (p<0.001) with bezafibrate treatment. Conversely, no significant difference in the %Change in eGFR was found between the non-CKD and CKD groups with pemafibrate treatment (p=0.775) ( Table 5 ) .
Table 5. Comparison of the non-CKD and CKD groups in eGFR reduction from baseline to the study endpoint after the 24-week treatment with bezafibrate or pemafibrate.
| Non-CKD group (n= 43) | CKD group (n= 17) | p value (%Change) | |||
|---|---|---|---|---|---|
| %Change | %Change | ||||
| Bezafibrate treatment | |||||
| Baseline | 76.3±12.4 | 54.3±7.9 | |||
| 24 weeks | 67.6±13.4§ | −11.3±11.5 | 44.2±7.9§ | −18.4±9.7 | 0.010 |
| Pemafibrate treatment | |||||
| Baseline | 76.4±14.1 | 59.0±6.6 | |||
| 24 weeks | 72.1±13.1‡ | −4.9±10.5 | 53.5±6.3* | −9.3±9.7 | 0.775 |
Data are presented as mean±standard deviation. *p<0.05; ‡p<0.005; §p<0.001 vs. baseline value. eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease.
Safety
As shown in Table 6 , the incidence rates of AEs were 37% (n=22) and 43% (n=26) with bezafibrate and pemafibrate treatments, respectively. Treatment-related AEs occurred in one patient in each treatment group: thickening of the gallbladder with bezafibrate treatment and hot flash with pemafibrate treatment. In the bezafibrate treatment group, SAEs occurred in five patients, of which two were related to the treatment: worsening renal function and acute cholecystitis. Conversely, in the pemafibrate treatment group, SAEs occurred in six patients, but none were related to the treatment. There were no treatment discontinuations because of AEs or SAEs.
Table 6. Safety assessment because of adverse events and abnormal laboratory values during the study.
| Bezafibrate treatment (n= 60) | Pemafibrate treatment (n= 60) | |
|---|---|---|
| Total AEs | 22 (37) | 26 (43) |
| Treatment-related AEs | 1 (2) | 1 (2) |
| Discontinuation due to AEs | 0 (0) | 0 (0) |
| SAEs | 5 (8) | 6 (10) |
| Treatment-related SAEs | 0 (0) | 0 (0) |
| Discontinuation due to SAEs | 0 (0) | 0 (0) |
| AEs of clinical interest | ||
| CK levels of >5×ULN | 1 (2) | 0 (0) |
| AST and/or ALT levels of >3×ULN | 0 (0) | 0 (0) |
| Increase in the creatinine levels of ≥ 0.5 mg/day and/or 25% | 14 (23) ‡ | 3 (5) |
| Symptoms related to rhabdomyolysis/myopathy | 0 (0) | 0 (0) |
| Symptoms related to gallbladder disease | 1 (2) | 0 (0) |
| Symptoms related to hepatic disease | 0 (0) | 0 (0) |
| Symptoms related to pancreatic disease | 0 (0) | 0 (0) |
| Symptoms related to gastrointestinal disease | 0 (0) | 0 (0) |
Data are presented as n (%). ‡p<0.005 vs. pemafibrate treatment. AEs, adverse events; SAEs, serious adverse events; CK, creatine kinase; ULN, upper limit of normal; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
One patient (2%) showed a >5-fold increase (from 103 U/L to 615 U/L) in serum CK level (ULN: 287 U/L) when treated with bezafibrate. No patients showed a >3-fold increase in serum AST and/or ALT levels in both treatments. The incidence of increased creatinine levels was significantly higher with bezafibrate treatment than with pemafibrate treatment (14 vs. 3 of 60 patients, p=0.004). Rhabdomyolysis/myopathy and hepatic, pancreatic, and gastrointestinal diseases were not observed in this 24-week study.
Discussion
This crossover study demonstrated that pemafibrate dose of 0.2 mg/day significantly reduced fasting serum TG levels compared with bezafibrate dose of 400 mg/day for 24 weeks in patients with statin-treated CAD with dyslipidemia and high TG and low HDL-C levels. Compared with bezafibrate, pemafibrate significantly increased serum Apo A-I levels and significantly decreased serum Apo B levels. Pemafibrate also improved glucose metabolism. These effects of pemafibrate on the lipid and glucose profiles suggest that it is more antiatherosclerotic than bezafibrate. Pemafibrate also significantly decreased serum ALT and γ-GT levels compared with bezafibrate. The increase in creatinine levels was significantly smaller in the pemafibrate treatment group than in the bezafibrate treatment group. Regarding safety, the use of pemafibrate was safer than that of bezafibrate in terms of liver and renal function.
In this study, the impact of pemafibrate and bezafibrate treatment on lipid and glucose metabolism was compared in patients with statin-treated CAD with dyslipidemia. To our knowledge, this is the first study to compare pemafibrate and bezafibrate. The TG-lowering effect of pemafibrate in this study (%Change from baseline, −46.1%) is consistent with that reported in the previous phase III clinical trial of pemafibrate (−46.2%) 11) . Likewise, pemafibrate was significantly more effective than bezafibrate in increasing Apo A-I levels and reducing Apo B levels. Because the Apo B/Apo A-I ratio is an atherogenic lipoprotein marker for ASCVD risk 19) , pemafibrate might be superior to bezafibrate in terms of reducing ASCVD events. Although the large-scale clinical trial PROMINENT study (NCT03071692) 20) has been stopped prematurely, further examination and discussion are required to evaluate the effect of pemafibrate on the prevention of ASCVD.
In this study, the increase in serum LDL-C levels by both fibrate treatments was consistent with the results of previous studies 15 , 21 , 22) , and this increase may be attributable to the conversion of very-low-density lipoprotein particles to LDL particles 23) . A previous study analyzed the cholesterol content of the subclasses of LDL particles and found that the LDL particle size was significantly increased in patients treated with fibrates 22) . Because the LDL-C/Apo B ratio represents the LDL-particle size, an increase in this marker in this study suggests that the fibrate treatment resulted in a shift in the LDL particle size from smaller, dense pattern to a larger, more buoyant pattern.
Postprandial hyperlipidemia, which plays a role in the progression or vulnerability of atheromatous plaques 24) , is characterized by an excess of Apo B-48–containing TG-rich lipoprotein. Our results are consistent with those of previous studies showing a reduction in serum Apo B-48 levels in response to fibrate treatment 15 , 21 , 22 , 25) . A previous clinical trial showed that pemafibrate was superior to fenofibrate in the improvement of postprandial lipid profile 15 , 22) . In this study, serum Apo B-48 levels markedly decreased after both pemafibrate and bezafibrate treatments, although the difference was not statistically significant. In addition, treatment with pioglitazone, a PPAR-γ agonist, has been shown to suppress postprandial changes in serum TG levels in overweight patients with CAD 26) . Because bezafibrate is a stronger PPAR-γ agonist than permafibrate 27) , the improvement of postprandial lipid profile by bezafibrate may be partly due to the stimulation of PPAR-γ in addition to that of PPAR-α.
Little is known about the effects of pemafibrate on serum adipocytokine levels in patients with dyslipidemia. To our knowledge, this is the first study to demonstrate the differences between pemafibrate and bezafibrate in their effects on circulating metabolic parameters related to insulin resistance in patients with dyslipidemia. In this study, an increase in serum adiponectin levels was observed with bezafibrate treatment, whereas no significant change was observed with pemafibrate treatment. These findings are consistent with those of previous studies linking bezafibrate treatment to an increase in serum adiponectin levels 28) . This increase by bezafibrate may be due to the activation of PPAR-γ rather than that of PPAR-α because PPAR-γ activation is strongly associated with control of various genes involved in adipose tissue metabolism 29) .
As for the effect of pemafibrate treatment on glucose metabolism, previous studies reported that pemafibrate improved both FPG and insulin sensitivity 21 , 30 , 31) , which is consistent with this study results showing that pemafibrate produced favorable effects on glucose markers, such as plasma glucose and serum insulin levels and HOMA-IR. In this study, compared with bezafibrate, the stronger PPAR-α agonist pemafibrate was noninferior in improving glucose metabolism. In addition to the amelioration of insulin sensitivity, pemafibrate has been reported to have the potential to increase the capacity of insulin secretion in the pancreas and glucose uptake in the liver 32 - 34) .
Regarding the effect of pemafibrate treatment on liver function, there are several reports linking pemafibrate to an improvement in the levels of hepatic enzymes, such as ALT and γ-GT in patients with dyslipidemia 11 , 15 , 21 , 25) , which is consistent with this study results. The mechanism of fibrate-induced improvement in liver dysfunction is attributed to the modulation of lipid turnover and energy metabolism in the liver 35 , 36) . A previous study demonstrated that pemafibrate decreased liver fat and suppressed hepatic fibrogenesis in mouse models of nonalcoholic steatohepatitis (NASH) 35) . Particularly, diseases with abnormal hepatic lipid metabolism, such as nonalcoholic fatty liver disease (NAFLD) and NASH, are closely associated with PPAR-α activation 36 , 37) . Our results showing the superiority of pemafibrate to bezafibrate in improving liver function suggest that the use of a more selective PPAR-α stimulant could be an effective treatment strategy for patients with NAFLD or NASH 38) . Although the patients in this study had no liver dysfunction, greater reductions in hepatic enzyme levels were observed in patients with higher baseline reference values in the clinical trial of pemafibrate 31) .
We also verified the safety aspect of pemafibrate associated with renal function over bezafibrate. As fibrates are known to aggravate its test values, such as serum creatinine levels and eGFR 39) , both bezafibrate and pemafibrate increased serum creatinine and decreased the eGFR. However, these changes were much smaller with pemafibrate treatment than with bezafibrate treatment. Bezafibrate is excreted from the kidney, whereas pemafibrate is primarily metabolized in the liver and then excreted into the bile 11 , 16) . The precise mechanism for the deterioration of renal function remains unknown; however, the metabolic processes of fibrates may be relevant to the etiology of the condition. Interestingly, the effects of bezafibrate treatment appear to differ from those of pemafibrate treatment in patients with renal impairment. In the comparison of patients with and without CKD, there were no significant differences in the %Change of the eGFR during the pemafibrate treatment, which was consistent with the findings of a long-term phase III trial of pemafibrate 16) . No incidence of SAEs related to renal dysfunction was observed with pemafibrate treatment, and the incidence of AEs due to increased creatinine levels was lower with pemafibrate treatment than with bezafibrate treatment. All enrolled patients had received statin treatment to reduce ASCVD events. Rhabdomyolysis and myopathy are also a major concern when using a statin–fibrate combination, especially in patients with CKD 40) . A serum CK level of >5×ULN was observed with bezafibrate treatment but not in the pemafibrate treatment, and none of the patients had symptoms related to rhabdomyolysis/myopathy. Therefore, our results and those of previous clinical studies 11 , 15 , 16 , 30) suggest that pemafibrate would be a safer therapeutic option in a wide range of patients with dyslipidemia, even in those with CKD, although the number of patients with CKD was limited in this study.
Pemafibrate, fenofibrate, and bezafibrate are commonly used as fibrates for patients with hypertriglyceridemia in real-world clinical settings. This study and the phase III clinical trial 11) suggest that pemafibrate is the most beneficial fibrate for these patients.
This study has several limitations. First, it was conducted at a single facility with a relatively low number of patients; thus, statistical biases may have been introduced, although our results were statistically significant in several endpoints. Second, most of the patients were Japanese men; therefore, the sex-related effects of fibrate treatment on lipid and glucose metabolism remain unclear. Furthermore, whether the findings from Japanese patients would be true for patients from different countries is unknown. Third, the maximum dose of pemafibrate of 0.4 mg/day was not administered to any patient; however, in Japan, 0.2 mg/day is a usual therapeutic dose for adult patients in a real-world setting.
Conclusion
In patients with CAD with hypertriglyceridemia, pemafibrate dose of 0.2 mg/day resulted in a significant reduction in serum TG levels compared with bezafibrate dose of 400 mg/day. The study results also demonstrated the superiority of pemafibrate over bezafibrate in terms of other lipid and glucose markers and liver enzymes. The good safety profile of pemafibrate, including mild deterioration of renal parameters and fewer AEs related to increased serum creatinine levels, may provide a promising treatment strategy for a wide range of patients including those with CKD.
Acknowledgements
The authors gratefully acknowledge Y. Sato, a medical clerk for data management with this study. The authors would also like to thank Enago (www.enago.jp) for English language editing.
Conflict of Interest
The authors declare no conflict of interests.
Notice of Grant Support
This study received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contribution Statement
A.N., Y.K., K.S., and H.E. conducted the research and performed clinical data analysis; A.N., H.S., M.K., M.M., and M.K. performed clinical work and data analysis; A.N. and H.E. designed the research; and A.N. wrote the manuscript.
References
- 1).Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, and Collins R: Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet, 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Tall AR, Thomas DG, Conzales-Cabodevilla AG, and Goldberg IJ: Addressing dyslipidemic risk beyond LDL-cholesterol. J Clin Invest, 2022; 132: e148559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Budoff M: Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol, 2016; 118: 138-145 [DOI] [PubMed] [Google Scholar]
- 4).Irawati D, Mamo JC, Soares MJ, Slivkoff-Clark KM, and James AP: Hypertriglyceridemic subjects exhibit an accumulation of small dense chylomicron particles in the fasting state. Atherosclerosis, 2015; 243: 236-241 [DOI] [PubMed] [Google Scholar]
- 5).Dallinga-Thie GM, Kroon J, Boren J, and Chapman MJ: Triglyceride-rich lipoproteins and remnants: targets for therapy? Curr Cardiol Rep, 2016; 18: 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, Ariga R, Thomas S, Francis J, Rodgers C, Clarke W, Sabharwal N, Antoniades C, Schneider J, Robson M, Clarke K, Karamitsos T, Rider O, and Neubauer S: Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol, 2016; 68: 53-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida Y, Rakugi H, Wakatsuki A, Yamashita S, and Committee for epidemiology and clinical management of atherosclerosis: Japan atherosclerosis society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O, and ESC Scientific Document Group: 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: The Task Force for the management of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J, 2019; 41: 111-188 [Google Scholar]
- 9).Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Derranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, and Yeboah J: 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019; 139: e1082-e1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Yamashita S, Masuda D, and Mstsuzaka Y: Pemafibrate, a new selective PPARα modulator: drug concept and its clinical applications for dyslipidemia and metabolic diseases. Curr Atheroscler Rep, 2020; 22: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Ishibashi S, Arai H, Yokote K, Araki E, Suganami H, Yamashita S, and K-877 Study Group: Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J Clin Lipidol, 2018; 12: 173-184 [DOI] [PubMed] [Google Scholar]
- 12).Teramoto T, Shirai K, Daida H, and Yamada N: Effects of bezafibrate on lipid and glucose metabolism in dyslipidemic patients with diabetes: the J-BENEFIT study. Cardiovasc Diabetol, 2012; 11:29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).The BIP Study Group: Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation, 2000; 102: 21-27 [DOI] [PubMed] [Google Scholar]
- 14).Yamagishi K, and Iso H: The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol Health, 2017; 39: e2017003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S, and K-877 Study Group: Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: a multicenter, placebo-controlled, double-blind, randomized trial. J Atheroscler Thromb, 2018; 25: 521-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Yokote K, Yamashita S, Arai H, Araki E, Suganami H, Ishibashi S, and K-877 Study Group: Long-term efficacy and safety of pemafibrate, a novel selective peroxisome proliferator-activated receptor-α modulator (SPPARMα), in dyslipidemic patients with renal impairment. Int J Mol Sci, 2019; 20: 706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, and Hishida A: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 18).Haffner SM, Miettinen H, and Stern MP: The homeostasis model in the San Antonio Heart Study. Diabetes Care, 1997; 20: 1087-1092 [DOI] [PubMed] [Google Scholar]
- 19).Walldius G, Jungner I, Aastveit AH, Holme I, Furberg CD, and Sniderman AD: The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin Chem Lab Med, 2004; 42: 1355-1363 [DOI] [PubMed] [Google Scholar]
- 20).Pradhan AD, Paynter NP, Everett BM, Glynn RJ, Amarenco P, Elam M, Ginsberg H, Hiatt WR, Ishibashi S, Koenig W, Nordestgaard BG, Fruchart JC, Libby P, and Ridker PM: Rationale and design of the pemafibrate to reduce cardiovascular outcomes by reducing triglycerides in patients with diabetes (PROMINENT) study. Am Heart J, 2018; 206: 80-93 [DOI] [PubMed] [Google Scholar]
- 21).Ishibashi S, Yamashita S, Arai H, Araki E, Yokote K, Suganami H, Fruchart JC, Kodama T, and K-877-04 Study Group: Effects of K-877, a novel selective PPARalpha modulator (SPPARMalpha), in dyslipidaemic patients: a randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis, 2016; 249: 36-43 [DOI] [PubMed] [Google Scholar]
- 22).Yamashita S, Arai H, Yokote K, Araki E, Matsushita M, Nojima T, Suganami H, and Ishibashi S: Efficacy and safety of pemafibrate, a novel selective peroxisome proliferation-activated receptor α modulator (SPPARMα): Pooled analysis of phase 2 and 3 studies in dyslipidemic patients with or without statin combination. Int J Mol Sci, 2019; 20: 5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Sigurdsson G, Nicoll A, and Lewis B: Conversion of very low density lipoprotein to low density lipoprotein. A metabolic study of apolipoprotein B kinetics in human subjects. J Clin Invest, 1975; 56: 1481-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Masuda D, and Yamashita S: Postprandial hyperlipidemia and remnant lipoproteins. J Atheroscler Thromb, 2017; 24: 95-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Arai H, Yamashita S, Yokote K, Araki E, Suganami H, Ishibashi S, and K-877 Study Group: Efficacy and safety of K-877, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), in combination with statin treatment: two randomized, double-blind, placebo-controlled clinical trials in patients with dyslipidaemia. Atherosclerosis, 2017; 261: 144-152 [DOI] [PubMed] [Google Scholar]
- 26).Mieszczanska H, Kaba NK, Francis CW, Gerich JE, Dodis R, Schwarz KQ, Phipps RP, Smith BH, Lee M, Messing S, and Taubman MB: Effects of pioglitazone on fasting and postprandial levels of lipid and hemostatic variables in overweight non-diabetic patients with coronary artery disease. J Thromb Haemost, 2007; 5: 942-949 [DOI] [PubMed] [Google Scholar]
- 27).Tenenbaum A, Motro M, Fisman EZ, Schwammenthal E, Adler Y, Goldenberg I, Leor J, Boyko V, Mandelzweig L, and Behar S: Peroxisome proliferator-activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation, 2004; 109: 2197-2202 [DOI] [PubMed] [Google Scholar]
- 28).Noguchi T, Kobayashi J, Yagi K, Nohara A, Yamaaki N, Sugihara M, Ito N, Oka R, Kawashiri MA, Tada H, Takata M, Inazu A, Yamagishi M, and Mabuchi H: Comparison of effects of bezafibrate and fenofibrate on circulating proprotein convertase subtilisin/kexin type 9 and adipocytokine levels in dyslipidemic subjects with impaired glucose tolerance or type 2 diabetes mellitus: results from a crossover study. Artheriosclerosis, 2011; 217: 165-170 [DOI] [PubMed] [Google Scholar]
- 29).Choi SS, Park J, and Choi JH: Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Rep, 2014; 47: 599-608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Araki E, Yamashita S, Arai H, Yokote K, Satoh J, Inoguchi T, Nakamura J, Maegawa H, Yoshioka N, Tanizawa Y, Watada H, Suganami H, and Ishibashi S: Effects of pemafibrate, a novel selective PPARα modulator, on lipid and glucose metabolism in patients with type 2 diabetes and hypertriglyceridemia: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care, 2018; 41: 538-546 [DOI] [PubMed] [Google Scholar]
- 31).Yokote K, Yamashita S, Arai H, Araki E, Matsushita M, Nojima T, Suganami H, and Ishibashi S: Effects of pemafibrate on glucose metabolism markers and liver function tests in patients of six phase 2 and phase 3 randomized double-blind placebo-controlled clinical trials. Cardiovasc Diabetol, 2021; 20: 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Araki M, Nakagawa Y, Oishi A, Han SI, Wang Y, Kumagai K, Ohno H, Mizunoe Y, Iwasaki H, Sekiya M, Matsuzaka T, and Shimano H: The peroxisome proliferator-activated receptor α (PPARα) agonist pemafibrate protects against diet-induced obesity in mice. Int J Mol Sci, 2018; 19: 2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Dong T, Lyu J, Imachi H, Kobayashi T, Fukunaga K, Sato S, Ibata T, Yoshimoto T, Yonezaki K, Iwama H, Zhang G, and Murao K: Selective peroxisome proliferator-activated receptor-α modulator K-877 regulates the expression of ATP-binding cassette transporter A1 in pancreatic beta cells. Eur J Pharmacol, 2018; 838: 78-84 [DOI] [PubMed] [Google Scholar]
- 34).Matsuba I, Matsuba R, Ishibashi S, Yamashita S, Arai H, Yokote K, Suganami H, and Araki E: Effects of a novel selective peroxisome proliferator-activated receptor-α modulator, pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance. J Diabetes Investig, 2018; 9: 1323-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Honda Y, Kessoku T, Ogawa Y, Tomeno W, Imajo K, Fujita K, Yoneda M, Takizawa T, Saito S, Nagashima Y, and Nakajima A: Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci Rep, 2017; 7: 42477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, and Leclercq I: Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology, 2003; 38: 123-132 [DOI] [PubMed] [Google Scholar]
- 37).Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, Endo H, Takahashi H, Inamori M, Kobayashi N, Kirikoshi H, Kubota K, Saito S, and Nakajima A: Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology, 2009; 50: 772-780 [DOI] [PubMed] [Google Scholar]
- 38).Nakajima A, Eguchi Y, Yoneda M, Imajo K, Tamaki N, Suganami H, Nojima T, Tanigawa R, Iizuka M, Iida Y, and Loomba R: Randomised clinical trial: pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther, 2021; 54: 1263-1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Davidson MH, Armani A, McKenney JM, and Jacobson TA: Safety considerations with fibrate therapy. Am J Cardiol, 2007; 99: 3C-18C [DOI] [PubMed] [Google Scholar]
- 40).Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ, and Platt R: Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA, 2004; 292: 2585-2590 [DOI] [PubMed] [Google Scholar]